Fgf21 Deficiency Delays Hair Follicle Cycling and Modulates miRNA–Target Gene Interactions in Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Models and Sample Collection
2.2. Preparation of Hair Follicle Cycle-Synchronized Mouse Model
2.3. Real-Time Quantitative PCR (RT-qPCR)
2.4. miRNA Library Construction and High-Throughput Sequencing
2.5. Data Quality Control and miRNA Identification
2.6. Differential Expression Analysis of miRNAs
2.7. Target Gene Enrichment Analysis and Network Construction
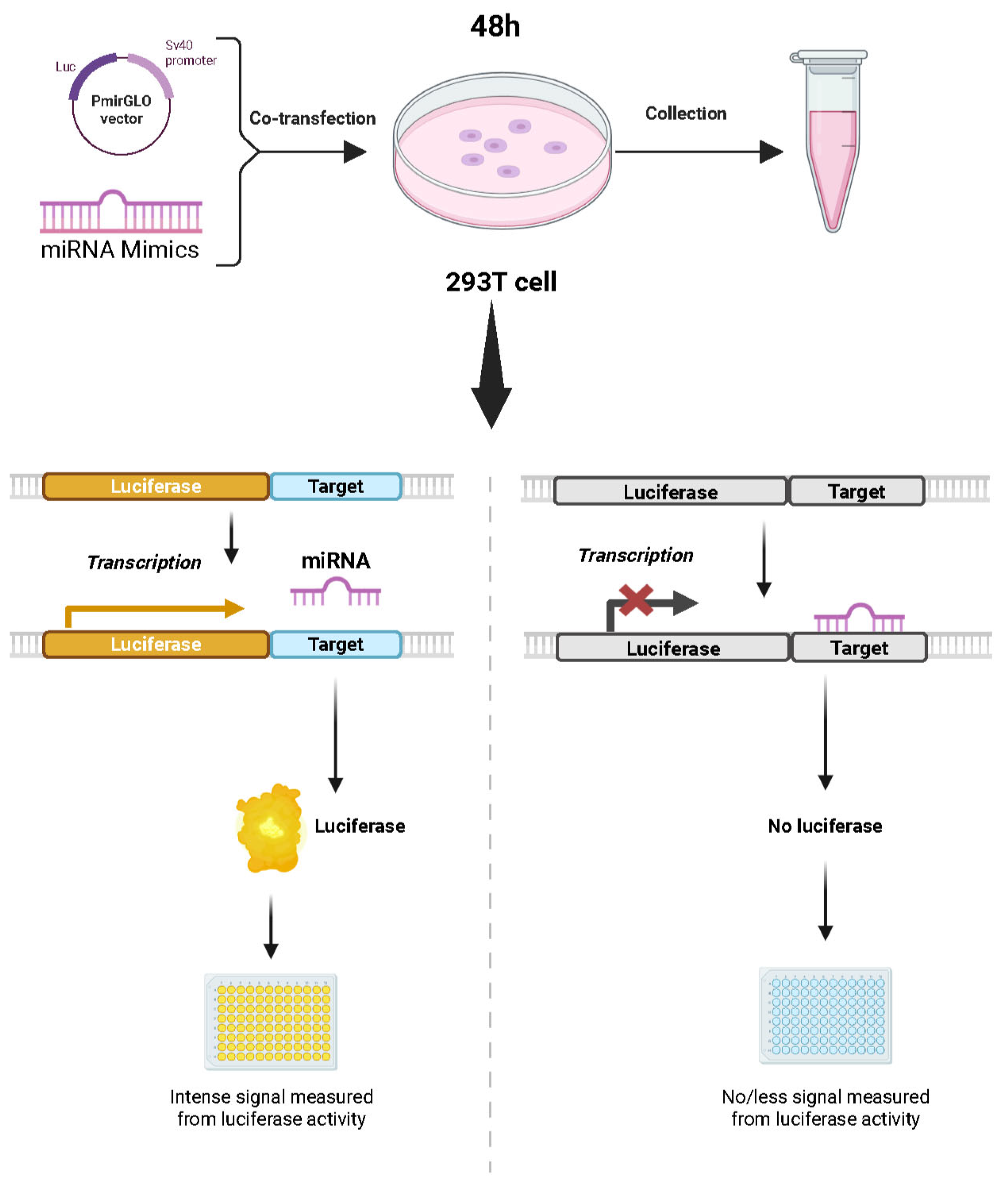
2.8. Validation of Target Relationships
2.9. Morphological Observation
2.10. Immunofluorescence Staining
2.11. Statistical Analysis
3. Results
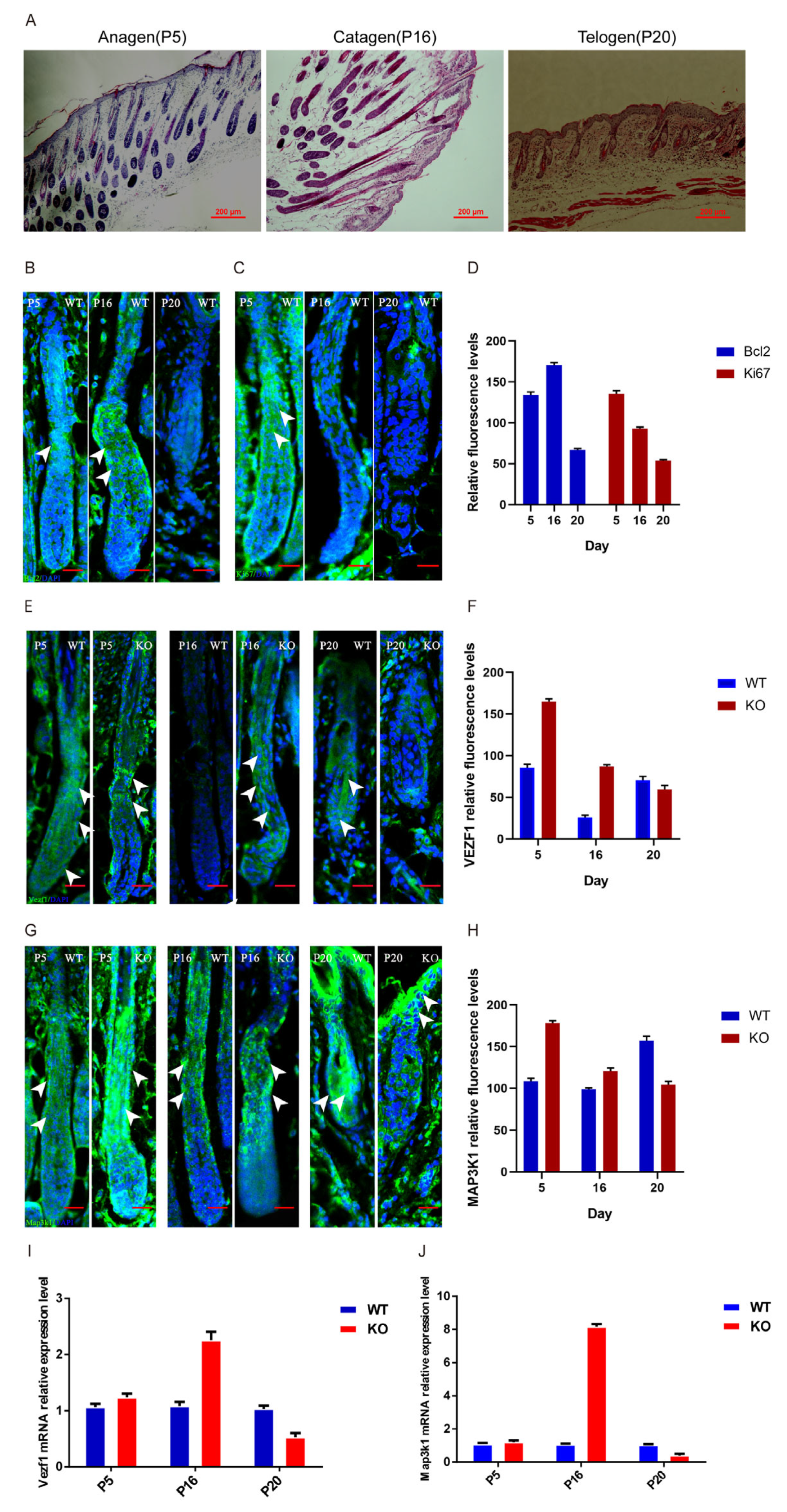
3.1. Fgf21 Knockout Delays the Transition from Telogen to Anagen in Hair Follicles
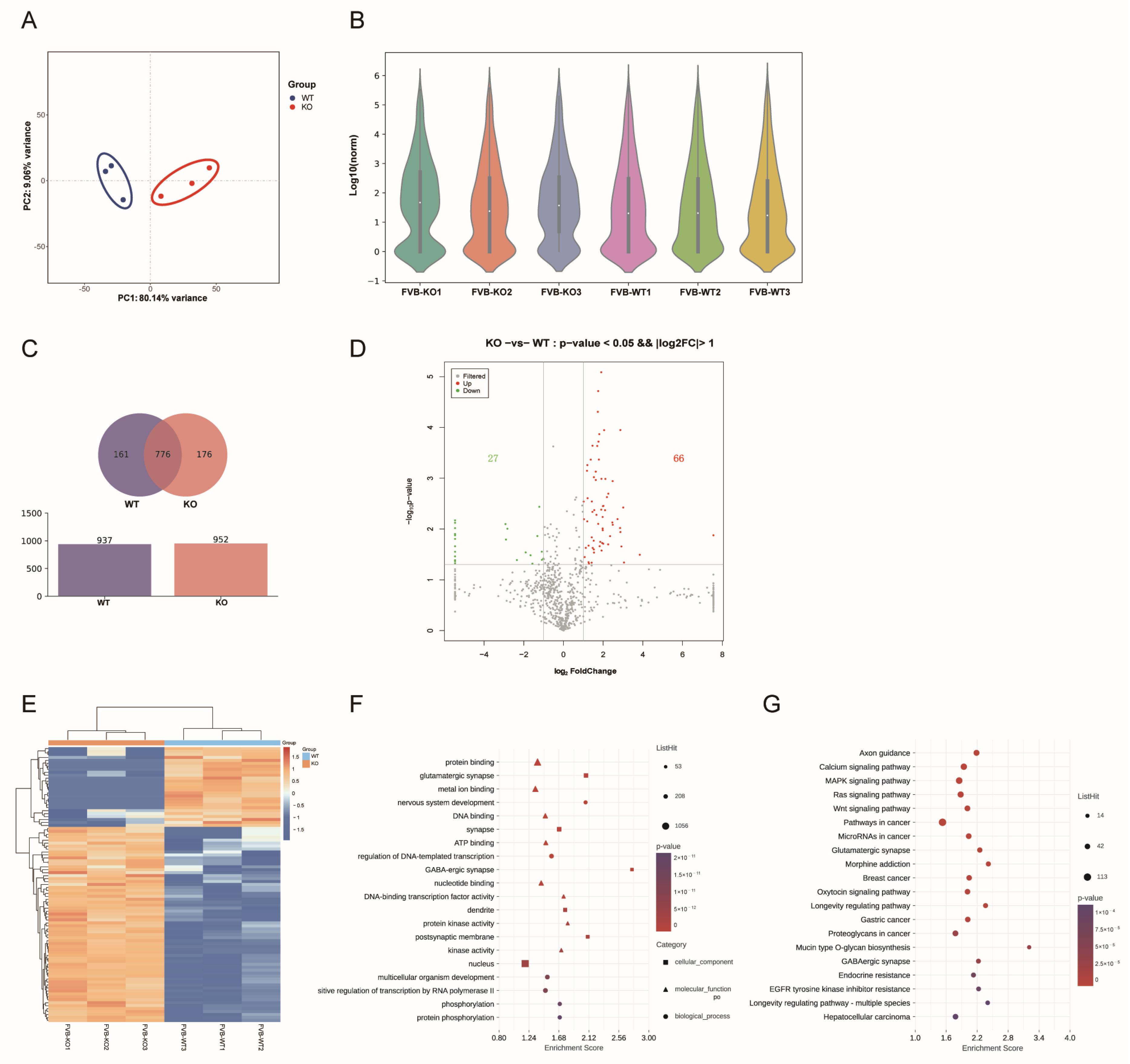
3.2. Expression Analysis and Functional Annotation of miRNAs
3.3. Clustering Analysis and Expression Level Detection of Hair Follicle-Related miRNAs
3.4. Clustering Analysis and Network Construction of Target Genes
3.5. Functional Implications of miRNAs in Hair Follicle Development
3.6. Fgf21 Regulates Expression and Localization of Vezf1 and Map3k1 in the Hair Follicle Growth Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | one-way analysis of variance |
| DPC | dermal papilla cell |
| EMT | epithelial–mesenchymal transition |
| FGF | fibroblast growth factor |
| Fgf21 | fibroblast growth factor 21 |
| FV1b | friend virus susceptibility 1b |
| FVB | friend virus B-type |
| GO | gene ontology |
| H&E | hematoxylin and eosin |
| HFSC | hair follicle stem cell |
| iPSC | induced pluripotent stem cell |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| Map3k1 | mitogen-activated protein kinase kinase kinase 1 |
| miRNA | microRNA |
| NC | negative control |
| PCA | principal component analysis |
| RT-qPCR | real-time quantitative PCR |
| shh | sonic hedgehog |
| TPM | transcripts per million |
| VEGF | vascular endothelial growth factor |
| Vezf1 | vascular endothelial zinc finger 1 |
| WT | wild-type |
References
- Sennett, R.; Rendl, M. Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol. 2012, 23, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.M.; Tsai, C.F.; Yen, C.M.; Lin, M.H.; Wang, W.H.; Chan, C.C.; Chen, C.L.; Phua, K.K.L.; Pan, S.H.; Plikus, M.V.; et al. Inducing hair follicle neogenesis with secreted proteins enriched in embryonic skin. Biomaterials 2018, 167, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, Y.; He, J.; Wang, J.; Chen, X.; Yang, R. Regulation of signaling pathways in hair follicle stem cells. Burn. Trauma 2022, 10, tkac022. [Google Scholar] [CrossRef]
- Kumar, V.; Goutam, R.S.; Park, S.; Lee, U.; Kim, J. Functional Roles of FGF Signaling in Early Development of Vertebrate Embryos. Cells 2021, 10, 2148. [Google Scholar] [CrossRef]
- Goutam, R.S.; Kumar, V.; Lee, U.; Kim, J. Exploring the Structural and Functional Diversity among FGF Signals: A Comparative Study of Human, Mouse, and Xenopus FGF Ligands in Embryonic Development and Cancer Pathogenesis. Int. J. Mol. Sci. 2023, 24, 7556. [Google Scholar] [CrossRef] [PubMed]
- Kimura-Ueki, M.; Oda, Y.; Oki, J.; Komi-Kuramochi, A.; Honda, E.; Asada, M.; Suzuki, M.; Imamura, T. Hair cycle resting phase is regulated by cyclic epithelial FGF18 signaling. J. Investig. Dermatol. 2012, 132, 1338–1345. [Google Scholar] [CrossRef]
- Krugluger, W.; Stiefsohn, K.; Moser, K.; Moser, C.; Laciak, K. Evaluation of Gene Expression Patterns in Micrografts Demonstrate Induction of Catagen-Like Processes During Storage. Dermatol. Surg. 2017, 43, 275–280. [Google Scholar] [CrossRef]
- Mok, K.W.; Saxena, N.; Heitman, N.; Grisanti, L.; Srivastava, D.; Muraro, M.J.; Jacob, T.; Sennett, R.; Wang, Z.; Su, Y.; et al. Dermal Condensate Niche Fate Specification Occurs Prior to Formation and Is Placode Progenitor Dependent. Dev. Cell 2019, 48, 32–48.e35. [Google Scholar] [CrossRef]
- Biggs, L.C.; Mäkelä, O.J.; Myllymäki, S.M.; Das Roy, R.; Närhi, K.; Pispa, J.; Mustonen, T.; Mikkola, M.L. Hair follicle dermal condensation forms via Fgf20 primed cell cycle exit, cell motility, and aggregation. Elife 2018, 7, e36468. [Google Scholar] [CrossRef]
- Jia, Q.; Zhang, S.; Wang, D.; Liu, J.; Luo, X.; Liu, Y.; Li, X.; Sun, F.; Xia, G.; Zhang, L. Regulatory Effects of FGF9 on Dermal Papilla Cell Proliferation in Small-Tailed Han Sheep. Genes 2023, 14, 1106. [Google Scholar] [CrossRef]
- Petiot, A.; Conti, F.J.; Grose, R.; Revest, J.M.; Hodivala-Dilke, K.M.; Dickson, C. A crucial role for Fgfr2-IIIb signalling in epidermal development and hair follicle patterning. Development 2003, 130, 5493–5501. [Google Scholar] [CrossRef]
- Harshuk-Shabso, S.; Dressler, H.; Niehrs, C.; Aamar, E.; Enshell-Seijffers, D. Fgf and Wnt signaling interaction in the mesenchymal niche regulates the murine hair cycle clock. Nat. Commun. 2020, 11, 5114. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Krishnaswami, S.R.; Cowing-Zitron, C.; Hung, N.J.; Reilly-Rhoten, H.; Burns, J.; Yu, B.D. Negative regulation of Shh levels by Kras and Fgfr2 during hair follicle development. Dev. Biol. 2013, 373, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Komi-Kuramochi, A.; Asada, M.; Suzuki, M.; Oki, J.; Jiang, J.; Imamura, T. Comprehensive analysis of FGF and FGFR expression in skin: FGF18 is highly expressed in hair follicles and capable of inducing anagen from telogen stage hair follicles. J. Investig. Dermatol. 2005, 124, 877–885. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Katoh, M.; Katoh, M. Evolutionary conservation of CCND1-ORAOV1-FGF19-FGF4 locus from zebrafish to human. Int. J. Mol. Med. 2003, 12, 45–50. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, F.; Zhao, M.; Ma, L.; Li, H.; Xie, Y.; Nai, R.; Che, T.; Su, R.; Zhang, Y.; et al. The intragenic mRNA-microRNA regulatory network during telogen-anagen hair follicle transition in the cashmere goat. Sci. Rep. 2018, 8, 14227. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhang, K.; Ye, J.X.; Lian, X.H.; Yang, T. Wnt10b promotes growth of hair follicles via a canonical Wnt signalling pathway. Clin. Exp. Dermatol. 2011, 36, 534–540. [Google Scholar] [CrossRef]
- Guo, M.; Jiang, J.; Zhang, A.; Yu, W.; Huang, X. Cholesterol promotes hair growth through activating sympathetic nerves and enhancing the proliferation of hair follicle stem cells. Mol. Med. 2025, 31, 86. [Google Scholar] [CrossRef]
- Veselá, B.; Matalová, E. Expression of apoptosis-related genes in the mouse skin during the first postnatal catagen stage, focused on localization of Bnip3L and caspase-12. Connect. Tissue Res. 2015, 56, 326–335. [Google Scholar] [CrossRef]
- Müller-Röver, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, W.-d.; Zhong, Z.-y.; Zhou, X.-b.; Shi, X.-r.; Wang, X. FGF7 secreted from dermal papillae cell regulates the proliferation and differentiation of hair follicle stem cell1. J. Integr. Agric. 2023, in press. [Google Scholar] [CrossRef]
- Woo, J.; Suh, W.; Sung, J.H. Hair Growth Regulation by Fibroblast Growth Factor 12 (FGF12). Int. J. Mol. Sci. 2022, 23, 9467. [Google Scholar] [CrossRef] [PubMed]
- Flippo, K.H.; Potthoff, M.J. Metabolic Messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Zhang, X.; Li, X.; Bai, Y.; Ao, Y.; Hexig, B.; Guo, X.; Liu, D. Fgf21 knockout mice generated using CRISPR/Cas9 reveal genetic alterations that may affect hair growth. Gene 2020, 733, 144242. [Google Scholar] [CrossRef]
- Xiong, J.W.; Leahy, A.; Lee, H.H.; Stuhlmann, H. Vezf1: A Zn finger transcription factor restricted to endothelial cells and their precursors. Dev. Biol. 1999, 206, 123–141. [Google Scholar] [CrossRef]
- Liang, J.; Huang, W.; Cai, W.; Wang, L.; Guo, L.; Paul, C.; Yu, X.Y.; Wang, Y. Inhibition of microRNA-495 Enhances Therapeutic Angiogenesis of Human Induced Pluripotent Stem Cells. Stem Cells 2017, 35, 337–350. [Google Scholar] [CrossRef]
- Kuschnerus, K.; Straessler, E.T.; Müller, M.F.; Lüscher, T.F.; Landmesser, U.; Kränkel, N. Increased Expression of miR-483-3p Impairs the Vascular Response to Injury in Type 2 Diabetes. Diabetes 2019, 68, 349–360. [Google Scholar] [CrossRef]
- Das, S.; Gupta, V.; Bjorge, J.; Shi, X.; Gong, W.; Garry, M.G.; Garry, D.J. ETV2 and VEZF1 interaction and regulation of the hematoendothelial lineage during embryogenesis. Front. Cell Dev. Biol. 2023, 11, 1109648. [Google Scholar] [CrossRef]
- Wang, F.; Yamauchi, M.; Muramatsu, M.; Osawa, T.; Tsuchida, R.; Shibuya, M. RACK1 regulates VEGF/Flt1-mediated cell migration via activation of a PI3K/Akt pathway. J. Biol. Chem. 2011, 286, 9097–9106. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kuhnert, F.; Campagnolo, L.; Xiong, J.W.; Lemons, D.; Fitch, M.J.; Zou, Z.; Kiosses, W.B.; Gardner, H.; Stuhlmann, H. Dosage-dependent requirement for mouse Vezf1 in vascular system development. Dev. Biol. 2005, 283, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, H.; Kanemura, M.; Yamazaki, T.; Abe, M.; Sato, Y. Vascular endothelial zinc finger 1 is involved in the regulation of angiogenesis: Possible contribution of stathmin/OP18 as a downstream target gene. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 878–884. [Google Scholar] [CrossRef]
- Parker, A.; Cross, S.H.; Jackson, I.J.; Hardisty-Hughes, R.; Morse, S.; Nicholson, G.; Coghill, E.; Bowl, M.R.; Brown, S.D. The goya mouse mutant reveals distinct newly identified roles for MAP3K1 in the development and survival of cochlear sensory hair cells. Dis. Models Mech. 2015, 8, 1555–1568. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, W.; Sun, C.; Liu, Q.; Wang, J.; Wang, M. miR-145-5p inhibits epithelial-mesenchymal transition via the JNK signaling pathway by targeting MAP3K1 in non-small cell lung cancer cells. Oncol. Lett. 2017, 14, 6923–6928. [Google Scholar] [CrossRef]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Ao, Y.; Xie, X.; Ulan, T.; Liu, D.; Guo, X. Fgf21 Deficiency Delays Hair Follicle Cycling and Modulates miRNA–Target Gene Interactions in Mice. Biology 2025, 14, 526. https://doi.org/10.3390/biology14050526
Li Y, Ao Y, Xie X, Ulan T, Liu D, Guo X. Fgf21 Deficiency Delays Hair Follicle Cycling and Modulates miRNA–Target Gene Interactions in Mice. Biology. 2025; 14(5):526. https://doi.org/10.3390/biology14050526
Chicago/Turabian StyleLi, Yana, Yue Ao, Xinru Xie, Tug Ulan, Dongjun Liu, and Xudong Guo. 2025. "Fgf21 Deficiency Delays Hair Follicle Cycling and Modulates miRNA–Target Gene Interactions in Mice" Biology 14, no. 5: 526. https://doi.org/10.3390/biology14050526
APA StyleLi, Y., Ao, Y., Xie, X., Ulan, T., Liu, D., & Guo, X. (2025). Fgf21 Deficiency Delays Hair Follicle Cycling and Modulates miRNA–Target Gene Interactions in Mice. Biology, 14(5), 526. https://doi.org/10.3390/biology14050526




