Supra-Physiological Levels of Magnesium Counteract the Inhibitory Effect of Zoledronate on RANKL-Dependent Osteoclastogenesis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Molecular Analysis
2.3. Flow Cytometry Analysis
2.4. Cell Visualization
2.5. Statistical Analysis
3. Results
3.1. Effects Determined by Treatment with MgCl2 and ZA on the Osteoclast Differentiation of THP-1 Cells, Induced by Stimulation with Phorbol Esters, M-CSF, and RANKL
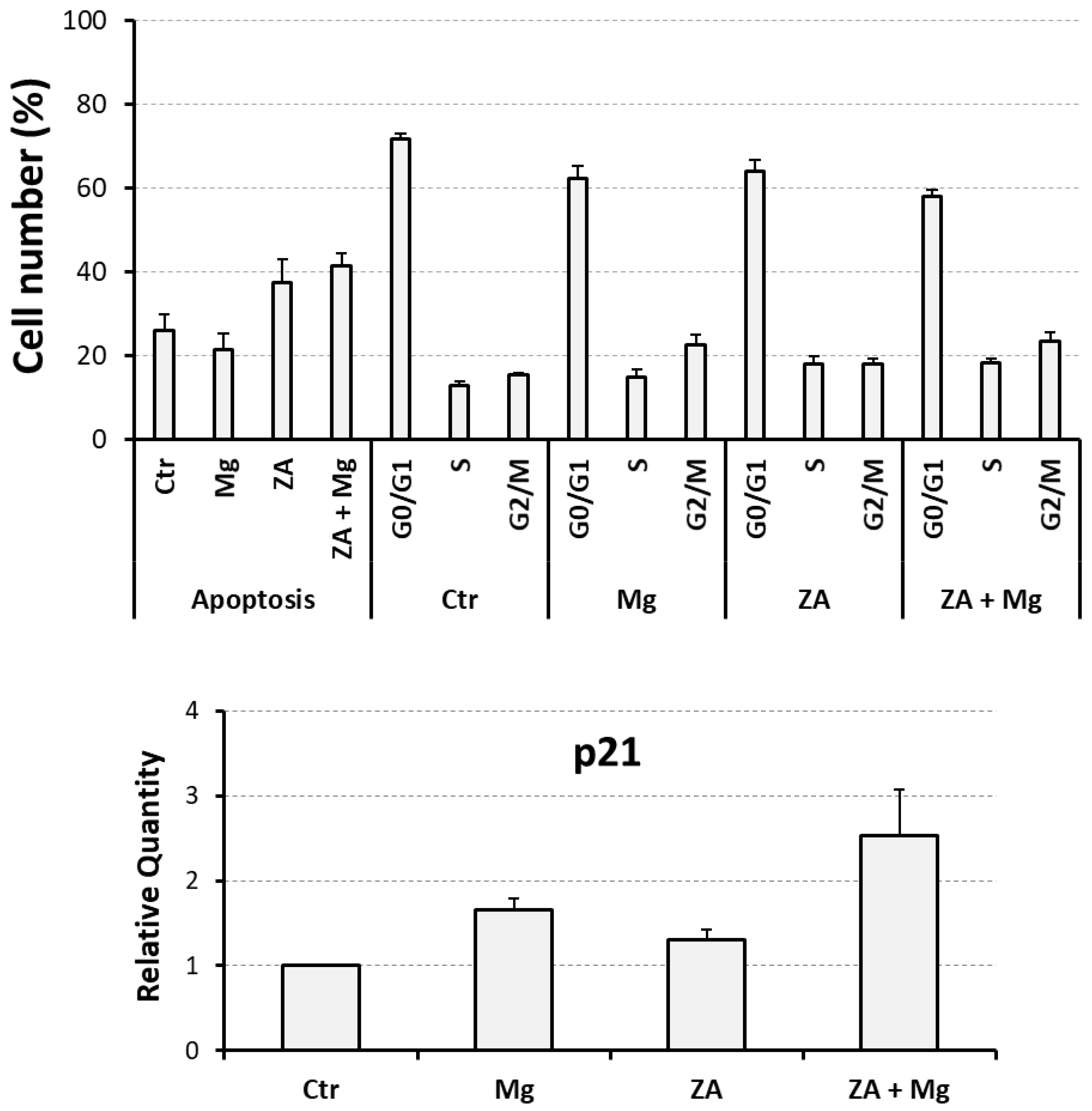
3.2. Proliferative and Apoptotic Effects Determined by Treatment with MgCl2 and ZA During the Osteoclast Differentiation of THP-1 Cells, Induced by Stimulation with Phorbol Esters, M-CSF, and RANKL
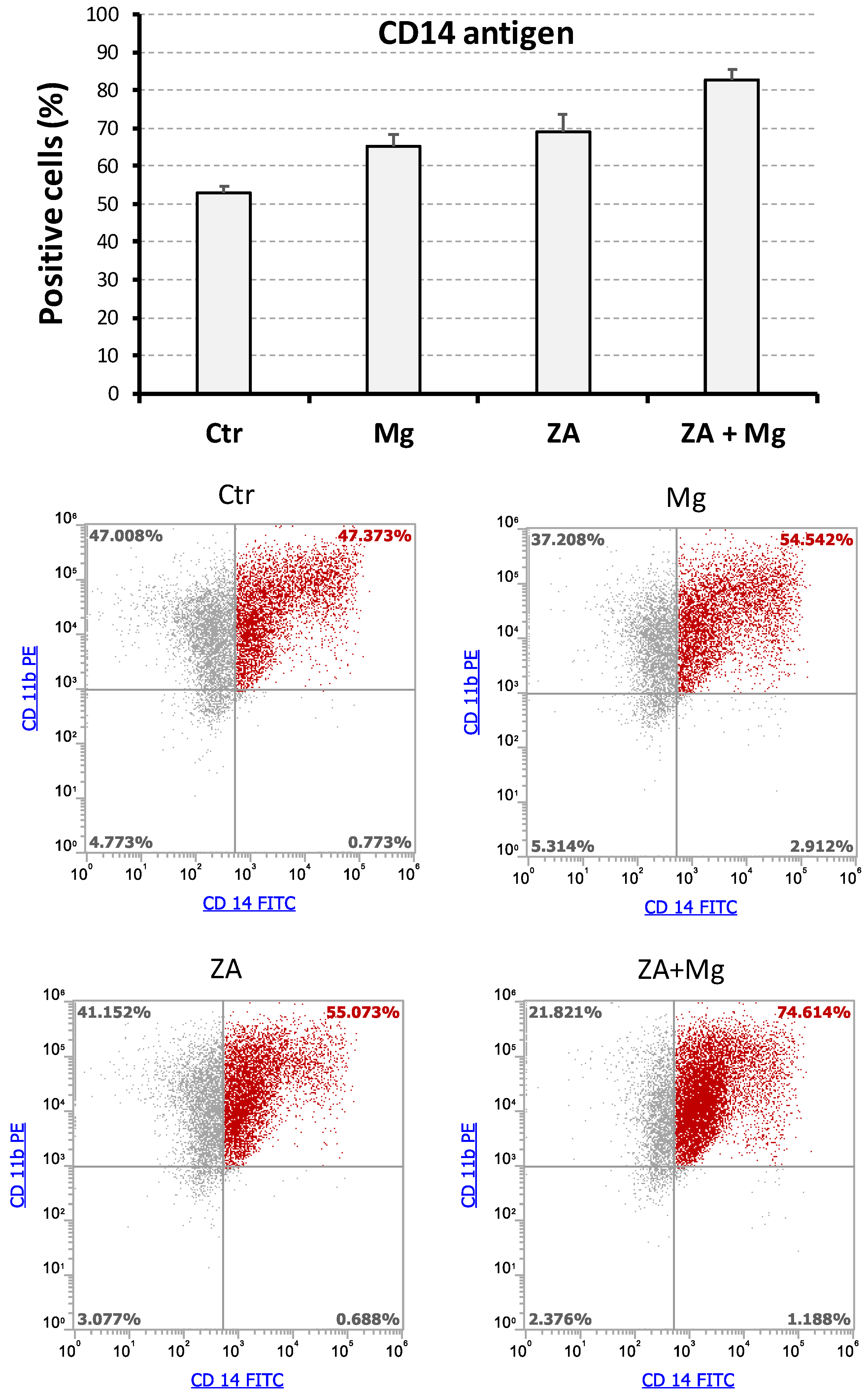
3.3. Effects Determined by Treatment with MgCl2 and ZA on the Expression of CD14 and CD11b Surface Antigens During the Osteoclast Differentiation of THP-1 Cells Induced by Stimulation with Phorbol Esters, M-CSF, and RANKL
3.4. Morphological and Cytochemical Changes Determined by Treatment with MgCl2 and ZA During the Osteoclast Differentiation of THP-1 Cells, Induced by Stimulation with Phorbol Esters, M-CSF, and RANKL
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buck, D.W., 2nd; Dumanian, G.A. Bone biology and physiology: Part I. The fundamentals. Plast. Reconstr. Surg. 2012, 129, 1314–1320. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef] [PubMed]
- Buck, D.W., 2nd; Dumanian, G.A. Bone biology and physiology: Part II. Clinical correlates. Plast. Reconstr. Surg. 2012, 129, 950e–956e. [Google Scholar] [CrossRef]
- Seemann, L.L.; Hanos, C.T.; Pujalte, G.G.A. Metabolic Bone Disease. Prim. Care 2024, 51, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Tian, Y.; Zhao, F.; Chen, Z.; Su, P.; Li, Y.; Qian, A. Bone Microenvironment and Osteosarcoma Metastasis. Int. J. Mol. Sci. 2020, 21, 6985. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Croucher, P.; Padhani, A.R.; Clézardin, P.; Chow, E.; Fallon, M.; Guise, T.; Colangeli, S.; Capanna, R.; Costa, L. Bone metastases. Nat. Rev. Dis. Primers 2020, 6, 83. [Google Scholar] [CrossRef]
- Bernstein, Z.S.; Kim, E.B.; Raje, N. Bone Disease in Multiple Myeloma: Biologic and Clinical Implications. Cells 2022, 11, 2308. [Google Scholar] [CrossRef]
- Panaroni, C.; Yee, A.J.; Raje, N.S. Myeloma and Bone Disease. Curr. Osteoporos. Rep. 2017, 15, 483–498. [Google Scholar] [CrossRef] [PubMed]
- Cremers, S.; Drake, M.T.; Ebetino, F.; Bilezikian, J.P.; Russell, R.G.G. Pharmacology of Bisphosphonates. Br. J. Clin. Pharmacol. 2019, 85, 1052–1062. [Google Scholar] [CrossRef]
- Burkiewicz, J.S.; Scarpace, S.L.; Bruce, S.P. Denosumab in osteoporosis and oncology. Ann. Pharmacother. 2009, 43, 1445–1455. [Google Scholar] [CrossRef]
- Bedatsova, L.; Drake, M.T. The skeletal impact of cancer therapies. Br. J. Clin. Pharmacol. 2019, 85, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Kimmel, D.B. Mechanism of Action, Pharmacokinetic and Pharmacodynamic Profile, and Clinical Applications of Nitrogen-Containing Bisphosphonates. J. Dent. Res. 2007, 86, 1022–1033. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and Zoledronate (Zometa) Induced Avascular Necrosis of the Jaws: A Growing Epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Kawahara, M.; Kuroshima, S.; Sawase, T. Clinical Considerations for Medication-Related Osteonecrosis of the Jaw: A Comprehensive Literature Review. Int. J. Implant. Dent. 2021, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.; Hazboun, R.; Tetradis, S. Pathophysiology of Osteonecrosis of the Jaws. Oral Maxillofac. Surg. Clin. N. Am. 2015, 27, 489–496. [Google Scholar] [CrossRef]
- Chang, J.; Hakam, A.E.; McCauley, L.K. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr. Osteoporos. Rep. 2018, 16, 584–595. [Google Scholar] [CrossRef]
- Anesi, A.; Generali, L.; Sandoni, L.; Pozzi, S.; Grande, A. From Osteoclast Differentiation to Osteonecrosis of the Jaw: Molecular and Clinical Insights. Int. J. Mol. Sci. 2019, 20, 4925. [Google Scholar] [CrossRef]
- Otto, S.; Tröltzsch, M.; Jambrovic, V.; Panya, S.; Probst, F.; Ristow, O.; Ehrenfeld, M.; Pautke, C. Tooth Extraction in Patients Receiving Oral or Intravenous Bisphosphonate Administration: A Trigger for BRONJ Development? J. Craniomaxillofac. Surg. 2015, 43, 847–854. [Google Scholar] [CrossRef]
- Yamazaki, T.; Yamori, M.; Ishizaki, T.; Asai, K.; Goto, K.; Takahashi, K.; Nakayama, T.; Bessho, K. Increased Incidence of Osteonecrosis of the Jaw after Tooth Extraction in Patients Treated with Bisphosphonates: A Cohort Study. Int. J. Oral Maxillofac. Surg. 2012, 41, 1397–1403. [Google Scholar] [CrossRef]
- Han, S.; Li, X.; Xia, Y.; Yu, Z.; Cai, N.; Malwal, S.R.; Han, X.; Oldfield, E.; Zhang, Y. Farnesyl Pyrophosphate Synthase as a Target for Drug Development: Discovery of Natural-Product-Derived Inhibitors and Their Activity in Pancreatic Cancer Cells. J. Med. Chem. 2019, 62, 10867–10896. [Google Scholar] [CrossRef]
- Fu, P.A.; Shen, C.Y.; Yang, S.R.; Lee, C.H.; Chen, H.W.; Lai, E.C.; Chung, W.P. Long-term use of denosumab and its association with skeletal-related events and osteonecrosis of the jaw. Sci. Rep. 2023, 13, 8403. [Google Scholar] [CrossRef] [PubMed]
- Querrer, R.; Ferrare, N.; Melo, N.; Stefani, C.M.; Dos Reis, P.E.D.; Mesquita, C.R.M.; Borges, G.A.; Leite, A.F.; Figueiredo, P.T. Differences between bisphosphonate-related and denosumab-related osteonecrosis of the jaws: A systematic review. Support. Care Cancer 2021, 29, 2811–2820. [Google Scholar] [CrossRef] [PubMed]
- Eleutherakis-Papaiakovou, E.; Bamias, A. Antiresorptive treatment-associated ONJ. Eur. J. Cancer Care 2017, 26. [Google Scholar] [CrossRef] [PubMed]
- Rupel, K.; Ottaviani, G.; Gobbo, M.; Contardo, L.; Tirelli, G.; Vescovi, P.; Di Lenarda, R.; Biasotto, M. A Systematic Review of Therapeutical Approaches in Bisphosphonates-Related Osteonecrosis of the Jaw (BRONJ). Oral Oncol. 2014, 50, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Fliefel, R.; Tröltzsch, M.; Kühnisch, J.; Ehrenfeld, M.; Otto, S. Treatment Strategies and Outcomes of Bisphosphonate-Related Osteonecrosis of the Jaw (BRONJ) with Characterization of Patients: A Systematic Review. Int. J. Oral Maxillofac. Surg. 2015, 44, 568–585. [Google Scholar] [CrossRef]
- Khan, A.; Morrison, A.; Cheung, A.; Hashem, W.; Compston, J. Osteonecrosis of the Jaw (ONJ): Diagnosis and Management in 2015. Osteoporos. Int. 2016, 27, 853–859. [Google Scholar] [CrossRef]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Miner. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef]
- Zanocco-Marani, T.; Ricchiuto, S.; Caselli, L.; Lorenzi, E.; Lettucci, E.; Grande, A. Local re-activation of osteoclast differentiation as a novel therapeutic strategy for osteonecrosis of the jaw. Front. Endocrinol. 2024, 15, 1447314. [Google Scholar] [CrossRef]
- Kwon, Y.D.; Kim, D.Y. Role of Teriparatide in Medication-Related Osteonecrosis of the Jaws (MRONJ). Dent. J. 2016, 4, 41. [Google Scholar] [CrossRef]
- Wong, S.K. Glycogen Synthase Kinase-3 Beta (GSK3β) as a Potential Drug Target in Regulating Osteoclastogenesis: An Updated Review on Current Evidence. Biomolecules 2024, 14, 502. [Google Scholar] [CrossRef]
- Otto, M.; Lux, C.; Schlittenbauer, T.; Halling, F.; Ziebart, T. Geranyl-geraniol addition affects potency of bisphosphonates-a comparison in vitro promising a therapeutic approach for bisphosphonate-associated osteonecrosis of the jaw and oral wound healing. Oral Maxillofac. Surg. 2022, 26, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Ricchiuto, S.; Palumbo, R.; Lami, F.; Gavioli, F.; Caselli, L.; Montanari, M.; Zappavigna, V.; Anesi, A.; Zanocco-Marani, T.; Grande, A. The Capacity of Magnesium to Induce Osteoclast Differentiation Is Greatly Enhanced by the Presence of Zoledronate. Biology 2023, 12, 1297. [Google Scholar] [CrossRef]
- Rude, R.K.; Singer, F.R.; Gruber, H.E. Skeletal and hormonal effects of magnesium deficiency. J. Am. Coll. Nutr. 2009, 28, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Belluci, M.M.; Schoenmaker, T.; Rossa-Junior, C.; Orrico, S.R.; de Vries, T.J.; Everts, V. Magnesium deficiency results in an increased formation of osteoclasts. J. Nutr. Biochem. 2013, 24, 1488–1498. [Google Scholar] [CrossRef]
- Belluci, M.M.; de Molon, R.S.; Rossa, C., Jr.; Tetradis, S.; Giro, G.; Cerri, P.S.; Marcantonio, E., Jr.; Orrico, S.R.P. Severe magnesium deficiency compromises systemic bone mineral density and aggravates inflammatory bone resorption. J. Nutr. Biochem. 2020, 77, 108301. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.Y.; Guo, J.; Yang, W.F.; Tao, Z.Y.; Lan, X.; Wang, L.; Xu, J.; Qin, L.; Su, Y.X. Biodegradable magnesium implant enhances angiogenesis and alleviates medication-related osteonecrosis of the jaw in rats. J. Orthop. Translat. 2022, 33, 153–161. [Google Scholar] [CrossRef]
- Zheng, L.Z.; Wang, J.L.; Xu, J.K.; Zhang, X.T.; Liu, B.Y.; Huang, L.; Zhang, R.; Zu, H.Y.; He, X.; Mi, J.; et al. Magnesium and vitamin C supplementation attenuates steroid-associated osteonecrosis in a rat model. Biomaterials 2020, 238, 119828. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef]
- Parenti, S.; Sandoni, L.; Montanari, M.; Zanocco-Marani, T.; Anesi, A.; Iotti, S.; Manfredini, R.; Frassineti, C.; Davalli, P.; Grande, A. Magnesium Favors the Capacity of Vitamin D3 to Induce the Monocyte Differentiation of U937 Cells. Magnes. Res. 2021, 34, 114–129. [Google Scholar]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J.C. Effects of Extracellular Magnesium Extract on the Proliferation and Differentiation of Human Osteoblasts and Osteoclasts in Coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Z.; Qu, X.; Li, H.; Yang, K.; Wan, P.; Tan, L.; Ouyang, Z.; Liu, X.; Tian, B.; Xiao, F.; et al. The effect of metallic magnesium degradation products on osteoclast-induced osteolysis and attenuation of NF-κB and NFATc1 signaling. Biomaterials 2014, 35, 6299–6310. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Luthringer, B.J.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Travers, R. Effects of magnesium and lactose supplementation on bone metabolism in the X-linked hypophosphatemic mouse. Metabolism 1983, 32, 165–171. [Google Scholar] [CrossRef]
- Wallach, S. Relation of magnesium to osteoporosis and calcium urolithiasis. Magnes. Trace Elem. 1991, 10, 281–286. [Google Scholar]
- Aydin, H.; Deyneli, O.; Yavuz, D.; Gözü, H.; Mutlu, N.; Kaygusuz, I.; Akalin, S. Short-term oral magnesium supplementation suppresses bone turnover in postmenopausal osteoporotic women. Biol. Trace Elem. Res. 2010, 133, 136–143. [Google Scholar] [CrossRef]
- Dimai, H.P.; Porta, S.; Wirnsberger, G.; Lindschinger, M.; Pamperl, I.; Dobnig, H.; Wilders-Truschnig, M.; Lau, K.H. Daily oral magnesium supplementation suppresses bone turnover in young adult males. J. Clin. Endocrinol. Metab. 1998, 83, 2742–2748. [Google Scholar] [CrossRef]
- Martini, L.A. Magnesium supplementation and bone turnover. Nutr. Rev. 1999, 57, 227–229. [Google Scholar] [CrossRef]
- Dall, R.D.; Cheung, M.M.; Shewokis, P.A.; Altasan, A.; Volpe, S.L.; Amori, R.; Singh, H.; Sukumar, D. Combined vitamin D and magnesium supplementation does not influence markers of bone turnover or glycemic control: A randomized controlled clinical trial. Nutr. Res. 2023, 110, 33–43. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Fuchigami, M.; Miwa, M. Dietary magnesium supplementation suppresses bone resorption via inhibition of parathyroid hormone secretion in rats fed a high-phosphorus diet. Magnes. Res. 2010, 23, 126–130. [Google Scholar]
- Marie, P.J.; Travers, R.; Delvin, E.E. Influence of magnesium supplementation on bone turnover in the normal young mouse. Calcif. Tissue Int. 1983, 35, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Marie, P.J.; Hott, M. Effect of calcitonin on the magnesium-induced bone resorption in the mouse. Magnesium 1987, 6, 100–108. [Google Scholar]
- Li, Z.H.; Si, Y.; Xu, G.; Chen, X.M.; Xiong, H.; Lai, L.; Zheng, Y.Q.; Zhang, Z.G. High-dose PMA with RANKL and MCSF induces THP-1 cell differentiation into human functional osteoclasts in vitro. Mol. Med. Rep. 2017, 16, 8380–8384. [Google Scholar] [CrossRef] [PubMed]
- Asagiri, M.; Takayanagi, H. The Molecular Understanding of Osteoclast Differentiation. Bone 2007, 40, 251–264. [Google Scholar] [CrossRef]
- Boyle, W.J.; Scott Simonet, W.; Lacey, D.L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Asagiri, M.; Sato, K.; Usami, T.; Ochi, S.; Nishina, H.; Yoshida, H.; Morita, I.; Wagner, E.F.; Mak, T.W.; Serfling, E.; et al. Autoamplification of NFATc1 Expression Determines Its Essential Role in Bone Homeostasis. Exp. Biol. Med. 2005, 202, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T. Regulators of Osteoclast Differentiation and Cell-Cell Fusion. Keio J. Med. 2011, 60, 101–105. [Google Scholar] [CrossRef]
- Pelekanou, V.; Villarroel-Espindola, F.; Schalper, K.A.; Pusztai, L.; Rimm, D.L. CD68, CD163, and Matrix Metalloproteinase 9 (MMP-9) Co-Localization in Breast Tumor Microenvironment Predicts Survival Differently in ER-Positive and -Negative Cancers. Breast Cancer Res. 2018, 20, 154. [Google Scholar] [CrossRef]
- Massey, H.M.; Flanagan, A.M. Human osteoclasts derive from CD14-positive monocytes. Br. J. Haematol. 1999, 106, 167–170. [Google Scholar] [CrossRef]
- Root, S.H.; Aguila, H.L. Novel population of human monocyte and osteoclast progenitors from pluripotent stem cells and peripheral blood. Blood Adv. 2021, 5, 4435–4446. [Google Scholar] [CrossRef]
- Hoefert, S.; Schmitz, I.; Weichert, F.; Gaspar, M.; Eufinger, H. Macrophages and bisphosphonate-related osteonecrosis of the jaw (BRONJ): Evidence of local immunosuppression of macrophages in contrast to other infectious jaw diseases. Clin. Oral Investig. 2015, 19, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Kozutsumi, R.; Kuroshima, S.; Al-Omari, F.A.; Hayano, H.; Nakajima, K.; Kakehashi, H.; Sawase, T. Depletion of macrophages deteriorates bisphosphonate-related osteonecrosis of the jaw-like lesions in mice. Bone 2023, 177, 116899. [Google Scholar] [CrossRef] [PubMed]
- Gkouveris, I.; Soundia, A.; Gouveris, P.; Zouki, D.; Hadaya, D.; Tetradis, S. Macrophage Involvement in Medication-Related Osteonecrosis of the Jaw (MRONJ): A Comprehensive, Short Review. Cancers 2022, 14, 330. [Google Scholar] [CrossRef] [PubMed]
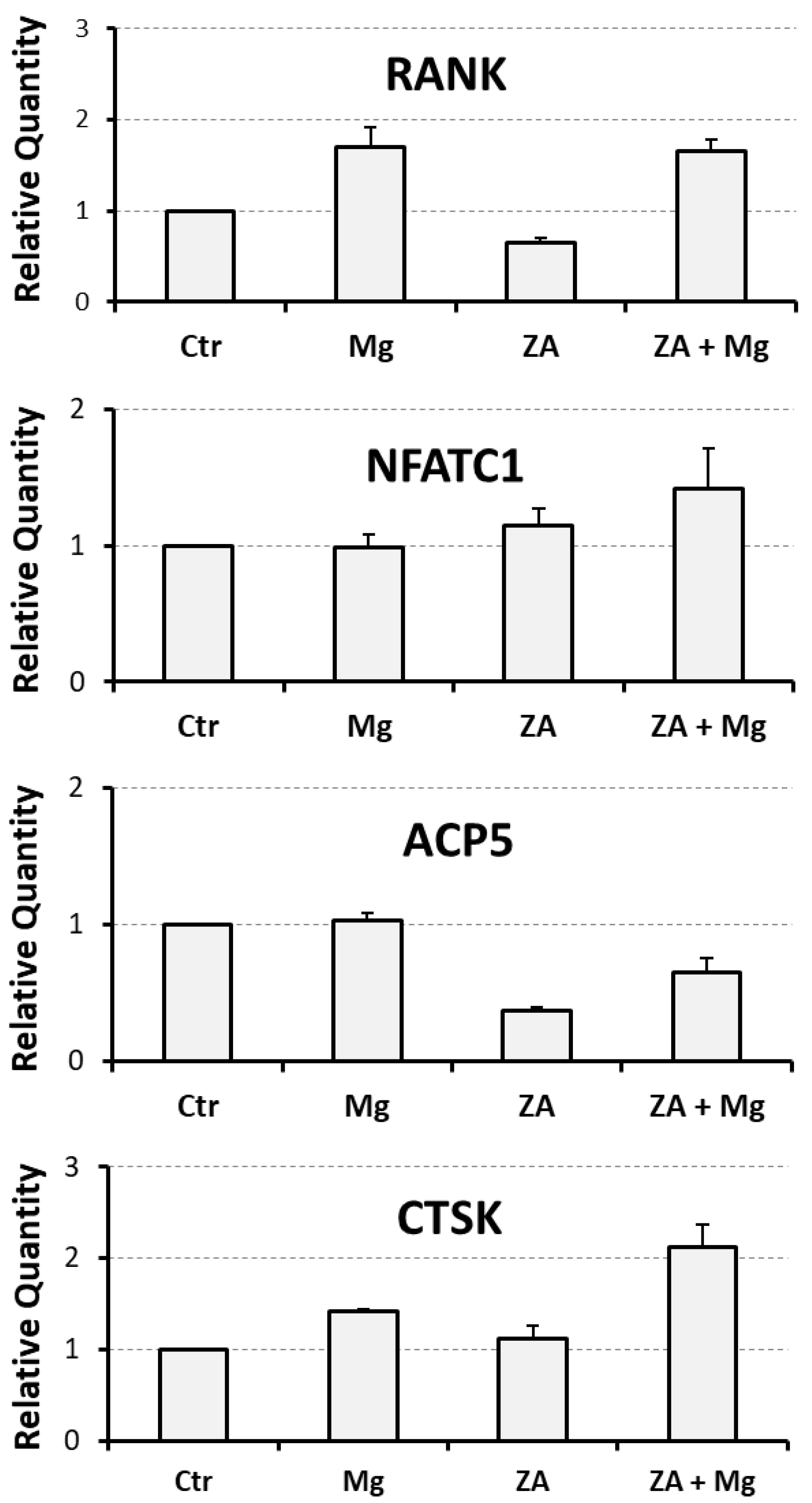
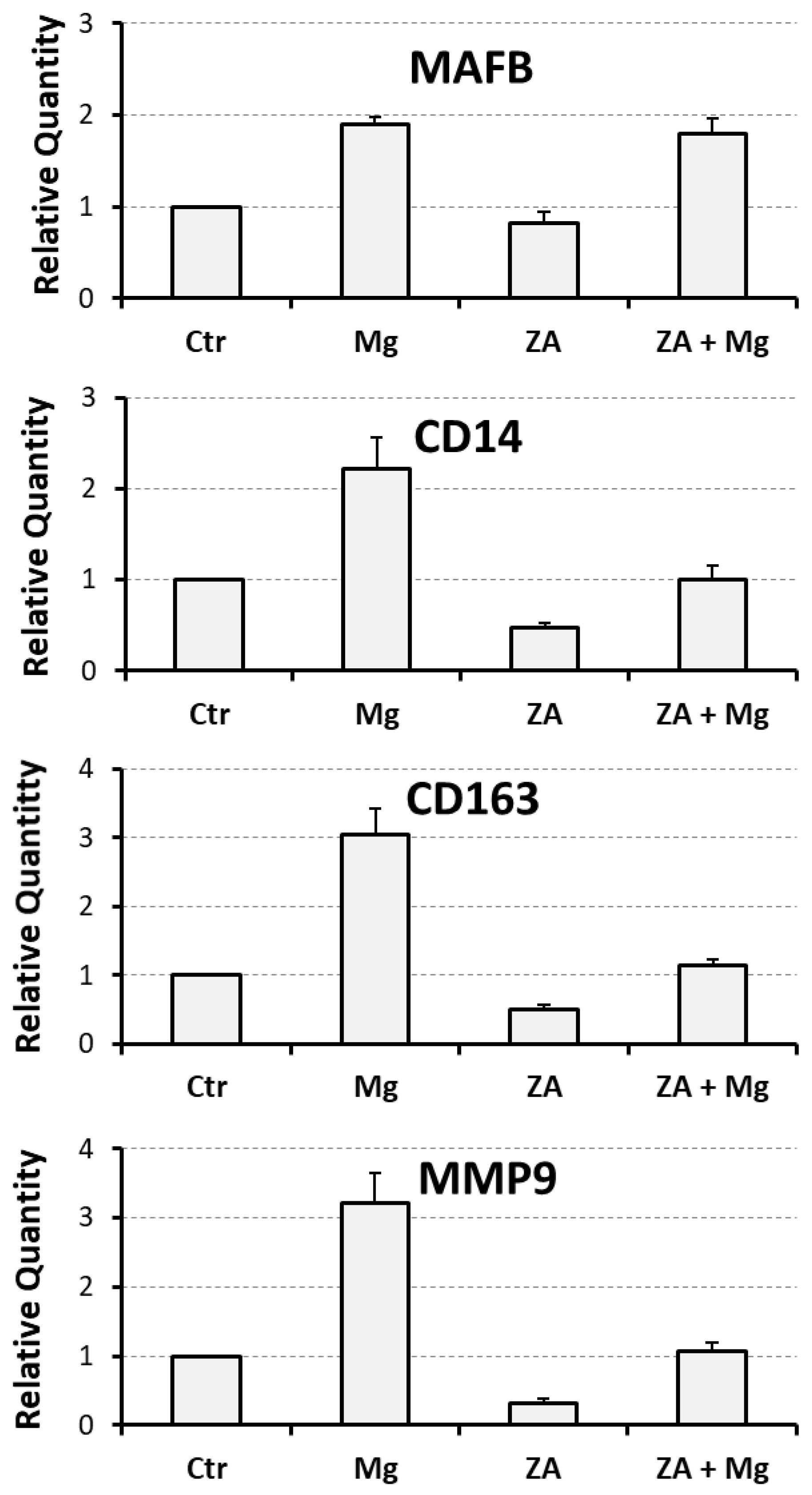
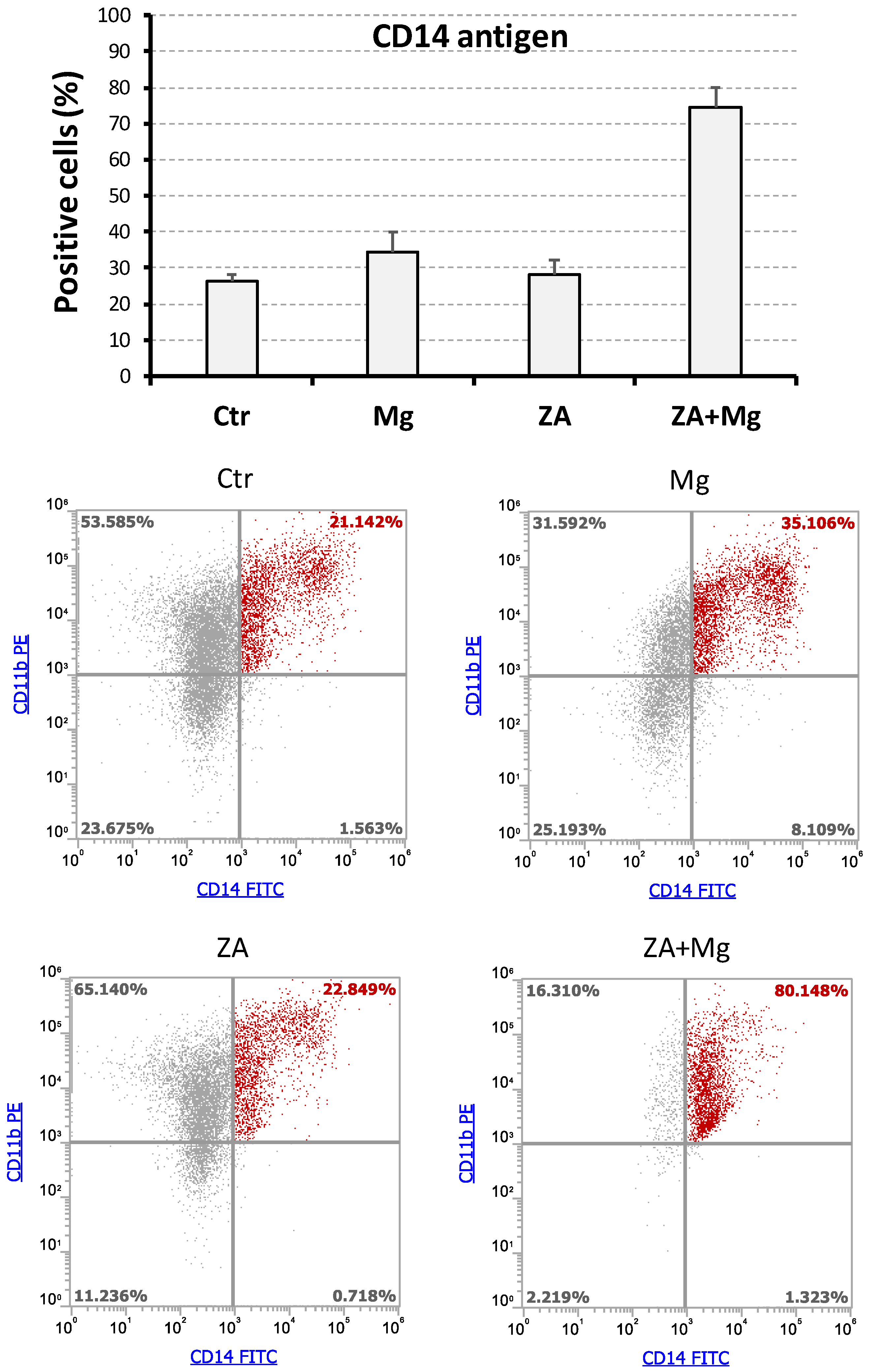
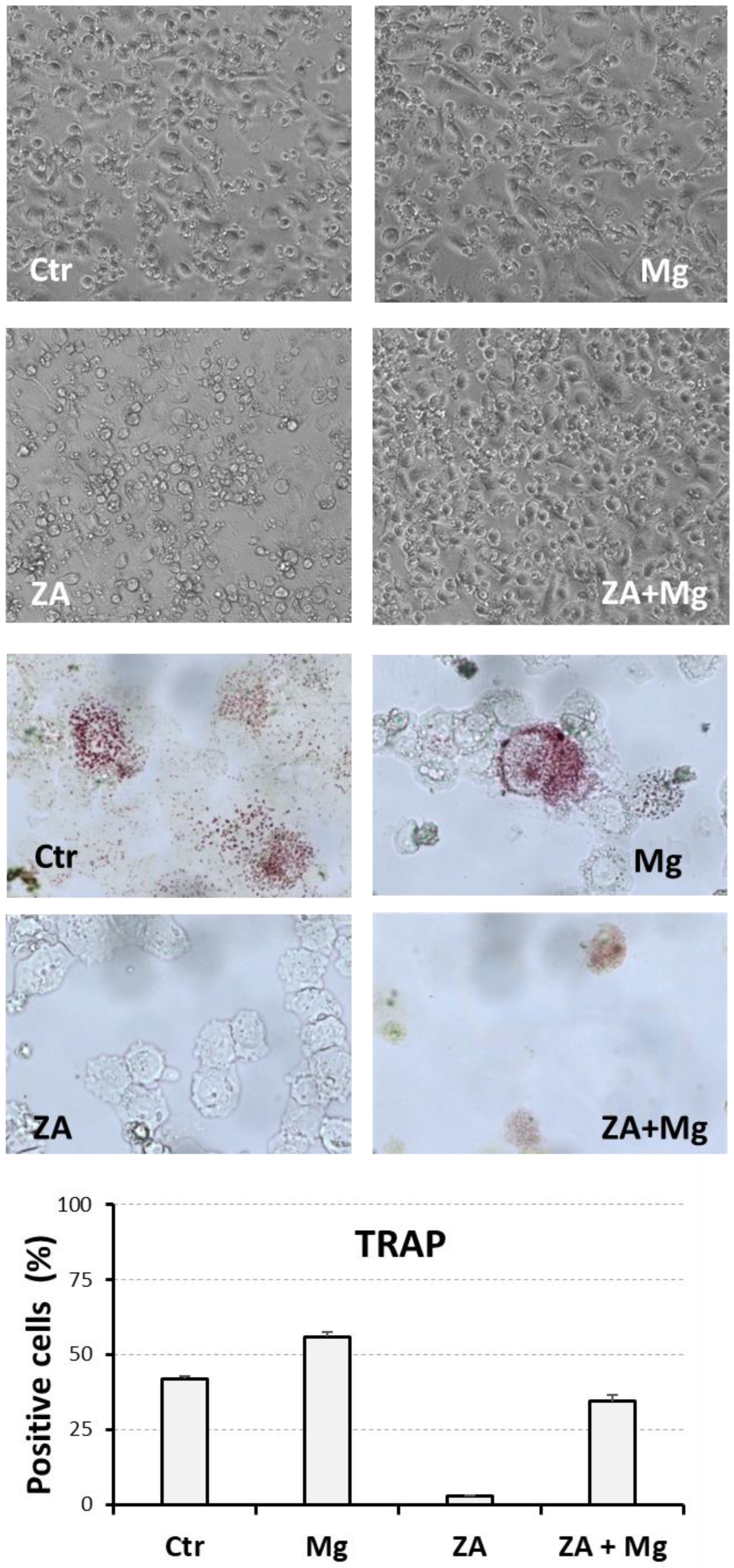
| Analyzed Marker | Ctr | Mg | ZA | ZA + Mg |
|---|---|---|---|---|
| RANK | 1 | 1.7 ± 0.2 | 0.7 ± 0.1 | 1.7 ± 0.1 |
| NFATC1 | 1 | 1.0 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.3 |
| ACP5 | 1 | 1.0 ± 0.1 | 0.4 ± 0.1 | 0.7 ± 0.1 |
| CTSK | 1 | 1.4 ± 0.1 | 1.1 ± 0.1 | 2.1 ± 0.3 |
| MMP9 | 1 | 3.2 ± 0.4 | 0.3 ± 0.1 | 1.1 ± 0.1 |
| MAFB | 1 | 1.9 ± 0.1 | 0.8 ± 0.1 | 1.8 ± 0.2 |
| CD14 | 1 | 2.2 ± 0.3 | 0.5 ± 0.1 | 1.0 ± 0.2 |
| CD163 | 1 | 3.1 ± 0.4 | 0.5 ± 0.1 | 1.2 ± 0.1 |
| Analyzed Parameter | Ctr | Mg | ZA | ZA + Mg |
|---|---|---|---|---|
| Apoptosis | 26.1 ± 3.9 | 21.5 ± 3.9 | 37.5 ± 5.7 | 41.6 ± 3.0 |
| G0/G1 | 71.7 ± 1.3 | 62.4 ± 3.0 | 64.1 ± 2.7 | 58.1 ± 1.6 |
| S | 12.8 ± 1.1 | 15.0 ± 1.6 | 18.0 ± 1.8 | 18.4 ± 1.8 |
| G2/M | 15.5 ± 0.5 | 22.6 ± 2.5 | 17.9 ± 1.3 | 23.5 ± 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caselli, L.; De Pasquale, L.; Palumbo, R.; Ricchiuto, S.; Montanari, M.; Rontauroli, S.; Ottani, A.; Norfo, R.; Zanocco-Marani, T.; Grande, A. Supra-Physiological Levels of Magnesium Counteract the Inhibitory Effect of Zoledronate on RANKL-Dependent Osteoclastogenesis. Biology 2025, 14, 533. https://doi.org/10.3390/biology14050533
Caselli L, De Pasquale L, Palumbo R, Ricchiuto S, Montanari M, Rontauroli S, Ottani A, Norfo R, Zanocco-Marani T, Grande A. Supra-Physiological Levels of Magnesium Counteract the Inhibitory Effect of Zoledronate on RANKL-Dependent Osteoclastogenesis. Biology. 2025; 14(5):533. https://doi.org/10.3390/biology14050533
Chicago/Turabian StyleCaselli, Lorenzo, Lisa De Pasquale, Rossella Palumbo, Silvia Ricchiuto, Monica Montanari, Sebastiano Rontauroli, Alessandra Ottani, Ruggiero Norfo, Tommaso Zanocco-Marani, and Alexis Grande. 2025. "Supra-Physiological Levels of Magnesium Counteract the Inhibitory Effect of Zoledronate on RANKL-Dependent Osteoclastogenesis" Biology 14, no. 5: 533. https://doi.org/10.3390/biology14050533
APA StyleCaselli, L., De Pasquale, L., Palumbo, R., Ricchiuto, S., Montanari, M., Rontauroli, S., Ottani, A., Norfo, R., Zanocco-Marani, T., & Grande, A. (2025). Supra-Physiological Levels of Magnesium Counteract the Inhibitory Effect of Zoledronate on RANKL-Dependent Osteoclastogenesis. Biology, 14(5), 533. https://doi.org/10.3390/biology14050533







