High-Pressure Microfluidic Homogenization Improves the Stability and Antioxidant Properties of Coenzyme Q10 Nanoliposomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Preparation of Liposomes
2.3. Characterization of Liposomes
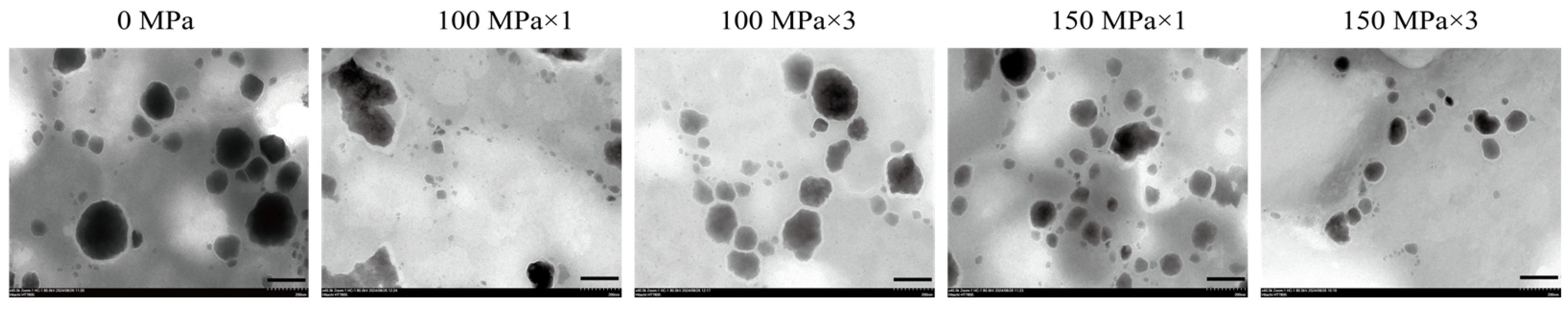
2.3.1. Transmission Electron Microscope (TEM) Observation
2.3.2. Dynamic Light Scattering (DLS) Measurement
2.3.3. Entrapment Efficiency (EE)
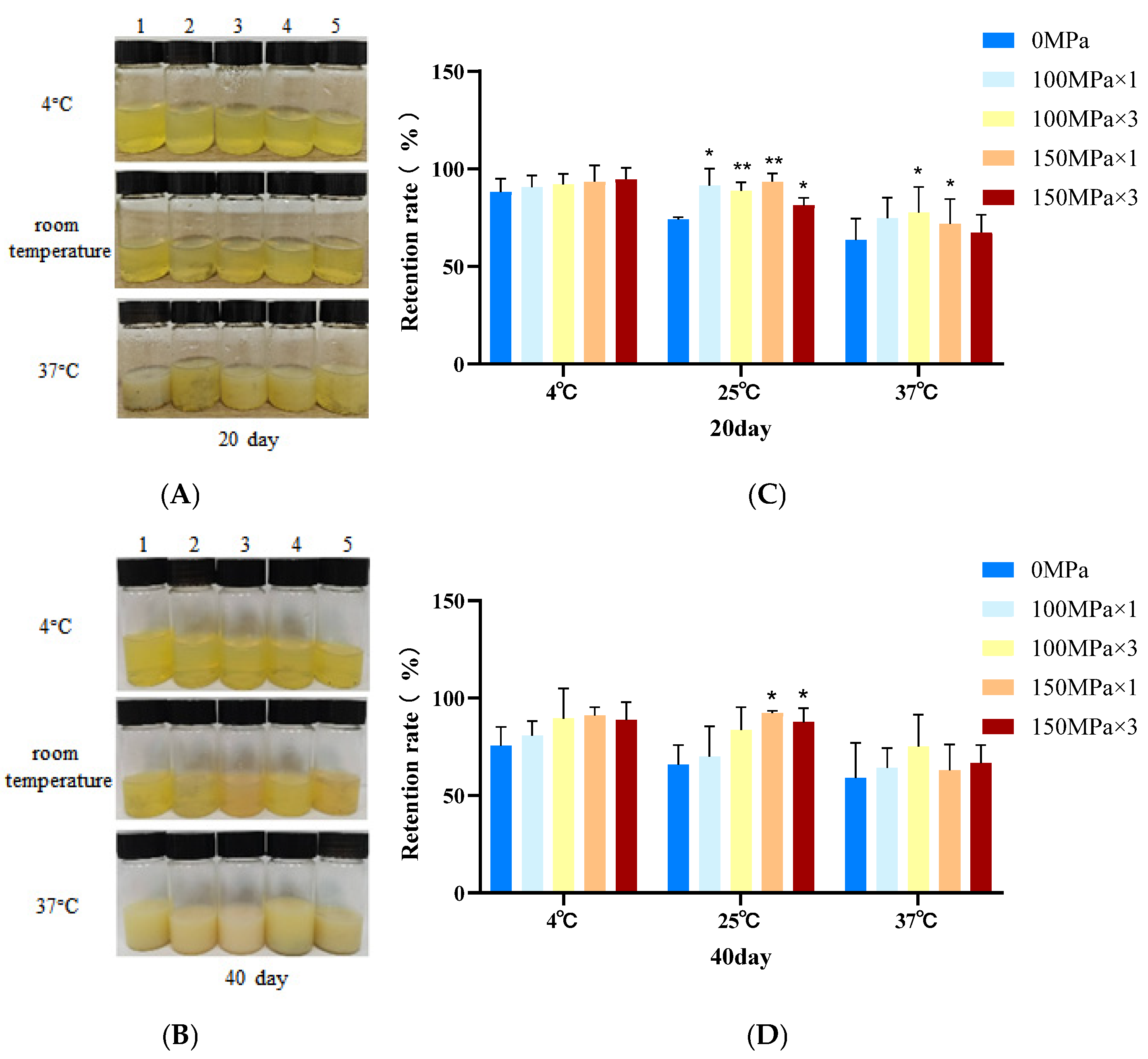
2.4. Storage Stability
2.5. Cell Culture and Treatment
2.5.1. Cell Viability Assay
2.5.2. Reactive Oxygen Species (ROS) Assay
2.6. Statistical Analysis
3. Results and Discussion
3.1. Microstructure of Coenzyme Q10 Nanoliposomes
3.2. Particle Size Distribution
3.3. Zeta Potential
3.4. Liposome Encapsulation Efficiency
3.5. Liposome Storage Stability
3.6. Antioxidant Properties in HepG2 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Molyneux, S.L.; Young, J.M.; Florkowski, C.M.; Lever, M.; George, P.M. Coenzyme Q10: Is there a clinical role and a case for measurement? Clin. Biochem. Rev. 2008, 29, 71–82. [Google Scholar] [PubMed]
- Crane, F.L. Biochemical functions of Coenzyme Q10. J. Am. Coll. Nutr. 2001, 20, 591–598. [Google Scholar] [CrossRef]
- Hargreaves, I.P. Ubiquinone: Cholesterol’s reclusive cousin. Ann. Clin. Biochem. 2003, 40, 207–218. [Google Scholar] [CrossRef]
- Di Lorenzo, A.; Iannuzzo, G.; Parlato, A.; Cuomo, G.; Testa, C.; Coppola, M.; D’Ambrosio, G.; Oliviero, D.A.; Sarullo, S.; Vitale, G.; et al. Clinical evidence for Q10 coenzyme supplementation in heart failure: From energetics to functional improvement. J. Clin. Med. 2020, 9, 1266. [Google Scholar] [CrossRef]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in cardiovascular and metabolic diseases: Current state of the problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Maffioli, P. Coenzyme Q10 liquid supplementation in dyslipidemic subjects with statin-related clinical symptoms: A double-blind, randomized, placebo-controlled study. Drug. Des. Devel. Ther. 2019, 13, 3647–3655. [Google Scholar] [CrossRef]
- Singh, R.B.; Niaz, M.A.; Rastogi, S.S.; Shukla, P.K.; Thakur, A.S. Effect of hydrosoluble Coenzyme Q10 on blood pressures and insulin resistance in hypertensive patients with coronary artery disease. J. Hum. Hypertens. 1999, 13, 203–208. [Google Scholar] [CrossRef]
- Kaikkonen, J.; Nyyssönen, K.; Tuomainen, T.P.; Ristonmaa, U.; Salonen, J.T. Determinants of plasma Coenzyme Q10 in humans. FEBS Lett. 1999, 443, 163–166. [Google Scholar] [CrossRef]
- Mancini, A.; Festa, R.; Raimondo, S.; Pontecorvi, A.; Littarru, G.P. Hormonal influence on Coenzyme Q(10) levels in blood plasma. Int. J. Mol. Sci. 2011, 12, 9216–9225. [Google Scholar] [CrossRef] [PubMed]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug. Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, T.; Torre, M.; Shao, R.; Zheng, Y.; Wang, D.; Li, X.; Liu, A.; Zhang, W.; Deng, X.; et al. Aromatized liposomes for sustained drug delivery. Nat. Commun. 2023, 14, 6659. [Google Scholar] [CrossRef]
- Wehbe, M.; Malhotra, A.; Anantha, M.; Roosendaal, J.; Leung, A.W.Y.; Plackett, D.; Edwards, K.; Gilabert-Oriol, R.; Bally, M.B. A simple passive equilibration method for loading carboplatin into pre-formed liposomes incubated with ethanol as a temperature dependent permeability enhancer. J. Control. Release 2017, 252, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Rodwattanagul, S.; Sasarom, M.; Riangjanapatee, P.; Anuchapreeda, S.; Okonogi, S. Antioxidant activity of sophora exigua and liposome development of its powerful extract. Drug Discov. Ther. 2024, 18, 150–159. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 155, 102–122. [Google Scholar] [CrossRef]
- Liu, W.; Liu, J.; Xie, M.; Liu, C.; Liu, W.; Wan, J. Characterization and high-pressure microfluidization-induced activation of polyphenoloxidase from Chinese pear (Pyrus pyrifolia Nakai). J. Agric. Food Chem. 2009, 57, 5376–5380. [Google Scholar] [CrossRef]
- Jafari, S.M.; He, Y.; Bhandari, B. Nano-emulsion production by sonication and microfluidization—A comparison. Int. J. Food Prop. 2006, 9, 475–485. [Google Scholar] [CrossRef]
- Schultz, S.; Wagner, G.; Urban, K.; Ulrich, J. High-pressure homogenization as a process for emulsion formation. Chem. Eng. Technol. 2004, 27, 361–368. [Google Scholar] [CrossRef]
- Tran, M.; Voronin, G.L.; Roberts, R.F.; Coupland, J.N.; Ziegler, G.R.; Harte, F.M. The effect of high-pressure jet processing on cocoa stability in chocolate milk. J. Dairy Sci. 2021, 104, 11432–11441. [Google Scholar] [CrossRef]
- Tarafdar, A.; Kumar, Y.; Kaur, B.P.; Badgujar, P.C. High-pressure microfluidization of sugarcane juice: Effect on total phenols, total flavonoids, antioxidant activity, and microbiological quality. J. Food Process. Preserv. 2021, 45, e15428. [Google Scholar] [CrossRef]
- Koley, T.K.; Nishad, J.; Kaur, C.; Su, Y.; Sethi, S.; Saha, S.; Sen, S.; Bhatt, B.P. Effect of high-pressure microfluidization onnutritional quality of carrot (Daucus carota L.) juice. J. Food Sci. Technol. 2020, 57, 2159–2168. [Google Scholar] [CrossRef]
- Romano, E.; Palladino, R.; Cannavale, M.; Lamparelli, E.P.; Maglione, B. Enhanced stability of oral vitamin C delivery: A novel large-scale method for liposomes production and encapsulation through dynamic high-pressure microfluidization. Nanomaterials 2024, 14, 516. [Google Scholar] [CrossRef]
- Xia, S.; Xu, S.; Zhang, X. Optimization in the preparation of Coenzyme Q10 nanoliposomes. J. Agric. Food. Chem. 2006, 54, 6358–6366. [Google Scholar] [CrossRef]
- Wagner, A.; Vorauer-Uhl, K. Liposome technology for industrial purposes. J. Drug Deliv. 2011, 2011, 591325. [Google Scholar] [CrossRef]
- Furini, S.; Domene, C. Computational studies of transport in ion channels using metadynamics. Biochim. Biophys. Acta 2016, 1858, 1733–1740. [Google Scholar] [CrossRef]
- Yoo, J.W.; Chambers, E.; Mitragotri, S. Factors that control the circulation time of nanoparticles in blood: Challenges, solutions and future prospects. Curr. Pharm. Des. 2010, 16, 2298–2307. [Google Scholar] [CrossRef]
- Shah, N.; Mohammad, A.S.; Saralkar, P.; Sprowls, S.A.; Vickers, S.D.; John, D.; Tallman, R.M.; Lucke-Wold, B.P.; Jarrell, K.E.; Pinti, M.; et al. Investigational chemotherapy and novel pharmacokinetic mechanisms for the treatment of breast cancer brain metastases. Pharmacol. Res. 2018, 132, 47–68. [Google Scholar] [CrossRef]
- Giambelluca, M.; Markova, E.; Louet, C.; Steinkjer, B.; Sundset, R.; Skalko-Basnet, N.; Hak, S. Liposomes—Human phagocytes interplay in whole blood: Effect of liposome design. Nanomedicine. 2023, 54, 102712. [Google Scholar] [CrossRef]
- Maritim, S.; Boulas, P.; Lin, Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int. J. Pharm. 2021, 592, 120051. [Google Scholar] [CrossRef]
- Maa, Y.F.; Hsu, C.C. Performance of sonication and microfluidization for liquid–liquid emulsification. Pharm. Dev. Technol. 1999, 4, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Plessis, J.; Ramachandran, C.; Weiner, N.; Muller, D.G. The influence of lipid composition and lamellarity of liposomes on the physical stability of liposomes upon storage. Int. J. Pharm. 1996, 127, 273–278. [Google Scholar] [CrossRef]
- Dong, Y.; Feng, S.S. Methoxy poly (ethylene glycol)-poly (lactide) (MPEG-PLA) nanoparticles for controlled delivery of anticancer drugs. Biomaterials 2004, 25, 2843–2849. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Elsayed, M.M.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef]
- Scherphof, G.L.; Kamps, J.A. The role of hepatocytes in the clearance of liposomes from the blood circulation. Prog. Lipid Res. 2001, 40, 149–166. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, M.L.; Rabasco, A.M. Charged liposomes as carriers to enhance the permeation through the skin. Expert Opin. Drug Deliv. 2011, 8, 857–871. [Google Scholar] [CrossRef]
- Sguizzato, M.; Mariani, P.; Spinozzi, F.; Benedusi, M.; Cervellati, F.; Cortesi, R.; Drechsler, M.; Prieux, R.; Valacchi, G.; Esposito, E. Ethosomes for Coenzyme Q10 cutaneous administration: From design to 3D skin tissue evaluation. Antioxidants 2020, 9, 485. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From bangham to supercritical fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B.; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical applications and potential for image-guided drug delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef]
- García, R.A.; Pantazatos, S.P.; Pantazatos, D.P.; MacDonald, R.C. Cholesterol stabilizes hemifused phospholipid bilayer vesicles. Biochim. Biophys. Acta 2001, 1511, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.N.A.H.; Sykes, B.D.; McElhaney, R.N. Thermotropic phase behavior of model membranes composed of phosphatidylcholines containing dl-methyl anteisobranched fatty acids. 1. Differential scanning calorimetric and phosphorus-31 NMR spectroscopic studies. Biochemistry 1987, 26, 4036–4044. [Google Scholar] [CrossRef]
- Cappellani, M.R.; Perinelli, D.R.; Pescosolido, L.; Schoubben, A.; Cespi, M.; Cossi, R.; Blasi, P. Injectable nanoemulsions prepared by high pressure homogenization: Processing, sterilization, and size evolution. Appl. Nanosci. 2018, 8, 1483–1491. [Google Scholar] [CrossRef]
- Chen, H.W.; Chen, S.D.; Wu, H.T.; Cheng, C.H.; Chiou, C.S.; Chen, W.T. Improvement in curcumin’s stability and release by formulation in flexible nano-liposomes. Nanomaterials 2024, 14, 1836. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, F.; Cheng, D.; Ma, Q.; Wang, W.; Wang, J.; Sun, J. Physical stability of oil-in-water multi-layered Coenzyme Q10 nano-emulsions. Food Chem. 2025, 464, 141860. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, L.; Han, H.K. TPGS-chitosome as an effective oral delivery system for improving the bioavailability of Coenzyme Q10. Eur. J. Pharm. Biopharm. 2015, 89, 339–346. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Tajes, M.; Ramos-Fernández, E.; Weng-Jiang, X.; Bosch-Morató, M.; Guivernau, B.; Eraso-Pichot, A.; Salvador, B.; Fernàndez-Busquets, X.; Roquer, J.; Muñoz, F.J. The blood-brain barrier: Structure, function and therapeutic approaches to cross it. Mol. Membr. Biol. 2014, 31, 152–167. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Z.; Huang, W.; Zhao, X.; Xu, L.; Teng, C.; Li, Y. Hyaluronic acid-decorated lipid nanocarriers as novel vehicles for curcumin: Improved stability, cellular absorption, and anti-inflammatory effects. Food Chem. 2025, 463, 141420. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Wang, Z.; Zhao, X.; Xu, L.; Xu, L.; Teng, C.; Liu, S.; Huang, W.; Li, Y. Advanced targeted curcumin delivery using biodegradable hierarchical microspheres with calcium pectinate matrix and hyaluronic acid moieties for enhancing colitis amelioration. Carbohyd. Polym. 2025, 353, 123273. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, S.; Li, Y.; Feng, J. Recent research advances in delivery systems based on the assembly of egg white proteins: Structure design and applications in the food industry. Food Hydrocolloid. 2024, 153, 110021. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, X.; Wang, J.; Xu, B.; Feng, J.; Huang, W. High-Pressure Microfluidic Homogenization Improves the Stability and Antioxidant Properties of Coenzyme Q10 Nanoliposomes. Biology 2025, 14, 568. https://doi.org/10.3390/biology14050568
Li X, Zhao X, Wang J, Xu B, Feng J, Huang W. High-Pressure Microfluidic Homogenization Improves the Stability and Antioxidant Properties of Coenzyme Q10 Nanoliposomes. Biology. 2025; 14(5):568. https://doi.org/10.3390/biology14050568
Chicago/Turabian StyleLi, Xinyu, Xingyu Zhao, Jing Wang, Baoshun Xu, Jin Feng, and Wuyang Huang. 2025. "High-Pressure Microfluidic Homogenization Improves the Stability and Antioxidant Properties of Coenzyme Q10 Nanoliposomes" Biology 14, no. 5: 568. https://doi.org/10.3390/biology14050568
APA StyleLi, X., Zhao, X., Wang, J., Xu, B., Feng, J., & Huang, W. (2025). High-Pressure Microfluidic Homogenization Improves the Stability and Antioxidant Properties of Coenzyme Q10 Nanoliposomes. Biology, 14(5), 568. https://doi.org/10.3390/biology14050568






