Future Climate Change Increases the Risk of Suitable Habitats for the Invasive Macrophyte Elodea nuttallii
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
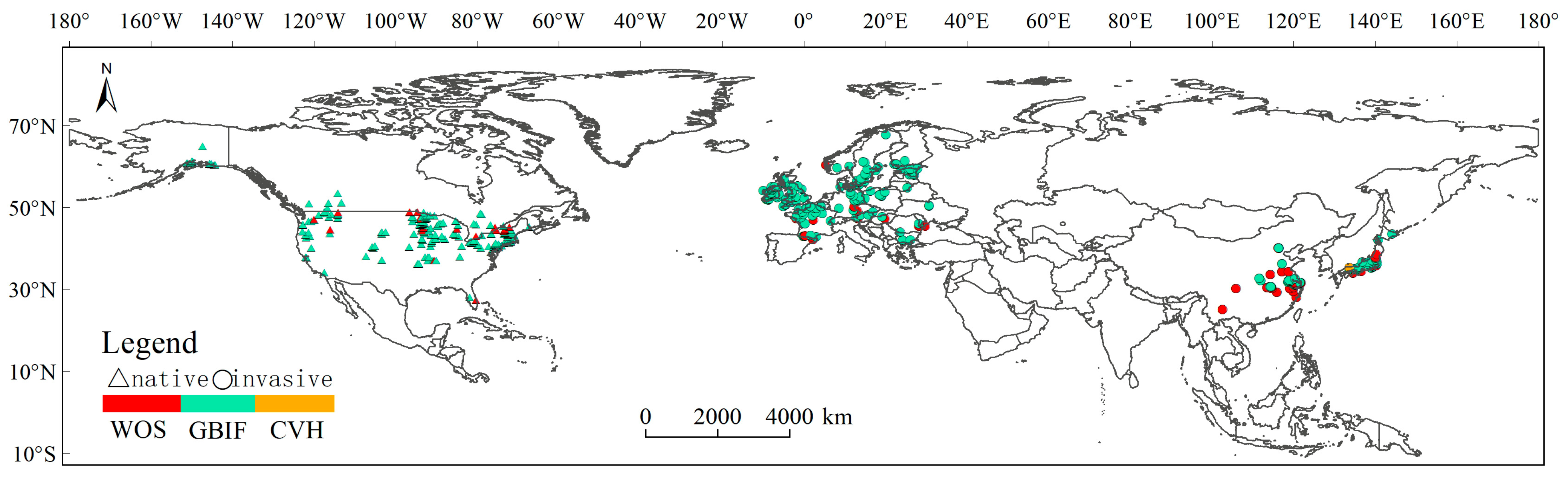
2.1. Occurrence Record Sources
2.2. Access to Impact Factors
2.3. Model Optimization and Precision Evaluation
2.4. Classification of Potential Suitable Habitats for Different Risk Levels
3. Results
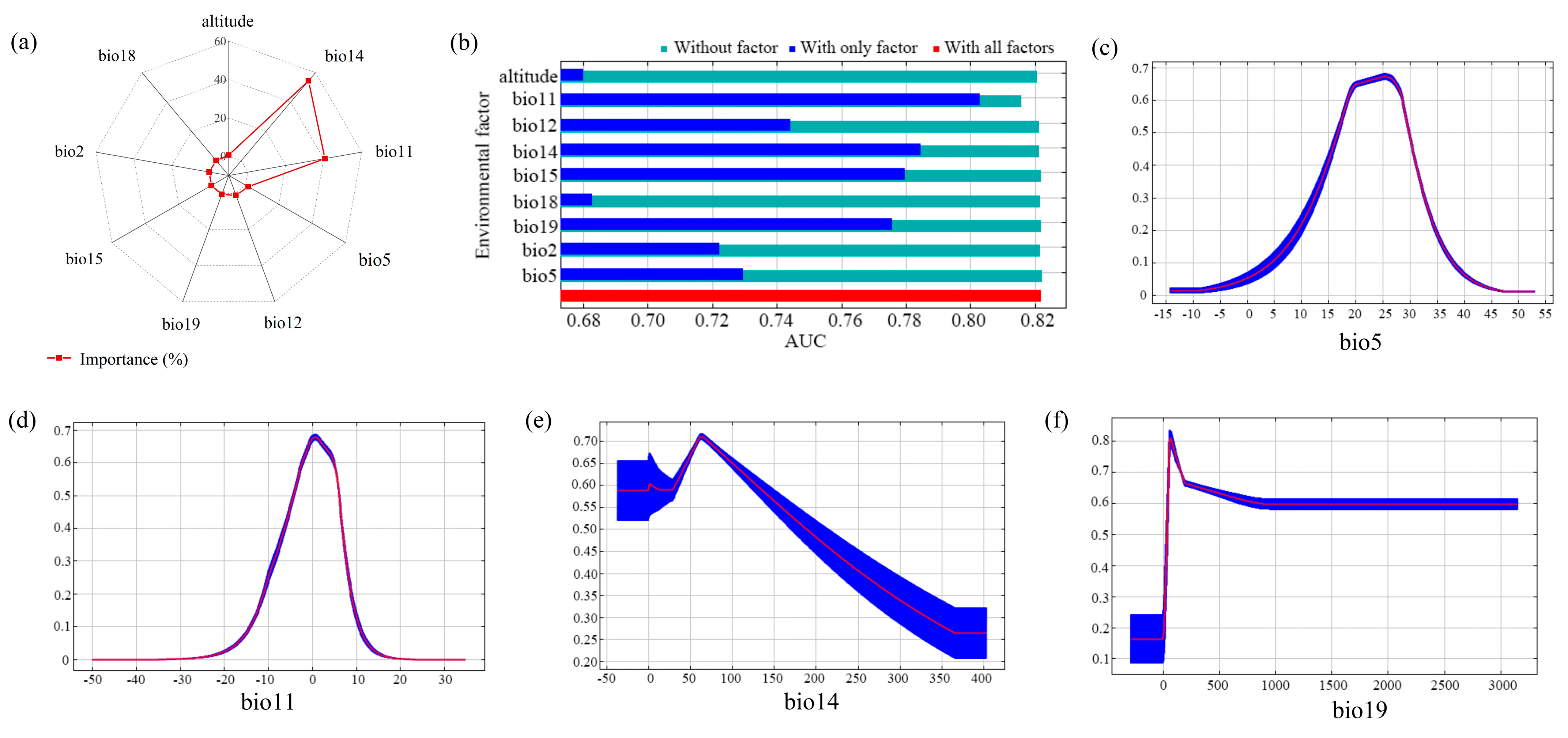
3.1. Significant Impact Factors
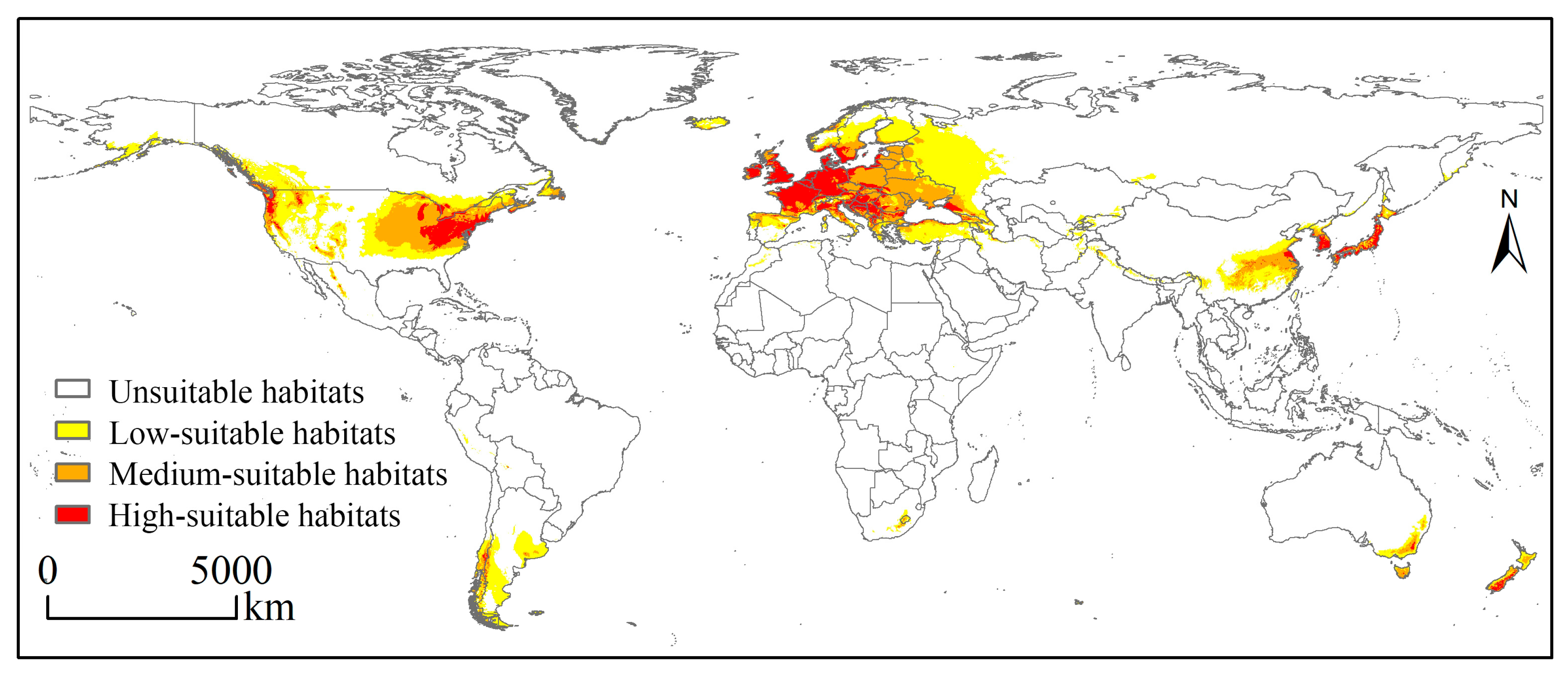
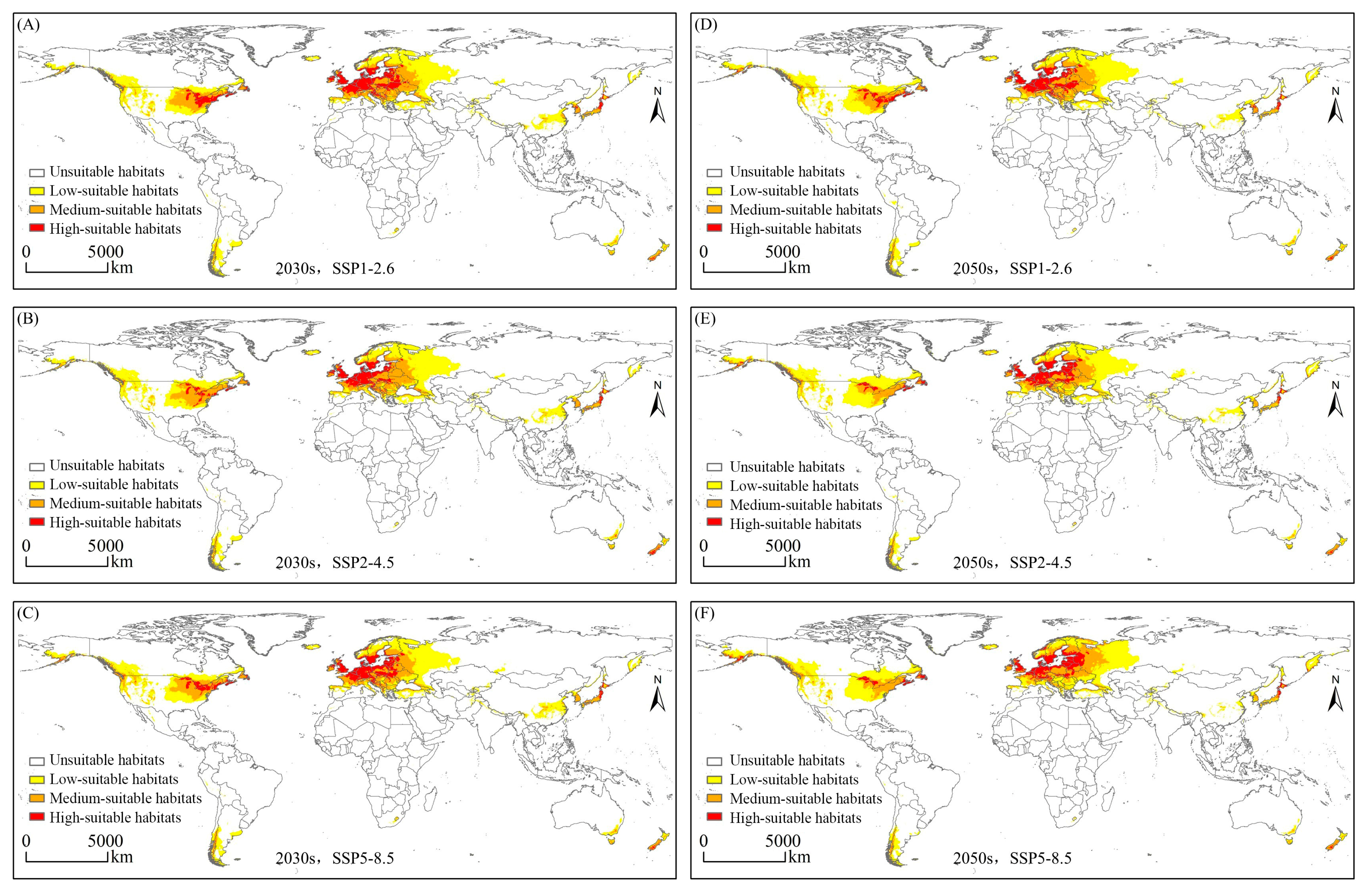
3.2. Globally Potential Suitable Habitats Currently and in the Future
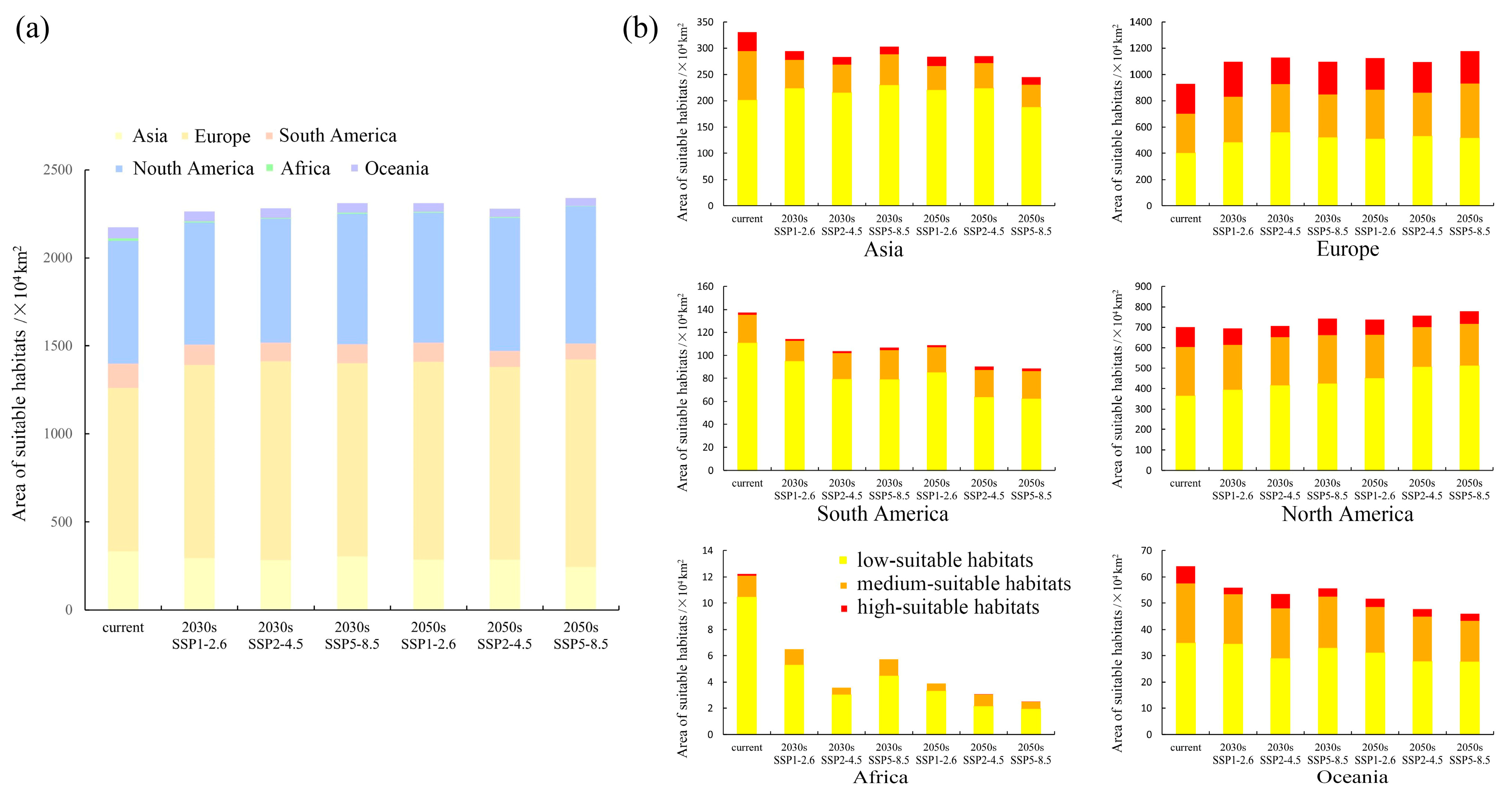
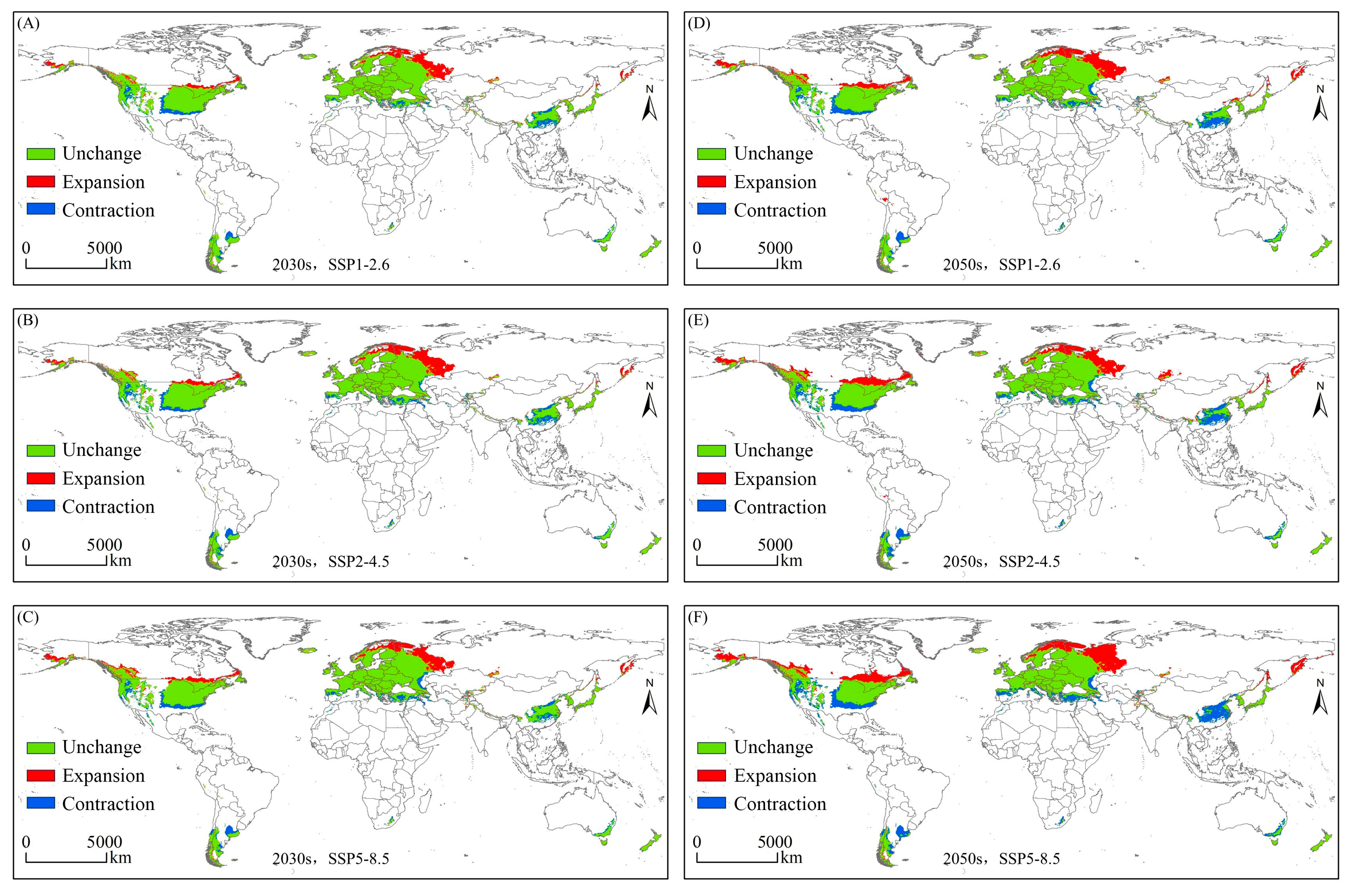
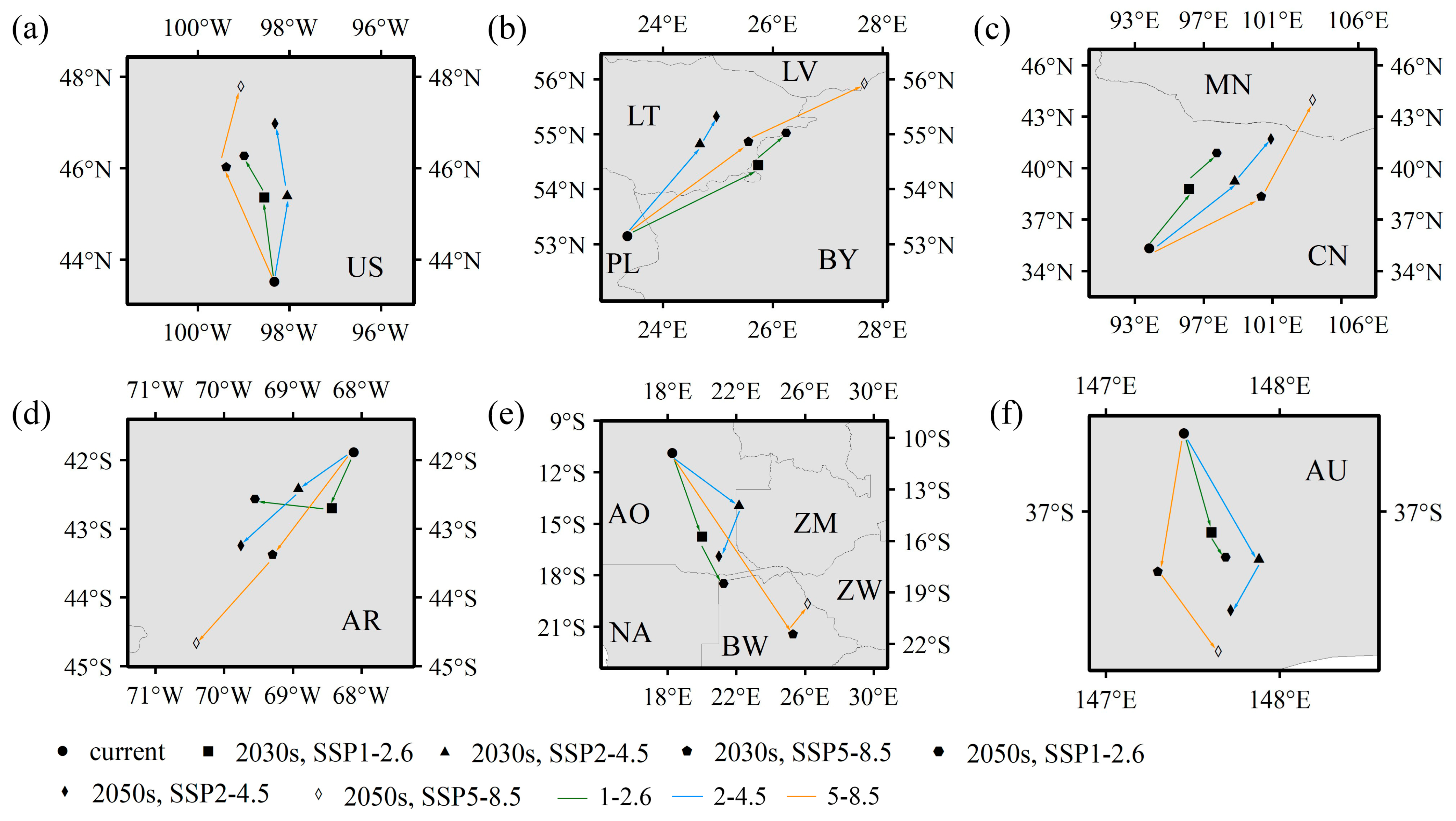
3.3. Variations in the Spatial Distribution of Suitable Habitats
4. Discussion
4.1. Influence of Impact Factors on Potentially Suitable Habitats
4.2. Variations in Potentially Suitable Habitat for Elodea nuttallii
4.3. Early Warning Recommendations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capers, R.S.; Selsky, R.; Bugbee, G.J.; White, J.C. Aquatic plant community invasibility and scale-dependent patterns in native and invasive species richness. Ecology 2007, 88, 3135–3143. [Google Scholar] [CrossRef] [PubMed]
- Pulzatto, M.M.; Cunha, E.R.; Dainez-Filho, M.S.; Thomaz, S.M. Association Between the Success of an Invasive Macrophyte, Environmental Variables and Abundance of a Competing Native Macrophyte. Front. Plant Sci. 2019, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Bando, F.M.; Figueiredo, B.R.S.; Moi, D.A.; Thomaz, S.M.; Michelan, T.S.; García-Girón, J.; Heino, J.; Alahuhta, J.; Romero, G.Q.; Mormul, R.P. Invasion by an exotic grass species homogenizes native freshwater plant communities. J. Ecol. 2023, 111, 799–813. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 750, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Harun, I.; Pushiri, H.; Amirul-Aiman, A.J.; Zulkeflee, Z. Invasive Water Hyacinth: Ecology, Impacts and Prospects for the Rural Economy. Plants 2021, 10, 1613. [Google Scholar] [CrossRef]
- Falasco, E.; Ector, L.; Ciaccio, E.; Hoffmann, L.; Bona, F. Alpine freshwater ecosystems in a protected area: A source of diatom diversity. Hydrobiologia 2012, 695, 233–251. [Google Scholar] [CrossRef]
- Lövei, G.L.; Lewinsohn, T.M. Megadiverse developing countries face huge risks from invasives. Trends Ecol. Evol. 2012, 27, 2–3. [Google Scholar] [CrossRef]
- Liu, X.; Huang, W.; Liu, Y.; Zhan, A. Perspectives of invasive alien species management in China. Ecol. Appl. 2024, 34, e2926. [Google Scholar] [CrossRef]
- Robinson, A.; Lehmann, J.; Barriopedro, D.; Rahmstorf, S.; Coumou, D. Increasing heat and rainfall extremes now far outside the historical climate. Npj Clim. Atmos. Sci. 2021, 4, 45. [Google Scholar] [CrossRef]
- Tabari, H. Climate change impact on flood and extreme precipitation increases with water availability. Sci. Rep. 2020, 10, 13768. [Google Scholar] [CrossRef]
- Omer, A.; Fristoe, T.; Yang, Q.; Razanajatovo, M.; Weigelt, P.; Kreft, H.; Dawson, W.; Dullinger, S.; Essl, F.; Pergl, J.; et al. The role of phylogenetic relatedness on alien plant success depends on the stage of invasion. Nat. Plants 2022, 8, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Pagad, S.; Bisset, S.; Genovesi, P.; Groom, Q.; Hirsch, T.; Jetz, W.; Ranipeta, A.; Schigel, D.; Sica, Y.V.; McGeoch, M.A. Country compendium of the global register of introduced and invasive species. Sci. Data 2022, 9, 391. [Google Scholar] [CrossRef]
- Woolway, R.I.; Kraemer, B.M.; Lenters, J.D.; Merchant, C.J.; O’Reilly, C.M.; Sharma, S. Global lake responses to climate change. Nat. Rev. Earth. Environ. 2020, 1, 388–403. [Google Scholar] [CrossRef]
- Smith, T.; Traxl, D.; Boers, N. Empirical evidence for recent global shifts in vegetation resilience. Nat. Clim. Chang. 2022, 12, 477–484. [Google Scholar] [CrossRef]
- CABI Database. Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.20761 (accessed on 1 April 2024).
- Kunii, H. Seasonal growth and profile structure development of Elodea nuttallii (Planch.) St. John in pond Ojaga-Ike, Japan. Aquat. Bot. 1984, 18, 239–247. [Google Scholar] [CrossRef]
- Xu, J.W.; Li, W.; Liu, G.H.; Zhang, L.J.; Liu, W.Z. Inter-specific competition between two submerged macrophytes, Elodea nuttallii and Hydrilla verticillata. J. Plant Ecol. 2007, 31, 83–92. [Google Scholar] [CrossRef]
- Rascio, N.; Mariani, P.; Vecchia, F.D.; Zanchin, A.L.; Pool, A.; Larcher, W.O. Ultrastructural and Photosynthetic Features of Leaves and Stems of Elodea canadensis. J. Plant Physiol. 1994, 144, 314–323. [Google Scholar] [CrossRef]
- Bučar, M.; Rimac, A.; Šegota, V.; Vuković, N.; Alegro, A. Ecology of Elodea canadensis Michx. and Elodea nuttallii (Planch.) H. St. John—Insights from National Water Monitoring in Croatia. Plants 2024, 13, 1624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, X.; Liu, J.; Hong, Y.; He, Q.; Yu, D.; Liu, C.; Dingshanbayi, H. Greater Performance of Exotic Elodea nuttallii in Response to Water Level May Make It a Better Invader Than Exotic Egeria densa During Winter and Spring. Front. Plant Sci. 2019, 10, 144. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, S.Z.; Bai, X.L.; Hu, W.; Wang, X.R. Evolution of community structure of aquatic macrophytes in East Taihu Lake and its wetlands. Acta Ecol. Sin. 2005, 25, 1541–1548. [Google Scholar] [CrossRef]
- EPPO Global Database. Available online: https://gd.eppo.int/taxon/ELDNU (accessed on 1 April 2024).
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Banerjee, A.K.; Feng, H.; Lin, Y.; Liang, X.; Wang, J.; Huang, Y. Setting the priorities straight—Species distribution models assist to prioritize conservation targets for the mangroves. Sci. Total Environ. 2022, 806, 150937. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum Entropy Modeling of Species Geographic Distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Peterson, A.T.; Papes, M.; Soberón, J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol. Model. 2008, 213, 63–72. [Google Scholar] [CrossRef]
- Javidan, N.; Kavian, A.; Pourghasemi, H.R.; Conoscenti, C.; Jafarian, Z.; Rodrigo-Comino, J. Evaluation of multi-hazard map produced using MaxEnt machine learning technique. Sci. Rep. 2021, 11, 6496. [Google Scholar] [CrossRef]
- Nikkel, E.; Clements, D.R.; Anderson, D.; Williams, J.L. Regional habitat suitability for aquatic and terrestrial invasive plant species may expand or contract with climate change. Biol. Invasions 2023, 25, 3805–3822. [Google Scholar] [CrossRef]
- Yasuno, N. Potential risk maps for invasive aquatic plants in Kanto region, Japan. Landsc. Ecol. Eng. 2022, 18, 299–305. [Google Scholar] [CrossRef]
- Kariyawasam, C.S.; Kumar, L.; Ratnayake, S.S. Potential distribution of aquatic invasive alien plants, Eichhornia crassipes and Salvinia molesta under climate change in Sri Lanka. Wetl. Ecol. Manag. 2021, 29, 531–545. [Google Scholar] [CrossRef]
- Pulparambil, H.; Nediyaparambu, S.P. Ecological niche modelling in identifying habitats for effective species conservation: A study on Endemic aquatic plant Crinum malabaricum. J. Nat. Conserv. 2023, 76, 126517. [Google Scholar] [CrossRef]
- GBIF. org (01 April 2024) GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.3qyuw4 (accessed on 1 April 2024).
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-Km Spatial Resolution Climate Surfaces for Global Land Areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Betts, M.G.; Yang, Z.; Hadley, A.S.; Smith, A.C.; Rousseau, J.S.; Northrup, J.M.; Nocera, J.J.; Gorelick, N.; Gerber, B.D. Forest degradation drives widespread avian habitat and population declines. Nat. Ecol. Evol. 2022, 6, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Bart, S.; Ana, D.J.; Konstantinos, T.; Karen, N.S.; Janina, E.; Eugenio, G. Modelling hot spot areas for the invasive alien plant Elodea nuttallii in the EU. Manag. Biol. Invasions 2019, 10, 151–170. [Google Scholar] [CrossRef]
- Cordeiro, P.F.; Goulart, F.F.; Macedo, D.R.; Campos, M.D.C.S.; Castro, S.R. Modeling of the potential distribution of Eichhornia crassipes on a global scale: Risks and threats to water ecosystems. Rev. Ambiente Água 2020, 15, e2421. [Google Scholar] [CrossRef]
- Buchan, L.A.J.; Padilla, D.K. Predicting the Likelihood of eurasian watermilfoil presence in lakes, a macrophyte monitoring tool. Ecol. Appl. 2000, 10, 1442–1455. [Google Scholar] [CrossRef]
- Ma, J.M.; Jin, T.X.; He, F.; Wu, J.; Cheng, S.; Wu, Z. Responses of Elodea nuttallii and Ceratophyllum demersum to high temperature. Fresenius Environ. Bull. 2009, 18, 1588–1596. [Google Scholar]
- Xu, W.W.; Hu, W.P.; Deng, J.C.; Zhu, J.G.; Li, Q.Q. How do water depth and harvest intensity affect the growth and reproduction of Elodea nuttallii (Planch.) St. John? J. Plant Ecol. 2016, 9, 212–223. [Google Scholar] [CrossRef][Green Version]
- Zehnsdorf, A.; Hussner, A.; Eismann, F.; Rönicke, H.; Melzer, H. Management options of invasive Elodea nuttallii and Elodea canadensis. Limnologica 2015, 51, 110–117. [Google Scholar] [CrossRef]
- Cao, Y.; Li, J.; Yin, W.; Li, W.; Han, Q.X. Two negatives make an affirmative: Can extreme flooding reduce the expansion of invasive submerged macrophyte in a large river? J. Environ. Manag. 2023, 346, 118964. [Google Scholar] [CrossRef]
- Atapaththu, K.S.S.; Asaeda, T. Growth and stress responses of Nuttall’s waterweed Elodea nuttallii (Planch) St. John to water movements. Hydrobiologia 2015, 747, 217–233. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Koppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Dore, M.H.I. Climate change and changes in global precipitation patterns: What do we know? Environ. Int. 2005, 31, 1167–1181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, D. Urban carbon emission scenario prediction and multi-objective land use optimization strategy under carbon emission constraints. J. Clean. Prod. 2023, 430, 139684. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, C.Z.; Singh, K.; Zhong, K.Y. Green technology innovation, trade deficit and carbon emission transfer in agriculture under the new “dual circulation” development pattern of China. Ecol. Indic. 2024, 159, 111757. [Google Scholar] [CrossRef]
- Caplat, P.; Edelaar, P.; Dudaniec, R.Y.; Green, A.J.; Okamura, B.; Cote, J.; Ekroos, J.; Jonsson, P.R.; Löndahl, J.; Tesson, S.V.M.; et al. Looking beyond the mountain: Dispersal barriers in a changing world. Front. Ecol. Environ. 2016, 14, 261–268. [Google Scholar] [CrossRef]
- Huang, R.; Oduor, A.M.O.; Yan, Y.; Yu, W.; Chao, C.; Dong, L.; Jin, S.; Li, F. Nutrient enrichment, propagule pressure, and herbivory interactively influence the competitive ability of an invasive alien macrophyte Myriophyllum aquaticum. Front. Plant Sci. 2024, 15, 1411767. [Google Scholar] [CrossRef]
- Hulme, P.E. Trade, transport and trouble: Managing invasive species pathways in an era of globalization. J. Appl. Ecol. 2009, 46, 10–18. [Google Scholar] [CrossRef]
- Guan, B.C.; Guo, H.J.; Chen, S.S.; Li, D.M.; Liu, X.; Gong, X. Shifting ranges of eleven invasive alien plants in China in the face of climate change. Ecol. Inform. 2019, 55, 101024. [Google Scholar] [CrossRef]
- Raymond, C.; Horton, R.M.; Zscheischler, J.; Martius, O.; AghaKouchak, A.; Balch, J.; Bowen, S.G.; Camargo, S.J.; Hess, J.; Kornhuber, K.; et al. Understanding and managing connected extreme events. Nat. Clim. Chang. 2020, 10, 611–621. [Google Scholar] [CrossRef]
- Qi, Y.; Xian, X.; Zhao, H.; Yang, M.; Zhang, Y.; Yu, W.; Liu, W. World Spread of Tropical Soda Apple (Solanum viarum) under Global Change: Historical Reconstruction, Niche Shift, and Potential Geographic Distribution. Biology 2023, 12, 1179. [Google Scholar] [CrossRef] [PubMed]
- Casal, C.M.V. Global documentation of fish introductions: The growing grisis and recommendations for action. Biol. Invasions 2006, 8, 3–11. [Google Scholar] [CrossRef]
- Zhao, H.; Xian, X.; Yang, N.; Chen, T.; Li, J.; Sheppard, A.; Wan, F.; Qi, G.; Liu, W. A Proposed Coupling Framework of Biological Invasions: Quantifying the Management Prioritization in Mealybugs Invasion. Glob. Chang. Biol. 2024, 30, e17583. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of World Fisheries and Aquaculture 2016; FAO: Rome, Italy, 2016; Available online: https://www.fao.org/family-farming/detail/en/c/465805/ (accessed on 1 April 2024).
| Abbreviations | Full Names | Abbreviations | Full Names |
|---|---|---|---|
| bio 1 | Annual Mean Temperature | bio 11 | Mean Temperature of Coldest Quarter |
| bio 2 | Mean Diurnal Range | bio 12 | Annual Precipitation |
| bio 3 | Isothermality | bio 13 | Precipitation of Wettest Month |
| bio 4 | Temperature Seasonality | bio 14 | Precipitation of Driest Month |
| bio 5 | Max Temperature of Warmest Month | bio 15 | Precipitation Seasonality |
| bio 6 | Min Temperature of Coldest Month | bio 16 | Precipitation of Wettest Quarter |
| bio 7 | Temperature Annual Range | bio 17 | Precipitation of Driest Quarter |
| bio 8 | Mean Temperature of Wettest Quarter | bio 18 | Precipitation of Warmest Quarter |
| bio 9 | Mean Temperature of Driest Quarter | bio 19 | Precipitation of Coldest Quarter |
| bio 10 | Mean Temperature of Warmest Quarter | Altitude | Altitude |
| Indicators | 2030s | 2050s | |||||
|---|---|---|---|---|---|---|---|
| SSP1-2.6 | SSP2-4.5 | SSP5-8.5 | SSP1-2.6 | SSP2-4.5 | SSP5-8.5 | ||
| Expansion | Area/×104 km2 | 331.26 | 392.13 | 413.24 | 465.19 | 513.54 | 656.28 |
| Proportion/% | 2.17 | 2.57 | 2.71 | 3.05 | 3.36 | 4.30 | |
| Contraction | Area/×104 km2 | 242.27 | 286.89 | 275.83 | 327.89 | 409.09 | 490.01 |
| Proportion/% | 1.59 | 1.88 | 1.81 | 2.15 | 2.68 | 3.21 | |
| Unchange | Area/×104 km2 | 1939.51 | 1894.90 | 1905.96 | 1853.90 | 1772.70 | 1691.77 |
| Proportion/% | 12.70 | 12.41 | 12.48 | 12.14 | 11.61 | 11.08 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Y.; Zhang, Y.; Xue, J.; Zhang, Z.; Cao, J.; Yang, N.; Wan, F.; Xian, X.; Liu, W. Future Climate Change Increases the Risk of Suitable Habitats for the Invasive Macrophyte Elodea nuttallii. Biology 2025, 14, 504. https://doi.org/10.3390/biology14050504
Qi Y, Zhang Y, Xue J, Zhang Z, Cao J, Yang N, Wan F, Xian X, Liu W. Future Climate Change Increases the Risk of Suitable Habitats for the Invasive Macrophyte Elodea nuttallii. Biology. 2025; 14(5):504. https://doi.org/10.3390/biology14050504
Chicago/Turabian StyleQi, Yuhan, Yu Zhang, Jiali Xue, Zhen Zhang, Jingjing Cao, Nianwan Yang, Fanghao Wan, Xiaoqing Xian, and Wanxue Liu. 2025. "Future Climate Change Increases the Risk of Suitable Habitats for the Invasive Macrophyte Elodea nuttallii" Biology 14, no. 5: 504. https://doi.org/10.3390/biology14050504
APA StyleQi, Y., Zhang, Y., Xue, J., Zhang, Z., Cao, J., Yang, N., Wan, F., Xian, X., & Liu, W. (2025). Future Climate Change Increases the Risk of Suitable Habitats for the Invasive Macrophyte Elodea nuttallii. Biology, 14(5), 504. https://doi.org/10.3390/biology14050504







