Emerging Multifunctional Biomaterials for Addressing Drug Resistance in Cancer
Simple Summary
Abstract
1. Introduction
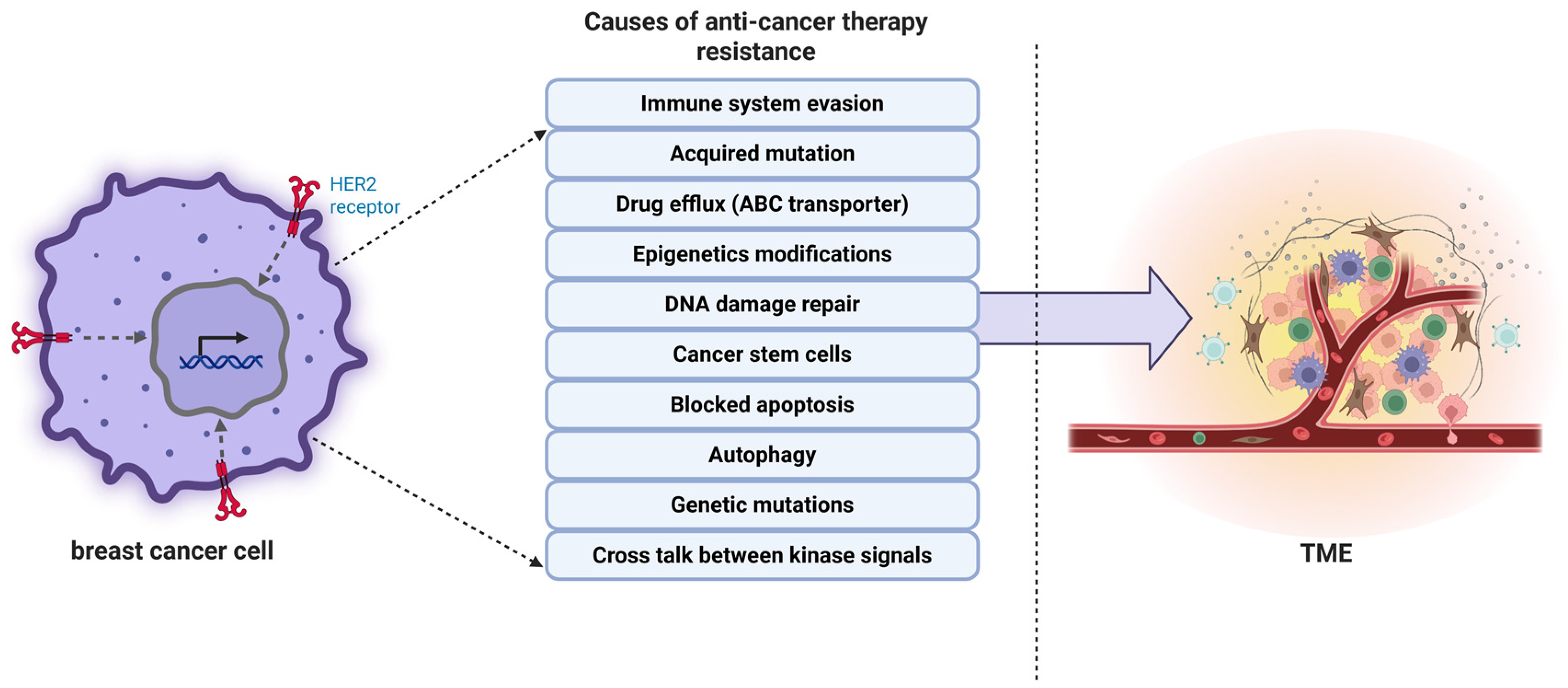
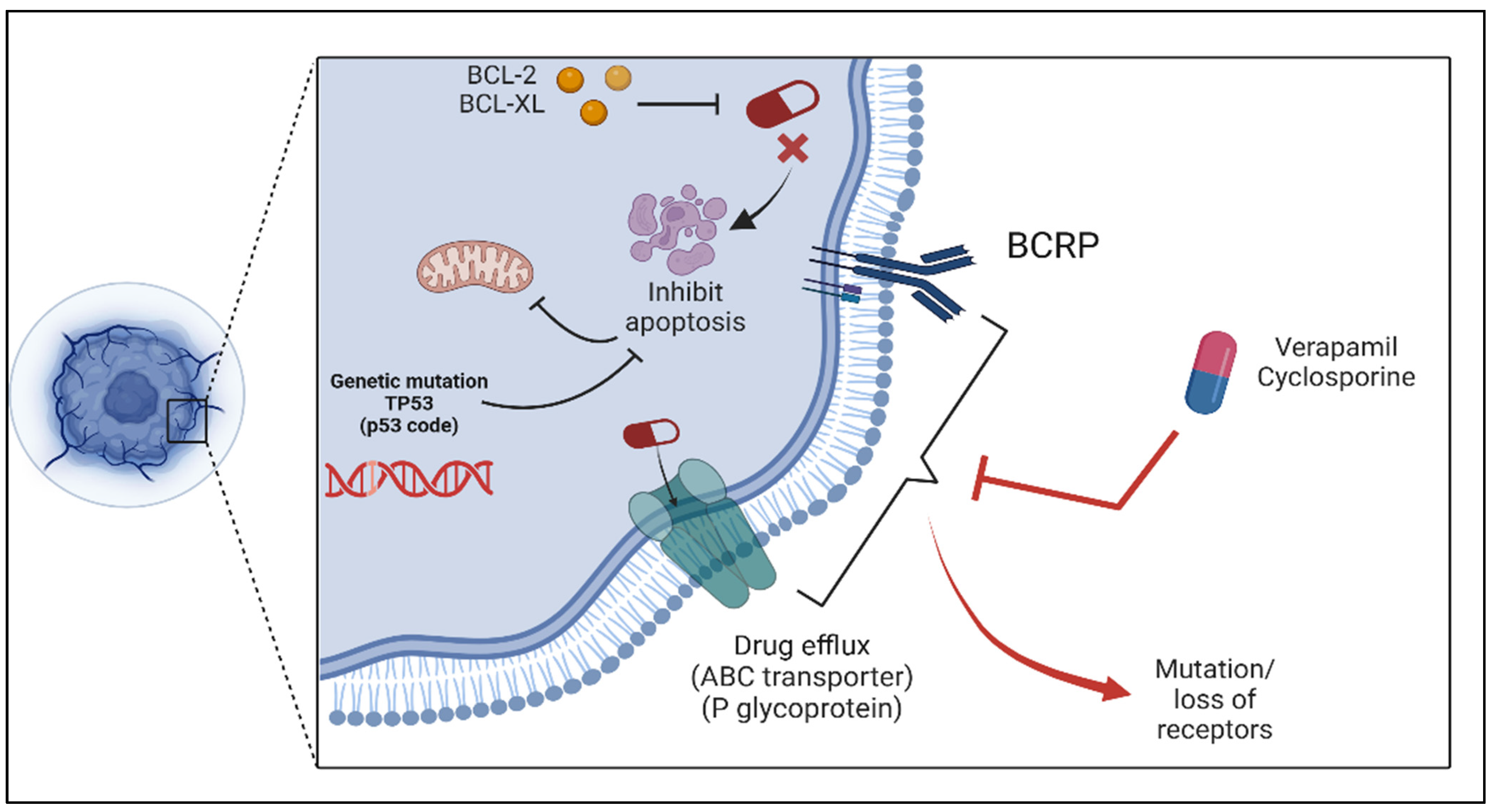
2. Drug Resistance Mechanisms in Cancer
2.1. Tumor Heterogeneity
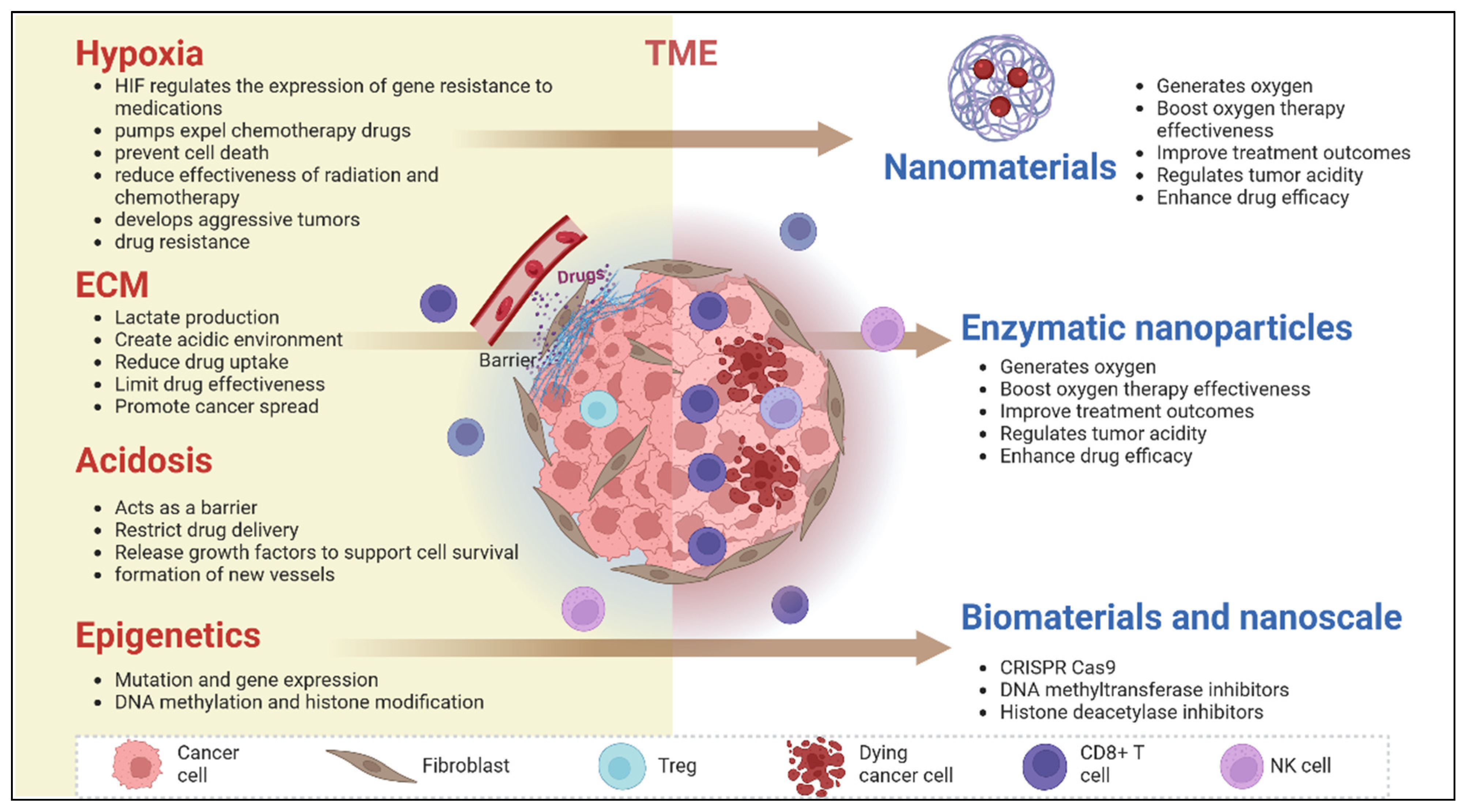
2.2. Role of Tumor Microenvironment (TME) in Drug Resistance
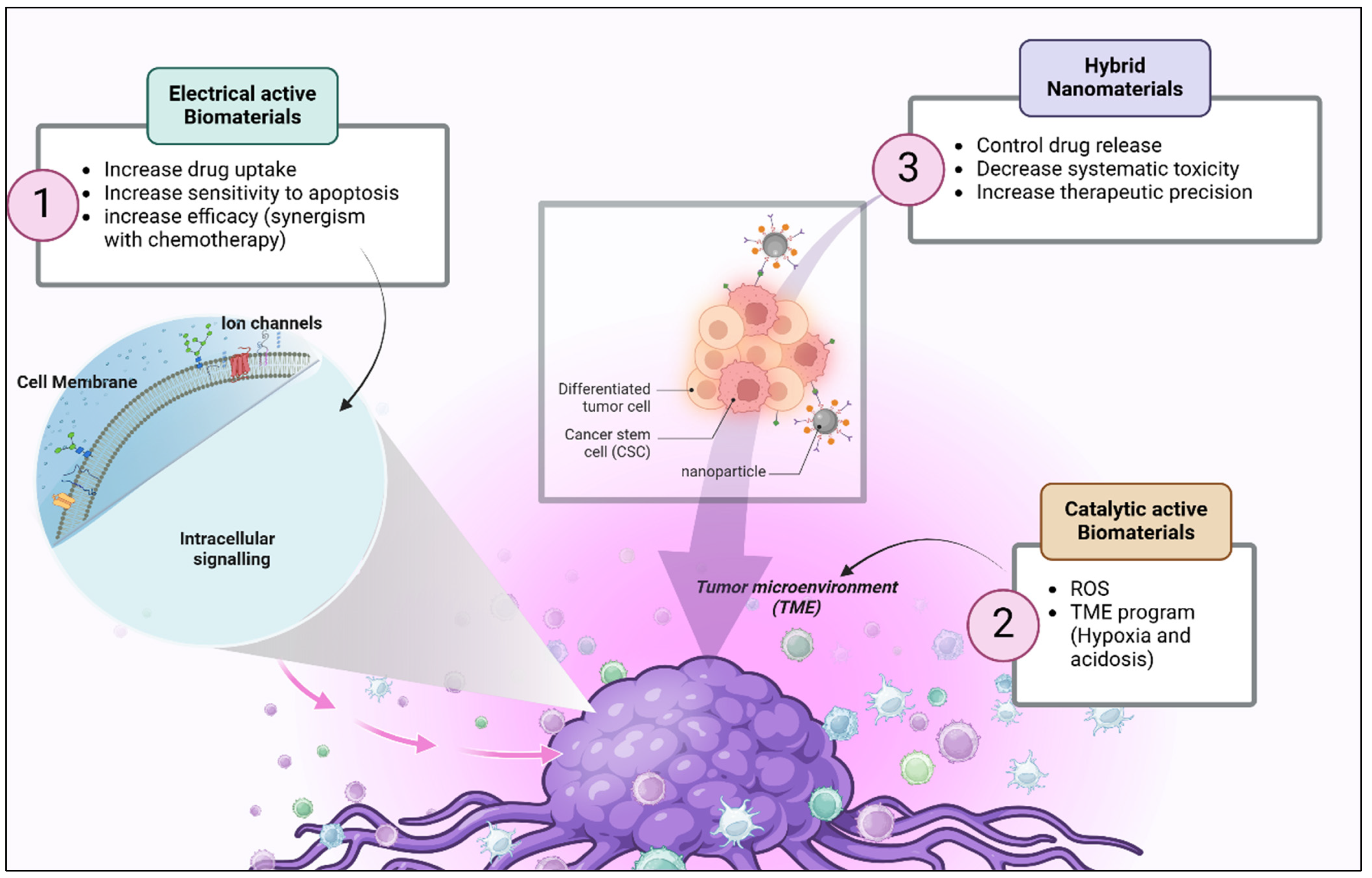
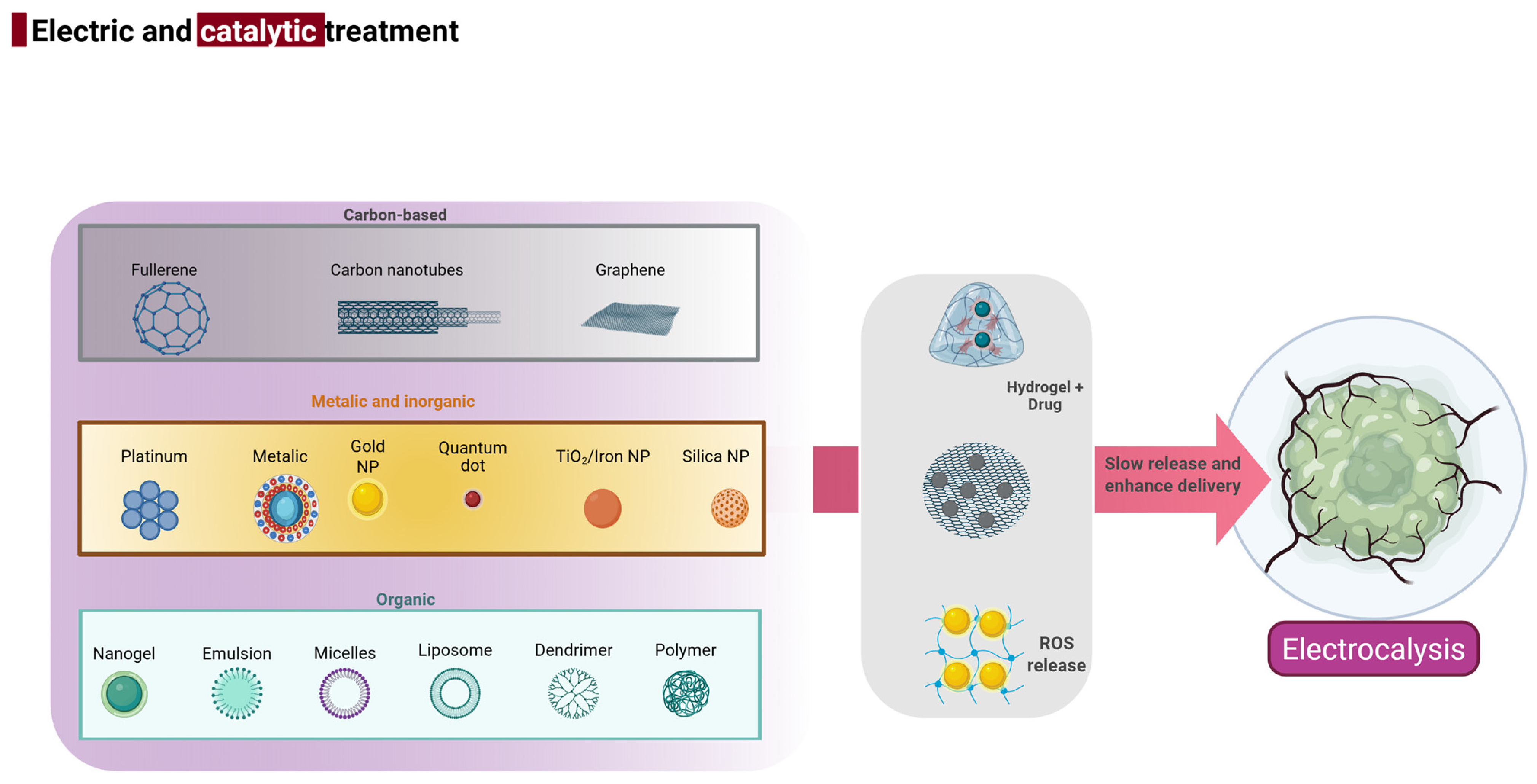
3. Multifunctional Biomaterials
4. Biomaterials and Their Potential in Overcoming Drug Resistance
4.1. Electrostimulation and Cancer Cells
4.1.1. Enhancing Drug Penetration into Cancer Cells by Disrupting the Cell Membrane
4.1.2. Sensitizing Cancer Cells to Therapy by Modulating Ion Channels and Membrane Potential
Regulation of Cellular Signaling Pathways Through Electrically Active Materials
- (a)
- Conductive hydrogels: Soft, biocompatible materials with electrical conductivity that can provide localized electrical stimulation to specific tumors. They can enhance the treatment of resistant tumors. For instance, conductive hydrogels incorporated with anticancer drugs can release both drugs and electrical signals, exerting a synergistic effect on resistant tumors [86].
- (b)
- Nanowires: Silicon and carbon nanowires have been used to transfer electrical pulses to cancer cells, while conductive nanowires have been employed to control cellular bioelectric properties due to their nanoscale dimensions. Studies have shown that stimulation with nanowires increases mitochondrial dysfunction in resistant cancer cells and thereby enhances apoptosis [87].
4.2. Synergizing Electrostimulation with Chemotherapy
4.3. Future Directions and Challenges
5. Catalytic Biomaterials and Their Potential in Overcoming Drug Resistance
5.1. Catalytic Biomaterial and ROS
5.2. Catalytic Enhancement of Drug Activation In Situ
5.3. Tumor Microenvironment Reprogramming
5.3.1. Catalysis-Driven Normalization of the Acidic or Hypoxic Microenvironment
5.3.2. Enzymatic Activity to Degrade Extracellular Matrix and Improve Drug Penetration
5.4. Case Studies
5.4.1. Catalysts Based on Platinum in Combination Therapies
5.4.2. Iron Oxide Nanoparticles as Ferroptosis Promoters
5.4.3. Iron–Sulfur Cluster-Based Catalysts for Ferroptosis Induction
5.4.4. Ruthenium and Platinum Hybrid Complexes for Evading Chemoresistance
6. Synergistic Role of Electrical and Catalytic Properties in Overcoming Drug Resistance
6.1. Mechanisms of Electrical and Catalytic Activity
6.2. Enhancing Drug Release
6.3. Improved Drug Delivery and Retention
7. Challenges and Future Perspectives
7.1. Current Challenges
7.1.1. Biocompatibility and Safety Issues
7.1.2. Scalability and Translation to Clinical Practice
7.2. Future Directions
7.2.1. Integration of Immunotherapy and Personalized Medicine with Multifunctional Biomaterials
7.2.2. Evolution of Intelligent Materials with Self-Regulating Characteristics
7.2.3. Tuning Nanomechanical and Rheological Properties for Improved Drug Delivery
7.2.4. Potential Effects
Innovative Influence on Cancer Treatment
Effect on Drug Resistance Management
The Possibility of Global Healthcare Improvements
7.2.5. Clinical Translation Status and Future Trials
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABC transporter | ATP-binding cassette transporter |
| BAX | B-cell lymphoma protein 2 (Bcl-2)-associated X protein |
| BCL | B-cell lymphoma 2 |
| BCRP | Breast cancer resistance protein |
| CMI | Chronic myeloid leukemia |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| Fe-NP | Iron nanoparticles |
| GSH | Glutathione |
| IONPs | Iron oxide nanoparticles |
| MDR | Resistance to multiple drugs |
| MOFs | Metal–organic frameworks |
| MRPs | Multidrug resistance-associated proteins |
| NK cells | Natural killer cells |
| NSCLC | Non-small cell lung cancer |
| PEDOT:PSS | Poly(3,4-ethylenedioxythiophene):polystyrene sulfonate |
| ROS | Reactive oxygen species |
| TiO2 | Titanium dioxide |
| TKIs | Tyrosine kinase inhibitors |
| TME | Tumor microenvironment |
| WHO | World Health Organization |
References
- Mundel, R.; Dhadwal, S.; Bharti, S.; Chatterjee, M. A comprehensive overview of various cancer types and their progression. In Handbook of Oncobiology: From Basic to Clinical Sciences; Springer: Singapore, 2023; pp. 1–17. [Google Scholar]
- Chen, G.; Lu, J.; Li, B.; Zhao, M.; Liu, D.; Yang, Z.; Liu, F. Efficacy and safety of Shenqi Fuzheng injection combined with chemotherapy for cancer: An overview of systematic reviews. Phytomedicine 2024, 125, 155293. [Google Scholar] [CrossRef] [PubMed]
- Vasan, N.; Baselga, J.; Hyman, D.M. A view on drug resistance in cancer. Nature 2019, 575, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, J.; Yang, M.; Xu, W.; Wang, J.; Hou, G.; Suo, A. Doxorubicin/cisplatin co-loaded hyaluronic acid/chitosan-based nanoparticles for in vitro synergistic combination chemotherapy of breast cancer. Carbohydr. Polym. 2019, 225, 115206. [Google Scholar] [CrossRef]
- Meng, L.; Zheng, Y.; Liu, H.; Fan, D. The tumor microenvironment: A key player in multidrug resistance in cancer. Oncologie 2024, 26, 41–58. [Google Scholar] [CrossRef]
- Ma, J.; Huang, L.; Han, C. Expert consensus on the use of third-generation EGFR-TKIs in EGFR-mutated advanced non-small cell lung cancer with various T790M mutations post-resistance to first-/second-generation EGFR-TKIs. Ther. Adv. Med. Oncol. 2024, 16, 17588359241289648. [Google Scholar] [CrossRef]
- Kohestani, A.A.; Xu, Z.; Baştan, F.E.; Boccaccini, A.R.; Pishbin, F. Electrically conductive coatings in tissue engineering. Acta Biomater. 2024, 186, 30–62. [Google Scholar] [CrossRef]
- Wang, H.; He, W.; Liao, J.; Wang, S.; Dai, X.; Yu, M.; Xie, Y.; Chen, Y. Catalytic Biomaterials-Activated In Situ Chemical Reactions: Strategic Modulation and Enhanced Disease Treatment. Adv. Mater. 2025, 37, 2411967. [Google Scholar] [CrossRef]
- Faridbod, F.; Ganjali, M.R.; Dinarvand, R.; Norouzi, P. Developments in the field of conducting and non-conducting polymer based potentiometric membrane sensors for ions over the past decade. Sensors 2008, 8, 2331–2412. [Google Scholar] [CrossRef]
- Nag, O.K.; Muroski, M.E.; Hastman, D.A.; Almeida, B.; Medintz, I.L.; Huston, A.L.; Delehanty, J.B. Nanoparticle-mediated visualization and control of cellular membrane potential: Strategies, progress, and remaining issues. ACS Nano 2020, 14, 2659–2677. [Google Scholar] [CrossRef]
- Peetla, C.; Vijayaraghavalu, S.; Labhasetwar, V. Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles. Adv. Drug Deliv. Rev. 2013, 65, 1686–1698. [Google Scholar] [CrossRef]
- Zhang, Q.; Luo, Q.; Liu, Z.; Sun, M.; Dong, X. Nano-ROS-generating approaches to cancer dynamic therapy: Lessons from nanoparticles. Chem. Eng. J. 2023, 457, 141225. [Google Scholar] [CrossRef]
- Wu, P.; Han, J.; Gong, Y.; Liu, C.; Yu, H.; Xie, N. Nanoparticle-based drug delivery systems targeting tumor microenvironment for cancer immunotherapy resistance: Current advances and applications. Pharmaceutics 2022, 14, 1990. [Google Scholar] [CrossRef]
- Majidinia, M.; Mirza-Aghazadeh-Attari, M.; Rahimi, M.; Mihanfar, A.; Karimian, A.; Safa, A.; Yousefi, B. Overcoming multidrug resistance in cancer: Recent progress in nanotechnology and new horizons. IUBMB Life 2020, 72, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Yn, L.D.; Beeraka, N.M.; Zhou, R.; Lu, P.; Song, R.; Sinelnikov, M.Y.; Chen, K.; Fan, R.; et al. Recent updates on the efficacy of mitocans in photo/radio-therapy for targeting metabolism in chemo/radio-resistant cancers: Nanotherapeutics. Curr. Med. Chem. 2025, 32, 2156–2182. [Google Scholar] [CrossRef]
- Goodman, L.S.; Wintrobe, M.M.; Dameshek, W.; Goodman, M.J.; Gilman, A.; McLennan, M.T. Nitrogen mustard therapy: Use of methyl-bis(beta-chloroethyl)amine hydrochloride and tris(beta-chloroethyl)amine hydrochloride for Hodgkin’s disease, lymphosarcoma, leukemia and certain allied and miscellaneous disorders. JAMA 1984, 251, 2255–2261. [Google Scholar] [CrossRef]
- Benner, S.E.; Wahl, G.M.; Von Hoff, D.D. Double minute chromosomes and homogeneously staining regions in tumors taken directly from patients versus in human tumor cell lines. Anticancer Drugs 1991, 2, 11–26. [Google Scholar] [CrossRef]
- Nathanson, D.A.; Gini, B.; Mottahedeh, J.; Visnyei, K.; Koga, T.; Gomez, G.; Eskin, A.; Hwang, K.; Wang, J.; Masui, K.; et al. Targeted therapy resistance mediated by dynamic regulation of extrachromosomal mutant EGFR DNA. Science 2014, 343, 72–76. [Google Scholar] [CrossRef]
- Junttila, M.R.; De Sauvage, F.J. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature 2013, 501, 346–354. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017, 7, 339. [Google Scholar] [CrossRef]
- El-Tanani, M.; Dakir, E.H.; Raynor, B.; Morgan, R. Mechanisms of nuclear export in cancer and resistance to chemotherapy. Cancers 2016, 8, 35. [Google Scholar] [CrossRef]
- Ravindranath, A.; Yuen, H.F.; Chan, K.K.; Grills, C.; Fennell, D.A.; Lappin, T.R.; El-Tanani, M. Wnt–β-catenin–Tcf-4 signalling-modulated invasiveness is dependent on osteopontin expression in breast cancer. Br. J. Cancer 2011, 105, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Hee Choi, Y.; Yu, A.M. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr. Pharm. Des. 2014, 20, 793–807. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Sethi, N.; Patel, P.; Shah, S.; Patel, K. Exploring the potential of P-glycoprotein inhibitors in the targeted delivery of anti-cancer drugs: A comprehensive review. Eur. J. Pharm. Biopharm. 2024, 198, 114267. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Gao, G.; Habaz, I.A.; Wang, Y. Mechanisms of resistance to tyrosine kinase inhibitor-targeted therapy and overcoming strategies. MedComm 2024, 5, e694. [Google Scholar] [CrossRef]
- Gorre, M.E.; Mohammed, M.; Ellwood, K.; Hsu, N.; Paquette, R.; Rao, P.N.; Sawyers, C.L. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 2001, 293, 876–880. [Google Scholar] [CrossRef]
- Fraser, M.; Leung, B.; Jahani-Asl, A.; Yan, X.; Thompson, W.E.; Tsang, B.K. Chemoresistance in human ovarian cancer: The role of apoptotic regulators. Reprod. Biol. Endocrinol. 2023, 1, 1–13. [Google Scholar] [CrossRef]
- Schneiderman, D.; Kim, J.M.; Senterman, M.; Tsang, B.K. Sustained suppression of Fas ligand expression in cisplatin-resistant human ovarian surface epithelial cancer cells. Apoptosis 1999, 4, 271–282. [Google Scholar] [CrossRef]
- Blombery, P.; Anderson, M.A.; Gong, J.N.; Thijssen, R.; Birkinshaw, R.W.; Thompson, E.R.; Teh, C.E.; Nguyen, T.; Xu, Z.; Flensburg, C.; et al. Acquisition of the recurrent Gly101Val mutation in BCL2 confers resistance to venetoclax in patients with progressive chronic lymphocytic leukemia. Cancer Discov. 2019, 9, 342–353. [Google Scholar] [CrossRef]
- Glover, H.L.; Schreiner, A.; Dewson, G.; Tait, S.W. Mitochondria and cell death. Nat. Cell Biol. 2024, 26, 1434–1446. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, Z.; Fu, Y.; Hou, Y.; Sun, J.; Hu, F.; Yu, S.; Gong, K.; Liu, Y.; Zhao, G. An overview of the functions of p53 and drugs acting either on wild- or mutant-type pEur. J. Med. Chem. 2024, 265, 116121. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Pan, T.; Jiang, D.; Jin, L.; Geng, Y.; Feng, X.; Shen, A.; Zhang, L. The lncRNA-GAS5/miR-221-3p/DKK2 axis modulates ABCB1-mediated adriamycin resistance of breast cancer via the Wnt/β-catenin signaling pathway. Mol. Ther. Nucleic Acids 2020, 19, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Jiang, X.; Tan, S.; Yang, Y.; Yang, W.; Han, Y.; Chen, Y.; Oyang, L.; Lin, J.; et al. Metabolic reprogramming and epigenetic modifications in cancer: From the impacts and mechanisms to the treatment potential. Exp. Mol. Med. 2023, 55, 1357–1370. [Google Scholar] [CrossRef]
- Yuen, H.F.; Gunasekharan, V.K.; Chan, K.K.; Zhang, S.D.; Platt-Higgins, A.; Gately, K.; El-Tanani, M. RanGTPase: A candidate for Myc-mediated cancer progression. J. Natl. Cancer Inst. 2013, 105, 475–488. [Google Scholar] [CrossRef]
- Nakanishi, T.; Ross, D.D. Breast cancer resistance protein (BCRP/ABCG2): Its role in multidrug resistance and regulation of its gene expression. Chin. J. Cancer 2012, 31, 73. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Shamsabadipour, A.; Bhatti, A.; Forouzanfar, M.; Rajabnejad, M.; Behzadmehr, R.; Díez-Pascual, A.M. Therapeutic performance of temozolomide-loaded nanomaterials: A state-of-the-art. J. Drug Deliv. Sci. Technol. 2023, 85, 104568. [Google Scholar] [CrossRef]
- Hussain, S.R.; Cheney, C.M.; Johnson, A.J.; Lin, T.S.; Grever, M.R.; Caligiuri, M.A.; Lucas, D.M.; Byrd, J.C. Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: Down-regulation enhances rituximab-mediated apoptosis and complement-dependent cytotoxicity. Clin. Cancer Res. 2007, 13, 2144–2150. [Google Scholar] [CrossRef]
- Dhanyamraju, P.K. Drug resistance mechanisms in cancers: Execution of pro-survival strategies. J. Biomed. Res. 2024, 38, 95. [Google Scholar] [CrossRef]
- Gu, Y.; Yang, R.; Zhang, Y.; Guo, M.; Takehiro, K.; Zhan, M.; Yang, L.; Wang, H. Molecular mechanisms and therapeutic strategies in overcoming chemotherapy resistance in cancer. Mol. Biomed. 2025, 6, 2. [Google Scholar] [CrossRef]
- Stefanski, C.D.; Keffler, K.; McClintock, S.; Milac, L.; Prosperi, J.R. APC loss affects DNA damage repair causing doxorubicin resistance in breast cancer cells. Neoplasia 2019, 21, 1143–1150. [Google Scholar] [CrossRef]
- Kettner, N.M.; Vijayaraghavan, S.; Durak, M.G.; Bui, T.; Kohansal, M.; Ha, M.J.; Liu, B.; Rao, X.; Wang, J.; Yi, M.; et al. Combined inhibition of STAT3 and DNA repair in palbociclib-resistant ER-positive breast cancer. Clin. Cancer Res. 2019, 25, 3996–4013. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liao, K.; Gross, N.; Wang, Z.; Li, G.; Zuo, W.; Zhong, S.; Zhang, Z.; Zhang, H.; Yang, J.; et al. Homologous recombination enhances radioresistance in hypopharyngeal cancer cell line by targeting DNA damage response. Oral Oncol. 2020, 100, 104469. [Google Scholar] [CrossRef] [PubMed]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.; Zouridis, H.; Wu, Y.; Cheng, L.L.; Tan, I.B.; Gopalakrishnan, V.; Ooi, C.H.; Lee, J.; Qin, L.; Wu, J.; et al. Integrated epigenomics identifies BMP4 as a modulator of cisplatin sensitivity in gastric cancer. Gut 2013, 62, 22–33. [Google Scholar] [CrossRef]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Y.; Xiang, F.; Li, M.; Li, H.; Chi, J.; Ren, K. Suppression of TGF-β1 enhances chemosensitivity of cisplatin-resistant lung cancer cells through the inhibition of drug-resistant proteins. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1505–1512. [Google Scholar] [CrossRef]
- Kang, D.; Han, Z.; Oh, G.H.; Joo, Y.; Choi, H.J.; Song, J.J. Down-regulation of TGF-β expression sensitizes the resistance of hepatocellular carcinoma cells to sorafenib. Yonsei Med. J. 2017, 58, 899–909. [Google Scholar] [CrossRef]
- Deng, Y.; Shi, M.; Yi, L.; Khan, M.N.; Xia, Z.; Li, X. Eliminating a barrier: Aiming at VISTA, reversing MDSC-mediated T cell suppression in the tumor microenvironment. Heliyon 2024, 10, e37060. [Google Scholar] [CrossRef]
- Penner-Goeke, S.; Lichtensztejn, Z.; Neufeld, M.; Ali, J.L.; Altman, A.D.; Nachtigal, M.W.; McManus, K.J. The temporal dynamics of chromosome instability in ovarian cancer cell lines and primary patient samples. PLoS Genet. 2017, 13, e1006707. [Google Scholar] [CrossRef]
- Replogle, J.M.; Zhou, W.; Amaro, A.E.; McFarland, J.M.; Villalobos-Ortiz, M.; Ryan, J.; Letai, A.; Yilmaz, O.; Sheltzer, J.; Lippard, S.J.; et al. Aneuploidy increases resistance to chemotherapeutics by antagonizing cell division. Proc. Natl. Acad. Sci. USA 2020, 117, 30566–30576. [Google Scholar] [CrossRef]
- Son, B.; Lee, S.; Youn, H.; Kim, E.; Kim, W.; Youn, B. The role of tumor microenvironment in therapeutic resistance. Oncotarget 2016, 8, 3933. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yang, F.; Shao, C.; Wei, K.; Xie, M.; Shen, H.; Shu, Y. Role of hypoxia in cancer therapy by regulating the tumor microenvironment. Mol. Cancer 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.R.; Hay, M.P. Targeting hypoxia in cancer therapy. Nat. Rev. Cancer 2011, 11, 393–410. [Google Scholar] [CrossRef]
- Bogdanov, A.; Bogdanov, A.; Chubenko, V.; Volkov, N.; Moiseenko, F.; Moiseyenko, V. Tumor acidity: From hallmark of cancer to target of treatment. Front. Oncol. 2022, 12, 979154. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular matrix in the tumor microenvironment and its impact on cancer therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Cheng, F.Y.; Chan, C.H.; Wang, B.J.; Yeh, Y.L.; Wang, Y.J.; Chiu, H.W. The oxygen-generating calcium peroxide-modified magnetic nanoparticles attenuate hypoxia-induced chemoresistance in triple-negative breast cancer. Cancers 2021, 13, 606. [Google Scholar] [CrossRef]
- Lin, M.; Wang, X. Natural Biopolymer-Based Delivery of CRISPR/Cas9 for Cancer Treatment. Pharmaceutics 2023, 16, 62. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; González-Cortés, A.; Campuzano, S.; Pingarrón, J.M. Multimodal/multifunctional nanomaterials in (bio)electrochemistry: Now and in the coming decade. Nanomaterials 2020, 10, 2556. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, Q.; Wang, C.; Hao, Y.; Yang, N.; Chen, M.; Ji, J.; Feng, L.; Liu, Z. Rational design of biomaterials to potentiate cancer thermal therapy. Chem. Rev. 2023, 123, 7326–7378. [Google Scholar] [CrossRef]
- Mousavi, A.; Vahdat, S.; Baheiraei, N.; Razavi, M.; Norahan, M.H.; Baharvand, H. Multifunctional conductive biomaterials as promising platforms for cardiac tissue engineering. ACS Biomater. Sci. Eng. 2020, 7, 55–82. [Google Scholar] [CrossRef]
- Ji, S.R.; Liu, C.; Zhang, B.; Yang, F.; Xu, J.; Long, J.; Jin, C.; Fu, D.L.; Ni, Q.X.; Yu, X.J. Carbon nanotubes in cancer diagnosis and therapy. Biochim. Biophys. Acta Rev. Cancer 2010, 1806, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Hada, V.; Malvi, D.; Mili, M.; Khan, M.M.; Chaturvedi, G.; Hashmi, S.A.; Srivastava, A.K.; Verma, S. MXenes: Promising 2D materials for wound dressing applications–A perspective review. Mater. Adv. 2022, 3, 7445–7462. [Google Scholar] [CrossRef]
- Haggag, Y.; Elshikh, M.; El-Tanani, M.; Bannat, I.M.; McCarron, P.; Tambuwala, M.M. Nanoencapsulation of sophorolipids in PEGylated poly (lactide-co-glycolide) as a novel approach to target colon carcinoma in the murine model. Drug Deliv. Transl. Res. 2020, 10, 1353–1366. [Google Scholar] [CrossRef]
- Guo, B.; Ma, P.X. Conducting polymers for tissue engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Yang, Y.W. Metal–organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Galski, H.; Fojo, A.; Willingham, M.; Lai, S.L.; Gazdar, A.; Pirker, R.; Green, A.; Crist, W.; Brodeur, G.M.; et al. Expression of multidrug resistance gene in human cancers. J. Natl. Cancer Inst. 1989, 81, 116–124. [Google Scholar] [CrossRef]
- Fu, L.; Liang, Y.; Deng, L.; Ding, Y.; Chen, L.; Ye, Y.; Yang, X.; Pan, Q. Characterization of tetrandrine, a potent inhibitor of P-glycoprotein-mediated multidrug resistance. Cancer Chemother. Pharmacol. 2004, 53, 349–356. [Google Scholar] [CrossRef]
- Etgar, L.; Leitus, G.; Fradkin, L.; Assaraf, Y.G.; Tannenbaum, R.; Lifshitz, E. Optical and magnetic properties of conjugate structures of PbSe quantum dots and γ-Fe2O3 nanoparticles. ChemPhysChem 2009, 10, 2235–2241. [Google Scholar] [CrossRef]
- Dawar, S.; Singh, N.; Kanwar, R.K.; Kennedy, R.L.; Veedu, R.N.; Zhou, S.F.; Krishnakumar, S.; Hazra, S.; Sasidharan, S.; Duan, W.; et al. Multifunctional and multitargeted nanoparticles for drug delivery to overcome barriers of drug resistance in human cancers. Drug Discov. Today 2013, 18, 1292–1300. [Google Scholar] [CrossRef]
- Yin, J.J.; Sharma, S.; Shumyak, S.P.; Wang, Z.X.; Zhou, Z.W.; Zhang, Y.; Guo, P.; Li, C.Z.; Kanwar, J.R.; Yang, T.; et al. Synthesis and biological evaluation of novel folic acid receptor-targeted, β-cyclodextrin-based drug complexes for cancer treatment. PLoS ONE 2013, 8, e62289. [Google Scholar] [CrossRef]
- Luqmani, Y.A. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 2005, 14, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Bogman, K.; Peyer, A.K.; Török, M.; Küsters, E.; Drewe, J. HMG-CoA reductase inhibitors and P-glycoprotein modulation. Br. J. Pharmacol. 2001, 132, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Swamy, V.S.; Prasad, R. Green synthesis of silver nanoparticles from the leaf extract of Santalum album and its antimicrobial activity. J. Optoelectron. Biomed. Mater. 2012, 4, 53–59. [Google Scholar]
- Yang, L.; Mao, H.; Wang, Y.A.; Cao, Z.; Peng, X.; Wang, X.; Duan, H.; Ni, C.; Yuan, Q.; Adams, G.; et al. Single chain epidermal growth factor receptor antibody conjugated nanoparticles for in vivo tumor targeting and imaging. Small 2009, 5, 235–243. [Google Scholar] [CrossRef]
- Kukowska-Latallo, J.F.; Candido, K.A.; Cao, Z.; Nigavekar, S.S.; Majoros, I.J.; Thomas, T.P.; Balogh, L.P.; Khan, M.K.; Baker, J.R. Nanoparticle targeting of anticancer drug improves therapeutic response in animal model of human epithelial cancer. Cancer Res. 2005, 65, 5317–5324. [Google Scholar] [CrossRef]
- Chen, T.J.; Cheng, T.H.; Chen, C.Y.; Hsu, S.C.; Cheng, T.L.; Liu, G.C.; Wang, Y.M. Targeted Herceptin–dextran iron oxide nanoparticles for noninvasive imaging of HER2/neu receptors using MRI. JBIC J. Biol. Inorg. Chem. 2009, 14, 253–260. [Google Scholar] [CrossRef]
- Dhar, S.; Gu, F.X.; Langer, R.; Farokhzad, O.C.; Lippard, S.J. Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA–PEG nanoparticles. Proc. Natl. Acad. Sci. USA 2008, 105, 17356–17361. [Google Scholar] [CrossRef]
- Janigro, D.; Perju, C.; Fazio, V.; Hallene, K.; Dini, G.; Agarwal, M.K.; Cucullo, L. Alternating current electrical stimulation enhanced chemotherapy: A novel strategy to bypass multidrug resistance in tumor cells. BMC Cancer 2006, 6, 72. [Google Scholar] [CrossRef]
- Kim, K.; Lee, W.G. Electroporation for nanomedicine: A review. J. Mater. Chem. B 2017, 5, 2726–2738. [Google Scholar] [CrossRef]
- Rembiałkowska, N.; Dubińska-Magiera, M.; Sikora, A.; Szlasa, W.; Szewczyk, A.; Czapor-Irzabek, H.; Daczewska, M.; Saczko, J.; Kulbacka, J. Doxorubicin assisted by microsecond; electroporation promotes irreparable morphological alternations in sensitive and resistant human breast adenocarcinoma cells. Appl. Sci. 2020, 10, 2765. [Google Scholar] [CrossRef]
- Yang, M.; Brackenbury, W.J. Membrane potential and cancer progression. Front. Physiol. 2013, 4, 185. [Google Scholar] [CrossRef] [PubMed]
- Hayes, A.J.; Melrose, J. Electro-Stimulation, a promising therapeutic treatment modality for tissue repair: Emerging roles of sulfated glycosaminoglycans as electro-regulatory mediators of intrinsic repair processes. Adv. Ther. 2020, 3, 2000151. [Google Scholar] [CrossRef]
- Tai, Y.; Banerjee, A.; Goodrich, R.; Jin, L.; Nam, J. Development and utilization of multifunctional polymeric scaffolds for the regulation of physical cellular microenvironments. Polymers 2021, 13, 3880. [Google Scholar] [CrossRef]
- Franklin, S. Combinatorial Effects of Functional Nanoparticles and Electromagnetic Stimulation on Cells; The University of Texas at San Antonio: San Antonio, TX, USA, 2016. [Google Scholar]
- Robby, A.I.; Kim, S.G.; Jo, H.J.; Lee, G.; Lee, H.S.; Lee, K.D.; Ryu, J.H.; Park, S.Y. Tumor microenvironment-responsive touch sensor-based pH-triggered controllable conductive hydrogel. Appl. Mater. Today 2021, 25, 101259. [Google Scholar] [CrossRef]
- Shashaani, H.; Faramarzpour, M.; Hassanpour, M.; Namdar, N.; Alikhani, A.; Abdolahad, M. Silicon nanowire based biosensing platform for electrochemical sensing of Mebendazole drug activity on breast cancer cells. Biosens. Bioelectron. 2016, 85, 363–370. [Google Scholar] [CrossRef]
- Löscher, W.; Potschka, H. Drug resistance in brain diseases and the role of drug efflux transporters. Nat. Rev. Neurosci. 2005, 6, 591–602. [Google Scholar] [CrossRef]
- Song, S.Y.; Park, J.H.; Lee, J.S.; Kim, J.R.; Sohn, E.H.; Jung, M.S.; Yoo, H.S. A randomized, placebo-controlled trial evaluating changes in peripheral neuropathy and quality of life by using low-frequency electrostimulation on breast cancer patients treated with chemotherapy. Integr. Cancer Ther. 2020, 19, 1534735420925519. [Google Scholar] [CrossRef]
- Xu, L.; Huang, J.; Liu, J.; Xi, Y.; Zheng, Z.; To, K.K.; Fu, L. CM082 enhances the efficacy of chemotherapeutic drugs by inhibiting the drug efflux function of ABCG2. Mol. Ther.-Oncolytics 2020, 16, 100–110. [Google Scholar] [CrossRef]
- Wu, H.; Yang, L.; Liu, H.; Zhou, D.; Chen, D.; Zheng, X.; Yang, H.; Li, C.; Chang, J.; Wu, A.; et al. Exploring the efficacy of tumor electric field therapy against glioblastoma: An in vivo and in vitro study. CNS Neurosci. Ther. 2021, 27, 1587–1604. [Google Scholar] [CrossRef]
- Sersa, G.; Miklavcic, D.; Cemazar, M.; Rudolf, Z.; Pucihar, G.; Snoj, M. Electrochemotherapy in treatment of tumours. Eur. J. Surg. Oncol. 2008, 34, 232–240. [Google Scholar] [CrossRef]
- Zeng, W.; Zhang, H.; Yuan, X.; Chen, T.; Pei, Z.; Ji, X. Two-dimensional nanomaterial-based catalytic medicine: Theories, advanced catalyst and system design. Adv. Drug Deliv. Rev. 2022, 184, 114241. [Google Scholar] [CrossRef] [PubMed]
- Chadha, U.; Selvaraj, S.K.; Ashokan, H.; Hariharan, S.P.; Mathew Paul, V.; Venkatarangan, V.; Paramasivam, V. Complex nanomaterials in catalysis for chemically significant applications: From synthesis and hydrocarbon processing to renewable energy applications. Adv. Mater. Sci. Eng. 2022, 2022, 1552334. [Google Scholar] [CrossRef]
- Zhou, X.; An, B.; Lin, Y.; Ni, Y.; Zhao, X.; Liang, X. Molecular mechanisms of ROS-modulated cancer chemoresistance and therapeutic strategies. Biomed. Pharmacother. 2023, 165, 115036. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Karakoti, A.S.; Self, W.; Seal, S.; Singh, S. Redox-sensitive cerium oxide nanoparticles protect human keratinocytes from oxidative stress induced by glutathione depletion. Langmuir 2016, 32, 12202–12211. [Google Scholar] [CrossRef]
- Zhao, Z.; Cao, Y.; Xu, R.; Fang, J.; Zhang, Y.; Xu, X.; Huang, L.; Li, R. Nanoparticles (NPs)-mediated targeted regulation of redox homeostasis for effective cancer therapy. Smart Mater. Med. 2024, 5, 291–320. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The next generation of platinum drugs: Targeted Pt(II) agents, nanoparticle delivery, and Pt(IV) prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Aghdash, A.K.; Ghobadi, H.; Karami, P.; Johari-Ahar, M. Highly sensitive electrochemiluminescent immunoassay for detecting neuron-specific enolase (NSE) based on polyluminol and glucose oxidase-conjugated glucose-encapsulating liposome. Microchem. J. 2022, 181, 107785. [Google Scholar] [CrossRef]
- Mahmudi, M.; Widiyastuti, W.; Nurlilasari, P.; Affandi, S.; Setyawan, H. Manganese dioxide nanoparticles synthesized by electrochemical method and its catalytic activity towards oxygen reduction reaction. J. Ceram. Soc. Jpn. 2018, 126, 906–913. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Y.; Wang, Z.; Yang, L.; Zhang, Y.; Zhang, Z.; Jia, L. Iron-based MOF with catalase-like activity improves the synergistic therapeutic effect of PDT/ferroptosis/starvation therapy by reversing the tumor hypoxic microenvironment. J. Nanobiotechnol. 2024, 22, 705. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Gao, Y.; Shaha, S.; Wang, L.L.; Park, K.S.; Nakajima, M.; Adebowale, O.; Mitragotri, S. Extracellular matrix degrading enzyme with stroma-targeting peptides enhance the penetration of liposomes into tumors. J. Control. Release 2022, 352, 1093–1103. [Google Scholar] [CrossRef]
- Grünwald, B.; Vandooren, J.; Locatelli, E.; Fiten, P.; Opdenakker, G.; Proost, P.; Krüger, A.; Lellouche, J.P.; Israel, L.L.; Shenkman, L.; et al. Matrix metalloproteinase-9 (MMP-9) as an activator of nanosystems for targeted drug delivery in pancreatic cancer. J. Control. Release 2016, 239, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-directed active tumor-targeting polymeric nanoparticles for cancer chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef] [PubMed]
- Čepelak, I.; Dodig, S.; Dodig, D. Ferroptosis: Regulated cell death. Arch. Ind. Hyg. Toxicol. 2020, 71, 99. [Google Scholar] [CrossRef]
- Sant’Angelo, D.; Descamps, G.; Lecomte, V.; Stanicki, D.; Penninckx, S.; Dragan, T.; Van Gestel, D.; Laurent, S.; Journe, F. Therapeutic approaches with iron oxide nanoparticles to induce ferroptosis and overcome radioresistance in cancers. Pharmaceuticals 2025, 18, 325. [Google Scholar] [CrossRef]
- Ko, M.J.; Min, S.; Hong, H.; Yoo, W.; Joo, J.; Zhang, Y.S.; Kang, H.; Kim, D.H. Magnetic nanoparticles for ferroptosis cancer therapy with diagnostic imaging. Bioact. Mater. 2024, 32, 66–97. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Chen, L.; Wu, Y.; Min, J.; Wang, F. Iron homeostasis and ferroptosis in human diseases: Mechanisms and therapeutic prospects. Signal Transduct. Target. Ther. 2024, 9, 271. [Google Scholar]
- Zhang, C.; Kang, T.; Wang, X.; Song, J.; Zhang, J.; Li, G. Stimuli-responsive platinum and ruthenium complexes for lung cancer therapy. Front. Pharmacol. 2022, 13, 1035217. [Google Scholar] [CrossRef]
- Katoh, K. Effects of electrical stimulation of the cell: Wound healing, cell proliferation, apoptosis, and signal transduction. Med. Sci. 2023, 11, 11. [Google Scholar] [CrossRef]
- Huang, Y.; Yao, K.; Zhang, Q.; Huang, X.; Chen, Z.; Zhou, Y.; Yu, X. Bioelectronics for electrical stimulation: Materials, devices and biomedical applications. Chem. Soc. Rev. 2024, 53, 8632–8712. [Google Scholar] [CrossRef]
- Rane, A.V.; Prajitha, V.; Jibin, K.P.; Moyo, M.; Abitha, V.K.; Kanny, K.; Thomas, S. Specific interactions in nanohybrid systems. In Hybrid Nanofillers for Polymer Reinforcement; Elsevier: Amsterdam, The Netherlands, 2024; pp. 73–133. [Google Scholar]
- Park, J.Y.; Kim, S. Preparation and electroactivity of polymer-functionalized graphene oxide-supported platinum nanoparticles catalysts. Int. J. Hydrogen Energy 2013, 38, 6275–6282. [Google Scholar] [CrossRef]
- Kankala, R.K.; Liu, C.G.; Yang, D.Y.; Wang, S.B.; Chen, A.Z. Ultrasmall platinum nanoparticles enable deep tumor penetration and synergistic therapeutic abilities through free radical species-assisted catalysis to combat cancer multidrug resistance. Chem. Eng. J. 2020, 383, 123138. [Google Scholar] [CrossRef]
- Jin, X.; Yao, S.; Qiu, F.; Mao, Z.; Wang, B. A multifunctional hydrogel containing gold nanorods and methylene blue for synergistic cancer phototherapy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126154. [Google Scholar] [CrossRef]
- Zheng, M.; Yao, S.; Zhao, Y.; Wan, X.; Hu, Q.; Tang, C.; Jiang, Z.; Wang, S.; Liu, Z.; Li, L. Self-driven electrical stimulation-promoted cancer catalytic therapy and chemotherapy based on an implantable nanofibrous patch. ACS Appl. Mater. Interfaces 2023, 15, 7855–7866. [Google Scholar] [CrossRef] [PubMed]
- Letko Khait, N.; Ho, E.; Shoichet, M.S. Wielding the double-edged sword of inflammation: Building biomaterial-based strategies for immunomodulation in ischemic stroke treatment. Adv. Funct. Mater. 2021, 31, 2010674. [Google Scholar] [CrossRef]
- Zhong, S.; Jeong, J.H.; Chen, Z.; Chen, Z.; Luo, J.L. Targeting tumor microenvironment by small-molecule inhibitors. Transl. Oncol. 2020, 13, 57–69. [Google Scholar] [CrossRef]
- Sharifi, S.; Behzadi, S.; Laurent, S.; Forrest, M.L.; Stroeve, P.; Mahmoudi, M. Toxicity of nanomaterials. Chem. Soc. Rev. 2012, 41, 2323–2343. [Google Scholar] [CrossRef]
- Xu, H.; Ci, S.; Ding, Y.; Wang, G.; Wen, Z. Recent advances in precious metal-free bifunctional catalysts for electrochemical conversion systems. J. Mater. Chem. A 2019, 7, 8006–8029. [Google Scholar] [CrossRef]
- Zheng, R.; Zhu, X.; Xiao, Y. Advances in CAR-T-cell therapy in T-cell malignancies. J. Hematol. Oncol. 2024, 17, 49. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart nanoparticles for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar]
- Grzelak, A.; Piotrowski, S. Mechanical Matching of Nanocarriers to Tumor Extracellular Matrix Enhances Drug Delivery. Biomedicines 2024, 12, 1393. [Google Scholar]
- Pan, J.; Liu, X. Nanomechanical Tuning of Hydrogels for Cancer Therapy. Polymers 2023, 15, 3280. [Google Scholar]
- Wang, H.; Li, S.; Yang, Y.; Zhang, L.; Zhang, Y.; Wei, T. Perspectives of metal-organic framework nanosystem to overcome tumor drug resistance. Cancer Drug Resist. 2022, 5, 954–970. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lammers, T.; Storm, G.; Hennink, W.E. Physico-Chemical Strategies to Enhance Stability and Drug Retention of Polymeric Micelles for Tumor-Targeted Drug Delivery. Macromol. Biosci. 2017, 17, 10.1002/mabi.201600160. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, T.; Qin, S.; Huang, Z.; Zhou, L.; Shi, J.; Nice, E.C.; Xie, N.; Huang, C.; Shen, Z. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J. Hematol. Oncol. 2022, 15, 132. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.A.; Kesari, S.; Steinberg, D.M.; Toms, S.A.; Taylor, L.P.; Lieberman, F.; Silvani, A.; Fink, K.L.; et al. Maintenance Therapy with Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015, 314, 2535–2543. [Google Scholar] [CrossRef]
- Strong, M.E.; Richards, J.R.; Torres, M.; Beck, C.M.; La Belle, J.T. Faradaic electrochemical impedance spectroscopy for enhanced analyte detection in diagnostics. Biosens. Bioelectron. 2021, 177, 112949. [Google Scholar] [CrossRef]
| Drug Resistance Mechanism | Pathway | Resistance from Drug | References |
|---|---|---|---|
| Drug efflux pump | Upregulation of ABC transporters: P-gp, BCRP, and MRP | Doxorubicin, axitinib, bisantrene, sunitinib malate | [36] |
| Overexpression of P-gp via TRPC5-mediated Ca2+ influx activating NFATc3 | Adriamycin, paclitaxel, temozolomide | [37] | |
| Inhibition of cell death | Mutations occur in anti-apoptotic genes: caspases, BCL-2 family, BCL-XL, BAX MCL-1, TP53 | Gemcitabine, rituximab | [38] |
| Overexpression of ABCB1 gene | Carboplatin, Taxol, VP-16 | [39] | |
| DNA damage repair | NHEJ pathway | Chemotherapy | [40] |
| Loss of APC | Doxorubicin | [41] | |
| Reduced ER protein and DNA repair mechanism; upregulation of the IL-6/STAT3 pathway | Palbociclib | [42] | |
| Upregulation of BRCA1, BRCA2, Rad51 gene | Radioresistance | [43] | |
| Drug target modification | BCR-ABL signaling | STI-571 (Abl tyrosine kinase inhibitor) | [27] |
| EGFR (T790M), HER2, | Tyrosine kinase inhibitors (TKIs), imatinib | [26] | |
| Mutation in β-tubulin | Paclitaxel | [44] | |
| Epigenetics | Overexpression of ABCB1 levels | Adriamycin | [33] |
| Overexpression of BMP4 | Cisplatin | [45] | |
| Downregulation of GAS5 levels | Adriamycin | [33] | |
| Tumor-promoting inflammation | Activation of STAT3 | Tyrosine kinase inhibitors | [46] |
| Elevated expression of P-gp | Cisplatin | [47] | |
| Elevated expression of TGF-β | Sorafenib | [48] | |
| Immune Evasion | PD-L1, MDSCs, Tregs | Immunotherapy (e.g., checkpoint inhibitors) | [49] |
| Genome mutations | Chromosomal instability | Paclitaxel, carboplatin | [50] |
| Aneuploidy | Cisplatin | [51] |
| Biomaterial | Drug Resistance Marker | Role of Markers | Mechanism | References |
|---|---|---|---|---|
| PLA and PEG nanoparticles | Transporter proteins (P-gp, MRP-1, MRP-2) | Efflux of drug from cancer cells, reducing drug concentration inside the cell | Deliver anticancer drugs without the use of P-gp | [67] |
| Nanotubes | HIF-1α | Induce hypoxia and decrease drug influx and promotes resistance | siRNA against HIF-1α reduces its activity | [68] |
| Dendrimers/magnetic nanoparticles | Survivin | Inhibits apoptosis and enhances cancer cell survival | Antisense survivin-loaded nanoparticles silence survivin expression to promote apoptosis | [69] |
| Mesoporous silica nanoparticles | Bcl-2 | Anti-apoptotic gene that prevents cell death | siRNA-loaded MSNs suppress Bcl-2, enhancing chemotherapy-induced apoptosis | [70] |
| Poly(D,L-lactide-co-glycolide) | p53 mutations | Loss of tumor suppressor function, leading to unchecked cell growth | Deliver wild-type p53 DNA for restoring tumor suppressor activity | [71] |
| Liposomes | Transferrin receptor | Overexpressed in MDR cancer cells, aiding drug resistance | Liposome-conjugated transferrin targets cancer cells for direct drug uptake | [72] |
| Poly(beta-amino ester) | Intracellular pH | Low pH reduces drug effectiveness and uptake | Enhanced drug delivery even in acidic conditions | [72] |
| PEO-modified poly(ε-caprolactone) | Ceramide levels | Reduced ceramide levels prevent apoptosis in cancer cells | Increase ceramide levels to induce cancer cell death | [73] |
| Functionalized quantum dots plus magnetic iron oxide nanoparticles | EGFR | Promotes tumor growth and drug resistance | Targeted systemic delivery of EGFR antibodies for cancer therapy | [74] |
| PAMAM dendrimers | Folate receptors | Overexpressed in breast, kidney, ovary, lung, and brain cancers | Use excessive folate receptors for targeted drug delivery | [75] |
| Herceptin-dextran iron oxide nanoparticles | HER2/neu receptors | Overexpression leads to resistance in breast cancer | High accumulation in tumors to reduce tumor volume | [76] |
| PLGA-PEG nanoparticles | PSMA | Causes prostate cancer progression | Internalized by PSMA-positive cells, leading to targeted therapy | [77] |
| PEG-poly(ε-caprolactone) nanoparticles | Lipoprotein receptor-related protein | Overexpressed in gliomas and brain cancers, aiding drug resistance | Enables drug penetration through the blood–brain barrier | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Tanani, M.; Rabbani, S.A.; Babiker, R.; El-Tanani, Y.; Satyam, S.M.; Porntaveetus, T. Emerging Multifunctional Biomaterials for Addressing Drug Resistance in Cancer. Biology 2025, 14, 497. https://doi.org/10.3390/biology14050497
El-Tanani M, Rabbani SA, Babiker R, El-Tanani Y, Satyam SM, Porntaveetus T. Emerging Multifunctional Biomaterials for Addressing Drug Resistance in Cancer. Biology. 2025; 14(5):497. https://doi.org/10.3390/biology14050497
Chicago/Turabian StyleEl-Tanani, Mohamed, Syed Arman Rabbani, Rasha Babiker, Yahia El-Tanani, Shakta Mani Satyam, and Thantrira Porntaveetus. 2025. "Emerging Multifunctional Biomaterials for Addressing Drug Resistance in Cancer" Biology 14, no. 5: 497. https://doi.org/10.3390/biology14050497
APA StyleEl-Tanani, M., Rabbani, S. A., Babiker, R., El-Tanani, Y., Satyam, S. M., & Porntaveetus, T. (2025). Emerging Multifunctional Biomaterials for Addressing Drug Resistance in Cancer. Biology, 14(5), 497. https://doi.org/10.3390/biology14050497








