Bolstering CD8+ T Cells’ Antitumor Immunity: A Promising Strategy to Improve the Response to Advanced Prostate Cancer Treatment
Simple Summary
Abstract
1. Introduction
2. Heterogeneity of CD8+ T Cells in PCa
2.1. Evolution and Development of CD8+ T Cells
2.2. Dysfunction of CD8+ T Cells in PCa
2.3. Prognostic Value of CD8+ T Cell Infiltration of Prostate Tumors
2.4. New CD8+ T-Cell Subsets in Prostate Cancer Identified by Single-Cell Omics
3. Factors and Mechanisms for the Dysfunction of CD8+ T Cells in PCa
3.1. Cellular Components in Tumors Contribute to CD8+ T-Cell Dysfunction
3.2. Noncellular Components in Tumors Contribute to CD8+ T-Cell Dysfunction
4. Strategies for Improving CD8+ T-Cell Function in PCa Treatment
4.1. Autologous Cellular Immunotherapy in PCa
4.2. The Microbiome and CD8+ T Cells in PCa
4.3. ICB Therapy in PCa
4.4. Chemotherapy Therapy in PCa
4.5. CAR T-Cell Therapies and Vaccines in PCa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.A.; Marchese, M.; Cole, A.P.; Mahal, B.A.; Friedlander, D.F.; Krimphove, M.; Kilbridge, K.L.; Lipsitz, S.R.; Nguyen, P.L.; Choueiri, T.K.; et al. Geographic Distribution of Racial Differences in Prostate Cancer Mortality. JAMA Netw. Open. 2020, 3, e201839. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.; Oh, H.; Lee, J.A.; Kim, E.J. Prostate cancer risk prediction based on clinical factors and prostate-specific antigen. BMC Urol. 2023, 23, 100. [Google Scholar] [CrossRef]
- Zhu, Y.; Mo, M.; Wei, Y.; Wu, J.; Pan, J.; Freedland, S.J.; Zheng, Y.; Ye, D. Epidemiology and genomics of prostate cancer in Asian men. Nat. Rev. Urol. 2021, 18, 282–301. [Google Scholar] [CrossRef]
- Schaeffer, E.M.; Srinivas, S.; Adra, N.; An, Y.; Barocas, D.; Bitting, R.; Bryce, A.; Chapin, B.; Cheng, H.H.; D’Amico, A.V.; et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2023, 21, 1067–1096. [Google Scholar] [CrossRef]
- Lenis, A.T.; Ravichandran, V.; Brown, S.; Alam, S.M.; Katims, A.; Truong, H.; Reisz, P.A.; Vasselman, S.; Nweji, B.; Autio, K.A.; et al. Microsatellite Instability, Tumor Mutational Burden, and Response to Immune Checkpoint Blockade in Patients with Prostate Cancer. Clin. Cancer Res. 2024, 30, 3894–3903. [Google Scholar] [CrossRef]
- Handy, C.E.; Antonarakis, E.S. Sipuleucel-T for the treatment of prostate cancer: Novel insights and future directions. Future Oncol. 2018, 14, 907–917. [Google Scholar] [CrossRef]
- Yechiel, Y.; Chicheportiche, A.; Keidar, Z.; Ben-Haim, S. Prostate Cancer Radioligand Therapy: Beta-labeled Radiopharmaceuticals. PET Clin. 2024, 19, 389–399. [Google Scholar] [CrossRef]
- Xue, J.; Chen, K.; Hu, H.; Gopinath, S.C.B. Progress in gene therapy treatments for prostate cancer. Biotechnol. Appl. Biochem. 2022, 69, 1166–1175. [Google Scholar] [CrossRef]
- Kamran, S.C.; D’Amico, A.V. Radiation Therapy for Prostate Cancer. Hematol. Oncol. Clin. N. Am. 2020, 34, 45–69. [Google Scholar] [CrossRef]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Wang, G.C.; Zhao, D.; Spring, D.J.; DePinho, R.A. Genetics and biology of prostate cancer. Gene Dev. 2018, 32, 1105–1140. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-H.; Zhong, S.; Li, F.; Huang, C.; Chen, X.; Liu, Q.; Peng, S.; Park, H.; Lee, Y.M.; Dhillon, J.; et al. Tumor-derived OBP2A promotes prostate cancer castration resistance. J. Exp. Med. 2023, 220, e20211546. [Google Scholar] [CrossRef]
- Zhong, S.; Peng, S.; Chen, Z.; Chen, Z.; Luo, J.-L. Choosing Kinase Inhibitors for Androgen Deprivation Therapy-Resistant Prostate Cancer. Pharmaceutics 2022, 14, 498. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, N.; Liu, Q.; Guo, J.; Pan, Q.; Cheng, B.; Xu, J.; Dong, B.; Yang, G.; Yang, B.; et al. Antiandrogen treatment induces stromal cell reprogramming to promote castration resistance in prostate cancer. Cancer Cell 2023, 41, 1345–1362.e9. [Google Scholar] [CrossRef]
- Zhong, S.; Jeong, J.-H.; Huang, C.; Chen, X.; Dickinson, S.I.; Dhillon, J.; Yang, L.; Luo, J.-L. Targeting INMT and interrupting its methylation pathway for the treatment of castration resistant prostate cancer. J. Exp. Clin. Cancer Res. 2021, 40, 307. [Google Scholar] [CrossRef]
- Fontana, F.; Anselmi, M.; Limonta, P. Molecular mechanisms and genetic alterations in prostate cancer: From diagnosis to targeted therapy. Cancer Lett. 2022, 534, 215619. [Google Scholar] [CrossRef]
- Jeong, J.-H.; Park, S.-J.; Dickinson, S.I.; Luo, J.-L. A Constitutive Intrinsic Inflammatory Signaling Circuit Composed of miR-196b, Meis2, PPP3CC, and p65 Drives Prostate Cancer Castration Resistance. Mol. Cell 2017, 65, 154–167. [Google Scholar] [CrossRef]
- Wei, G.; Zhu, H.; Zhou, Y.; Pan, Y.; Yi, B.; Bai, Y. Single-cell sequencing revealed metabolic reprogramming and its transcription factor regulatory network in prostate cancer. Transl. Oncol. 2024, 44, 101925. [Google Scholar] [CrossRef]
- Ahmad, F.; Cherukuri, M.K.; Choyke, P.L. Metabolic reprogramming in prostate cancer. Br. J. Cancer 2021, 125, 1185–1196. [Google Scholar] [CrossRef]
- Fang, B.W.; Lu, Y.; Li, X.M.; Wei, Y.; Ye, D.W.; Wei, G.H.; Zhu, Y. Targeting the tumor microenvironment, a new therapeutic approach for prostate cancer. Prostate Cancer Prostatic Dis. 2024, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Karthaus, W.R.; Lee, Y.S.; Gao, V.R.; Wu, C.; Russo, J.W.; Liu, M.; Mota, J.M.; Abida, W.; Linton, E.; et al. Tumor Microenvironment-Derived NRG1 Promotes Antiandrogen Resistance in Prostate Cancer. Cancer Cell 2020, 38, 279–296.e9. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Kang, Y.; Zeng, Y. Targeting tumor and bone microenvironment: Novel therapeutic opportunities for castration-resistant prostate cancer patients with bone metastasis. Biochim. Biophys. Acta Rev. Cancer 2024, 1879, 189033. [Google Scholar] [CrossRef]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef]
- Pan, J.; Tong, F.; Ren, N.; Ren, L.; Yang, Y.; Gao, F.; Xu, Q. Role of N6-methyladenosine in the pathogenesis, diagnosis and treatment of prostate cancer (Review). Oncol. Rep. 2024, 51, 88. [Google Scholar] [CrossRef]
- Khan, S.; Baligar, P.; Tandon, C.; Nayyar, J.; Tandon, S. Molecular heterogeneity in prostate cancer and the role of targeted therapy. Life Sci. 2024, 336, 122270. [Google Scholar] [CrossRef]
- Zhong, S.W.; Jeong, J.H.; Chen, Z.K.; Chen, Z.H.; Luo, J.L. Targeting Tumor Microenvironment by Small-Molecule Inhibitors. Transl. Oncol. 2020, 13, 57–69. [Google Scholar] [CrossRef]
- Boedtkjer, E.; Pedersen, S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020, 82, 103–126. [Google Scholar] [CrossRef]
- Mohamed, O.A.A.; Tesen, H.S.; Hany, M.; Sherif, A.; Abdelwahab, M.M.; Elnaggar, M.H. The role of hypoxia on prostate cancer progression and metastasis. Mol. Biol. Rep. 2023, 50, 3873–3884. [Google Scholar] [CrossRef]
- Hirz, T.; Mei, S.; Sarkar, H.; Kfoury, Y.; Wu, S.; Verhoeven, B.M.; Subtelny, A.O.; Zlatev, D.V.; Wszolek, M.W.; Salari, K.; et al. Dissecting the immune suppressive human prostate tumor microenvironment via integrated single-cell and spatial transcriptomic analyses. Nat. Commun. 2023, 14, 663. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.S.; Weil, R.; Dovey, Z.; Davis, A.; Tewari, A.K. The Tumor Microenvironment and Immunotherapy in Prostate and Bladder Cancer. Urol. Clin. N. Am. 2020, 47, e17–e54. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Myers, K.V.; Pienta, K.J. Understanding the tumor-immune microenvironment in prostate cancer. Curr. Opin. Oncol. 2021, 33, 231–237. [Google Scholar] [CrossRef]
- Feng, D.; Xiong, Q.; Wei, Q.; Yang, L. Cellular landscape of tumour microenvironment in prostate cancer. Immunology 2023, 168, 199–202. [Google Scholar] [CrossRef]
- Stultz, J.; Fong, L. How to turn up the heat on the cold immune microenvironment of metastatic prostate cancer. Prostate Cancer Prostatic Dis. 2021, 24, 697–717. [Google Scholar] [CrossRef]
- Sun, B.L. Immunotherapy in treatment of metastatic prostate cancer: An approach to circumvent immunosuppressive tumor microenvironment. Prostate 2021, 81, 1125–1134. [Google Scholar] [CrossRef]
- Porter, L.H.; Zhu, J.J.; Lister, N.L.; Harrison, S.G.; Keerthikumar, S.; Goode, D.L.; Urban, R.Q.; Byrne, D.J.; Azad, A.; Vela, I.; et al. Low-dose carboplatin modifies the tumor microenvironment to augment CAR T cell efficacy in human prostate cancer models. Nat. Commun. 2023, 14, 5346. [Google Scholar] [CrossRef]
- Zhao, D.; Cai, L.; Lu, X.; Liang, X.; Li, J.; Chen, P.; Ittmann, M.; Shang, X.; Jiang, S.; Li, H.; et al. Chromatin Regulator CHD1 Remodels the Immunosuppressive Tumor Microenvironment in PTEN-Deficient Prostate Cancer. Cancer Discov. 2020, 10, 1374–1387. [Google Scholar] [CrossRef]
- Kfoury, Y.; Baryawno, N.; Severe, N.; Mei, S.; Gustafsson, K.; Hirz, T.; Brouse, T.; Scadden, E.W.; Igolkina, A.A.; Kokkaliaris, K.; et al. Human prostate cancer bone metastases have an actionable immunosuppressive microenvironment. Cancer Cell 2021, 39, 1464–1478.e8. [Google Scholar] [CrossRef]
- Palena, C.; Gulley, J.L. A rare insight into the immunosuppressive landscape of prostate cancer bone metastases. Cancer Cell 2021, 39, 1450–1452. [Google Scholar] [CrossRef]
- Souza-Fonseca-Guimaraes, F.; Rossi, G.R.; Dagley, L.F.; Foroutan, M.; McCulloch, T.R.; Yousef, J.; Park, H.-Y.; Gunter, J.H.; Beavis, P.A.; Lin, C.-Y.; et al. TGFbeta and CIS Inhibition Overcomes NK-cell Suppression to Restore Antitumor Immunity. Cancer Immunol. Res. 2022, 10, 1047–1054. [Google Scholar] [CrossRef]
- Wu, W.; Wang, X.A.; Le, W.; Lu, C.; Li, H.; Zhu, Y.; Chen, X.; An, W.; Xu, C.; Wu, Q.; et al. Immune microenvironment infiltration landscape and immune-related subtypes in prostate cancer. Front. Immunol. 2022, 13, 1001297. [Google Scholar] [CrossRef] [PubMed]
- Febles, V.R.A.; Hao, Y.; Ahsan, A.; Wu, J.S.; Qian, Y.Z.; Zhong, H.; Loeb, S.; Makarov, D.V.; Lepor, H.; Wysock, J.; et al. Single-cell analysis of localized prostate cancer patients links high Gleason score with an immunosuppressive profile. Prostate 2023, 83, 840–849. [Google Scholar] [CrossRef]
- Modena, A.; Ciccarese, C.; Iacovelli, R.; Brunelli, M.; Montironi, R.; Fiorentino, M.; Tortora, G.; Massari, F. Immune Checkpoint Inhibitors and Prostate Cancer: A New Frontier? Oncol. Rev. 2016, 10, 293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bancaro, N.; Calì, B.; Troiani, M.; Elia, A.R.; Arzola, R.A.; Attanasio, G.; Lai, P.; Crespo, M.; Gurel, B.; Pereira, R.; et al. Apolipoprotein E induces pathogenic senescent-like myeloid cells in prostate cancer. Cancer Cell 2023, 41, 602–619. [Google Scholar] [CrossRef]
- Zheng, W.; Wu, J.; Peng, Y.; Sun, J.; Cheng, P.; Huang, Q. Tumor-Associated Neutrophils in Colorectal Cancer Development, Progression and Immunotherapy. Cancers 2022, 14, 4755. [Google Scholar] [CrossRef]
- Novysedlak, R.; Guney, M.; Al Khouri, M.; Bartolini, R.; Foley, L.K.; Benesova, I.; Ozaniak, A.; Novak, V.; Vesely, S.; Pacas, P.; et al. The Immune Microenvironment in Prostate Cancer: A Comprehensive Review. Oncology 2024, 16, 1544882. [Google Scholar] [CrossRef] [PubMed]
- Muralidhar, A.; Hernandez, R.; Morris, Z.S.; Comas Rojas, H.; Bio Idrissou, M.; Weichert, J.P.; McNeel, D.G. Myeloid-derived suppressor cells attenuate the antitumor efficacy of radiopharmaceutical therapy using 90Y-NM600 in combination with androgen deprivation therapy in murine prostate tumors. J. Immunother. Cancer 2024, 12, e008760. [Google Scholar] [CrossRef]
- Wang, J.; Guo, T.; Zhang, X.M.; Guo, J.C.; Meng, X.Y.; Yan, S.; Wang, Y.; Xiao, Y.T.; Xu, W.D.; Wei, X.D.; et al. Comprehensive investigation in oncogenic functions and immunological roles of NCBP2 and its validation in prostate cancer. Transl. Oncol. 2024, 47, 102049. [Google Scholar] [CrossRef]
- Li, D.; Zhou, X.; Xu, W.; Chen, Y.; Mu, C.; Zhao, X.; Yang, T.; Wang, G.; Wei, L.; Ma, B. Prostate cancer cells synergistically defend against CD8+ T cells by secreting exosomal PD-L1. Cancer Med. 2023, 12, 16405–16415. [Google Scholar] [CrossRef]
- Ness, N.; Andersen, S.; Valkov, A.; Nordby, Y.; Donnem, T.; Al-Saad, S.; Busund, L.-T.; Bremnes, R.M.; Richardsen, E. Infiltration of CD8+ lymphocytes is an independent prognostic factor of biochemical failure-free survival in prostate cancer. Prostate 2014, 74, 1452–1461. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, X.; Huang, Y.; Zhang, X.; Sun, W.; Du, Y.; Xu, Z.; Kou, H.; Zhu, S.; Liu, C.; et al. Prostate cancer cell-derived exosomal IL-8 fosters immune evasion by disturbing glucolipid metabolism of CD8+ Tcell. Cell Rep. 2023, 42, 113424. [Google Scholar] [CrossRef]
- Ozbek, B.; Ertunc, O.; Erickson, A.; Vidal, I.D.; Gomes-Alexandre, C.; Guner, G.; Hicks, J.L.; Jones, T.; Taube, J.M.; Sfanos, K.S.; et al. Multiplex immunohistochemical phenotyping of T cells in primary prostate cancer. Prostate 2022, 82, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ge, Y.; Niu, K.; Li, Y.; Qi, L.-W.; Zhu, H.; Ma, G. MLXIPL associated with tumor-infiltrating CD8+ T cells is involved in poor prostate cancer prognosis. Fron. Immunol. 2024, 15, 1364329. [Google Scholar] [CrossRef] [PubMed]
- McNeel, D.G.; Eickhoff, J.C.; Wargowski, E.; Johnson, L.E.; Kyriakopoulos, C.E.; Emamekhoo, H.; Lang, J.M.; Brennan, M.J.; Liu, G. Phase 2 trial of T-cell activation using MVI-816 and pembrolizumab in patients with metastatic, castration-resistant prostate cancer (mCRPC). J. Immunother. Cancer 2022, 10, e004198. [Google Scholar] [CrossRef]
- Park, H.-M.; Cho, H.-I.; Shin, C.-A.; Shon, H.-J.; Kim, T.-G. Zoledronic acid induces dose-dependent increase of antigen-specific CD8 T-cell responses in combination with peptide/poly-IC vaccine. Vaccine 2016, 34, 1275–1281. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Wen, J.; Liu, S.; Huang, T.; Hatial, I.; Peng, X.; Al Janabi, H.; Huang, G.; Mittlesteadt, J.; et al. Targeting the chromatin effector Pygo2 promotes cytotoxic T cell responses and overcomes immunotherapy resistance in prostate cancer. Sci. Immunol. 2023, 8, eade4656. [Google Scholar] [CrossRef]
- Zhou, X.; Zou, L.; Liao, H.; Luo, J.; Yang, T.; Wu, J.; Chen, W.; Wu, K.; Cen, S.; Lv, D.; et al. Abrogation of HnRNP L enhances anti-PD-1 therapy efficacy via diminishing PD-L1 and promoting CD8+ T cell-mediated ferroptosis in castration-resistant prostate cancer. Acta Pharm. Sin. B 2022, 12, 692–707. [Google Scholar] [CrossRef]
- Morel, K.L.; Sheahan, A.V.; Burkhart, D.L.; Baca, S.C.; Boufaied, N.; Liu, Y.; Qiu, X.; Canadas, I.; Roehle, K.; Heckler, M.; et al. EZH2 inhibition activates a dsRNA-STING-interferon stress axis that potentiates response to PD-1 checkpoint blockade in prostate cancer. Nat. Cancer 2021, 2, 444–456. [Google Scholar] [CrossRef]
- Xu, W.; Li, Y.; Liu, L.; Xie, J.; Hu, Z.; Kuang, S.; Fu, X.; Li, B.; Sun, T.; Zhu, C.; et al. Icaritin-curcumol activates CD8+ T cells through regulation of gut microbiota and the DNMT1/IGFBP2 axis to suppress the development of prostate cancer. J. Exp. Clin. Cancer Res. 2024, 43, 149. [Google Scholar] [CrossRef]
- Mukherjee, D.; Romano, E.; Walshaw, R.; Zeef, L.A.H.; Banyard, A.; Kitcatt, S.J.; Cheadle, E.J.; Tuomela, K.; Pendharkar, S.; Al-Deka, A.; et al. Reprogramming the immunosuppressive tumor microenvironment results in successful clearance of tumors resistant to radiation therapy and anti-PD-1/PD-L1. Oncoimmunology 2023, 12, 2223094. [Google Scholar] [CrossRef] [PubMed]
- Zahm, C.D.; Moseman, J.E.; Delmastro, L.E.; G Mcneel, D. PD-1 and LAG-3 blockade improve anti-tumor vaccine efficacy. Oncoimmunology 2021, 10, 1912892. [Google Scholar] [CrossRef] [PubMed]
- Wolf, P.; Alzubi, J.; Gratzke, C.; Cathomen, T. The potential of CAR T cell therapy for prostate cancer. Nat. Rev. Urol. 2021, 18, 556–571. [Google Scholar] [CrossRef]
- Schepisi, G.; Cursano, M.C.; Casadei, C.; Menna, C.; Altavilla, A.; Lolli, C.; Cerchione, C.; Paganelli, G.; Santini, D.; Tonini, G.; et al. CAR-T cell therapy: A potential new strategy against prostate cancer. J. Immunother. Cancer 2019, 7, 258. [Google Scholar] [CrossRef]
- Thoma, C. Prostate cancer: Developing CAR T cell therapy. Nat. Rev. Urol. 2018, 15, 138. [Google Scholar]
- Perera, M.P.J.; Thomas, P.B.; Risbridger, G.P.; Taylor, R.; Azad, A.; Hofman, M.S.; Williams, E.D.; Vela, I. Chimeric Antigen Receptor T-Cell Therapy in Metastatic Castrate-Resistant Prostate Cancer. Cancers 2022, 14, 503. [Google Scholar] [CrossRef]
- Gorchakov, A.A.; Kulemzin, S.V.; Kochneva, G.V.; Taranin, A.V. Challenges and Prospects of Chimeric Antigen Receptor T-cell Therapy for Metastatic Prostate Cancer. Eur. Urol. 2020, 77, 299–308. [Google Scholar] [CrossRef]
- Saleh, O.M.; Albakri, K.A.; Alabdallat, Y.J.; Dajani, M.H.; El Gazzar, W.B. The safety and efficacy of CAR-T cells in the treatment of prostate cancer: Review. Biomarkers 2022, 27, 22–34. [Google Scholar] [CrossRef]
- Kelly, W.K.; Danila, D.C.; Lin, C.C.; Lee, J.L.; Matsubara, N.; Ward, P.J.; Armstrong, A.J.; Pook, D.; Kim, M.; Dorff, T.B.; et al. Xaluritamig, a STEAP1 × CD3 XmAb 2+1 Immune Therapy for Metastatic Castration-Resistant Prostate Cancer: Results from Dose Exploration in a First-in-Human Study. Cancer Discov. 2024, 14, 76–89. [Google Scholar] [CrossRef]
- Nolan-Stevaux, O.; Li, C.; Liang, L.; Zhan, J.; Estrada, J.; Osgood, T.; Li, F.; Zhang, H.; Case, R.; Murawsky, C.M.; et al. AMG 509 (Xaluritamig), an Anti-STEAP1 XmAb 2+1 T-cell Redirecting Immune Therapy with Avidity-Dependent Activity against Prostate Cancer. Cancer Discov. 2024, 14, 90–103. [Google Scholar] [CrossRef]
- Xie, J.; Guo, Z.; Zhu, Y.; Ma, M.; Jia, G. Peripheral blood inflammatory indexes in breast cancer: A review. Medicine 2023, 102, e36315. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Xie, G.; Cai, J.; Zhao, J.; Liu, J.; Yang, B.; Cui, J.; Chu, C. Expression and Clinical Significance of Immune Cell Function Detection in Peripheral Blood of Malignant Tumor Patients. Biomedical 2017, 7, 54–58. [Google Scholar]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Reina-Campos, M.; Scharping, N.E.; Goldrath, A.W. CD8+ T cell metabolism in infection and cancer. Nat. Rev. Immunol. 2021, 21, 718–738. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar]
- Dolina, J.S.; Van Braeckel-Budimir, N.; Thomas, G.D.; Salek-Ardakani, S. CD8+ T Cell Exhaustion in Cancer. Front. Immunol. 2021, 12, 715234. [Google Scholar] [CrossRef]
- Philip, M.; Schietinger, A. CD8+ T cell differentiation and dysfunction in cancer. Nat. Rev. Immunol. 2022, 22, 209–223. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, Y.; Li, B. CD8+ T cell exhaustion and cancer immunotherapy. Cancer Lett 2023, 559, 216043. [Google Scholar] [CrossRef]
- Mercader, M.; Bodner, B.K.; Moser, M.T.; Kwon, P.S.; Park, E.S.; Manecke, R.G.; Ellis, T.M.; Wojcik, E.M.; Yang, D.; Flanigan, R.C.; et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc. Natl. Acad. Sci. USA 2001, 98, 14565–14570. [Google Scholar] [CrossRef]
- Sorrentino, C.; Musiani, P.; Pompa, P.; Cipollone, G.; Di Carlo, E. Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory T lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin. Cancer Res. 2011, 17, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, W.; Dong, B.; Xin, Z.; Ji, Y.; Su, R.; Shen, K.; Pan, J.; Wang, Q.; Xue, W. Docetaxel remodels prostate cancer immune microenvironment and enhances checkpoint inhibitor-based immunotherapy. Theranostics 2022, 12, 4965–4979. [Google Scholar] [CrossRef]
- Yu, X.; Liu, R.; Gao, W.; Wang, X.; Zhang, Y. Single-cell omics traces the heterogeneity of prostate cancer cells and the tumor microenvironment. Cell Mol. Biol. Lett. 2023, 28, 38. [Google Scholar] [CrossRef] [PubMed]
- Bhinder, B.; Ferguson, A.; Sigouros, M.; Uppal, M.; Elsaeed, A.G.; Bareja, R.; Alnajar, H.; Eng, K.W.; Conteduca, V.; Sboner, A.; et al. Immunogenomic Landscape of Neuroendocrine Prostate Cancer. Clin. Cancer Res. 2023, 29, 2933–2943. [Google Scholar] [CrossRef]
- Mougola Bissiengou, P.; Montcho Comlan, J.G.; Atsame Ebang, G.; Sylla Niang, M.; Djoba Siawaya, J.F. Prostate malignant tumor and benign prostatic hyperplasia microenvironments in black African men: Limited infiltration of CD8+ T lymphocytes, NK-cells, and high frequency of CD73+ stromal cells. Cancer Rep. 2023, 6 (Suppl. S1), e1817. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Jing, N.; Wang, D.; Xu, P.; Wang, J.; Chen, X.; Cheng, C.; Xin, Z.; He, Y.; Zhao, H.; et al. A novel mouse model for liver metastasis of prostate cancer reveals dynamic tumour-immune cell communication. Cell Prolif. 2021, 54, e13056. [Google Scholar] [CrossRef]
- Petitprez, F.; Fossati, N.; Vano, Y.; Freschi, M.; Becht, E.; Luciano, R.; Calderaro, J.; Guedet, T.; Lacroix, L.; Rancoita, P.M.V.; et al. PD-L1 Expression and CD8+ T-cell Infiltrate are Associated with Clinical Progression in Patients with Node-positive Prostate Cancer. Eur. Urol. Focus 2019, 5, 192–196. [Google Scholar] [CrossRef]
- Goulielmaki, M.; Stokidis, S.; Anagnostou, T.; Voutsas, I.F.; Gritzapis, A.D.; Baxevanis, C.N.; Fortis, S.P. Frequencies of an Immunogenic HER-2/neu Epitope of CD8+ T Lymphocytes Predict Favorable Clinical Outcomes in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 5954. [Google Scholar] [CrossRef]
- Yang, Y.; Attwood, K.; Bshara, W.; Mohler, J.L.; Guru, K.; Xu, B.; Kalinski, P.; Chatta, G. High intratumoral CD8+ T-cell infiltration is associated with improved survival in prostate cancer patients undergoing radical prostatectomy. Prostate 2021, 81, 20–28. [Google Scholar] [CrossRef]
- Tuong, Z.K.; Loudon, K.W.; Berry, B.; Richoz, N.; Jones, J.; Tan, X.; Nguyen, Q.; George, A.; Hori, S.; Field, S.; et al. Resolving the immune landscape of human prostate at a single-cell level in health and cancer. Cell Rep. 2021, 37, 110132. [Google Scholar] [CrossRef]
- Wong, H.Y.; Sheng, Q.; Hesterberg, A.B.; Croessmann, S.; Rios, B.L.; Giri, K.; Jackson, J.; Miranda, A.X.; Watkins, E.; Schaffer, K.R.; et al. Single cell analysis of cribriform prostate cancer reveals cell intrinsic and tumor microenvironmental pathways of aggressive disease. Nat. Commun. 2022, 13, 6036. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wang, W.; Abudurexiti, M.; Zhang, X.; Ma, W.; Shi, G.; Du, L.; Xu, M.; Wang, X.; Tan, C.; et al. Integration Analysis of Single-Cell Multi-Omics Reveals Prostate Cancer Heterogeneity. Adv. Sci. 2024, 11, e2305724. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhu, G.; Yang, Y.; Wang, F.; Xiao, Y.-T.; Zhang, N.; Bian, X.; Zhu, Y.; Yu, Y.; Liu, F.; et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nat. Cell Biol. 2021, 23, 87–98. [Google Scholar] [CrossRef]
- De Vargas Roditi, L.; Jacobs, A.; Rueschoff, J.H.; Bankhead, P.; Chevrier, S.; Jackson, H.W.; Hermanns, T.; Fankhauser, C.D.; Poyet, C.; Chun, F.; et al. Single-cell proteomics defines the cellular heterogeneity of localized prostate cancer. Cell Rep. Med. 2022, 3, 100604. [Google Scholar] [CrossRef]
- Xin, S.; Liu, X.; Li, Z.; Sun, X.; Wang, R.; Zhang, Z.; Feng, X.; Jin, L.; Li, W.; Tang, C.; et al. ScRNA-seq revealed an immunosuppression state and tumor microenvironment heterogeneity related to lymph node metastasis in prostate cancer. Exp. Hematol. Oncol. 2023, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pachynski, R.K.; Narayan, V.; Flechon, A.; Gravis, G.; Galsky, M.D.; Mahammedi, H.; Patnaik, A.; Subudhi, S.K.; Ciprotti, M.; et al. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell 2020, 38, 489–499.e3. [Google Scholar] [CrossRef]
- Sridaran, D.; Bradshaw, E.; DeSelm, C.; Pachynski, R.; Mahajan, K.; Mahajan, N.P. Prostate cancer immunotherapy: Improving clinical outcomes with a multi-pronged approach. Cell Rep. Med. 2023, 4, 101199. [Google Scholar] [CrossRef]
- Hawley, J.E.; Obradovic, A.Z.; Dallos, M.C.; Lim, E.A.; Runcie, K.; Ager, C.R.; McKiernan, J.; Anderson, C.B.; Decastro, G.J.; Weintraub, J.; et al. Anti-PD-1 immunotherapy with androgen deprivation therapy induces robust immune infiltration in metastatic castration-sensitive prostate cancer. Cancer Cell 2023, 41, 1972–1988.e5. [Google Scholar] [CrossRef]
- Lyu, A.; Fan, Z.; Clark, M.; Lea, A.; Luong, D.; Setayesh, A.; Starzinski, A.; Wolters, R.; Arias-Badia, M.; Allaire, K.; et al. Evolution of myeloid-mediated immunotherapy resistance in prostate cancer. Nature 2024, 637, 1207–1217. [Google Scholar] [CrossRef]
- Tang, S.; Moore, M.L.; Grayson, J.M.; Dubey, P. Increased CD8+ T-cell function following castration and immunization is countered by parallel expansion of regulatory T cells. Cancer Res. 2012, 72, 1975–1985. [Google Scholar] [CrossRef]
- Lu, X.; Horner, J.W.; Paul, E.; Shang, X.; Troncoso, P.; Deng, P.; Jiang, S.; Chang, Q.; Spring, D.J.; Sharma, P.; et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature 2017, 543, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Yang, J.C.; Chen, B.; Nip, C.; Van Dyke, J.E.; Zhang, X.; Chen, H.-W.; Evans, C.P.; Murphy, W.J.; Liu, C. Androgen receptor blockade resistance with enzalutamide in prostate cancer results in immunosuppressive alterations in the tumor immune microenvironment. J. Immunother. Cancer 2023, 11, e006581. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Polesso, F.; Wang, C.; Sehrawat, A.; Hawkins, R.M.; Murray, S.E.; Thomas, G.V.; Caruso, B.; Thompson, R.F.; Wood, M.A.; et al. Androgen receptor activity in T cells limits checkpoint blockade efficacy. Nature 2022, 606, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, S.; Twardowski, P.; Lau, C.; Chan, Y.S.; Wong, K.; Xiao, S.; Wang, J.; Wu, X.; Frankel, P.; et al. Reduction of myeloid-derived suppressor cells in prostate cancer murine models and patients following white button mushroom treatment. Clin. Transl. Med. 2024, 14, e70048. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, F.; Wang, Y.-N.; Liu, B.; Zhou, P.; Wang, F.-X.; Zhou, H.-F.; Ge, Y.; Yue, T.-T.; Luo, J.-H.; et al. UBC9 deficiency enhances immunostimulatory macrophage activation and subsequent antitumor T cell response in prostate cancer. J. Clin. Investig. 2023, 133, e158352. [Google Scholar] [CrossRef]
- Ma, L.; Lin, Y. Expression and Clinical Significance of S100A11 and Autophagy Gene Beclin1 in Gastric Cancer Tissues. World Chin. J. Digestol. 2012, 20, 3266–3271. [Google Scholar] [CrossRef]
- Han, D.; Guo, C.; Cheng, H.; Lu, J.; Hou, Z.; Zhang, X.; Luo, Y.; Zhang, B.; Zhao, W.; Shang, P. Downregulation of S100A11 promotes T cell infiltration by regulating cancer-associated fibroblasts in prostate cancer. Int. Immunopharmacol. 2024, 128, 111323. [Google Scholar] [CrossRef]
- Abusamra, A.J.; Zhong, Z.; Zheng, X.; Li, M.; Ichim, T.E.; Chin, J.L.; Min, W.-P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 2005, 35, 169–173. [Google Scholar] [CrossRef]
- Chang, M.; He, Y.; Liu, C.; Lin, R.; Huang, X.; Liang, D.; Zhang, J.; Lu, Y. Downregulation of SEPTIN5 inhibits prostate cancer progression by increasing CD8+ T cell infiltration. Int. J. Biol. Sci. 2022, 18, 6035–6051. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Ferro, M.; Buonerba, C. Sipuleucel-T (Provenge®) for castration-resistant prostate cancer. BJU Int. 2012, 110 Pt 2, E99–E104. [Google Scholar] [CrossRef]
- Kantoff, P.W.; Higano, C.S.; Shore, N.D.; Berger, E.R.; Small, E.J.; Penson, D.F.; Redfern, C.H.; Ferrari, A.C.; Dreicer, R.; Sims, R.B.; et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 2010, 363, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Runcie, K.D.; Dallos, M.C. Prostate Cancer Immunotherapy-Finally in From the Cold? Curr. Oncol. Rep. 2021, 23, 88. [Google Scholar] [CrossRef]
- Luo, Z.-W.; Xia, K.; Liu, Y.-W.; Liu, J.-H.; Rao, S.-S.; Hu, X.-K.; Chen, C.-Y.; Xu, R.; Wang, Z.-X.; Xie, H. Extracellular Vesicles from Akkermansia muciniphila Elicit Antitumor Immunity Against Prostate Cancer via Modulation of CD8+ T Cells and Macrophages. Int. J. Nanomed. 2021, 16, 2949–2963. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, A.; Wang, Y.; Zhang, Y. Intratumoral microbiota: Roles in cancer initiation, development and therapeutic efficacy. Signal Transduct. Target. Ther. 2023, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Bachem, A.; Makhlouf, C.; Binger, K.J.; de Souza, D.P.; Tull, D.; Hochheiser, K.; Whitney, P.G.; Fernandez-Ruiz, D.; Dähling, S.; Kastenmüller, W.; et al. Microbiota-Derived Short-Chain Fatty Acids Promote the Memory Potential of Antigen-Activated CD8+ T Cells. Immunity 2019, 51, 285–297.e5. [Google Scholar] [CrossRef]
- Vardeu, A.; Davis, C.; McDonald, I.; Stahlberg, G.; Thapa, B.; Piotrowska, K.; Marshall, M.A.; Evans, T.; Wheeler, V.; Sebastian, S.; et al. Intravenous administration of viral vectors expressing prostate cancer antigens enhances the magnitude and functionality of CD8+ T cell responses. J. Immunother. Cancer 2022, 10, e005398. [Google Scholar] [CrossRef]
- Burbach, B.J.; O’Flanagan, S.D.; Shao, Q.; Young, K.M.; Slaughter, J.R.; Rollins, M.R.; Street, T.J.L.; Granger, V.E.; Beura, L.K.; Azarin, S.M.; et al. Irreversible electroporation augments checkpoint immunotherapy in prostate cancer and promotes tumor antigen-specific tissue-resident memory CD8+ T cells. Nat. Commun. 2021, 12, 3862. [Google Scholar] [CrossRef]
- Frijlink, E.; Bosma, D.M.; Busselaar, J.; Battaglia, T.W.; Staal, M.D.; Verbrugge, I.; Borst, J. PD-1 or CTLA-4 blockade promotes CD86-driven Treg responses upon radiotherapy of lymphocyte-depleted cancer in mice. J. Clin. Investig. 2024, 134, e171154. [Google Scholar] [CrossRef]
- Lo, B.C.; Kryczek, I.; Yu, J.; Vatan, L.; Caruso, R.; Matsumoto, M.; Sato, Y.; Shaw, M.H.; Inohara, N.; Xie, Y.; et al. Microbiota-dependent activation of CD4+ T cells induces CTLA-4 blockade-associated colitis via Fcγ receptors. Science 2024, 383, 62–70. [Google Scholar] [CrossRef]
- Arndt, C.; Feldmann, A.; Topfer, K.; Koristka, S.; Cartellieri, M.; Temme, A.; Ehninger, A.; Ehninger, G.; Bachmann, M. Redirection of CD4+ and CD8+ T lymphocytes via a novel antibody-based modular targeting system triggers efficient killing of PSCA+ prostate tumor cells. Prostate 2014, 74, 1347–1358. [Google Scholar] [CrossRef]
- Song, H.T.; Lu, T.; Han, D.H.; Zhang, J.Y.; Gan, L.B.; Xu, C.; Liu, S.J.; Li, P.; Zhang, K.Y.; Hu, Z.H.; et al. YAP1 Inhibition Induces Phenotype Switching of Cancer-Associated Fibroblasts to Tumor Suppressive in Prostate Cancer. Cancer Res. 2024, 84, 3728–3742. [Google Scholar] [CrossRef] [PubMed]
- Shenderov, E.; De Marzo, A.M.; Lotan, T.L.; Wang, H.; Chan, S.; Lim, S.J.; Ji, H.; Allaf, M.E.; Chapman, C.; Moore, P.A.; et al. Neoadjuvant enoblituzumab in localized prostate cancer: A single-arm, phase 2 trial. Nat. Med. 2023, 29, 888–897. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, M.; Chen, M.; Sun, Z.; Jiao, Y.; Ye, G.; Pan, J.; Ye, W.; Zhao, J.; Zhang, D. Zoledronic acid and thymosin alpha1 elicit antitumor immunity against prostate cancer by enhancing tumor inflammation and cytotoxic T cells. J. Immunother. Cancer 2023, 11, e006381. [Google Scholar] [CrossRef]
- Su, S.; You, S.; Wang, Y.; Tamukong, P.; Quist, M.J.; Grasso, C.S.; Kim, H.L. PAK4 inhibition improves PD1 blockade immunotherapy in prostate cancer by increasing immune infiltration. Cancer Lett. 2023, 555, 216034. [Google Scholar] [CrossRef] [PubMed]
- Petrylak, D.P.; Ratta, R.; Matsubara, N.; Korbenfeld, E.; Gafanov, R.; Mourey, L.; Todenhöfer, T.; Gurney, H.; Kramer, G.; Bergman, A.M.; et al. Pembrolizumab Plus Docetaxel Versus Docetaxel for Previously Treated Metastatic Castration-Resistant Prostate Cancer: The Randomized, Double-Blind, Phase III KEYNOTE-921 Trial. J. Clin. Oncol. 2025, 43, 1638–1649. [Google Scholar] [CrossRef]
- Smith, J.A.; Lee, K.; Patel, R. Prostate cancer immunotherapy: Improving clinical outcomes with a multi-pronged approach. Cancer Immunol. Res. 2025, 13, 456–468. [Google Scholar]
- Chen, Q.; Bao, Y.; Burner, D.; Kaushal, S.; Zhang, Y.; Mendoza, T.; Bouvet, M.; Ozkan, C.; Minev, B.; Ma, W. Tumor growth inhibition by mSTEAP peptide nanovaccine inducing augmented CD8+ T cell immune responses. Drug Deliv. Transl. Res. 2019, 9, 1095–1105. [Google Scholar] [CrossRef]
- Zelba, H.; Rabsteyn, A.; Bartsch, O.; Kyzirakos, C.; Kayser, S.; Seibold, M.; Harter, J.; Latzer, P.; Hadaschik, D.; Battke, F.; et al. Case Report: Targeting of individual somatic tumor mutations by multipeptide vaccination tailored for HLA class I and II presentation induces strong CD4 and CD8 T-cell responses in a patient with metastatic castration sensitive prostate cancer. Front. Immunol. 2023, 14, 1271449. [Google Scholar] [CrossRef]
- Abdul Sater, H.; Marte, J.L.; Donahue, R.N.; Walter-Rodriguez, B.; Heery, C.R.; Steinberg, S.M.; Cordes, L.M.; Chun, G.; Karzai, F.; Bilusic, M.; et al. Neoadjuvant PROSTVAC prior to radical prostatectomy enhances T-cell infiltration into the tumor immune microenvironment in men with prostate cancer. J. Immunother. Cancer 2020, 8, e000655. [Google Scholar] [CrossRef]
- Noguchi, M.; Arai, G.; Egawa, S.; Ohyama, C.; Naito, S.; Matsumoto, K.; Uemura, H.; Nakagawa, M.; Nasu, Y.; Eto, M.; et al. Mixed 20-peptide cancer vaccine in combination with docetaxel and dexamethasone for castration-resistant prostate cancer: A randomized phase II trial. Cancer Immunol. Immunother. 2020, 69, 847–857. [Google Scholar] [CrossRef]
- Schuhmacher, J.; Heidu, S.; Balchen, T.; Richardson, J.R.; Schmeltz, C.; Sonne, J.; Schweiker, J.; Rammensee, H.-G.; Thor Straten, P.; Roder, M.A.; et al. Vaccination against RhoC induces long-lasting immune responses in patients with prostate cancer: Results from a phase I/II clinical trial. J. Immunother. Cancer 2020, 8, e001157. [Google Scholar] [CrossRef] [PubMed]
- Obradovic, A.Z.; Dallos, M.C.; Zahurak, M.L.; Partin, A.W.; Schaeffer, E.M.; Ross, A.E.; Allaf, M.E.; Nirschl, T.R.; Liu, D.; Chapman, C.G.; et al. T-Cell Infiltration and Adaptive Treg Resistance in Response to Androgen Deprivation with or Without Vaccination in Localized Prostate Cancer. Clin. Cancer Res. 2020, 26, 3182–3192. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Kamat, N.V.; Pariva, T.E.; Wu, L.-T.; Tsao, A.; Sasaki, K.; Sun, H.; Javier, G.; Nutt, S.; Coleman, I.; et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat. Commun. 2023, 14, 2041. [Google Scholar] [CrossRef] [PubMed]
- Ajmal, I.; Farooq, M.A.; Duan, Y.; Yao, J.; Gao, Y.; Hui, X.; Ge, Y.; Chen, Y.; Ren, Y.; Du, B.; et al. Intrinsic ADRB2 inhibition improves CAR-T cell therapy efficacy against prostate cancer. Mol. Ther. 2024, 32, 3539–3557. [Google Scholar] [CrossRef]
- Narayan, V.; Barber-Rotenberg, J.S.; Jung, I.-Y.; Lacey, S.F.; Rech, A.J.; Davis, M.M.; Hwang, W.-T.; Lal, P.; Carpenter, E.L.; Maude, S.L.; et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: A phase 1 trial. Nat. Med. 2022, 28, 724–734. [Google Scholar] [CrossRef]
- Dorff, T.B.; Blanchard, M.S.; Adkins, L.N.; Luebbert, L.; Leggett, N.; Shishido, S.N.; Macias, A.; Del Real, M.M.; Dhapola, G.; Egelston, C.; et al. PSCA-CAR T cell therapy in metastatic castration-resistant prostate cancer: A phase 1 trial. Nat. Med. 2024, 30, 1636–1644. [Google Scholar] [CrossRef]
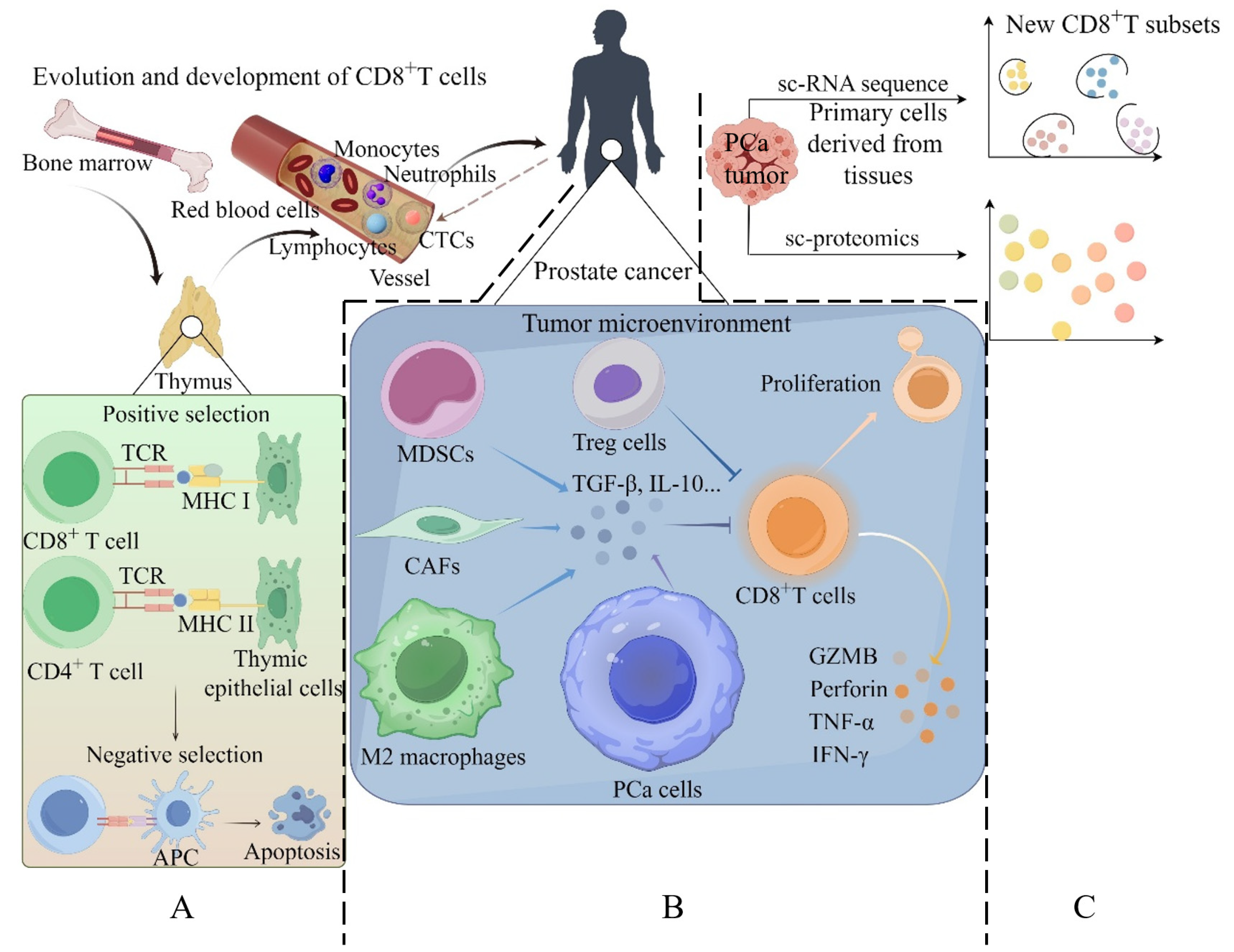
 : suppress; >>: secrecte.
: suppress; >>: secrecte.
 : suppress; >>: secrecte.
: suppress; >>: secrecte.
 : suppress; >>: secrecte;
: suppress; >>: secrecte;  : OBP2A;
: OBP2A;  : CXCL15/IL8;
: CXCL15/IL8;  : CCL20 derived from MDSCs;
: CCL20 derived from MDSCs;  : IL2;
: IL2;  : IL12;
: IL12;  : CCL20 derived from TAMs; and
: CCL20 derived from TAMs; and  : exosome containing indicated proteins. (2) PCa: prostate cancer; CAFs: cancer-associated fibroblasts; TAMs: tumor-associated macrophages; MDSCs: myeloid-derived suppressor cells; and Tregs: regulatory T cells.
: exosome containing indicated proteins. (2) PCa: prostate cancer; CAFs: cancer-associated fibroblasts; TAMs: tumor-associated macrophages; MDSCs: myeloid-derived suppressor cells; and Tregs: regulatory T cells.
 : suppress; >>: secrecte;
: suppress; >>: secrecte;  : OBP2A;
: OBP2A;  : CXCL15/IL8;
: CXCL15/IL8;  : CCL20 derived from MDSCs;
: CCL20 derived from MDSCs;  : IL2;
: IL2;  : IL12;
: IL12;  : CCL20 derived from TAMs; and
: CCL20 derived from TAMs; and  : exosome containing indicated proteins. (2) PCa: prostate cancer; CAFs: cancer-associated fibroblasts; TAMs: tumor-associated macrophages; MDSCs: myeloid-derived suppressor cells; and Tregs: regulatory T cells.
: exosome containing indicated proteins. (2) PCa: prostate cancer; CAFs: cancer-associated fibroblasts; TAMs: tumor-associated macrophages; MDSCs: myeloid-derived suppressor cells; and Tregs: regulatory T cells.
| CD8+ T Subset | The Role in PCa Progression | Reference |
|---|---|---|
| KLK3-high T-cell clusters (Cluster 5) | Its activity is significantly suppressed with the low level of cell metabolism and antitumor immune pathways, and is associated with micro-metastases of PCa. | [93] |
| TC03 subset and TC04 subset (apoptotic CD8+ T cells and Ki67highCD8+ T cells, respectively) | They are significantly enriched in prostate tumors compared to adjacent samples. | [94] |
| CD8+CXCR6+ T cells | It functions as effector T cells and its abundance is markedly declined in patients with malignant PCa. | [92] |
| CD8+GZMK+ T cells (CTL-1) | It is an intermediate state in the CD8+ T cell transition process from activation to exhaustion in PCa. | [95] |
| CCR7+CD8+ T cells | It is more common in lymphatic metastases compared with that in primary lesions, and promotes PCa metastasis and tumor growth. | [95] |
| IL7R+CD8+ T cells | It is more common in lymphatic metastases and is associated with T-cell proliferation and inhibition of tumor progression. | [95] |
| Therapy Modality | Mechanism Overview | Advantages | Disadvantages |
|---|---|---|---|
| Immune Checkpoint Inhibitors (ICIs) | Monoclonal antibodies against PD-1/PD-L1 or CTLA-4 relieve tumor-mediated suppression of CD8+ T cells | - Established approvals in multiple cancers - Can reinvigorate existing tumor-specific T cells - Convenient intravenous dosing | - Generally low response rate as monotherapy in PCa - irAEs (e.g., dermatitis, colitis) - Limited predictive biomarkers |
| Cancer Vaccines | Delivery of prostate-cancer antigens (e.g., PAP, PSA, PSMA) to prime antigen-specific CD8⁺ T cells | - Good safety profile, low toxicity - Elicit de novo antigen-specific responses - Be amenable to combination with ICIs or chemotherapy | - Often weak immunogenicity alone, low objective response rates - Require adjuvants or multiple doses - Subject to patient immune tolerance |
| Adoptive Cell Therapy (CAR T/TCR T) | Ex vivo expansion and genetic engineering of patient CD8+ T cells (e.g., CAR-PSMA), then reinfusion | - Generate high-affinity, tumor-specific effector cells - Potential for long-term persistence and immunosurveillance - Some striking clinical responses | - High manufacturing cost and lengthy production - Risk of cytokine release syndrome and neurotoxicity - Solid-tumor microenvironment barriers |
| Cytokine Therapy (IL-2, IL-15, IL-12) | Systemic or localized delivery of cytokines to boost CD8+ T cell proliferation and functionality | - Potent systemic enhancement of T cell activity - Certain cytokines improve tumor infiltration - Synergize with vaccines or ICIs | - Severe systemic toxicities (e.g., capillary leak syndrome) - Limited efficacy as monotherapy - Require targeted delivery to reduce side effects |
| Bispecific T-cell Engagers (BiTEs) | Dual-binding antibodies that link CD3 on T cells with prostate tumor antigens (e.g., PSMA), activating cytotoxicity | - Mobilize endogenous CD8+ T cells without ex vivo manipulation - Flexible dosing - Early clinical signs of antitumor activity | - Short half-life often necessitating continuous infusion - Risk of cytokine release syndrome - Sensitivity to antigen heterogeneity |
| Antibody–Drug Conjugates (ADCs) | Tumor-antigen–targeted antibody delivers cytotoxic payload and promotes antigen release to activate CD8+ T cells | - Dual killing mechanisms: direct cytotoxicity plus immune activation - Focused toxicity at tumor site - Some ADCs in PCa have approvals/trials | - Limited T-cell activation when used alone; may require combination with immunotherapy - ADC-associated toxicities (e.g., thrombocytopenia, hepatotoxicity) |
| Combination Strategies | Rational combinations of the above (e.g., ICI + vaccine, CAR T + ICI, chemotherapy + ICI) | - Overcome resistance to single agents - Synergistically enhance CD8+ T-cell recruitment, activation, and persistence - Improved response rates in early studies | - Increased regimen complexity and overlapping toxicities - Optimal sequencing and dosing remain undefined - Higher cost and logistical challenges |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, B.; Liang, L.; Li, Z.; Luo, J.; Zhong, S. Bolstering CD8+ T Cells’ Antitumor Immunity: A Promising Strategy to Improve the Response to Advanced Prostate Cancer Treatment. Biology 2025, 14, 544. https://doi.org/10.3390/biology14050544
Dang B, Liang L, Li Z, Luo J, Zhong S. Bolstering CD8+ T Cells’ Antitumor Immunity: A Promising Strategy to Improve the Response to Advanced Prostate Cancer Treatment. Biology. 2025; 14(5):544. https://doi.org/10.3390/biology14050544
Chicago/Turabian StyleDang, Beijing, Lixin Liang, Zhijun Li, Junli Luo, and Shangwei Zhong. 2025. "Bolstering CD8+ T Cells’ Antitumor Immunity: A Promising Strategy to Improve the Response to Advanced Prostate Cancer Treatment" Biology 14, no. 5: 544. https://doi.org/10.3390/biology14050544
APA StyleDang, B., Liang, L., Li, Z., Luo, J., & Zhong, S. (2025). Bolstering CD8+ T Cells’ Antitumor Immunity: A Promising Strategy to Improve the Response to Advanced Prostate Cancer Treatment. Biology, 14(5), 544. https://doi.org/10.3390/biology14050544






