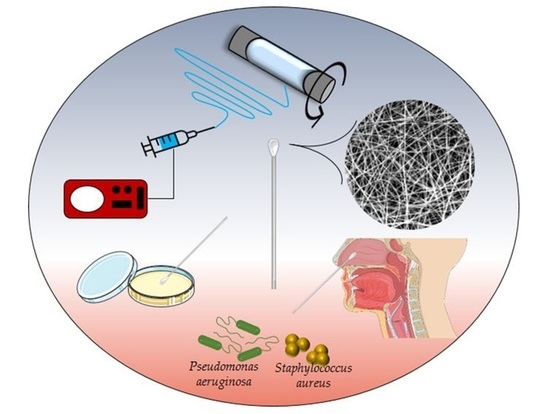
Development of PVA Electrospun Nanofibers for Fabrication of Bacteriological Swabs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospun PVA Mats
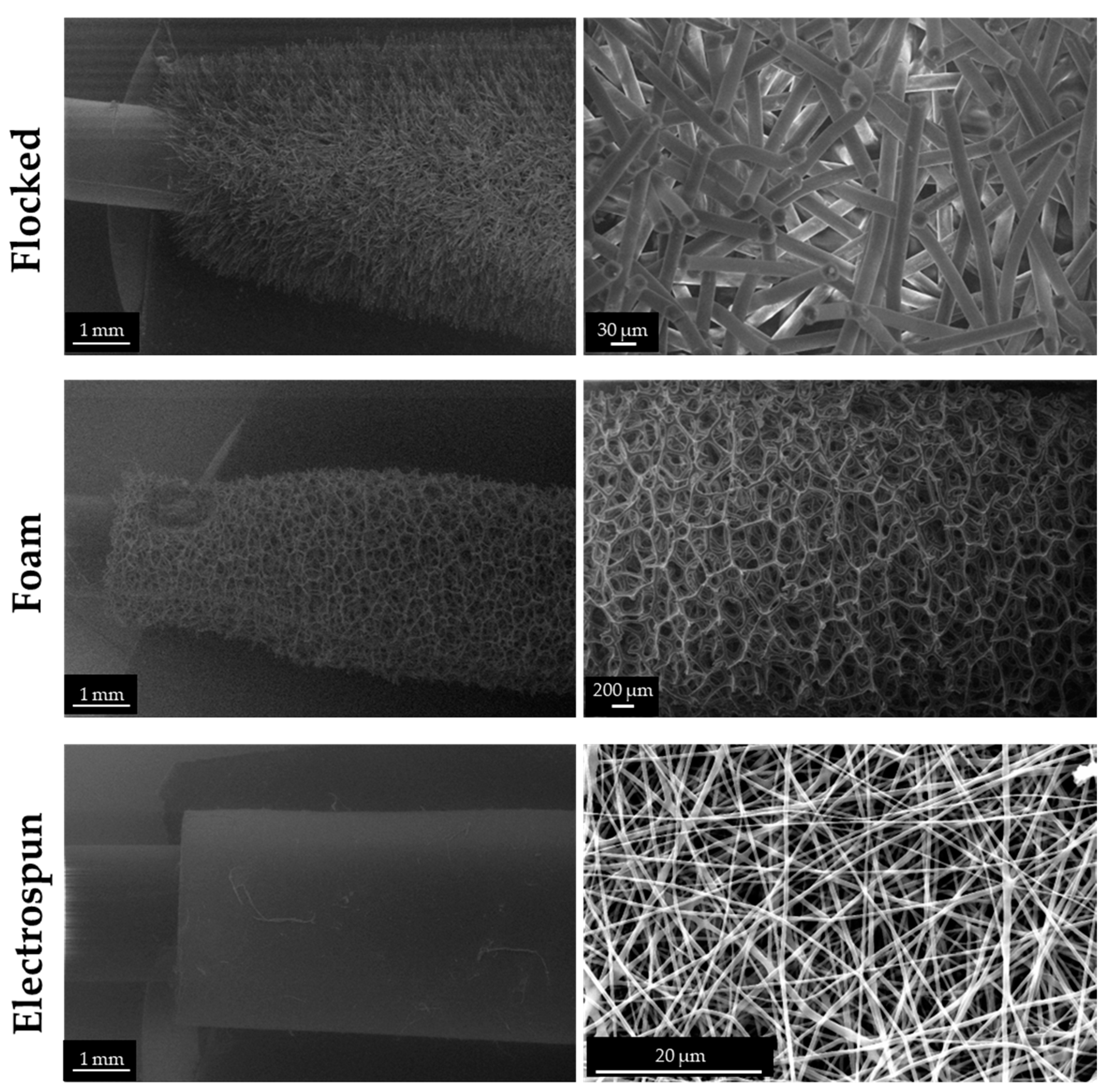
2.3. Swab Prototypes
2.4. PVA Mats Characterisation
2.4.1. Morphological Investigations
2.4.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.3. Swelling
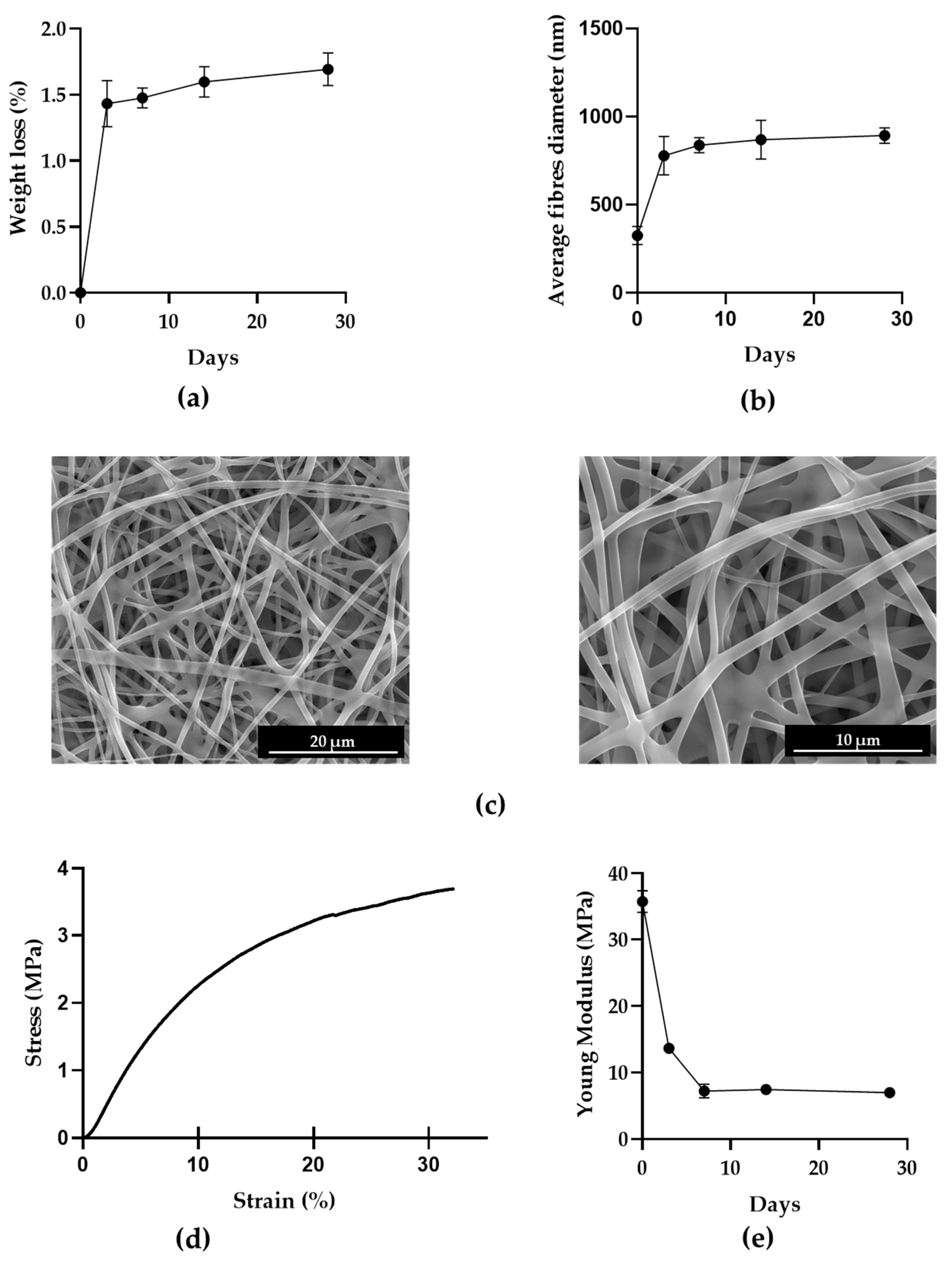
2.4.4. Stability Test
2.4.5. Mechanical Properties
2.5. Swab Prototypes Volume Uptake and Release
2.5.1. Water and PBS Uptake
2.5.2. Bovine Serum Albumin (BSA) Uptake and Release
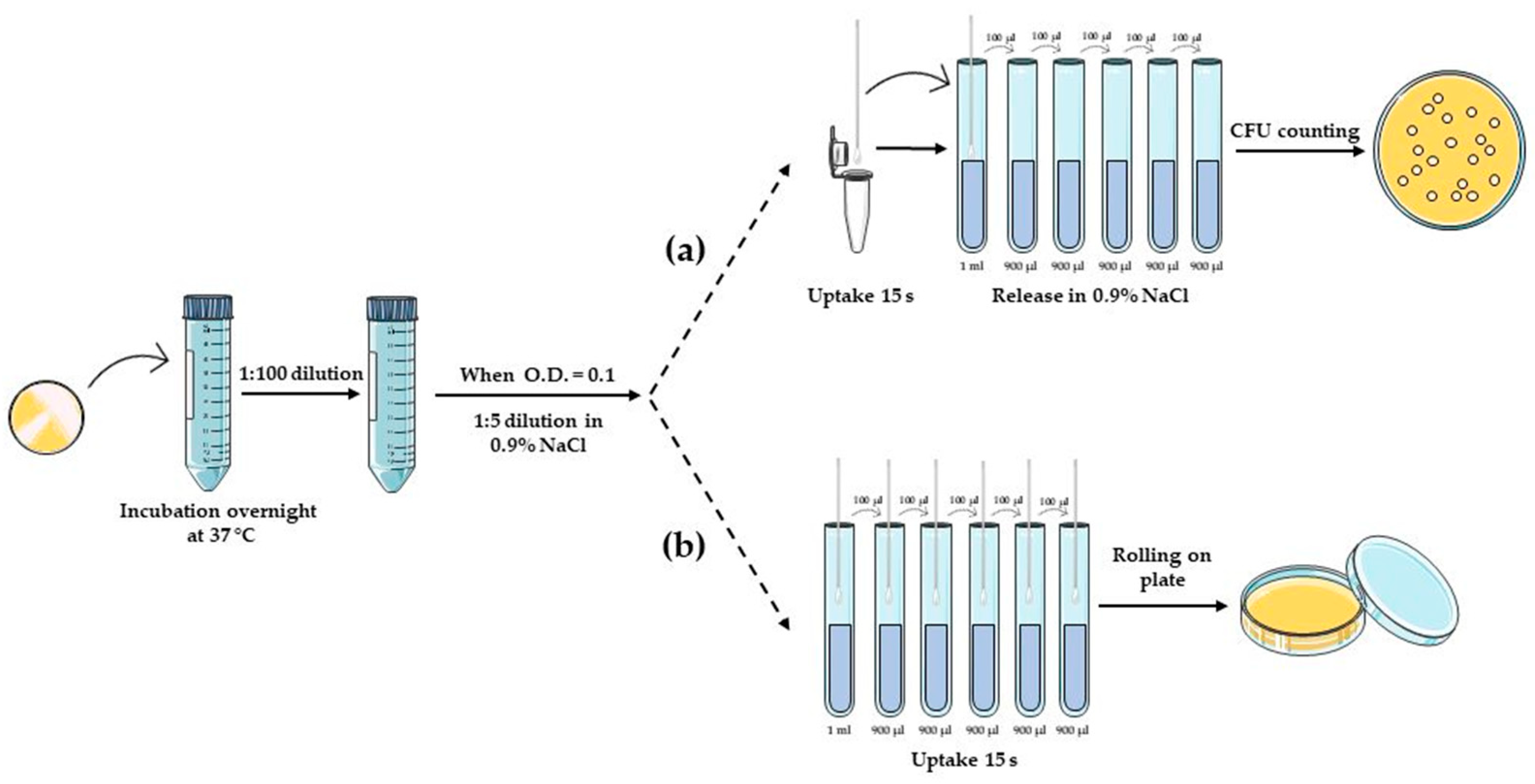
2.6. Detection of Bacteria
2.7. Statistical Analysis
3. Results
3.1. PVA Mats
3.2. Electrospun Swab Uptake and Release Capacity
3.3. Electrospun Prototypes for the Collection of Biological Specimens
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasiri, K.; Dimitrova, A. Comparing saliva and nasopharyngeal swab specimens in the detection of COVID-19: A systematic review and meta-analysis. J. Dent. Sci. 2021, 16, 799–805. [Google Scholar] [CrossRef]
- Yared, N.; Horvath, K.; Fashanu, O.; Zhao, R.; Baker, J.; Kulasingam, S. Optimizing Screening for Sexually Transmitted Infections in Men Using Self-Collected Swabs: A Systematic Review. Sex. Transm. Dis. 2018, 45, 294–300. [Google Scholar] [CrossRef]
- Bruijns, B.; Knotter, J.; Tiggelaar, R. A Systematic Review on Commercially Available Integrated Systems for Forensic DNA Analysis. Sensors 2023, 23, 1075. [Google Scholar] [CrossRef] [PubMed]
- Bonham, P.A. Swab cultures for diagnosing wound infections: A literature review and clinical guideline. J. Wound Ostomy Cont. Nurs. 2009, 36, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Whulanza, Y.; Supriadi, S.; Chalid, M.; Kreshanti, P.; Agus, A.; Napitupulu, P.; Supriyanto, J.; Rivai, E.; Purnomo, A. Setting Acceptance Criteria for a National Flocked Swab for Biological Specimens during the COVID-19 Pandemic. Int. J. Technol. 2020, 11, 888. [Google Scholar] [CrossRef]
- Warnke, P.; Warning, L.; Podbielski, A. Some Are More Equal—A Comparative Study on Swab Uptake and Release of Bacterial Suspensions. PLoS ONE 2014, 9, e102215. [Google Scholar] [CrossRef]
- Hameed, S.; Xie, L.; Ying, Y. Conventional and emerging detection techniques for pathogenic bacteria in food science: A review. Trends Food Sci. Technol. 2018, 81, 61–73. [Google Scholar] [CrossRef]
- Kim, S.-O.; Kim, S.-S. Bacterial pathogen detection by conventional culture-based and recent alternative (polymerase chain reaction, isothermal amplification, enzyme linked immunosorbent assay, bacteriophage amplification, and gold nanoparticle aggregation) methods in food samples: A review. J. Food Saf. 2021, 41, e12870. [Google Scholar]
- Allmann, M.; Höfelein, C.; Köppel, E.; Lüthy, J.; Meyer, R.; Niederhauser, C.; Wegmüller, B.; Candrian, U. Polymerase chain reaction (PCR) for detection of pathogenic microorganisms in bacteriological monitoring of dairy products. Res. Microbiol. 1995, 146, 85–97. [Google Scholar] [CrossRef]
- Allerton, F.; Nuttall, T. Antimicrobial use: Importance of bacterial culture and susceptibility testing. Practice 2021, 43, 500–510. [Google Scholar] [CrossRef]
- Boonham, N.; Kreuze, J.; Winter, S.; van der Vlugt, R.; Bergervoet, J.; Tomlinson, J.; Mumford, R. Methods in virus diagnostics: From ELISA to next generation sequencing. Virus Res. 2014, 186, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.; Frickmann, H.; Ottl, P.; Podbielski, A. Nasal Screening for MRSA: Different Swabs—Different Results! PLoS ONE 2014, 9, e111627. [Google Scholar] [CrossRef] [PubMed]
- Jansson, L.; Akel, Y.; Eriksson, R.; Lavander, M.; Hedman, J. Impact of swab material on microbial surface sampling. J. Microbiol. Methods 2020, 176, 106006. [Google Scholar] [CrossRef]
- Nitti, P.; Narayanan, A.; Pellegrino, R.; Villani, S.; Madaghiele, M.; Demitri, C. Cell-Tissue Interaction: The Biomimetic Approach to Design Tissue Engineered Biomaterials. Bioengineering 2023, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Zasada, A.A.; Zacharczuk, K.; Woźnica, K.; Główka, M.; Ziółkowski, R.; Malinowska, E. The influence of a swab type on the results of point-of-care tests. AMB Express 2020, 10, 46. [Google Scholar] [CrossRef]
- Vashist, V.; Banthia, N.; Kumar, S.; Agrawal, P. A systematic review on materials, design, and manufacturing of swabs. Ann. 3d Print. Med. 2023, 9, 100092. [Google Scholar] [CrossRef]
- Probst, A.; Facius, R.; Wirth, R.; Moissl-Eichinger, C. Validation of a nylon-flocked-swab protocol for efficient recovery of bacterial spores from smooth and rough surfaces. Appl. Environ. Microbiol. 2010, 76, 5148–5158. [Google Scholar] [CrossRef]
- Garnett, L.; Bello, A.; Tran, K.N.; Audet, J.; Leung, A.; Schiffman, Z.; Griffin, B.D.; Tailor, N.; Kobasa, D.; Strong, J.E. Comparison analysis of different swabs and transport mediums suitable for SARS-CoV-2 testing following shortages. J. Virol. Methods 2020, 285, 113947. [Google Scholar] [CrossRef]
- Bolgen, S.W. Flocking Technology. J. Coat. Fabr. 1991, 21, 123–131. [Google Scholar] [CrossRef]
- Mischnik, A.; Mieth, M.; Busch, C.; Hofer, S.; Zimmermann, S. First Evaluation of Automated Specimen Inoculation for Wound Swab Samples by Use of the Previ Isola System Compared to Manual Inoculation in a Routine Laboratory: Finding a Cost-Effective and Accurate Approach. J. Clin. Microbiol. 2012, 50, 2732–2736. [Google Scholar] [CrossRef]
- Jones, S.L.; Gibson, K.E. Characterization of Polyurethane Foam Environmental Monitoring Tools for the Recovery and Release of Viruses. Food Environ. Virol. 2020, 12, 158–166. [Google Scholar] [CrossRef]
- Gama, N.V.; Ferreira, A.; Barros-Timmons, A. Polyurethane Foams: Past, Present, and Future. Materials 2018, 11, 1841. [Google Scholar] [CrossRef]
- Keeratipibul, S.; Laovittayanurak, T.; Pornruangsarp, O.; Chaturongkasumrit, Y.; Takahashi, H.; Techaruvichit, P. Effect of swabbing techniques on the efficiency of bacterial recovery from food contact surfaces. Food Control 2017, 77, 139–144. [Google Scholar] [CrossRef]
- Park, J.-C.; Ito, T.; Kim, K.-O.; Kim, K.-W.; Kim, B.-S.; Khil, M.-S.; Kim, H.-Y.; Kim, I.-S. Electrospun poly(vinyl alcohol) nanofibers: Effects of degree of hydrolysis and enhanced water stability. Polym. J. 2010, 42, 273–276. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Guo, H. Research Progress of Polyvinyl Alcohol Water-Resistant Film Materials. Membranes 2022, 12, 347. [Google Scholar] [CrossRef]
- Kumar, A.; Han, S.S. PVA-based hydrogels for tissue engineering: A review. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 159–182. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, S.; Feng, W. PVA hydrogel properties for biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1228–1233. [Google Scholar] [CrossRef]
- Kobayashi, M.; Toguchida, J.; Oka, M. Preliminary study of polyvinyl alcohol-hydrogel (PVA-H) artificial meniscus. Biomaterials 2003, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, Z.W.; Dong, Y.; Davies, I.J.; Barbhuiya, S. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polym.-Plast. Technol. Eng. 2017, 56, 1307–1344. [Google Scholar] [CrossRef]
- Al-Taie, A.; Han, X.; Williams, C.M.; Abdulwhhab, M.; Abbott, A.P.; Goddard, A.; Wegrzyn, M.; Garton, N.J.; Barer, M.R.; Pan, J. 3-D printed polyvinyl alcohol matrix for detection of airborne pathogens in respiratory bacterial infections. Microbiol. Res. 2020, 241, 126587. [Google Scholar] [CrossRef]
- Huang, D.; Hu, Z.-D.; Liu, T.-Y.; Lu, B.; Zhen, Z.-C.; Wang, G.-X.; Ji, J.-H. Seawater degradation of PLA accelerated by water-soluble PVA. e-Polymers 2020, 20, 759–772. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Mallapragada, S.K.; Peppas, N.A. Dissolution mechanism of semicrystalline poly(vinyl alcohol) in water. J. Polym. Sci. Part B Polym. Phys. 1996, 34, 1339–1346. [Google Scholar] [CrossRef]
- Agarwal, S.; Wendorff, J.H.; Greiner, A. Use of electrospinning technique for biomedical applications. Polymer 2008, 49, 5603–5621. [Google Scholar] [CrossRef]
- Teo, W. A Review on Electrospinning Design and Nanofibre Assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Akhouy, G.; Aziz, K.; Gebrati, L.; El Achaby, M.; Akgul, Y.; Yap, P.-S.; Agustiono Kurniawan, T.; Aziz, F. Recent applications on biopolymers electrospinning: Strategies, challenges and way forwards. Polym.-Plast. Technol. Mater. 2023, 62, 1754–1775. [Google Scholar] [CrossRef]
- Nitti, P.; Gallo, N.; Palazzo, B.; Sannino, A.; Polini, A.; Verri, T.; Barca, A.; Gervaso, F. Effect of L-Arginine treatment on the in vitro stability of electrospun aligned chitosan nanofiber mats. Polym. Test. 2020, 91, 106758. [Google Scholar] [CrossRef]
- Reynolds, D.; Kollef, M. The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update. Drugs 2021, 81, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging Strategies to Combat ESKAPE Pathogens in the Era of Antimicrobial Resistance: A Review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef] [PubMed]
- Kunjalukkal Padmanabhan, S.; Lamanna, L.; Friuli, M.; Sannino, A.; Demitri, C.; Licciulli, A. Carboxymethylcellulose-Based Hydrogel Obtained from Bacterial Cellulose. Molecules 2023, 28, 829. [Google Scholar] [CrossRef]
- Padmanabhan, S.K.; Nitti, P.; Stanca, E.; Rochira, A.; Siculella, L.; Raucci, M.G.; Madaghiele, M.; Licciulli, A.; Demitri, C. Mechanical and biological properties of magnesium-and silicon-substituted hydroxyapatite scaffolds. Materials 2021, 14, 6942. [Google Scholar] [CrossRef] [PubMed]
- Nitti, P.; Palazzo, B.; Gallo, N.; Scalera, F.; Sannino, A.; Gervaso, F. Smooth-rough asymmetric PLGA structure made of dip coating membrane and electrospun nanofibrous scaffolds meant to be used for guided tissue regeneration of periodontium. Polym. Eng. Sci. 2022, 62, 2061–2069. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- CLSI. Quality Control of Microbiological Transport Systems; Approved Standard—Second Edition; CLSI Document M40-A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014. [Google Scholar]
- Deleanu, I.; Stoica, A.; M, S.; Dobre, L.; Dobre, T.; Jinga, S.; Tardei, C. Potassium sorbate release from poly(vinyl alcohol)–bacterial cellulose films. Chem. Pap. 2012, 66, 138–143. [Google Scholar] [CrossRef]
- Forbes, B.A.; Sahm, D.F.; Weissfeld, A.S. Bailey & Scott’s Diagnostic Microbiology, 12th ed.; Mosby Elsevier: Maryland Heights, MO, USA, 2007; pp. 10–840. [Google Scholar]



| Swabs Uptake (%) | |||
|---|---|---|---|
| Flocked Swab | Foam Swab | Electrospun Swab | |
| Water | 14.73 ± 0.90 | 3.54 ± 0.54 | 10.52 ± 2.05 |
| PBS | 12.62 ± 0.57 | 4.54 ± 0.51 | 6.08 ± 0.99 |
| BSA | 35.57 ± 1.01 | 19.68 ± 0.15 | 19.92 ± 3.99 |
| Swabs Release (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Flocked Swab | Foam Swab | Electrospun Swab | |||||||
| 15 min | 30 min | 60 min | 15 min | 30 min | 60 min | 15 min | 30 min | 60 min | |
| BSA | 34.10 ± 0.11 | 36.88 ± 2.91 | 36.48 ± 0.84 | 31.75 ± 4.43 | 33.57 ± 0.31 | 33.71 ± 0.37 | 23.99 ± 0.66 | 34.06 ± 5.24 | 41.10 ± 4.37 |
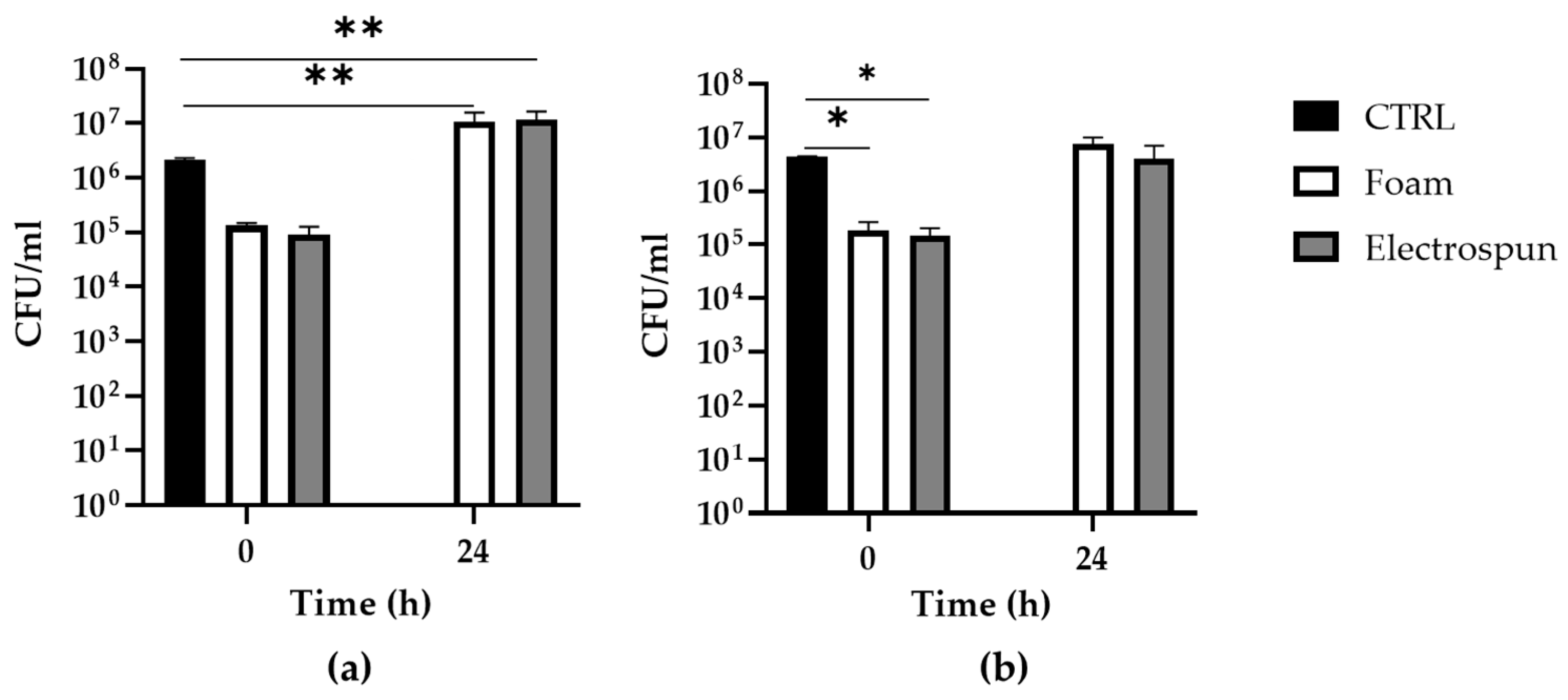
| Bacteria Detection (%) | ||||
|---|---|---|---|---|
| Foam Swab | Electrospun Swab | |||
| T0 | T24 | T0 | T24 | |
| P. aeruginosa | 1.3 105 ± 0.2 105 | 1.1 107 ± 0.5 107 | 0.9 105 ± 0.4 105 | 1.2 107 ± 0.5 107 |
| S. aureus | 1.9 105 ± 0.8 105 | 7.5 106 ± 2.5 106 | 1.5 105 ± 0.6 105 | 4.0 106 ± 3.1 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellegrino, R.; Villani, S.; Spagnolo, D.; Carofalo, I.; Carrino, N.; Calcagnile, M.; Alifano, P.; Madaghiele, M.; Demitri, C.; Nitti, P. Development of PVA Electrospun Nanofibers for Fabrication of Bacteriological Swabs. Biology 2023, 12, 1404. https://doi.org/10.3390/biology12111404
Pellegrino R, Villani S, Spagnolo D, Carofalo I, Carrino N, Calcagnile M, Alifano P, Madaghiele M, Demitri C, Nitti P. Development of PVA Electrospun Nanofibers for Fabrication of Bacteriological Swabs. Biology. 2023; 12(11):1404. https://doi.org/10.3390/biology12111404
Chicago/Turabian StylePellegrino, Rebecca, Stefania Villani, Daniela Spagnolo, Irene Carofalo, Nico Carrino, Matteo Calcagnile, Pietro Alifano, Marta Madaghiele, Christian Demitri, and Paola Nitti. 2023. "Development of PVA Electrospun Nanofibers for Fabrication of Bacteriological Swabs" Biology 12, no. 11: 1404. https://doi.org/10.3390/biology12111404
APA StylePellegrino, R., Villani, S., Spagnolo, D., Carofalo, I., Carrino, N., Calcagnile, M., Alifano, P., Madaghiele, M., Demitri, C., & Nitti, P. (2023). Development of PVA Electrospun Nanofibers for Fabrication of Bacteriological Swabs. Biology, 12(11), 1404. https://doi.org/10.3390/biology12111404