Comparative Analysis of Cytokine Expression Profiles in Prostate Cancer Patients
Simple Summary
Abstract
1. Background
2. Materials and Methods
2.1. Subjects and Samples
2.2. Multiplex ELISA (Luminex)
2.3. Statistical Analysis
3. Results
3.1. Clinicopathological Features of PCa Patients
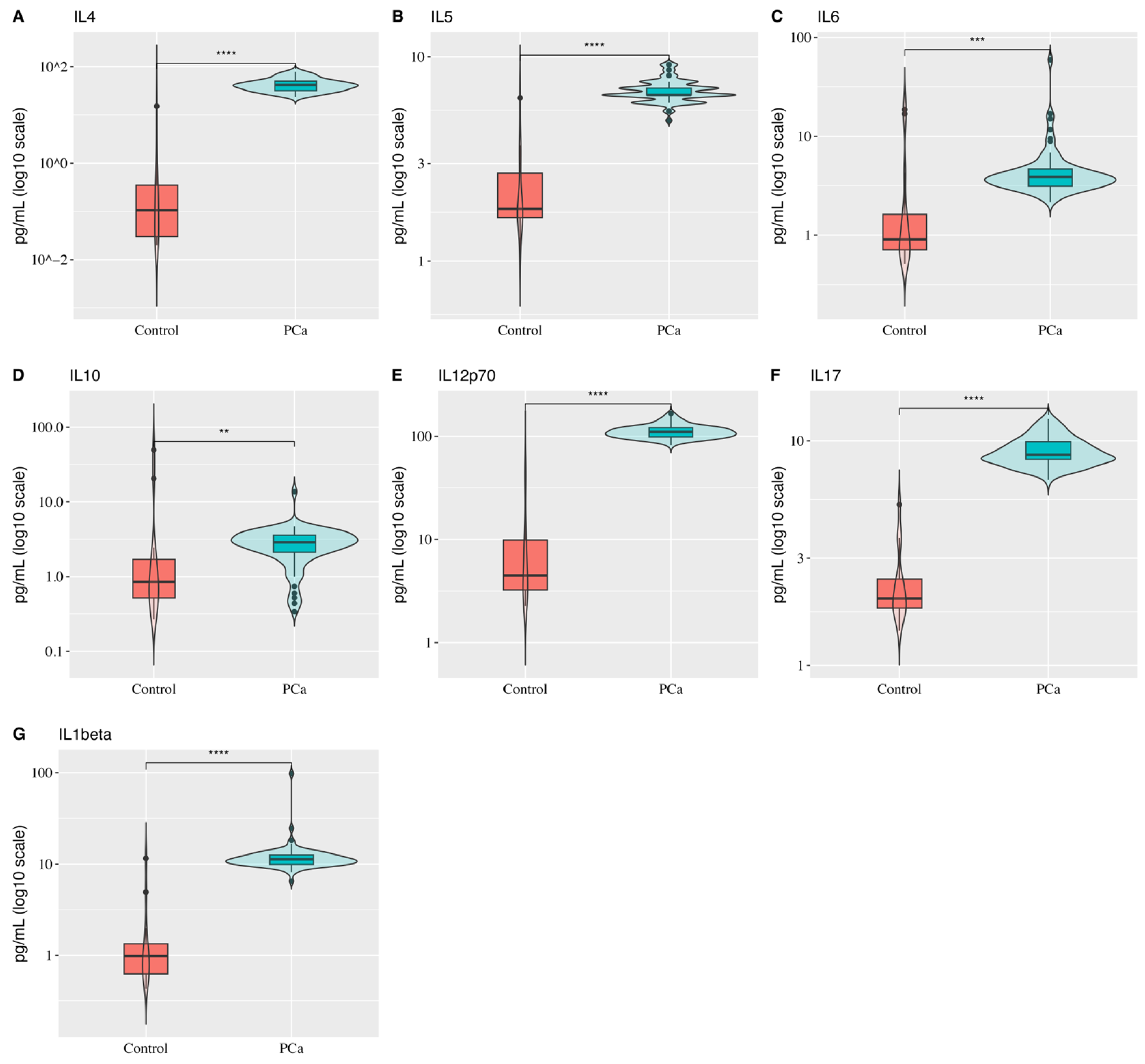
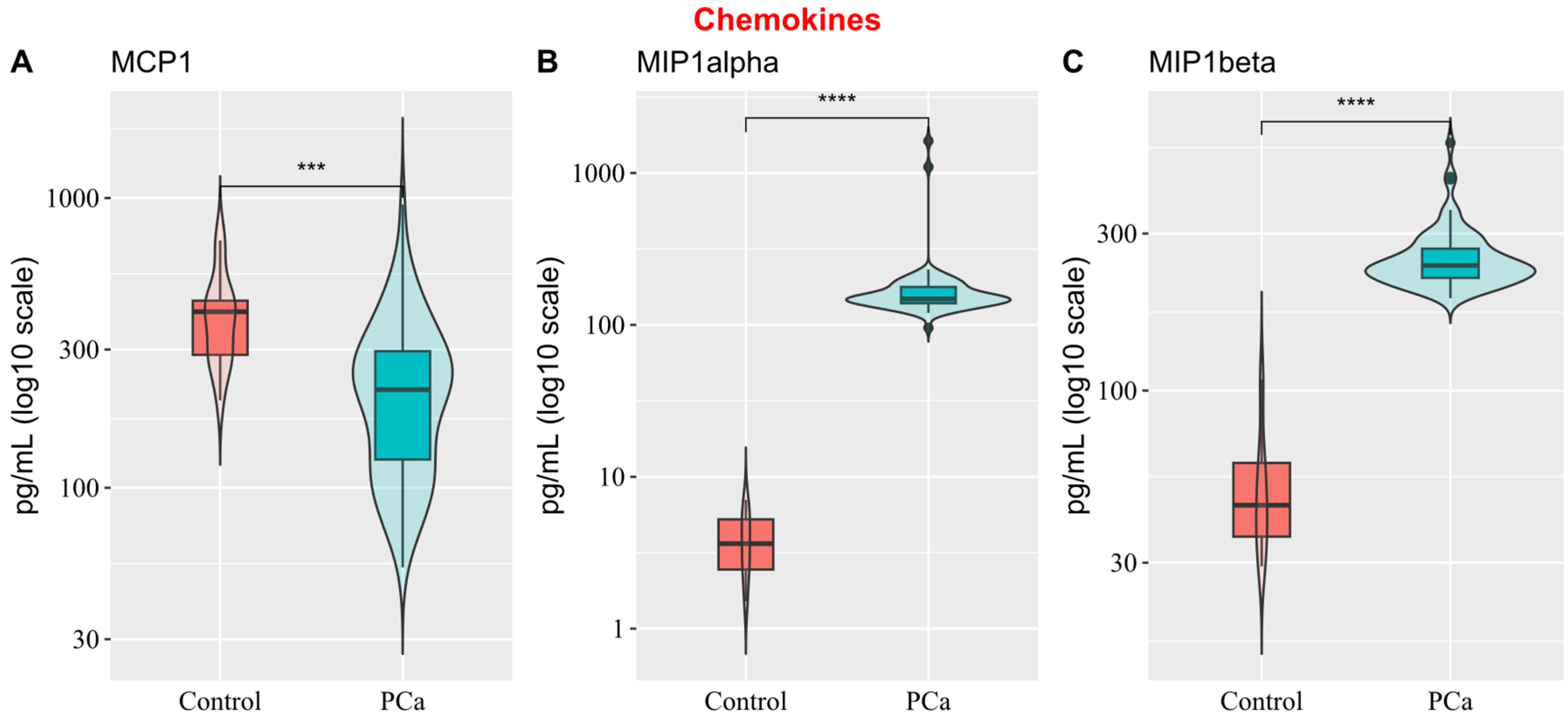
3.2. Comparison of Cytokine Expression Profiles Between Healthy and PCa Men
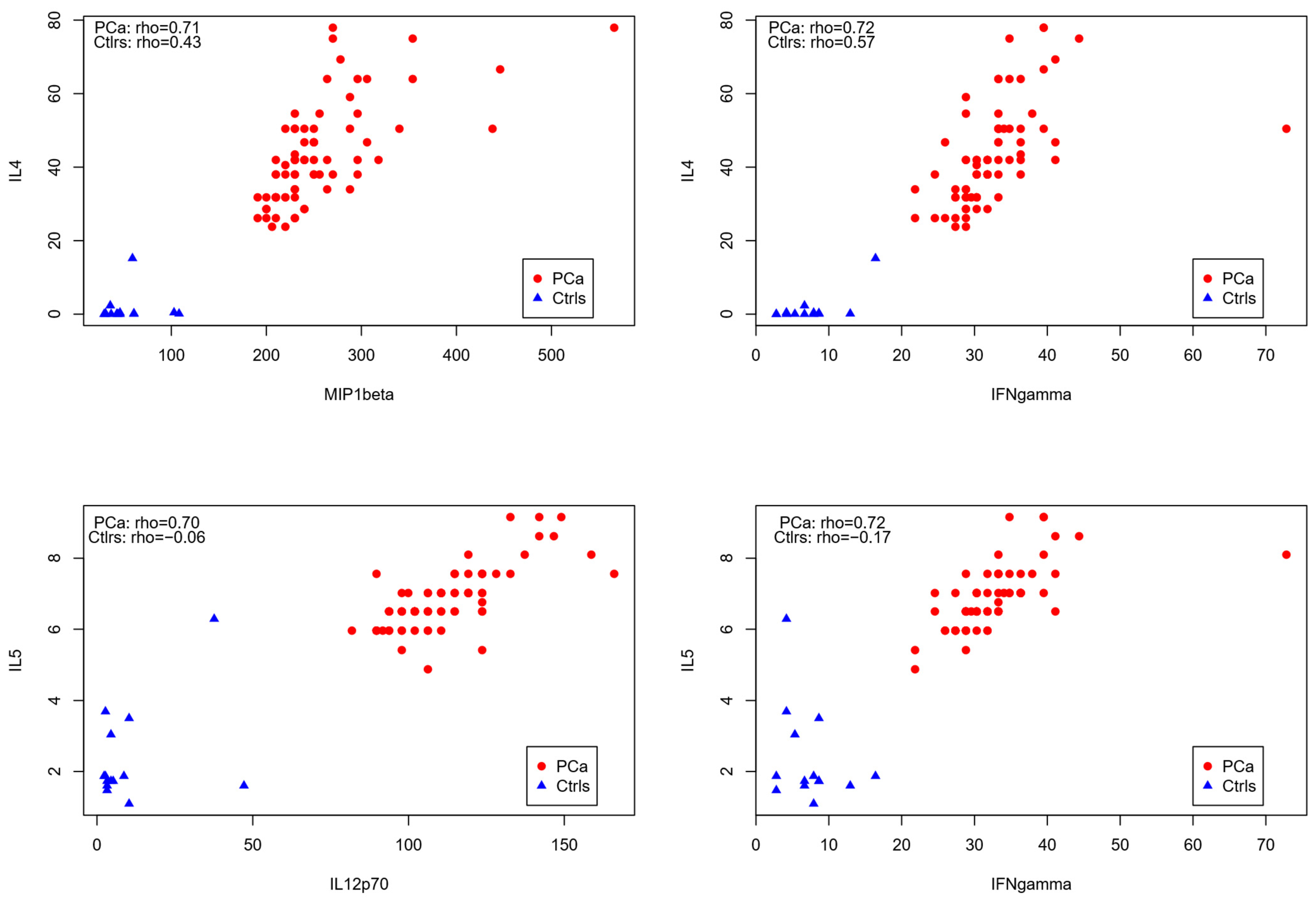
3.3. Correlation Analysis of Cytokines in PCa Patients
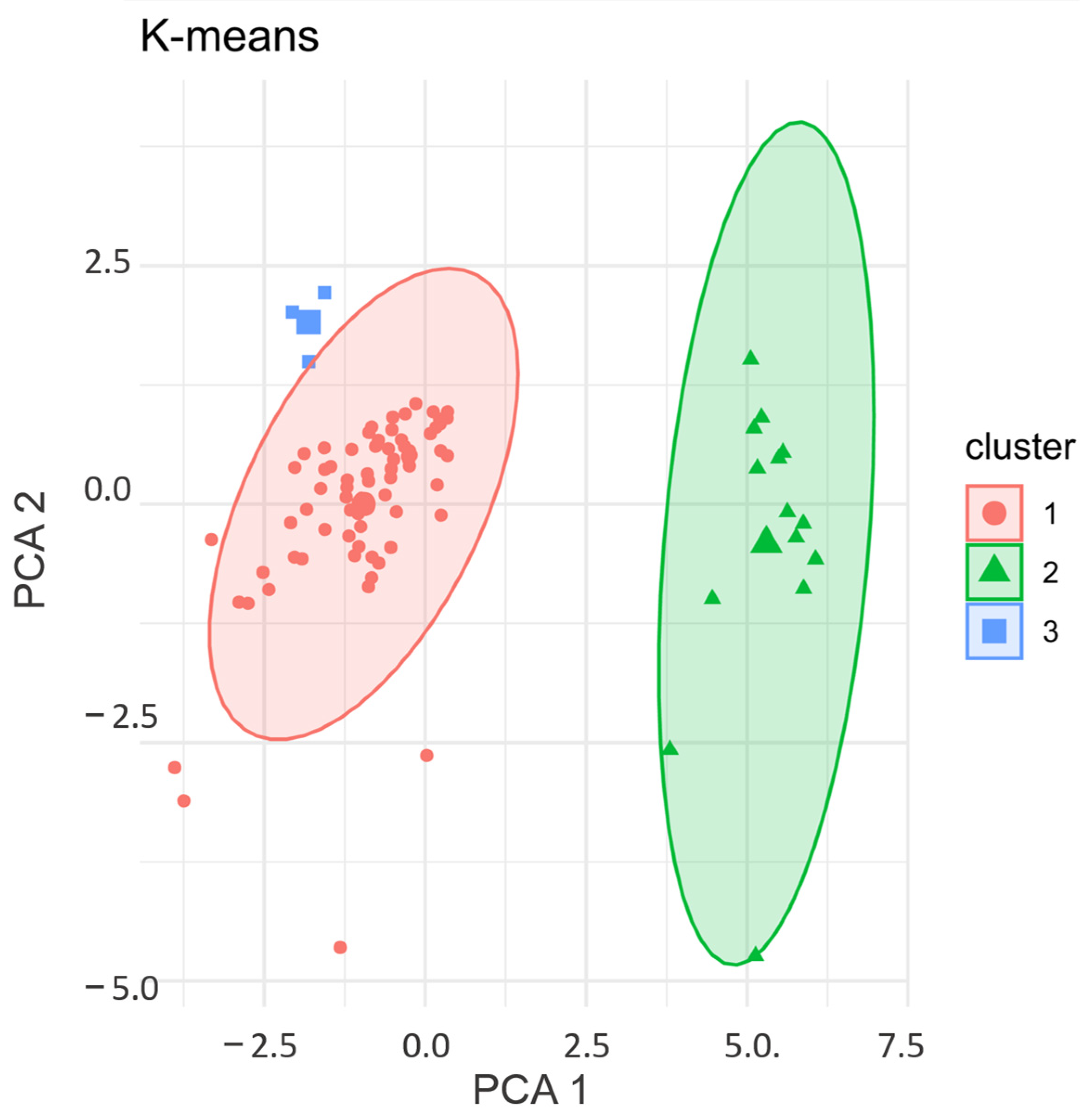
3.4. K-Means Clustering: Validation of Cytokine Expression Patterns in PCa Patients
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Cancer Facts & Figures 2024; American Cancer Society: Atlanta, GA, USA, 2024; Available online: https://www.cancer.org (accessed on 30 March 2025).
- Borda, V.; Loesch, D.P.; Guo, B.; Laboulaye, R.; Veliz-Otani, D.; French, J.N.; Leal, T.P.; Gogarten, S.M.; Ikpe, S.; Gouveia, M.H.; et al. Genetics of Latin American Diversity Project: Insights into population genetics and association studies in admixed groups in the Americas. Cell Genom. 2024, 4, 100692. [Google Scholar] [CrossRef] [PubMed]
- Peña, S.D.J.; Di Pietro, G.; Fuchshuber-Moraes, M.; Pasqualini Genro, J.; Hutz, M.H. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS ONE 2011, 6, e17063. [Google Scholar] [CrossRef] [PubMed]
- Jalalizadeh, M.; Roesch, H.R.M.; Korkes, F.; Dien-Trinh, Q.; Reis, L.O. Prostate cancer temporal and regional trends in Brazil. Oncol. Res. 2024, 32, 1565–1573. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2011, 2, 98. [Google Scholar] [CrossRef]
- Mughees, M.; Sengupta, A.; Khowal, S.; Wajid, S. Mechanism of tumour microenvironment in the progression and development of oral cancer. Mol. Biol. Rep. 2021, 48, 1773–1786. [Google Scholar] [CrossRef]
- Yang, K.Q.; Liu, Y.; Huang, Q.H.; Mo, N.; Zhang, Q.Y.; Meng, Q.G.; Cheng, J.W. Bone marrow-derived mesenchymal stem cells induced by inflammatory cytokines produce angiogenetic factors and promote prostate cancer growth. BMC Cancer 2017, 17, 878. [Google Scholar] [CrossRef]
- Cecchinato, V.; D’Agostino, G.; Raeli, L.; Uguccioni, M. Chemokine interaction with synergy-inducing molecules: Fine tuning modulation of cell trafficking. J. Leukoc. Biol. 2016, 99, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Haffner, M.C.; Zwart, W.; Roudier, M.P.; True, L.D.; Nelson, W.G.; Epstein, J.I.; De Marzo, A.M.; Nelson, P.S.; Yegnasubramanian, S. Genomic and phenotypic heterogeneity in prostate cancer. Nat. Rev. Urol. 2021, 18, 79–92. [Google Scholar] [CrossRef]
- Adekoya, T.O.; Richardson, R.M. Cytokines and Chemokines as Mediators of Prostate Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 4449. [Google Scholar] [CrossRef]
- Leber, M.F.; Efferth, T. Molecular principles of cancer invasion and metastasis (review). Int. J. Oncol. 2009, 34, 881–895. [Google Scholar]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Mughees, M.; Kaushal, J.B.; Sharma, G.; Wajid, S.; Batra, S.K.; Siddiqui, J.A. Chemokines and cytokines: Axis and allies in prostate cancer pathogenesis. Semin. Cancer Biol. 2022, 86 Pt 3, 497–512. [Google Scholar] [CrossRef]
- Dranoff, G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer 2004, 4, 11–22. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the eye of the cytokine storm. Microbiol. Mol. Biol. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Dasgupta, P.; Murphy, J.J. Prostate Cancer: The Role of Inflammation and Chemokines. Am. J. Pathol. 2019, 189, 2119–2137. [Google Scholar] [CrossRef]
- Pestka, S.; Krause, C.D.; Walter, M.R. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004, 202, 8–32. [Google Scholar] [CrossRef]
- Maimela, N.R.; Liu, S.; Zhang, Y. Fates of CD8+ T cells in Tumor Microenvironment. Comput. Struct. Biotechnol. J. 2019, 17, 1–13. [Google Scholar] [CrossRef]
- Mojic, M.; Takeda, K.; Hayakawa, Y. The Dark Side of IFN-γ: Its Role in Promoting Cancer Immunoevasion. Int. J. Mol. Sci. 2017, 19, 89. [Google Scholar] [CrossRef]
- Hansen, T.F.; Nederby, L.; Zedan, A.H.; Mejlholm, I.; Henriksen, J.R.; Steffensen, K.D.; Thomsen, C.B.; Raunkilde, L.; Jensen, L.H.; Jakobsen, A. Correlation Between Natural Killer Cell Activity and Treatment Effect in Patients with Disseminated Cancer. Transl. Oncol. 2019, 12, 968–972. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Michalaki, V.; Syrigos, K.; Charles, P.; Waxman, J. Serum levels of IL-6 and TNF-alpha correlate with clinicopathological features and patient survival in patients with prostate cancer. Br. J. Cancer 2004, 90, 2312–2316. [Google Scholar] [CrossRef] [PubMed]
- Maolake, A.; Izumi, K.; Natsagdorj, A.; Iwamoto, H.; Kadomoto, S.; Makino, T.; Naito, R.; Shigehara, K.; Kadono, Y.; Hiratsuka, K.; et al. Tumor necrosis factor-α induces prostate cancer cell migration in lymphatic metastasis through CCR7 upregulation. Cancer Sci. 2018, 109, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Todaro, M.; Alea, M.P.; Di Stefano, A.B.; Medema, J.P.; Stassi, G. IL-4-mediated drug resistance in colon cancer stem cells. Cancer Res. 2007, 67, 8216–8223. [Google Scholar] [CrossRef]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated eosinophils exert antitumor activity and prolong survival in tumor-bearing mice. Cancer Immunol. Immunother. 2019, 68, 455–466. [Google Scholar]
- Culig, Z.; Steiner, H.; Bartsch, G.; Hobisch, A. Interleukin-6 regulation of prostate cancer cell growth. Mol. Cell Endocrinol. 2005, 234, 55–61. [Google Scholar] [CrossRef]
- Shariat, S.F.; Kattan, M.W.; Traxel, E.; Andrews, B.; Zhu, K.; Wheeler, T.M.; Slawin, K.M. Association of preoperative plasma levels of interleukin-6 and its soluble receptor with prostate cancer prognosis. Clin. Cancer Res. 2001, 7, 2716–2722. [Google Scholar]
- Viola, M.V.; Fromowitz, F.; Oravez, S.; Deb, S.; Finkel, G.; Lundy, J.; Hand, P.; Thor, A.; Schlom, J. Expression of ras oncogene p21 in prostate cancer. N. Engl. J. Med. 1986, 314, 133–137. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Bruno, T.C.; Maris, C.H.; Xu, L.; Thoburn, C.J.; DeMarzo, A.M.; Meeker, A.K.; Isaacs, W.B.; Drake, C.G. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Prostate 2009, 70, 276–289. [Google Scholar] [CrossRef]
- Hodge, J.W.; Sabzevari, H.; Yafal, A.G.; Gritz, L.; Lorenz, M.G.; Schlom, J. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Immunol. Immunother. 2006, 55, 1320–1329. [Google Scholar]
- Lu, Y.; Cai, Z.; Galson, D.L.; Xiao, G.; Liu, Y.; George, D.E.; Melhem, M.F.; Yao, Z.; Zhang, J. Monocyte chemotactic protein-1 (MCP-1/CCL2) acts as a paracrine and autocrine factor for prostate cancer growth and invasion. J. Clin. Investig. 2006, 116, 2920–2930. [Google Scholar]
- Loberg, R.D.; Ying, C.; Craig, M.; Yan, L.I.; Snyder, L.A.; Pienta, K.J. CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Clin. Cancer Res. 2007, 13, 5287–5293. [Google Scholar] [CrossRef] [PubMed]
- Izumi, K.; Mizokami, A. Suppressive Role of Androgen/Androgen Receptor Signaling via Chemokines on Prostate Cancer Cells. J. Clin. Med. 2019, 8, 354. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Chen, J.Q.; Liu, C.; Malu, S.; Creasy, C.; Tetzlaff, M.T.; Xu, C.; McKenzie, J.A.; Zhang, C.; Liang, X.; et al. Loss of PTEN promotes resistance to T cell–mediated immunotherapy. Nature 2016, 539, 530–534. [Google Scholar] [CrossRef]
- Chen, R.; Liu, L.; Chen, H.; Xing, C.; Zhang, T.; Pang, Y.; Yang, X. Evaluation of the clinical application value of cytokine expression profiles in the differential diagnosis of prostate cancer. Cancer Immunol. Immunother. 2024, 73, 139. [Google Scholar] [CrossRef] [PubMed]
- Johnke, R.M.; Edwards, J.M.; Evans, M.J.; Nangami, G.N.; Bakken, N.T.G.; Kilburn, J.M.; Lee, T.K.; Allison, R.R.; Karlsson, U.L.; Arastu, H.H. Circulating cytokine levels in prostate cancer patients undergoing radiation therapy: Influence of neoadjuvant total androgen suppression. Vivo 2009, 23, 827–833. [Google Scholar]
- Ullah, A.; Jiao, W.; Shen, B. The role of proinflammatory cytokines and CXC chemokines (CXCL1-CXCL16) in the progression of prostate cancer: Insights on their therapeutic management. Cell Mol. Biol. Lett. 2024, 29, 73. [Google Scholar] [CrossRef]
- Rosenberg-Hasson, Y.; Hansmann, L.; Liedtke, M.; Herschmann, I.; Maecker, H.T. Effects of serum and plasma matrices on multiplex immunoassays. Immunol. Res. 2014, 58, 224–233. [Google Scholar] [CrossRef]
- Katongole, P.; Sande, O.J.; Nabweyambo, S.; Joloba, M.; Kajumbula, H.; Kalungi, S.; Reynolds, S.J.; Ssebambulidde, K.; Atuheirwe, M.; Orem, J.; et al. IL-6 and IL-8 cytokines are associated with elevated prostate-specific antigen levels among patients with adenocarcinoma of the prostate at the Uganda Cancer Institute. Future Oncol. 2022, 18, 661–667. [Google Scholar] [CrossRef]
- Deichaite, I.; Sears, T.J.; Sutton, L.; Rebibo, D.; Morgan, K.; Nelson, T.; Rose, B.; Tamayo, P.; Ferrara, N.; Asimakopoulos, F.; et al. Differential regulation of TNFα and IL-6 expression contributes to immune evasion in prostate cancer. J. Transl. Med. 2022, 20, 527. [Google Scholar] [CrossRef]
- Fizazi, K.; De Bono, J.S.; Flechon, A.; Heidenreich, A.; Voog, E.; Davis, N.B.; Qi, M.; Bandekar, R.; Vermeulen, J.T.; Cornfeld, M.; et al. Randomised phase II study of siltuximab (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur. J. Cancer 2012, 48, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Kiełb, P.; Kaczorowski, M.; Kowalczyk, K.; Piotrowska, A.; Nowak, Ł.; Krajewski, W.; Chorbińska, J.; Dudek, K.; Dzięgiel, P.; Hałoń, A.; et al. Role of IL-17A and IL-17RA in Prostate Cancer with Lymph Nodes Metastasis: Expression Patterns and Clinical Significance. Cancers 2023, 15, 4578. [Google Scholar] [CrossRef]
- Wise, G.J.; Marella, V.K.; Talluri, G.; Shirazian, D. Cytokine variations in patients with hormone treated prostate cancer. J. Urol. 2000, 164 Pt 1, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Ding, Y.; Xu, N. A Double-Edged Sword Role of Cytokines in Prostate Cancer Immunotherapy. Front. Oncol. 2021, 11, 688489. [Google Scholar] [CrossRef]
- Tong, Y.; Cao, Y.; Jin, T.; Huang, Z.; He, Q.; Mao, M. Role of Interleukin-1 family in bone metastasis of prostate cancer. Front. Oncol. 2022, 12, 951167. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Izumi, K.; Nakagawa, R.; Toriumi, R.; Aoyama, S.; Shimada, T.; Kano, H.; Makino, T.; Kadomoto, S.; Yaegashi, H.; et al. Usefulness of serum CCL2 as prognostic biomarker in prostate cancer: A long-term follow-up study. Jpn. J. Clin. Oncol. 2022, 52, 1337–1344. [Google Scholar] [CrossRef]
- Izumi, K.; Mizokami, A.; Lin, H.P.; Ho, H.M.; Iwamoto, H.; Maolake, A.; Natsagdorj, A.; Kitagawa, Y.; Kadono, Y.; Miyamoto, H.; et al. Serum chemokine (CC motif) ligand 2 level as a diagnostic, predictive, and prognostic biomarker for prostate cancer. Oncotarget 2016, 7, 8389–8398. [Google Scholar] [CrossRef]
- Fang, L.Y.; Izumi, K.; Lai, K.P.; Liang, L.; Li, L.; Miyamoto, H.; Lin, W.J.; Chang, C. Infiltrating Macrophages Promote Prostate Tumorigenesis via Modulating Androgen Receptor-Mediated CCL4–STAT3 Signaling. Cancer Res. 2013, 73, 5633–5646. [Google Scholar] [CrossRef]
- Tazaki, E.; Shimizu, N.; Tanaka, R.; Yoshizumi, M.; Kamma, H.; Imoto, S.; Goya, T.; Kozawa, K.; Nishina, A.; Kimura, H. Serum cytokine profiles in patients with prostate carcinoma. Exp. Ther. Med. 2011, 2, 887–891. [Google Scholar] [CrossRef]
- Paludan, S.R. Interleukin-4 and interferon-gamma: The quintessence of a mutual antagonistic relationship. Scand. J. Immunol. 1998, 48, 459–468. [Google Scholar] [CrossRef]
- Capibaribe, D.M.; Avilez, N.D.; Truzzi, N.C.C.; Truzzi, J.C.C.I.; Jalalizadeh, M.; Reis, L.O. Over 48,000 baseline prostate-specific antigen measurements in young men: A 16-year time-analysis. Cent. Eur. J. Urol. 2024, 77, 411–417. [Google Scholar] [CrossRef]

| Cytokines | Roles in Prostate Cancer | Mechanisms/Effects | Key Reference |
|---|---|---|---|
| IL-4 | Tumor cell survival and resistance | PI3K/AKT pathway; enhances chemo-resistance | [25] |
| IL-5 | Th2-skewed immunity | Reduces cytotoxic responses | [26] |
| IL-6 | PCa progression | AR signaling and STAT3; angiogenesis, castration resistance | [27] |
| IL-10 | Immune-suppressive/dual role | Inhibits T-cell activation; correlates with poor survival | [28] |
| IL-1β | Invasiveness and treatment resistance | Neuroendocrine differentiation and TME inflammation | [29] |
| IL-17A | Inflammation/metastasis | Angiogenesis/neutrophil infiltration | [30] |
| IL-12p70 | Anti-tumor immunity | Th1 cells and cytotoxic lymphocytes | [31] |
| MCP-1/CCL2 | Pro-tumoral macrophages | Bone metastasis/recurrence | [32] |
| MIP-1α/CCL3 | Bone metastasis | Osteoclastogenesis | [33] |
| MIP-1β/CCL4 | Immune suppression in TME | Immune cell recruitment to tumor | [13] |
| TNF-α | Chronic inflammation and resistance | AR-independent tumor growth | [34] |
| IFN-γ | Antitumor response (context-dependent) | Antigen presentation; PD-L1 upregulation in prolonged exposure | [35] |
| Variables | Patients N = 75 1 |
|---|---|
| Age at diagnosis | 70 (65, 72) |
| BMI | 26.2 (24.0, 30.9) |
| 1st PSA | 10 (7, 21) |
| Stage T | |
| T2 | 20 (57%) |
| T3 | 11 (31%) |
| T4 | 4 (11%) |
| Stage N | |
| N0 | 28 (80%) |
| N1 | 3 (8.6%) |
| NX | 4 (11%) |
| Stage M | |
| M0 | 31 (86%) |
| M1 | 5 (14%) |
| Family History | |
| Absent | 20 (41%) |
| Present | 29 (59%) |
| ISUP Grade Group | |
| 1 | 17 (23%) |
| 2 | 13 (17%) |
| 3 | 17 (23%) |
| 4 | 17 (23%) |
| 5 | 11 (15%) |
| Control | PCa | |
|---|---|---|
| N = 14 1 | N = 75 1 | |
| MCP1 | 405 (281, 442) | 218 (125, 296) |
| MIP1alpha | 4 (2, 5) | 148 (139, 178) |
| MIP1beta | 45 (36, 61) | 240 (220, 270) |
| IFNgamma | 7 (4, 9) | 32 (29, 35) |
| IL10 | 0.86 (0.50, 1.84) | 2.88 (2.12, 3.64) |
| IL12p70 | 4 (3, 10) | 110 (98, 124) |
| IL4 | 0 (0, 0) | 42 (32, 50) |
| IL5 | 1.80 (1.60, 3.04) | 6.50 (6.50, 7.02) |
| IL6 | 0.9 (0.7, 1.7) | 3.9 (3.1, 4.7) |
| TNFalpha | 14.9 (11.6, 18.1) | 6.9 (6.0, 7.9) |
| IL1beta | 1.0 (0.6, 1.4) | 11.3 (9.9, 12.7) |
| IL17 | 1.99 (1.80, 2.46) | 8.66 (8.26, 9.90) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, K.B.C.A.; Wosniaki, D.K.; Pereira, J.L.; Luz, M.; Albrecht, L.; Nardin, J.M.; Aoki, M.N.; Reis, L.O.; dos Reis, R.B.; Zanette, D.L. Comparative Analysis of Cytokine Expression Profiles in Prostate Cancer Patients. Biology 2025, 14, 505. https://doi.org/10.3390/biology14050505
Coelho KBCA, Wosniaki DK, Pereira JL, Luz M, Albrecht L, Nardin JM, Aoki MN, Reis LO, dos Reis RB, Zanette DL. Comparative Analysis of Cytokine Expression Profiles in Prostate Cancer Patients. Biology. 2025; 14(5):505. https://doi.org/10.3390/biology14050505
Chicago/Turabian StyleCoelho, Karoline Brito Caetano Andrade, Denise Kusma Wosniaki, Jonatas Luiz Pereira, Murilo Luz, Letusa Albrecht, Jeanine Marie Nardin, Mateus Nobrega Aoki, Leonardo O. Reis, Rodolfo Borges dos Reis, and Dalila Lucíola Zanette. 2025. "Comparative Analysis of Cytokine Expression Profiles in Prostate Cancer Patients" Biology 14, no. 5: 505. https://doi.org/10.3390/biology14050505
APA StyleCoelho, K. B. C. A., Wosniaki, D. K., Pereira, J. L., Luz, M., Albrecht, L., Nardin, J. M., Aoki, M. N., Reis, L. O., dos Reis, R. B., & Zanette, D. L. (2025). Comparative Analysis of Cytokine Expression Profiles in Prostate Cancer Patients. Biology, 14(5), 505. https://doi.org/10.3390/biology14050505






