HGF Aggravated Periodontitis-Associated Gut Barrier and Microbial Dysfunction: Implications for Oral–Gut Axis Regulation
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Periodontitis Model
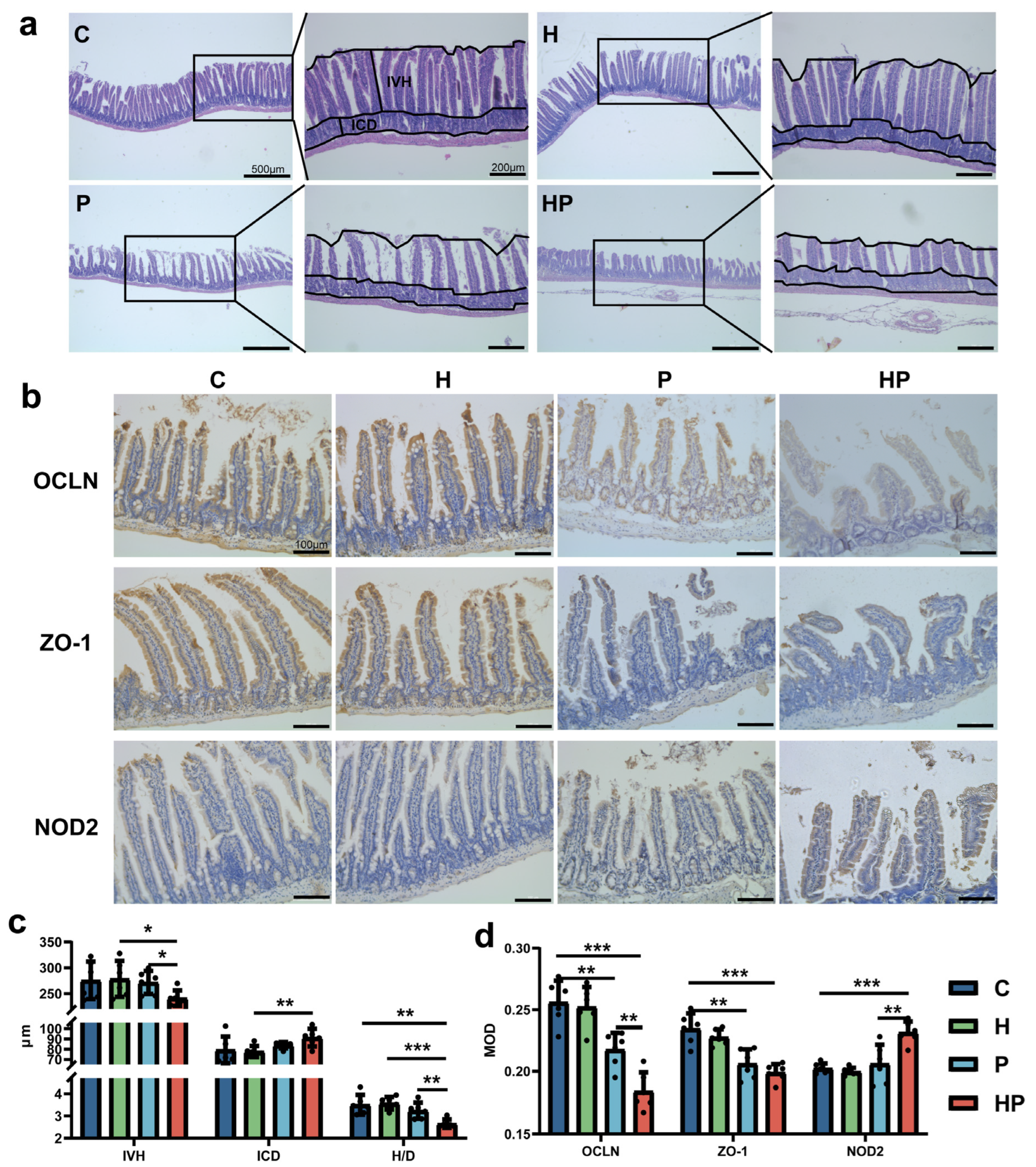
2.2. Histologic Analyses and Immunohistochemistry Staining
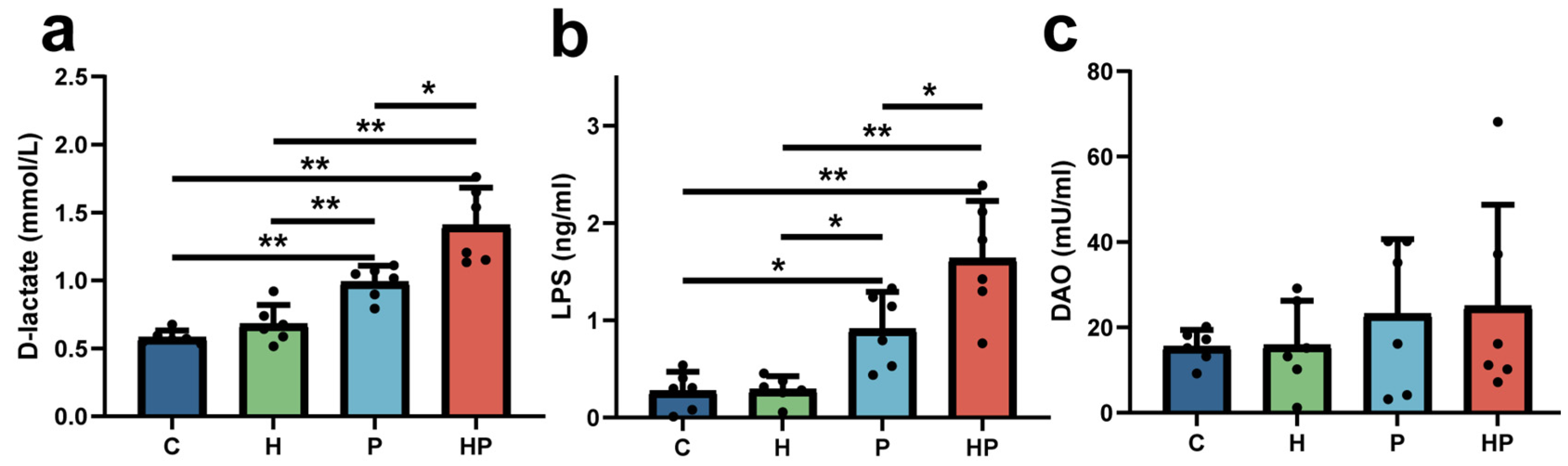
2.3. Intestinal Permeability Evaluation
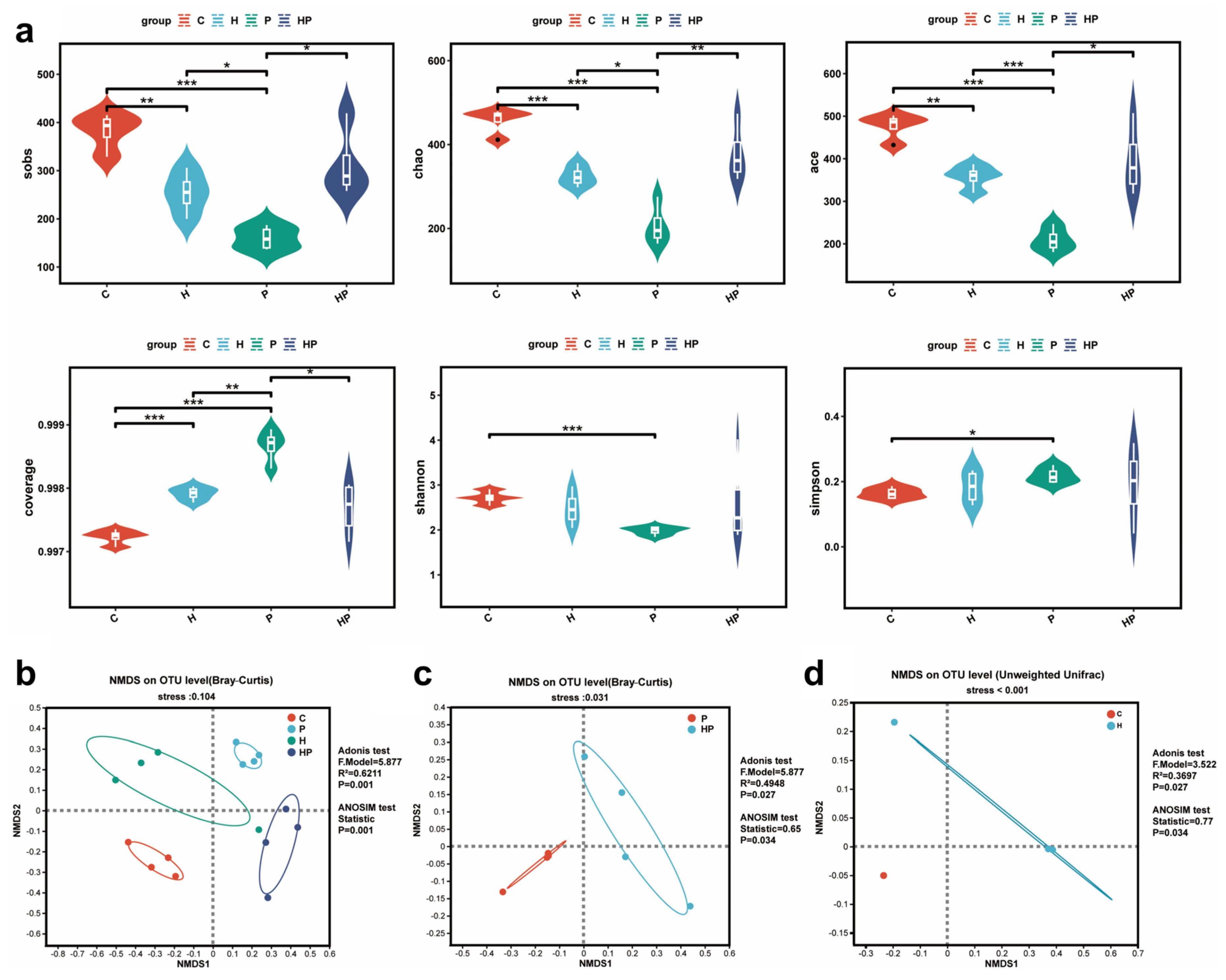
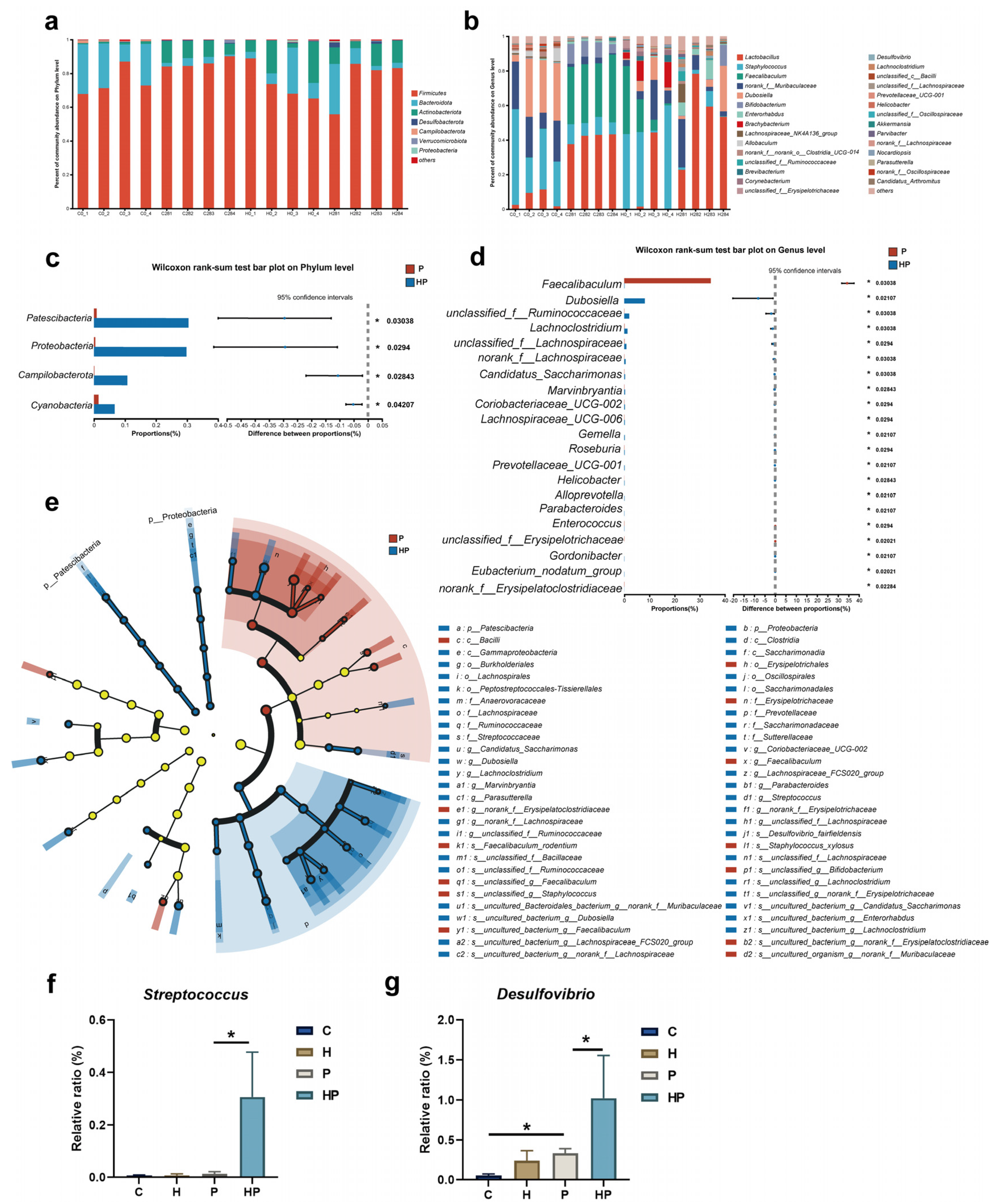
2.4. Sequencing of 16s rRNA and Bioinformatics
2.5. Statistical Analysis
3. Results
3.1. HGF May Increase Intestinal Permeability and Escalate Gut Barrier Disorder During the Periodontitis Period
3.2. HGF Altered Gut Microbiota Diversity in Mice
3.3. HGF Modulated Intestinal Microbiological Compositions When Periodontitis Occurred
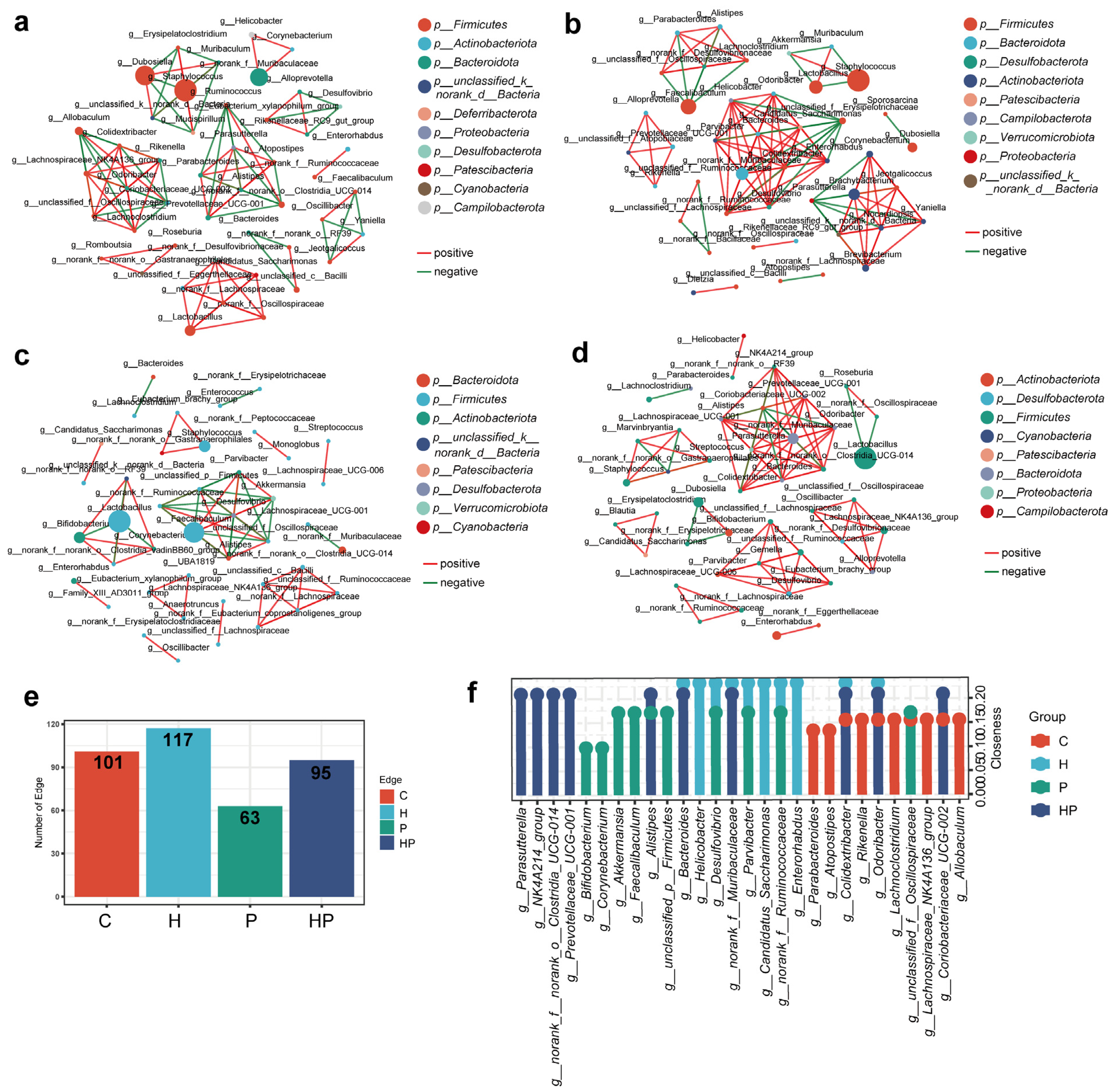
3.4. HGF Facilitated Different Co-Occurrence Network of Gut Bacterial Taxa
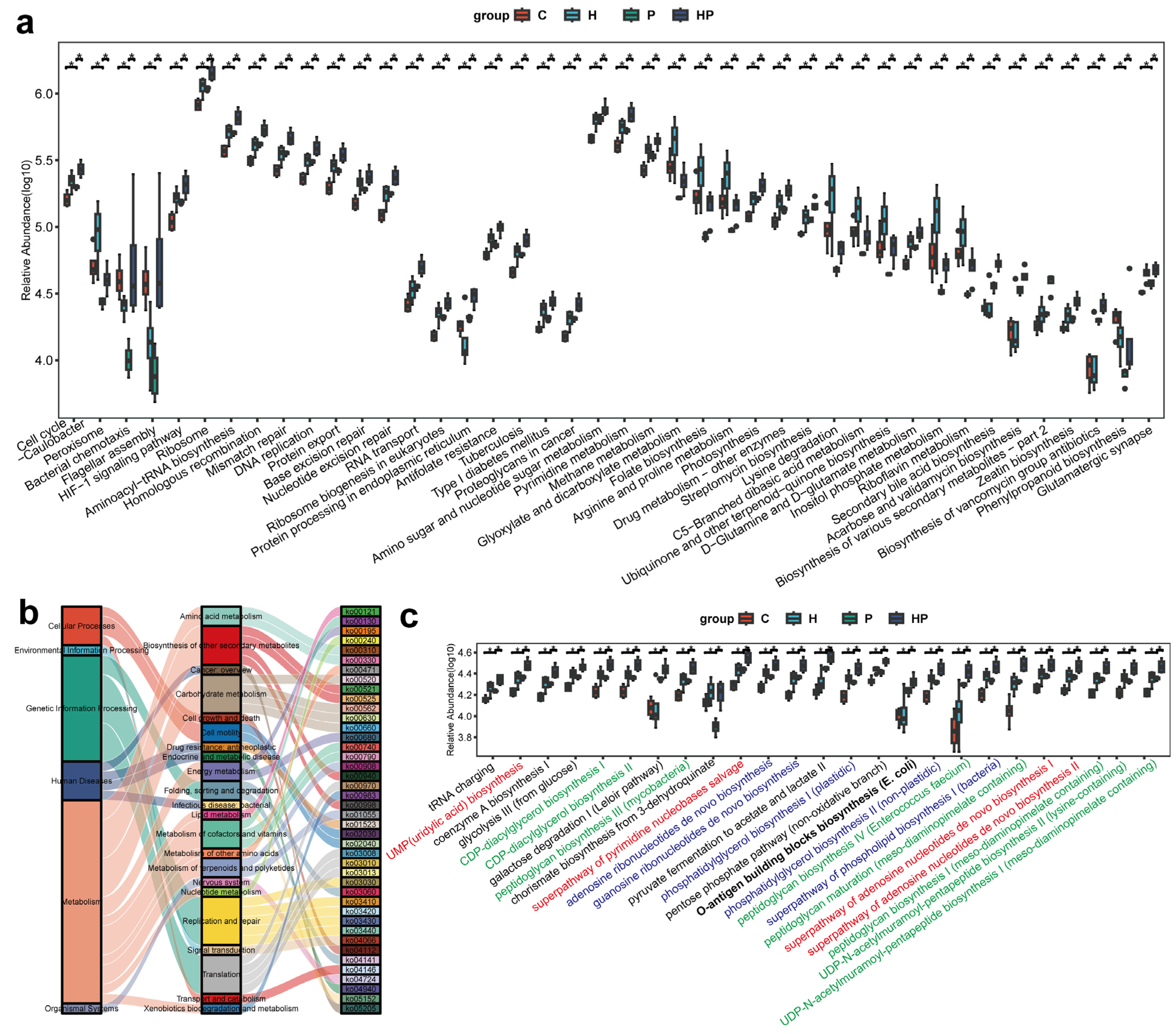
3.5. HGF Transformed Microbial Functions in Mice with Periodontitis
3.6. Identification of Key Gut Microbiota Associated with Intestinal Barrier
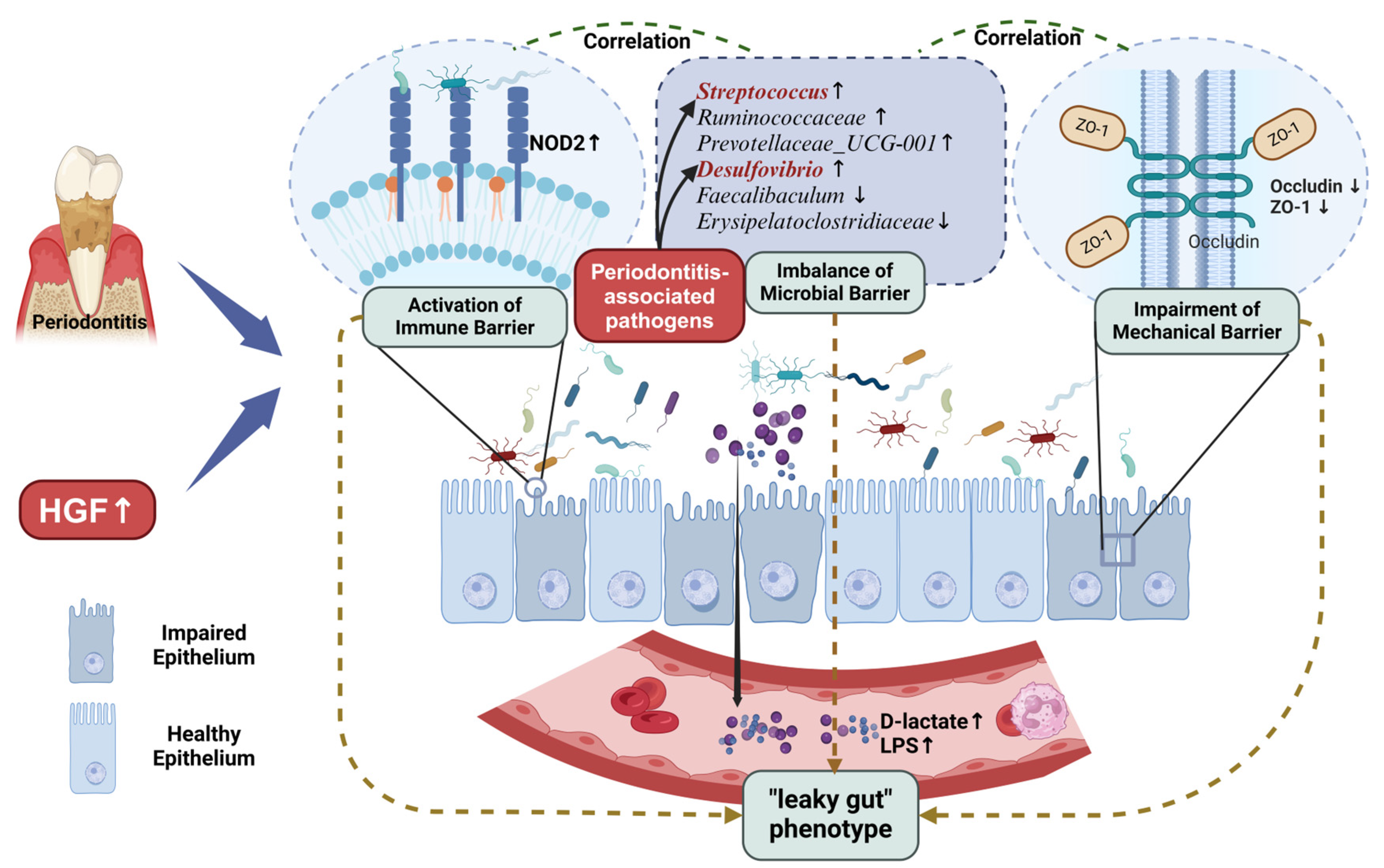
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HGF | Hepatocyte Growth Factor |
| HGF-Tg | Hepatocyte Growth Factor High-Expression Transgenic |
| LPS | Lipopolysaccharide |
| DAO | Diamine Oxidase |
| ZO-1 | Zonula Occludens-1 |
| NOD2 | Nucleotide-binding Oligomerization Domain-containing Protein 2 |
| c-Met | Cellular-Mesenchymal Epithelial Transition Factor |
| WT | Wild Type |
| C | WT Mice |
| H | HGF-Tg Mice |
| P | WT Mice with Periodontitis |
| HP | HGF-Tg Mice with Periodontitis |
| HE | Hematoxylin and Eosin |
| IVH | Intestinal Villus Height |
| ICD | Intestinal Crypt Depth |
| H/D | Ratio of Villus Height to Crypt Depth |
| MOD | Mean Density |
| IOD | Integrated Density |
| OTU | Operational Taxonomic Units |
| NMDS | Non-metric Multidimensional Scaling |
| LDA | Linear Discriminant Analysis |
| LEfSe | Linear Discriminant Analysis Effect Size |
| PICRUSt | Phylogenetic Investigation of Communities by Reconstruction of Unobserved States |
| RDA | Redundancy Analysis |
| db-RDA | Distance-based Redundancy Analysis |
| MetaCyc | Metabolic Pathways From all Domains of Life |
| TJ | Tight junction |
References
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.L.; Marcenes, W. Global burden of severe periodontitis in 1990–2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, Y.; Cui, Q.; Liu, N.; Chu, F.; Cong, B.; Wu, Y. Effect of Psoralen on the Intestinal Barrier and Alveolar Bone Loss in Rats With Chronic Periodontitis. Inflammation 2021, 44, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Liu, Y.; Cheng, H.; Bai, M.; Chen, J.; Ji, H.; He, M.; Chen, K. Ligature induced periodontitis in rats causes gut dysbiosis leading to hepatic injury through SCD1/AMPK signalling pathway. Life Sci. 2022, 288, 120162. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Zou, X.; Yang, X.-Q.; Peng, F.; Yu, D.-K.; Du, J.-R. Chronic periodontitis induces microbiota-gut-brain axis disorders and cognitive impairment in mice. Exp. Neurol. 2020, 326, 113176. [Google Scholar] [CrossRef]
- Morton, A.M.; Sefik, E.; Upadhyay, R.; Weissleder, R.; Benoist, C.; Mathis, D. Endoscopic photoconversion reveals unexpectedly broad leukocyte trafficking to and from the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 6696–6701. [Google Scholar] [CrossRef]
- Park, S.-Y.; Hwang, B.-O.; Lim, M.; Ok, S.-H.; Lee, S.-K.; Chun, K.-S.; Park, K.-K.; Hu, Y.; Chung, W.-Y.; Song, N.-Y. Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef]
- Ruan, Q.; Guan, P.; Qi, W.; Li, J.; Xi, M.; Xiao, L.; Zhong, S.; Ma, D.; Ni, J. Porphyromonas gingivalis regulates atherosclerosis through an immune pathway. Front. Immunol. 2023, 14, 1103592. [Google Scholar] [CrossRef]
- Yamazaki, K. Oral-gut axis as a novel biological mechanism linking periodontal disease and systemic diseases: A review. Jpn. Dent. Sci. Rev. 2023, 59, 273–280. [Google Scholar] [CrossRef]
- Bao, J.; Li, L.; Zhang, Y.; Wang, M.; Chen, F.; Ge, S.; Chen, B.; Yan, F. Periodontitis may induce gut microbiota dysbiosis via salivary microbiota. Int. J. Oral. Sci. 2022, 14, 32. [Google Scholar] [CrossRef]
- Wang, X.; Chen, L.; Teng, Y.; Xie, W.; Huang, L.; Wu, J.; Wang, H.; Xie, S. Effect of three oral pathogens on the TMA-TMAO metabolic pathway. Front. Cell Infect. Microbiol. 2024, 14, 1413787. [Google Scholar] [CrossRef]
- Yao, S.; Liu, X.; Feng, Y.; Li, Y.; Xiao, X.; Han, Y.; Xia, S. Unveiling the Role of HGF/c-Met Signaling in Non-Small Cell Lung Cancer Tumor Microenvironment. Int. J. Mol. Sci. 2024, 25, 9101. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, W.; Wu, Z.; He, X.; Tang, Y.; He, Q.; Lin, C.; Chen, Y.; Luo, G.; Yu, T.; et al. Hepatocyte growth factor is protective in early stage but bone-destructive in late stage of experimental periodontitis. J. Periodontal Res. 2024, 59, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Cui, Q.; Lu, W.; Yang, S.; Shi, M.; Li, Z.; Gao, P.; Xu, B.; Li, Z. Hepatocyte growth factor plays a dual role in tendon-derived stem cell proliferation, migration, and differentiation. J. Cell Physiol. 2019, 234, 17382–17391. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Huang, C.; Zhong, M.; Zhong, H.; Ruan, R.; Xiong, J.; Yao, Y.; Zhou, J.; Deng, J. Targeting HGF/c-MET signaling to regulate the tumor microenvironment: Implications for counteracting tumor immune evasion. Cell Commun. Signal 2025, 23, 46. [Google Scholar] [CrossRef]
- Zhao, Y.; Ye, W.; Wang, Y.D.; Chen, W.D. HGF/c-Met: A Key Promoter in Liver Regeneration. Front. Pharmacol. 2022, 13, 808855. [Google Scholar] [CrossRef]
- Nagaraja, C.; Pradeep, A.R. Hepatocyte growth factor levels in gingival crevicular fluid in health, disease, and after treatment. J. Periodontol. 2007, 78, 742–747. [Google Scholar] [CrossRef]
- Suzuki, S.; Aoki, A.; Katagiri, S.; Maekawa, S.; Ejiri, K.; Kong, S.; Nagata, M.; Yamaguchi, Y.; Ohshima, M.; Izumi, Y. Detection of hepatocyte growth factor in oral rinses using water for possible periodontal diagnosis. J. Oral. Sci. 2020, 62, 250–255. [Google Scholar] [CrossRef]
- D’Angelo, F.; Bernasconi, E.; Schäfer, M.; Moyat, M.; Michetti, P.; Maillard, M.H.; Velin, D. Macrophages promote epithelial repair through hepatocyte growth factor secretion. Clin. Exp. Immunol. 2013, 174, 60–72. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Bolder, U.; Finnerty, C.C.; Przkora, R.; Müller, U.; Maihöfer, R.; Thompson, J.C.; Wolf, S.E.; Herndon, D.N. The effect of hepatocyte growth factor on gut mucosal apoptosis and proliferation, and cellular mediators after severe trauma. Surgery 2005, 138, 482–489. [Google Scholar] [CrossRef]
- Stakenborg, M.; Verstockt, B.; Meroni, E.; Goverse, G.; De Simone, V.; Verstockt, S.; Di Matteo, M.; Czarnewski, P.; Villablanca, E.J.; Ferrante, M.; et al. Neutrophilic HGF-MET Signalling Exacerbates Intestinal Inflammation. J. Crohn’s Colitis 2020, 14, 1748–1758. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Y.; Shen, R.; Chen, J.; Chen, G.; Luo, G.; Ge, L. Hepatocyte growth factor (HGF) optimizes oral traumatic ulcer healing of mice by reducing inflammation. Cytokine 2017, 99, 275–280. [Google Scholar] [CrossRef]
- Festing, M.F. On determining sample size in experiments involving laboratory animals. Lab. Anim. 2018, 52, 341–350. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Wang, L.; Xu, W.; He, M.; Dong, H.; Shi, H.-Y.; Chen, Y.-Q.; Huang, Z.-L. Paraventricular thalamus controls consciousness transitions during propofol anaesthesia in mice. Br. J. Anaesth. 2023, 130, 698–708. [Google Scholar] [CrossRef]
- Jia, X.; Jia, L.; Mo, L.; Yuan, S.; Zheng, X.; He, J.; Chen, V.; Guo, Q.; Zheng, L.; Yuan, Q.; et al. Berberine Ameliorates Periodontal Bone Loss by Regulating Gut Microbiota. J. Dent. Res. 2019, 98, 107–116. [Google Scholar] [CrossRef]
- Yan, L.; He, X.; Tang, Y.; Zhao, X.; Luo, G.; Wang, X. HGF can reduce accumulation of inflammation and regulate glucose homeostasis in T2D mice. J. Physiol. Biochem. 2021, 77, 613–624. [Google Scholar] [CrossRef]
- Guan, H.; Pu, Y.; Liu, C.; Lou, T.; Tan, S.; Kong, M.; Sun, Z.; Mei, Z.; Qi, Q.; Quan, Z.; et al. Comparison of Fecal Collection Methods on Variation in Gut Metagenomics and Untargeted Metabolomics. mSphere 2021, 6, e0063621. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, D.; Ma, W.; Guo, Y.; Wang, A.; Wang, Q.; Lee, D.-J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016, 100, 1421–1426. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar]
- Barberán, A.; Bates, S.T.; Casamayor, E.O.; Fierer, N. Using network analysis to explore co-occurrence patterns in soil microbial communities. ISME J. 2012, 6, 343–351. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for prediction of metagenome functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’hara, B.; Stevens, M.; Oksanen, M.; Suggests, M. The vegan package: Community ecology package. R Package Version 2007, 1, 1–190. [Google Scholar]
- Wang, H.; Sun, R.-T.; Li, Y.; Yang, Y.-F.; Xiao, F.-J.; Zhang, Y.-K.; Wang, S.-X.; Sun, H.-Y.; Zhang, Q.-W.; Wu, C.-T.; et al. HGF Gene Modification in Mesenchymal Stem Cells Reduces Radiation-Induced Intestinal Injury by Modulating Immunity. PLoS ONE 2015, 10, e0124420. [Google Scholar] [CrossRef]
- Martin, T.A.; Watkins, G.; Mansel, R.E.; Jiang, W.G. Hepatocyte growth factor disrupts tight junctions in human breast cancer cells. Cell Biol. Int. 2004, 28, 361–371. [Google Scholar] [CrossRef]
- Jiang, W.G.; Martin, T.A.; Matsumoto, K.; Nakamura, T.; Mansel, R.E. Hepatocyte growth factor/scatter factor decreases the expression of occludin and transendothelial resistance (TER) and increases paracellular permeability in human vascular endothelial cells. J. Cell. Physiol. 1999, 181, 319–329. [Google Scholar] [CrossRef]
- Nasu, Y.; Ido, A.; Tanoue, S.; Hashimoto, S.; Sasaki, F.; Kanmura, S.; Setoyama, H.; Numata, M.; Funakawa, K.; Moriuchi, A.; et al. Hepatocyte growth factor stimulates the migration of gastric epithelial cells by altering the subcellular localization of the tight junction protein ZO-1. J. Gastroenterol. 2013, 48, 193–202. [Google Scholar] [CrossRef]
- Philpott, D.J.; Sorbara, M.T.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD proteins: Regulators of inflammation in health and disease. Nat. Rev. Immunol. 2014, 14, 9–23. [Google Scholar] [CrossRef]
- Panigrahy, D.; Gilligan, M.M.; Serhan, C.N.; Kashfi, K. Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol. Ther. 2021, 227, 107879. [Google Scholar] [CrossRef]
- Sasaki, K.; Inoue, J.; Sasaki, D.; Hoshi, N.; Shirai, T.; Fukuda, I.; Azuma, T.; Kondo, A.; Osawa, R. Construction of a Model Culture System of Human Colonic Microbiota to Detect Decreased Lachnospiraceae Abundance and Butyrogenesis in the Feces of Ulcerative Colitis Patients. Biotechnol. J. 2019, 14, e1800555. [Google Scholar] [CrossRef]
- Lin, H.; Meng, L.; Sun, Z.; Sun, S.; Huang, X.; Lin, N.; Zhang, J.; Lu, W.; Yang, Q.; Chi, J.; et al. Yellow Wine Polyphenolic Compound Protects Against Doxorubicin-Induced Cardiotoxicity by Modulating the Composition and Metabolic Function of the Gut Microbiota. Circ. Heart Fail. 2021, 14, e008220. [Google Scholar] [CrossRef]
- Hall, A.B.; Yassour, M.; Sauk, J.; Garner, A.; Jiang, X.; Arthur, T.; Lagoudas, G.K.; Vatanen, T.; Fornelos, N.; Wilson, R.; et al. A novel Ruminococcus gnavus clade enriched in inflammatory bowel disease patients. Genome Med. 2017, 9, 103. [Google Scholar] [CrossRef]
- Ley, R.E. Gut microbiota in 2015: Prevotella in the gut: Choose carefully. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 69–70. [Google Scholar] [CrossRef]
- Nie, Y.; Xie, X.-Q.; Zhou, L.; Guan, Q.; Ren, Y.; Mao, Y.; Shi, J.-S.; Xu, Z.-H.; Geng, Y. Desulfovibrio fairfieldensis-Derived Outer Membrane Vesicles Damage Epithelial Barrier and Induce Inflammation and Pyroptosis in Macrophages. Cells 2022, 12, 89. [Google Scholar] [CrossRef]
- Quaglio, A.E.V.; Grillo, T.G.; De Oliveira, E.C.S.; Di Stasi, L.C.; Sassaki, L.Y. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 4053–4060. [Google Scholar] [CrossRef]
- Bai, X.-B.; Xu, S.; Zhou, L.-J.; Meng, X.-Q.; Li, Y.-L.; Chen, Y.-L.; Jiang, Y.-H.; Lin, W.-Z.; Chen, B.-Y.; Du, L.-J.; et al. Oral pathogens exacerbate Parkinson’s disease by promoting Th1 cell infiltration in mice. Microbiome 2023, 11, 254. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chopra, A.; Karmakar, S.; Bhat, S.G. Periodontitis increases the risk of gastrointestinal dysfunction: An update on the plausible pathogenic molecular mechanisms. Crit. Rev. Microbiol. 2025, 51, 187–217. [Google Scholar] [CrossRef]
- Hao, Y.; Ji, Z.; Shen, Z.; Xue, Y.; Zhang, B.; Yu, D.; Liu, T.; Luo, D.; Xing, G.; Tang, J.; et al. Increase Dietary Fiber Intake Ameliorates Cecal Morphology and Drives Cecal Species-Specific of Short-Chain Fatty Acids in White Pekin Ducks. Front. Microbiol. 2022, 13, 853797. [Google Scholar] [CrossRef]
- Heinken, A.; Khan, M.T.; Paglia, G.; Rodionov, D.A.; Harmsen, H.J.M.; Thiele, I. Functional metabolic map of Faecalibacterium prausnitzii, a beneficial human gut microbe. J. Bacteriol. 2014, 196, 3289–3302. [Google Scholar] [CrossRef]
- Cao, Y.G.; Bae, S.; Villarreal, J.; Moy, M.; Chun, E.; Michaud, M.; Lang, J.K.; Glickman, J.N.; Lobel, L.; Garrett, W.S. Faecalibaculum rodentium remodels retinoic acid signaling to govern eosinophil-dependent intestinal epithelial homeostasis. Cell Host Microbe 2022, 30, 1295–1310.e8. [Google Scholar] [CrossRef]
- Li, J.; Lu, H.; Wu, H.; Huang, S.; Chen, L.; Gui, Q.; Zhou, W.; Yang, Y.; Wu, Y.; Zhang, H.; et al. Periodontitis in elderly patients with type 2 diabetes mellitus: Impact on gut microbiota and systemic inflammation. Aging 2020, 12, 25956–25980. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-W.; Rhee, K.-J.; Eom, Y.-B. Zerumbone Restores Gut Microbiota Composition in ETBF Colonized AOM/DSS Mice. J. Microbiol. Biotechnol. 2020, 30, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Rohmer, L.; Hocquet, D.; Miller, S.I. Are pathogenic bacteria just looking for food? Metabolism and microbial pathogenesis. Trends Microbiol. 2011, 19, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Sicard, J.-F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef]
- Bao, C.; Wu, L.; Wang, D.; Chen, L.; Jin, X.; Shi, Y.; Li, G.; Zhang, J.; Zeng, X.; Chen, J.; et al. Acupuncture improves the symptoms, intestinal microbiota, and inflammation of patients with mild to moderate Crohn’s disease: A randomized controlled trial. EClinicalMedicine 2022, 45, 101300. [Google Scholar] [CrossRef]
- Kunath, B.J.; De Rudder, C.; Laczny, C.C.; Letellier, E.; Wilmes, P. The oral-gut microbiome axis in health and disease. Nat. Rev. Microbiol. 2024, 22, 791–805. [Google Scholar] [CrossRef]
- Xu, S.; Lu, F.; Gao, J.; Yuan, Y. Inflammation-mediated metabolic regulation in adipose tissue. Obes. Rev. 2024, 25, e13724. [Google Scholar] [CrossRef]

| RDA1 | RDA2 | R2 | p Value | |
|---|---|---|---|---|
| D-lactate | 0.9698 | 0.244 | 0.7149 | 0.001 ** |
| LPS | 0.933 | 0.3598 | 0.6751 | 0.005 ** |
| DAO | 0.8887 | 0.4586 | 0.2643 | 0.118 |
| OCLN | −0.9715 | −0.2371 | 0.4466 | 0.028 * |
| ZO-1 | −0.9999 | −0.0116 | 0.5769 | 0.003 ** |
| NOD2 | 0.8939 | 0.4482 | 0.4821 | 0.02 * |
| IVH | −0.8418 | −0.5398 | 0.3366 | 0.079 |
| ICD | 0.6777 | 0.7354 | 0.1712 | 0.299 |
| H/D | −0.8107 | −0.5855 | 0.4562 | 0.027 * |
| CAP1 | CAP2 | R2 | p Value | |
|---|---|---|---|---|
| D-lactate | 0.6531 | 0.7573 | 0.3892 | 0.035 * |
| LPS | 0.8435 | 0.5371 | 0.3842 | 0.039 * |
| DAO | −0.0091 | 1 | 0.0267 | 0.857 |
| OCLN | −0.7471 | −0.6647 | 0.3335 | 0.063 |
| ZO-1 | −0.3224 | −0.9466 | 0.2213 | 0.19 |
| NOD2 | 0.7889 | 0.6145 | 0.1223 | 0.45 |
| IVH | −0.4423 | −0.8969 | 0.3318 | 0.094 |
| ICD | 0.8073 | −0.5902 | 0.2308 | 0.188 |
| H/D | −0.9128 | −0.4084 | 0.2126 | 0.223 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhong, Y.; Chen, L.; Liu, W.; Lin, C.; Chen, Y.; Wang, X. HGF Aggravated Periodontitis-Associated Gut Barrier and Microbial Dysfunction: Implications for Oral–Gut Axis Regulation. Biology 2025, 14, 496. https://doi.org/10.3390/biology14050496
Chen Z, Zhong Y, Chen L, Liu W, Lin C, Chen Y, Wang X. HGF Aggravated Periodontitis-Associated Gut Barrier and Microbial Dysfunction: Implications for Oral–Gut Axis Regulation. Biology. 2025; 14(5):496. https://doi.org/10.3390/biology14050496
Chicago/Turabian StyleChen, Zhen, Yang Zhong, Lu Chen, Weijia Liu, Chuyin Lin, Yannan Chen, and Xinhong Wang. 2025. "HGF Aggravated Periodontitis-Associated Gut Barrier and Microbial Dysfunction: Implications for Oral–Gut Axis Regulation" Biology 14, no. 5: 496. https://doi.org/10.3390/biology14050496
APA StyleChen, Z., Zhong, Y., Chen, L., Liu, W., Lin, C., Chen, Y., & Wang, X. (2025). HGF Aggravated Periodontitis-Associated Gut Barrier and Microbial Dysfunction: Implications for Oral–Gut Axis Regulation. Biology, 14(5), 496. https://doi.org/10.3390/biology14050496





