Efficacy and Safety of Visible and Near-Infrared Photobiomodulation Therapy on Astenospermic Human Sperm: Wavelength-Dependent Regulation of Nitric Oxide Levels and Mitochondrial Energetics
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
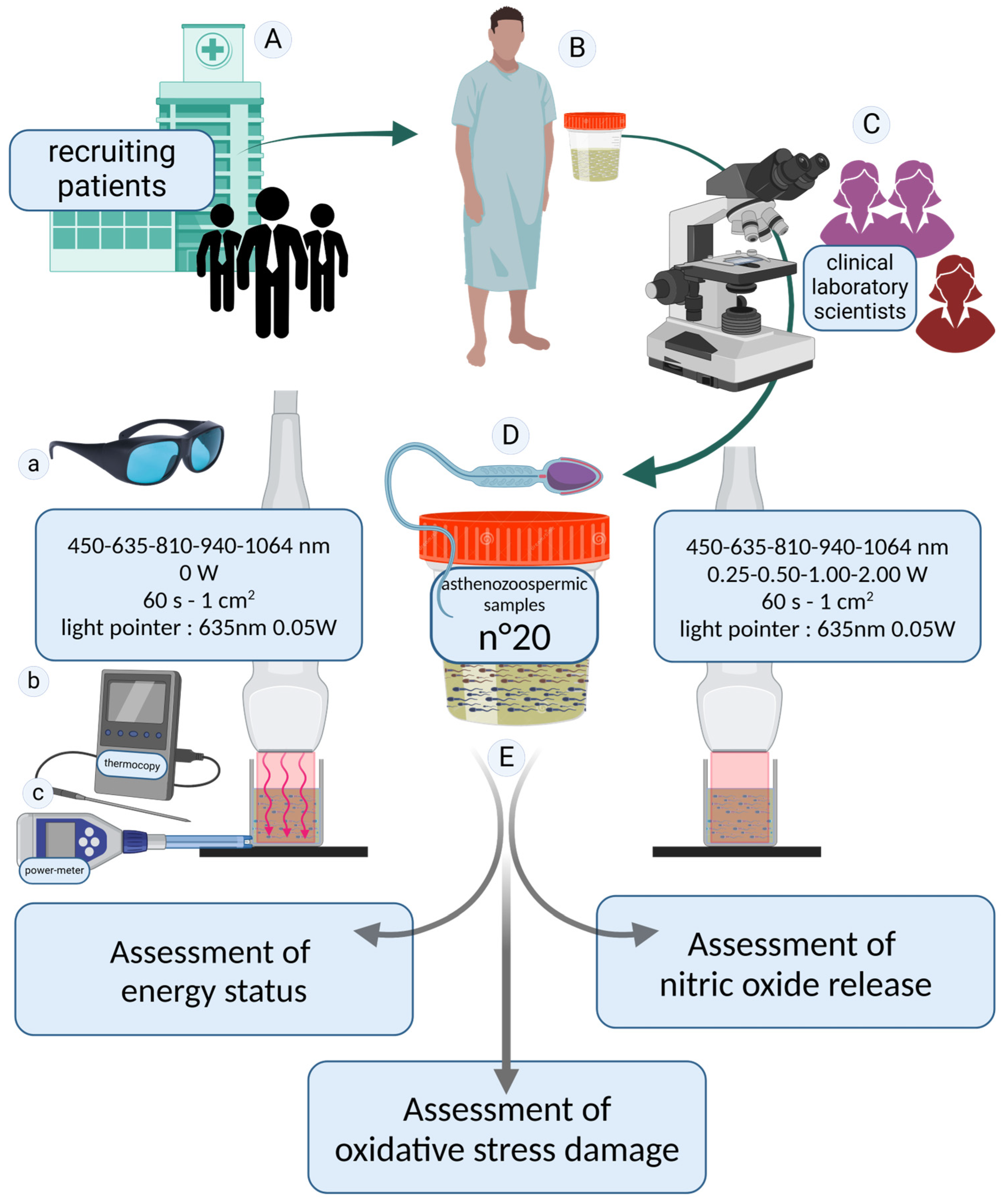
2.1. Patient Recruitment and Experimental Design
- 450 nm at 0.25, 0.50, 1.00, and 2.00 W; control at 0 W
- 635 nm at 0.25, 0.50, 1.00, and 2.00 W; control at 0 W
- 810 nm at 0.25, 0.50, 1.00, and 2.00 W; control at 0 W
- 940 nm at 0.25, 0.50, 1.00, and 2.00 W; control at 0 W
- 1064 nm at 0.25, 0.50, 1.00, and 2.00 W; control at 0 W
2.2. Technical Specifications of the Equipment Utilized for Spermatozoa Irradiation
2.3. Detection of Adenosine Triphosphate in Spermatozoa
2.4. Detection of Adenosine Monophosphate in Spermatozoa
2.5. Detection of Oxidative Stress Damage in Spermatozoa
2.5.1. Malondialdehyde Assay
2.5.2. 8-Hydroxy-2′-deoxyguanosine Assay
2.6. Detection of Nitric Oxide in Spermatozoa
2.7. Statistical Analysis
3. Results
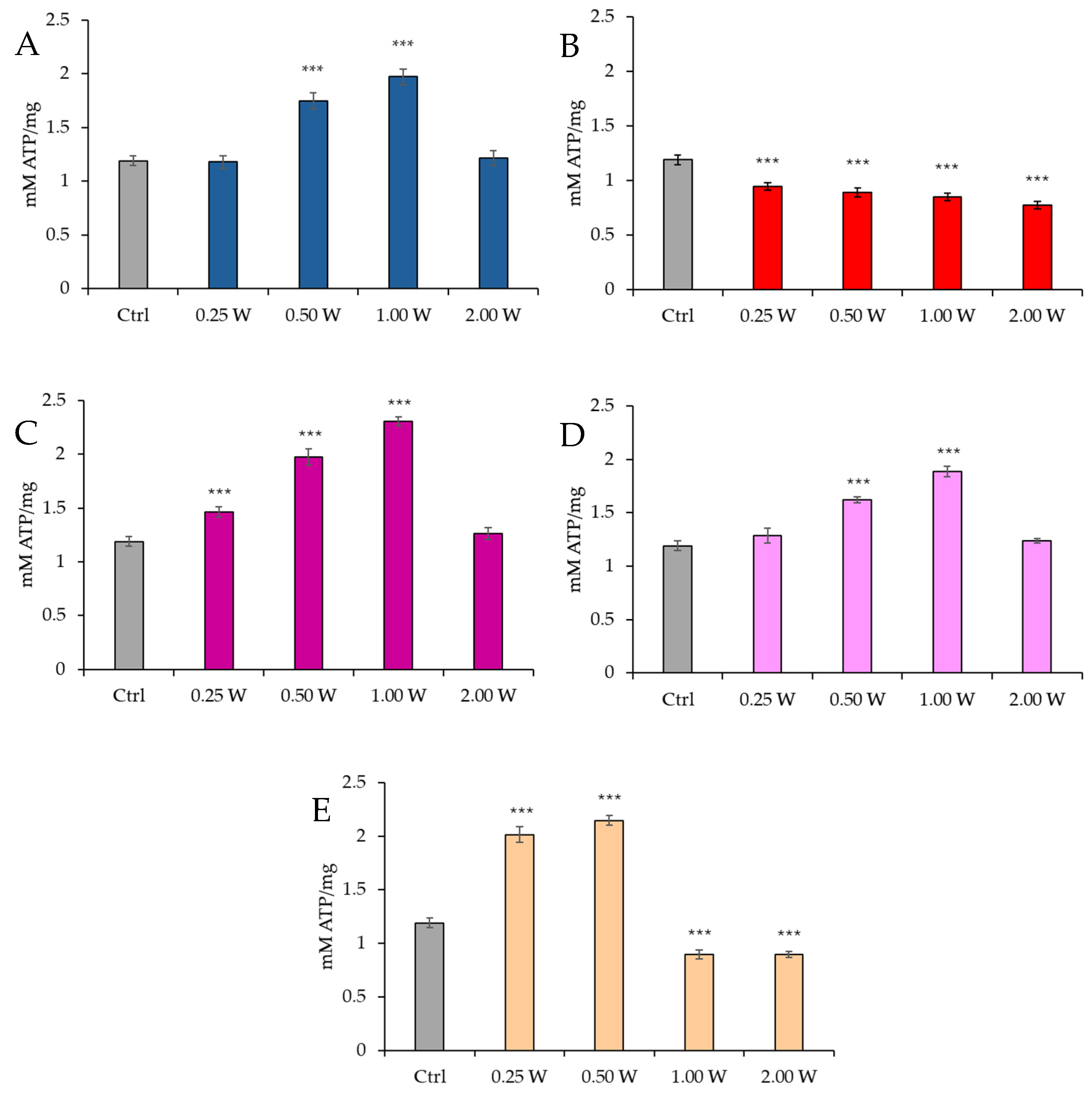
3.1. ATP Evaluation in Spermatozoa in Response to Photobiomodulation
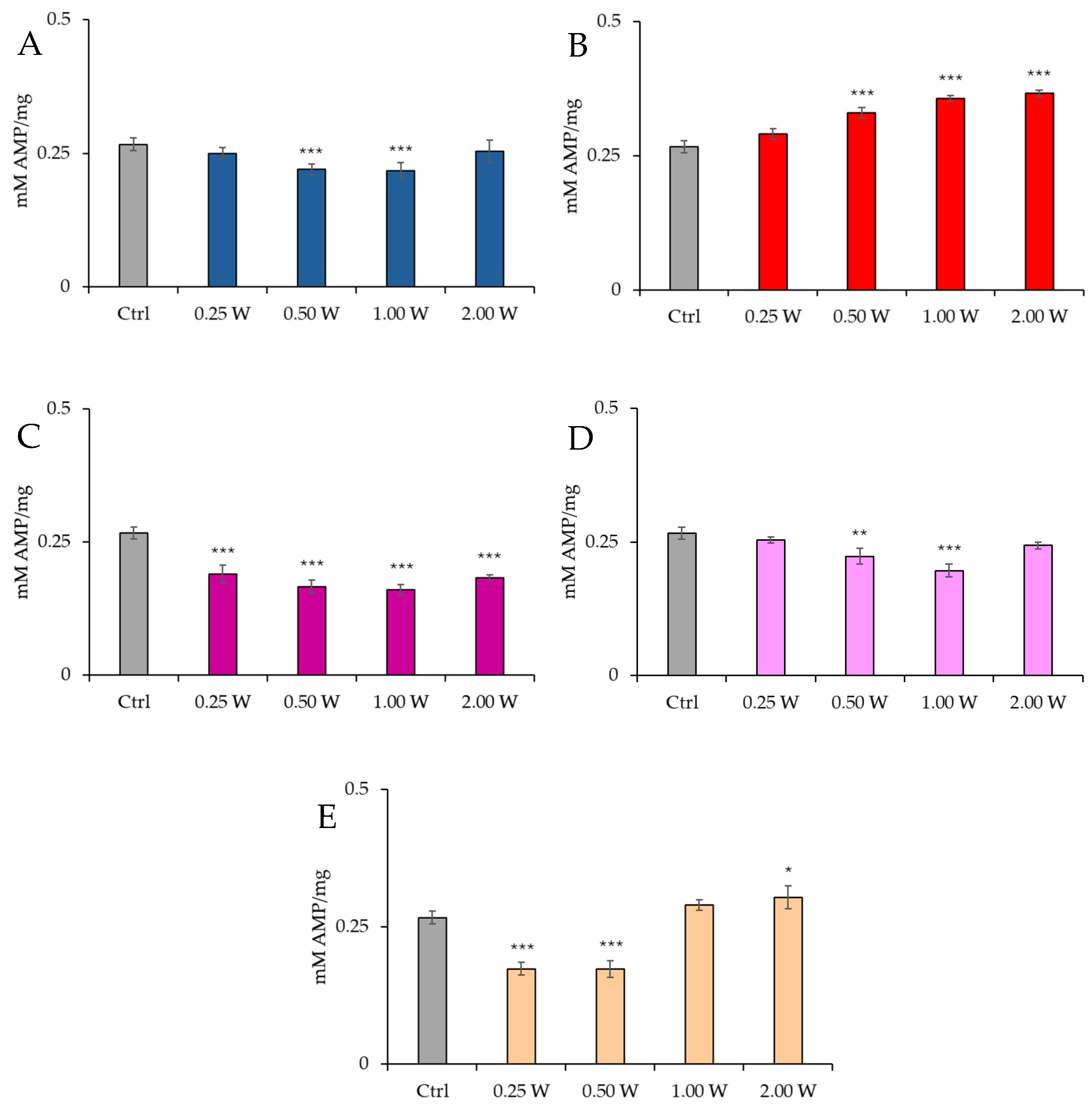
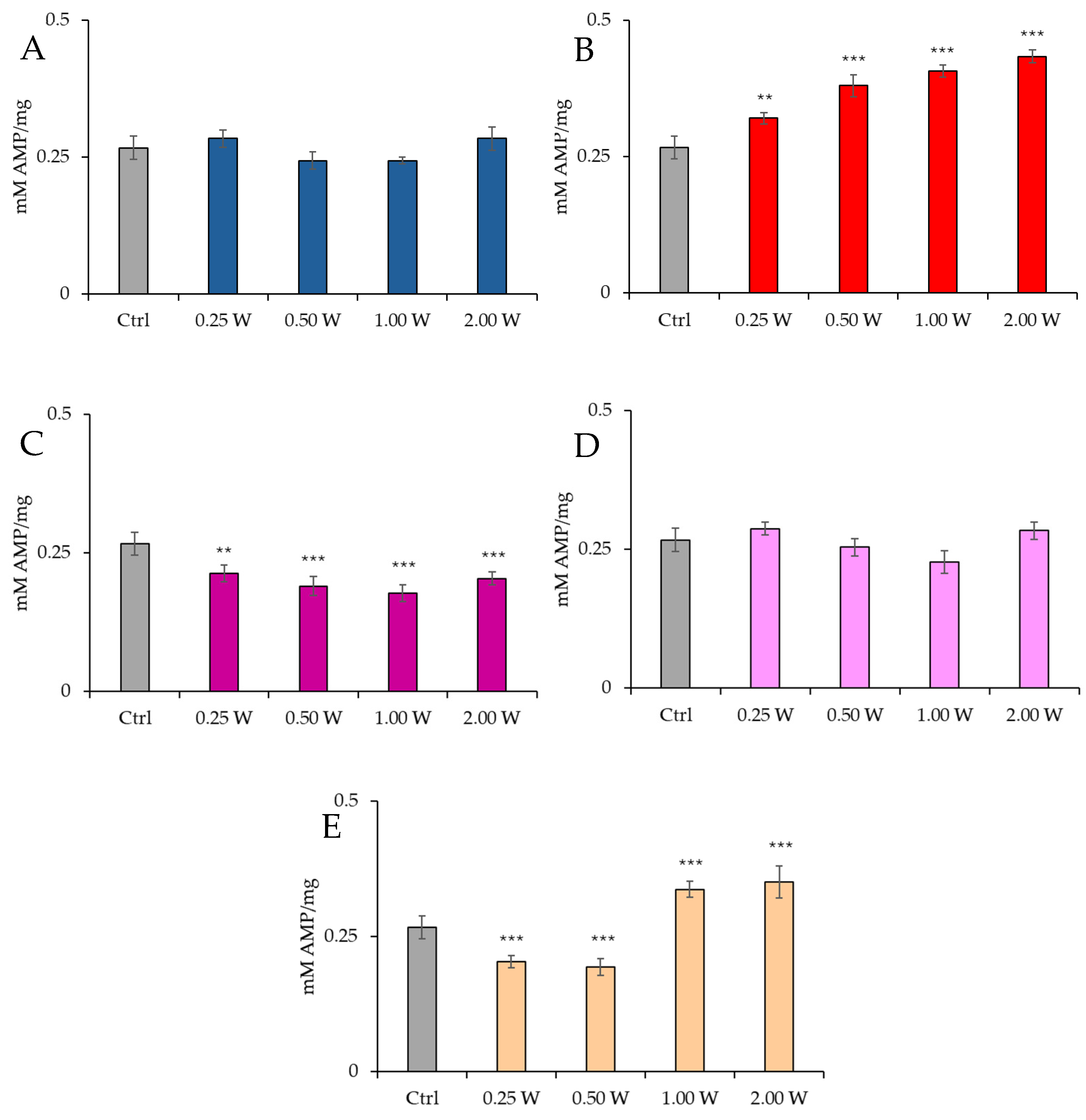
3.2. AMP Evaluation in Spermatozoa in Response to Photobiomodulation
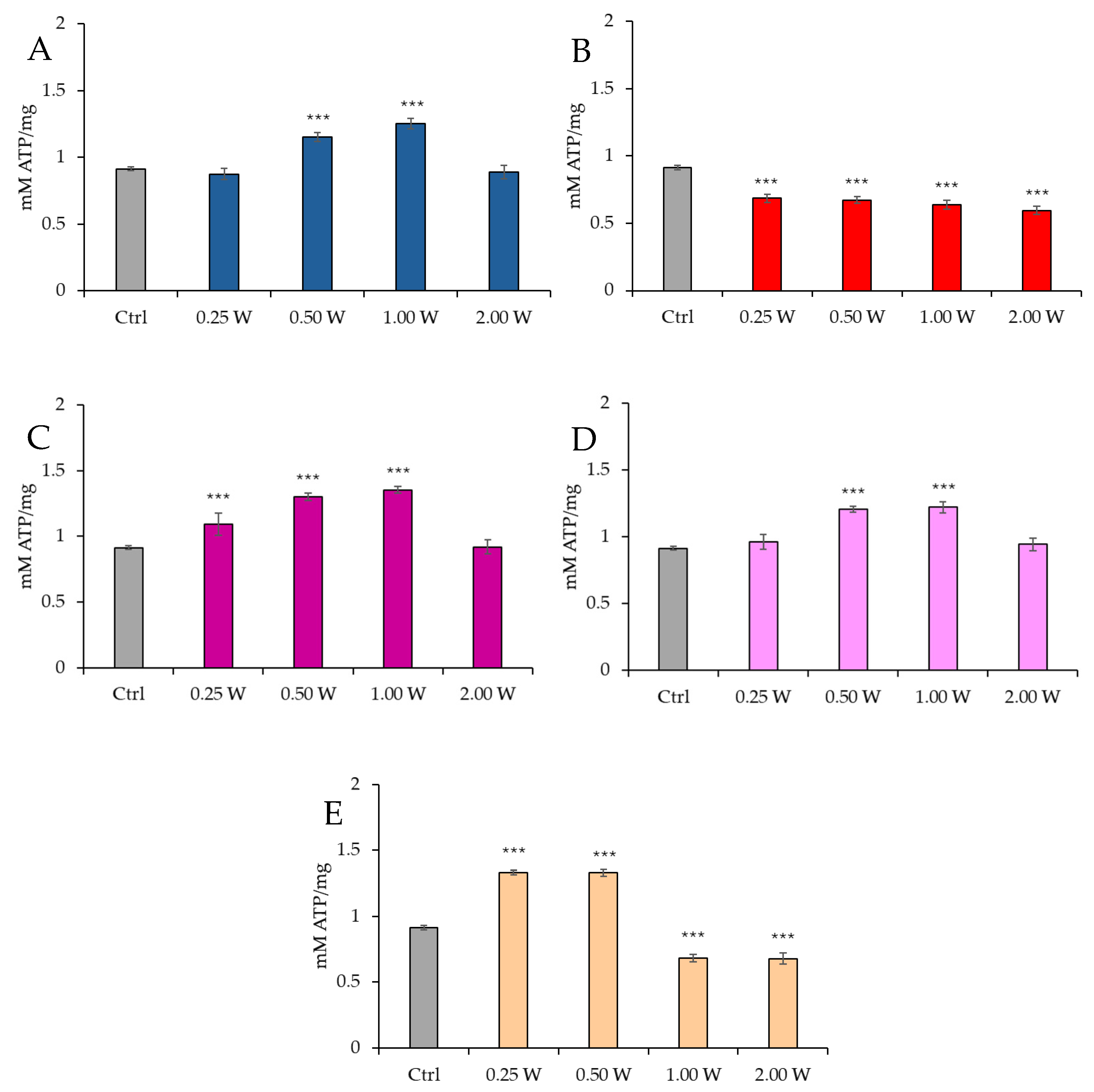
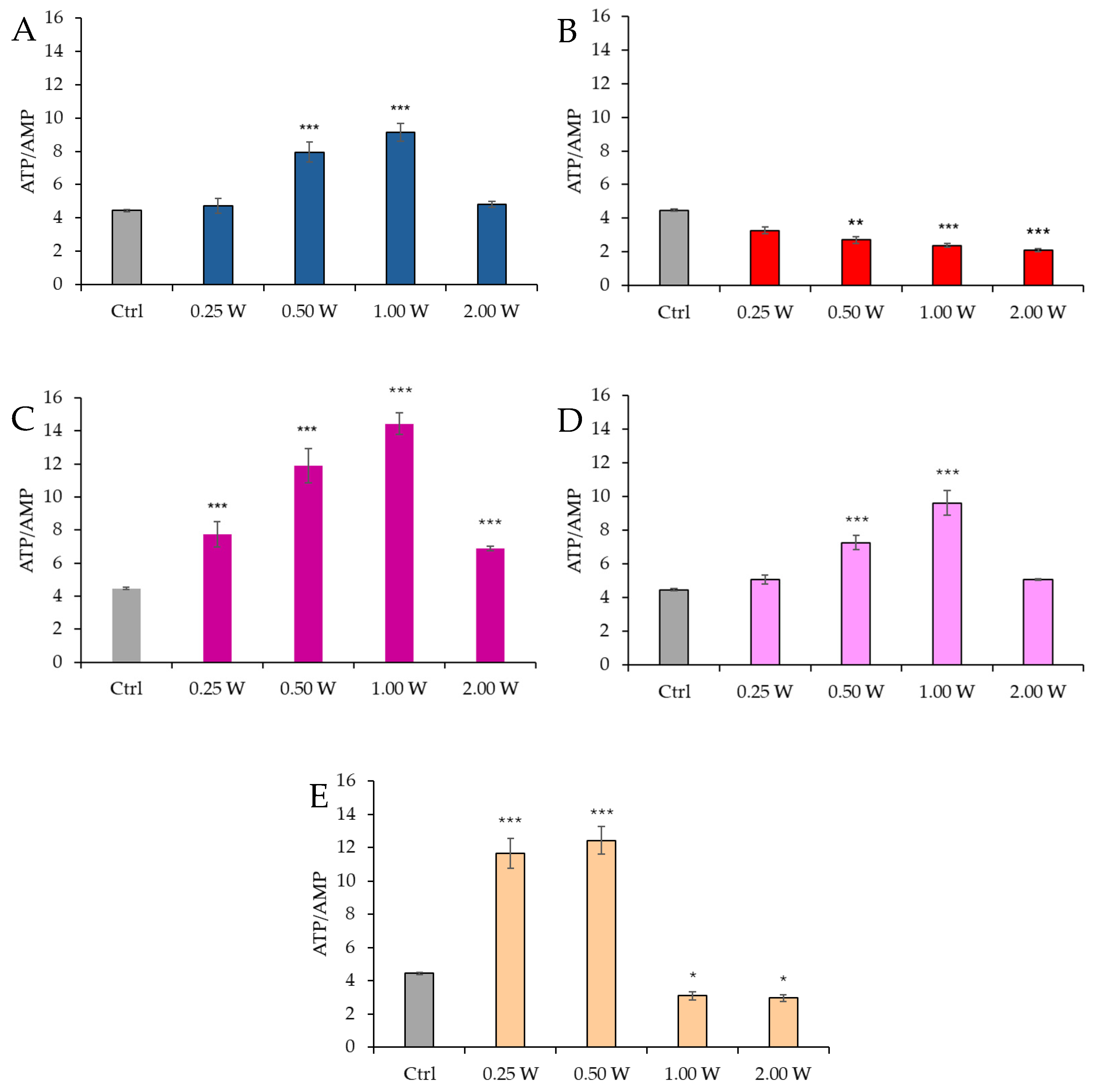
3.3. Energy Status (ATP/AMP Ratio) Evaluation in Spermatozoa in Response to Photobiomodulation
3.4. Evaluation of Oxidative Stress Damage in Spermatozoa in Response to Photobiomodulation
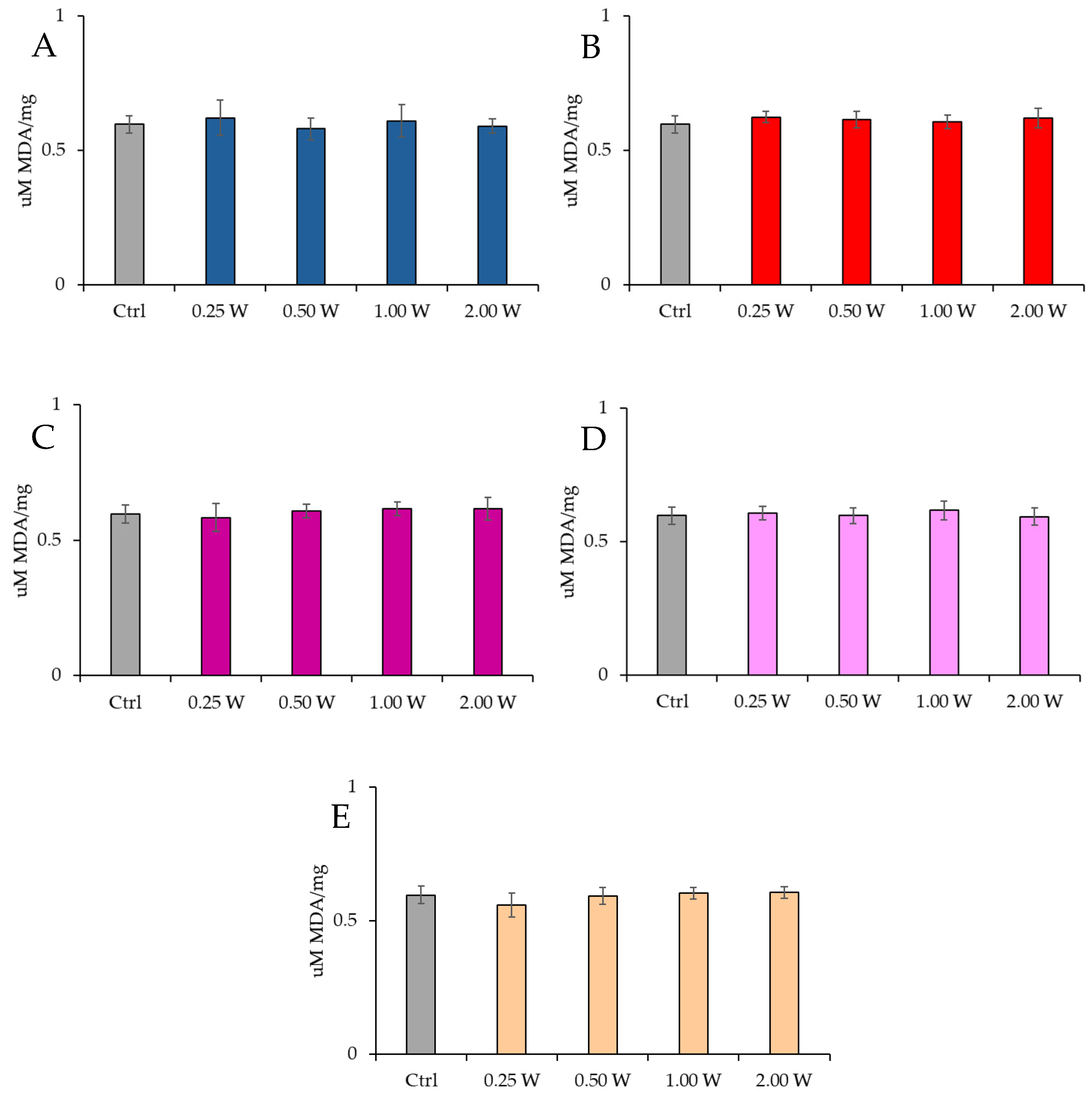
3.4.1. Evaluation of Malondialdehyde
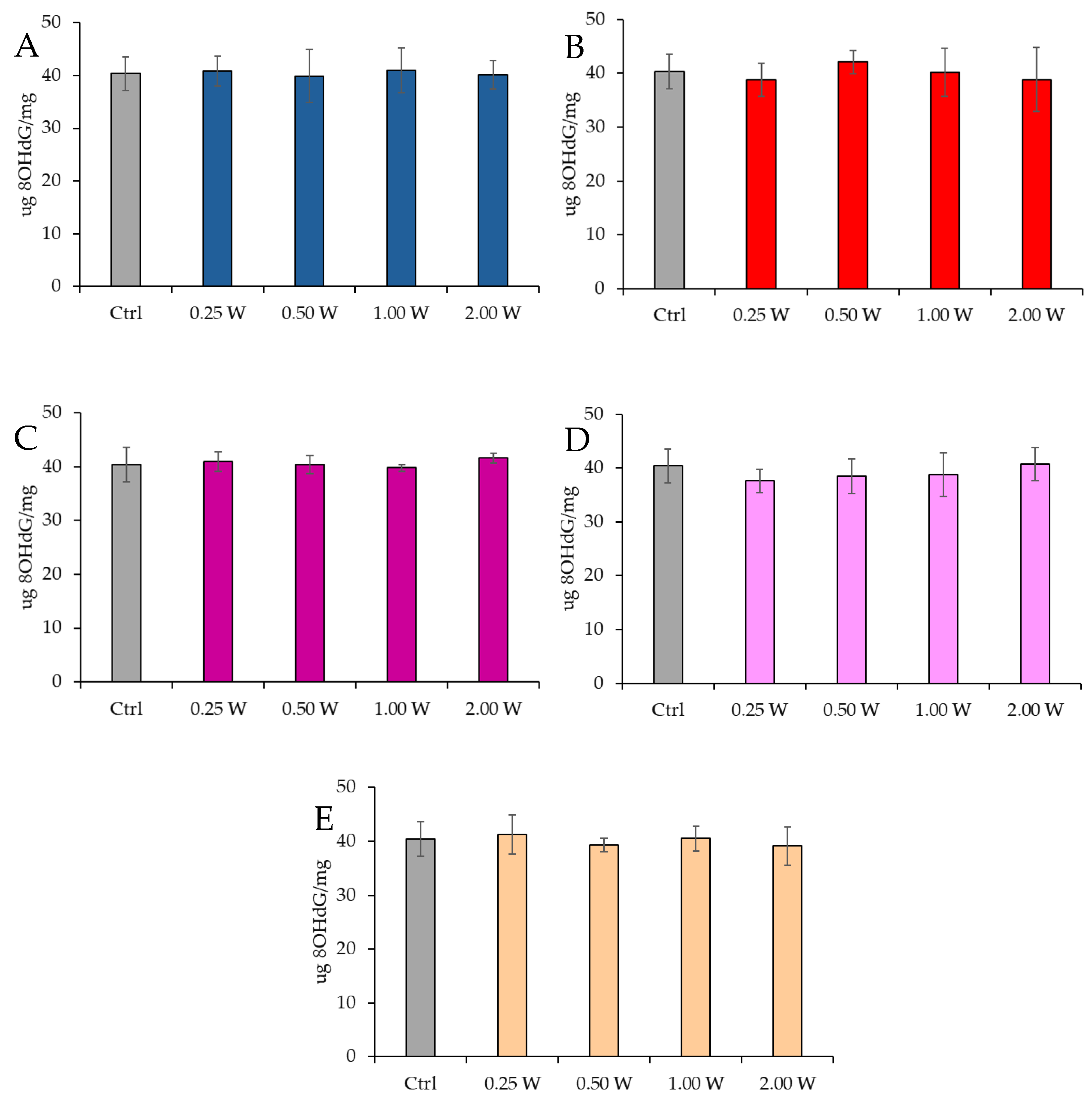
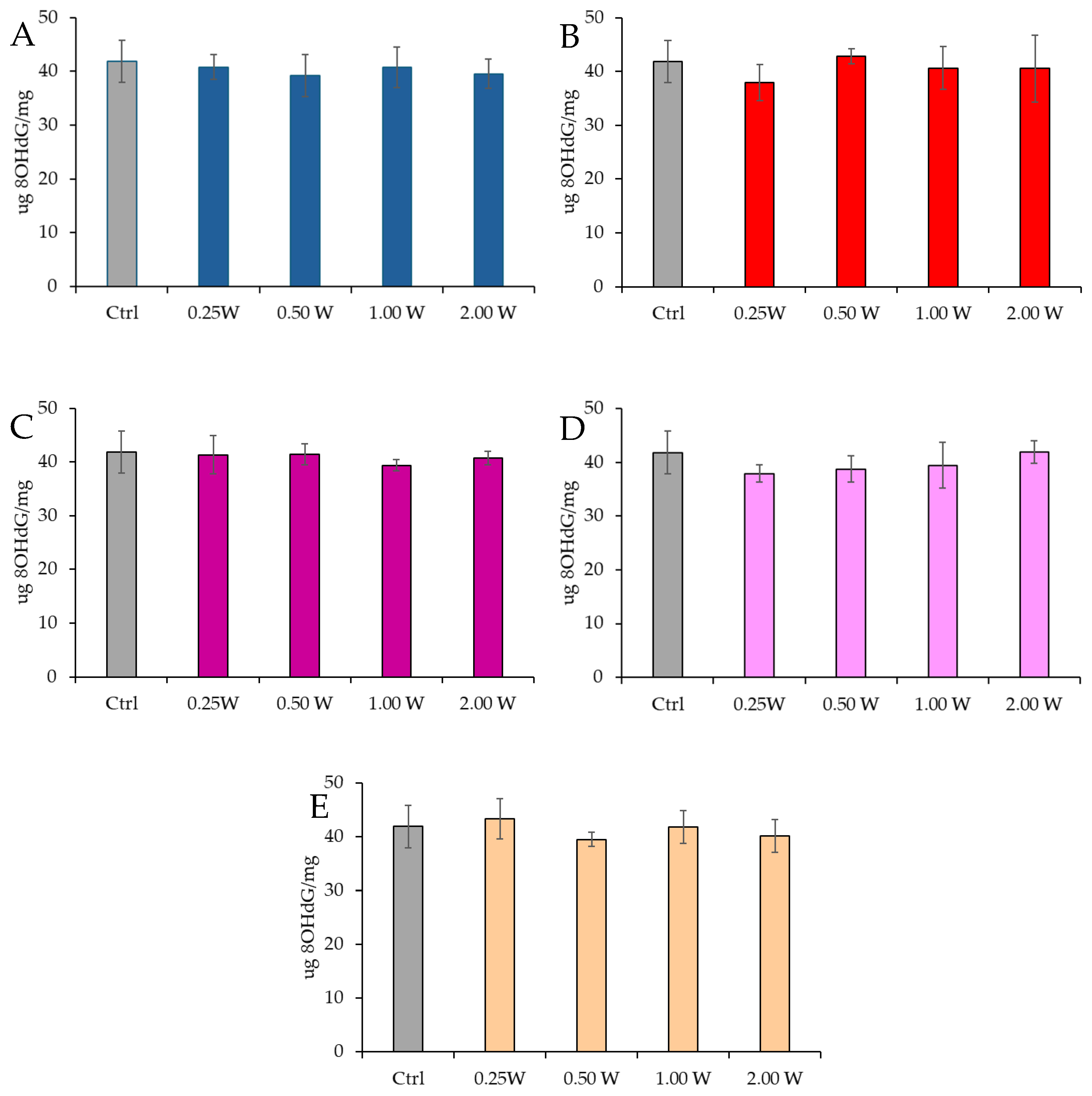
3.4.2. Evaluation of 8-Hydroxy-2′-Deoxyguanosine
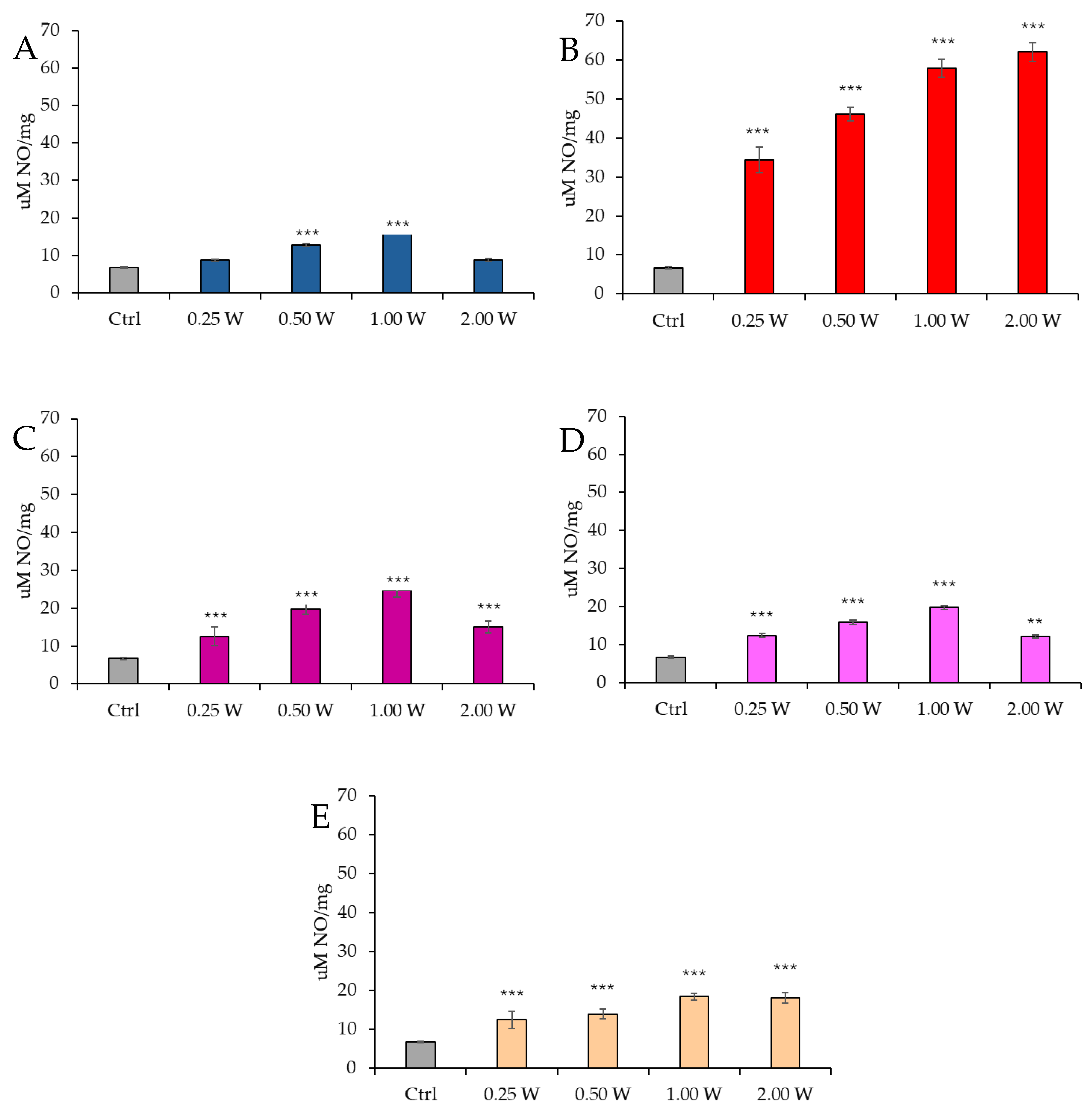
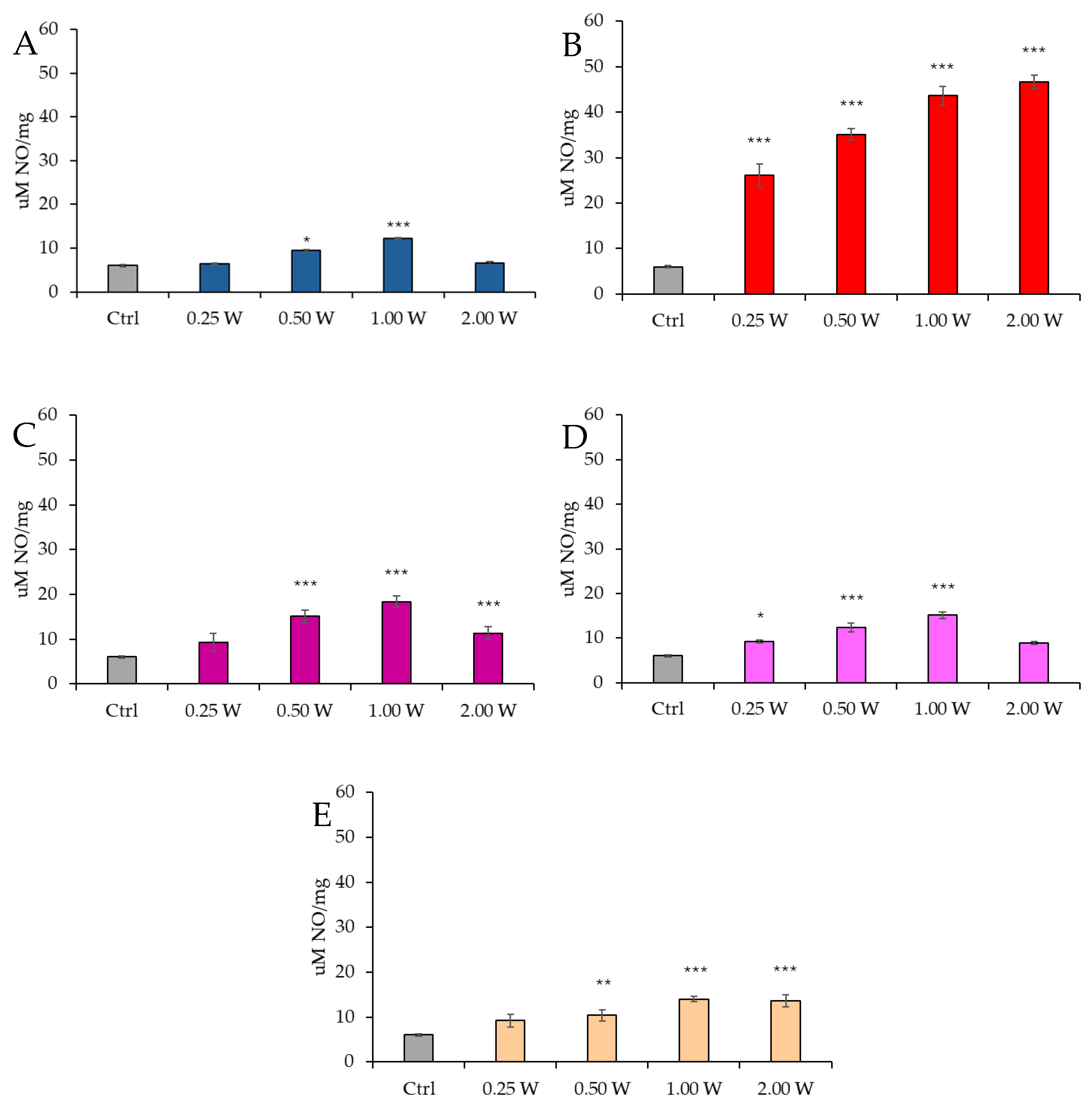
3.5. Evaluation of Nitric Oxide in Spermatozoa in Response to Photobiomodulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis of Samples Collected Globally in the 20th and 21st Centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef]
- Osadchuk, L.; Kleshchev, M.; Osadchuk, A. Effects of Cigarette Smoking on Semen Quality, Reproductive Hormone Levels, Metabolic Profile, Zinc and Sperm DNA Fragmentation in Men: Results from a Population-Based Study. Front. Endocrinol. 2023, 14, 1255304. [Google Scholar] [CrossRef]
- Agarwal, A.; Mulgund, A.; Hamada, A.; Chyatte, M.R. A Unique View on Male Infertility around the Globe. Reprod. Biol. Endocrinol. 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Singh, A.K. Impact of Environmental Factors on Human Semen Quality and Male Fertility: A Narrative Review. Environ. Sci. Eur. 2022, 34, 6. [Google Scholar] [CrossRef]
- Sudhakaran, G.; Kesavan, D.; Kandaswamy, K.; Guru, A.; Arockiaraj, J. Unravelling the Epigenetic Impact: Oxidative Stress and Its Role in Male Infertility-Associated Sperm Dysfunction. Reprod. Toxicol. 2024, 124, 108531. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Sharma, S.S.; Majumdar, S.S. Etiology of Male Infertility: An Update. Reprod. Sci. 2024, 31, 942–965. [Google Scholar] [CrossRef]
- Durairajanayagam, D.; Singh, D.; Agarwal, A.; Henkel, R. Causes and Consequences of Sperm Mitochondrial Dysfunction. Andrologia 2021, 53, e13666. [Google Scholar] [CrossRef] [PubMed]
- Vahedi Raad, M.; Firouzabadi, A.M.; Tofighi Niaki, M.; Henkel, R.; Fesahat, F. The Impact of Mitochondrial Impairments on Sperm Function and Male Fertility: A Systematic Review. Reprod. Biol. Endocrinol. 2024, 22, 83. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, S.J.; Guo, L.Y.; Zhang, Z.; Zhang, J.B.; Wang, X.M.; Meng, X.B.; Zhang, M.Y.; Zhang, K.K.; Chen, L.L.; et al. Nitric oxide synthase and its function in animal reproduction: An update. Front Physiol. 2023, 14, 1288669. [Google Scholar] [CrossRef]
- Ramya, T.; Misro, M.M.; Sinha, D.; Nandan, D.; Mithal, S. Altered Levels of Seminal Nitric Oxide, Nitric Oxide Synthase, and Enzymatic Antioxidants and Their Association with Sperm Function in Infertile Subjects. Fertil. Steril. 2011, 95, 135–140. [Google Scholar] [CrossRef]
- Kullisaar, T.; Türk, S.; Kilk, K.; Ausmees, K.; Punab, M.; Mändar, R. Increased Levels of Hydrogen Peroxide and Nitric Oxide in Male Partners of Infertile Couples. Andrology 2013, 1, 850–858. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria Functionality and Sperm Quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The Role of Mitochondria in Energy Production for Human Sperm Motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Doshi, S.B.; Khullar, K.; Sharma, R.K.; Agarwal, A. Role of Reactive Nitrogen Species in Male Infertility. Reprod. Biol. Endocrinol. 2012, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Liu, Q.; Qin, Y.; Qin, W.; Zhu, Z.; Sun, L.; Jiang, M.; Adu-Amankwaah, J.; Gao, F.; Tan, R.; et al. Mechanism of Mitochondrial Oxidative Phosphorylation Disorder in Male Infertility. Chin. Med. J. 2024. [Google Scholar] [CrossRef]
- Palma, F.R.; Gantner, B.N.; Sakiyama, M.J.; Kayzuka, C.; Shukla, S.; Lacchini, R.; Cunniff, B.; Bonini, M.G. ROS Production by Mitochondria: Function or Dysfunction? Oncogene 2023, 43, 295–303. [Google Scholar] [CrossRef]
- Venditti, P.; Di Stefano, L.; Di Meo, S. Mitochondrial Metabolism of Reactive Oxygen Species. Mitochondrion 2013, 13, 71–82. [Google Scholar] [CrossRef]
- Song, J.; Xiao, L.; Zhang, Z.; Wang, Y.; Kouis, P.; Rasmussen, L.J.; Dai, F. Effects of Reactive Oxygen Species and Mitochondrial Dysfunction on Reproductive Aging. Front. Cell Dev. Biol. 2024, 12, 1347286. [Google Scholar] [CrossRef]
- Chianese, R.; Pierantoni, R. Mitochondrial Reactive Oxygen Species (ROS) Production Alters Sperm Quality. Antioxidants 2021, 10, 92. [Google Scholar] [CrossRef]
- Luo, Y.; Zhu, Y.; Basang, W.; Wang, X.; Li, C.; Zhou, X. Roles of Nitric Oxide in the Regulation of Reproduction: A Review. Front. Endocrinol. 2021, 12, 752410. [Google Scholar] [CrossRef]
- Stigliani, S.; Ravera, S.; Maccarini, E.; Rizzo, C.; Massarotti, C.; Anserini, P.; Bozzo, M.; Amaroli, A.; Scaruffi, P. The Power of 810 Nm Near-Infrared Photobiomodulation Therapy for Human Asthenozoospermia. Sci. Rep. 2024, 14, 26819. [Google Scholar] [CrossRef] [PubMed]
- Safian, F.; Ghaffari Novin, M.; Karimi, M.; Kazemi, M.; Zare, F.; Ghoreishi, S.K.; Bayat, M. Photobiomodulation with 810 Nm Wavelengths Improves Human Sperms’ Motility and Viability In Vitro. Photobiomodul. Photomed. Laser Surg. 2020, 38, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Espey, B.T.; Kielwein, K.; van der Ven, H.; Steger, K.; Allam, J.P.; Paradowska-Dogan, A.; van der Ven, K. Effects of Pulsed-Wave Photobiomodulation Therapy on Human Spermatozoa. Lasers Surg. Med. 2022, 54, 540–553. [Google Scholar] [CrossRef]
- Poorhassan, M.; Gholaminejhad, M.; Ahmadi, H.; Mehboudi, L.; Kameh, M.C.; Pirani, M.; Hassanzadeh, G. Preclinical and Clinical Applications of Photobiomodulation Therapy in Sperm Motility: A Narrative Review. J. Lasers Med. Sci. 2022, 13, e75. [Google Scholar] [CrossRef]
- Eghbaldoost, A.; Mashhadsari, S.P.S.; Ghadirzadeh, E.; Ghoreifi, A.; Allameh, F. Therapeutic Effects of Low-Level Laser on Male Infertility: A Systematic Review. J. Lasers Med. Sci. 2023, 14, e36. [Google Scholar] [CrossRef]
- Moradi, A.; Ghaffari Novin, M.; Bayat, M. A Comprehensive Systematic Review of the Effects of Photobiomodulation Therapy in Different Light Wavelength Ranges (Blue, Green, Red, and Near-Infrared) on Sperm Cell Characteristics in Vitro and in Vivo. Reprod. Sci. 2024, 31, 3275–3302. [Google Scholar] [CrossRef]
- Karu, T.I. Lasers in Infertility Treatment: Irradiation of Oocytes and Spermatozoa. Photomed. Laser Surg. 2012, 30, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Iaffaldano, N.; Paventi, G.; Pizzuto, R.; Passarella, S.; Cerolini, S.; Zaniboni, L.; Marzoni, M.; Castillo, A.; Rosato, M.P. The Post-Thaw Irradiation of Avian Spermatozoa with He–Ne Laser Differently Affects Chicken, Pheasant and Turkey Sperm Quality. Anim. Reprod. Sci. 2013, 142, 168–172. [Google Scholar] [CrossRef]
- Xue, Y.; Xiong, Y.; Cheng, X.; Li, K. Applications of Laser Technology in the Manipulation of Human Spermatozoa. Reprod. Biol. Endocrinol. 2023, 21, 93. [Google Scholar] [CrossRef]
- Drozdov, A.L.; Karu, T.I.; Chudnovskii, V.M.; Yusupov, V.I.; Bagratashvili, V.N. Influence of Low-Intensity Red Diode and Laser Radiation on the Locomotor Activity of Sea Urchin Sperm. Dokl. Biochem. Biophys. 2014, 457, 146–148. [Google Scholar] [CrossRef]
- Lenzi, A.; Claroni, F.; Gandini, L.; Lombardo, F.; Barbieri, C.; Lino, A.; Dondero, F. Laser Radiation and Motility Patterns of Human Sperm. Arch. Androl. 1989, 23, 229–234. [Google Scholar] [CrossRef]
- Zan-Bar, T.; Bartoov, B.; Segal, R.; Yehuda, R.; Lavi, R.; Lubart, R.; Avtalion, R.R. Influence of Visible Light and Ultraviolet Irradiation on Motility and Fertility of Mammalian and Fish Sperm. Photomed. Laser Surg. 2005, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Landthaler, M.; Haina, D.; Schill, W.-B. The Effects of Laser Light on Sperm Motility and Velocity in Vitro. Andrologia 1984, 16, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.H.C.; De Tarso Camillo De Carvalho, P.; Serra, A.J.; Crespilho, A.M.; Schatzman Peron, J.P.; Rossato, C.; Leal-Junior, E.C.P.; Albertini, R. The Effect of Low-Level Laser Irradiation on Sperm Motility, and Integrity of the Plasma Membrane and Acrosome in Cryopreserved Bovine Sperm. PLoS ONE 2015, 10, e0121487. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, T.; Hristova, E.; Petrova, N. Low-Dose Ionizing Radiation Exposure on Human Male Gametes: Damage or Benefit. Life 2024, 14, 830. [Google Scholar] [CrossRef]
- Firestone, R.S.; Esfandiari, N.; Moskovtsev, S.I.; Burstein, E.; Videna, G.T.; Librach, C.; Bentov, Y.; Casper, R.F. The Effects of Low-Level Laser Light Exposure on Sperm Motion Characteristics and DNA Damage. J. Androl. 2012, 33, 469–473. [Google Scholar] [CrossRef]
- Iaffaldano, N.; Paventi, G.; Pizzuto, R.; Di Iorio, M.; Bailey, J.L.; Manchisi, A.; Passarella, S. Helium-Neon Laser Irradiation of Cryopreserved Ram Sperm Enhances Cytochrome c Oxidase Activity and ATP Levels Improving Semen Quality. Theriogenology 2016, 86, 778–784. [Google Scholar] [CrossRef]
- Salman Yazdi, R.; Bakhshi, S.; Jannat Alipoor, F.; Akhoond, M.R.; Borhani, S.; Farrahi, F.; Lotfi Panah, M.; Sadighi Gilani, M.A. Effect of 830-nm diode laser irradiation on human sperm motility. Lasers Med. Sci. 2014, 29, 97–104. [Google Scholar] [CrossRef]
- Lubart, R.; Friedmann, H.; Sinyakov, M.; Cohen, N.; Breitbart, H. Changes in Calcium Transport in Mammalian Sperm Mitochondria and Plasma Membranes Caused by 780 Nm Irradiation. Lasers Surg. Med. 1997, 21, 493–499. [Google Scholar] [CrossRef]
- Amaroli, A.; Gambardella, C.; Ferrando, S.; Hanna, R.; Benedicenti, A.; Gallus, L.; Faimali, M.; Benedicenti, S. The Effect of Photobiomodulation on the Sea Urchin Paracentrotus Lividus (Echinodermata) Using Higher-Fluence on Fertilization, Embryogenesis, and Larval Development: An in Vitro Study. Photomed. Laser Surg. 2017, 35. [Google Scholar] [CrossRef]
- Saylan, A.; Firat, T.; Yis, O.M. Effects of Photobiomodulation Therapy on Human Sperm Function. Rev. Int. Androl. 2023, 21. [Google Scholar] [CrossRef] [PubMed]
- Zupin, L.; Pascolo, L.; Luppi, S.; Ottaviani, G.; Crovella, S.; Ricci, G. Photobiomodulation Therapy for Male Infertility. Lasers Med. Sci. 2020, 35, 1671–1680. [Google Scholar] [CrossRef]
- Ahmed, R.; Hamdy, O.; Elattar, S.; Soliman, A.A. Improving Human Sperm Motility via Red and Near-Infrared Laser Irradiation: In-Vitro Study. Photochem. Photobiol. Sci. 2024, 23, 377–385. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-Nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef]
- Passarella, S.; Karu, T. Absorption of Monochromatic and Narrow Band Radiation in the Visible and near IR by Both Mitochondrial and Non-Mitochondrial Photoacceptors Results in Photobiomodulation. J. Photochem. Photobiol. B 2014, 140, 344–358. [Google Scholar] [CrossRef]
- Hamblin, M.R. Mechanisms and Mitochondrial Redox Signaling in Photobiomodulation. Photochem. Photobiol. 2018, 94, 199–212. [Google Scholar] [CrossRef]
- Amaroli, A.; Clemente Vargas, M.R.; Pasquale, C.; Raffetto, M.; Ravera, S. Photobiomodulation on Isolated Mitochondria at 810 Nm: First Results on the Efficiency of the Energy Conversion Process. Sci. Rep. 2024, 14, 11060. [Google Scholar] [CrossRef]
- Amaroli, A.; Benedicenti, S.; Bianco, B.; Bosco, A.; Clemente Vargas, M.R.; Hanna, R.; Kalarickel Ramakrishnan, P.; Raffetto, M.; Ravera, S. Electromagnetic Dosimetry for Isolated Mitochondria Exposed to Near-Infrared Continuous-Wave Illumination in Photobiomodulation Experiments. Bioelectromagnetics 2021, 42, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Signore, A.; Ravera, S. Photobiomodulation and Oxidative Stress: 980 Nm Diode Laser Light Regulates Mitochondrial Activity and Reactive Oxygen Species Production. Oxid. Med. Cell Longev. 2021, 2021, 6626286. [Google Scholar] [CrossRef]
- Ravera, S.; Ferrando, S.; Agas, D.; De Angelis, N.; Raffetto, M.; Sabbieti, M.G.; Signore, A.; Benedicenti, S.; Amaroli, A. 1064 Nm Nd:YAG Laser Light Affects Transmembrane Mitochondria Respiratory Chain Complexes. J. Biophotonics 2019, 12. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. The Protozoan, Paramecium Primaurelia, as a Non-Sentient Model to Test Laser Light Irradiation: The Effects of an 808nm Infrared Laser Diode on Cellular Respiration. Altern. Lab. Anim. 2015, 43, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Colombo, E.; Signore, A.; Aicardi, S.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Amaroli, A. Experimental and Clinical Applications of Red and Near-Infrared Photobiomodulation on Endothelial Dysfunction: A Review. Biomedicines 2021, 9, 274. [Google Scholar] [CrossRef] [PubMed]
- Keszler, A.; Lindemer, B.; Hogg, N.; Weihrauch, D.; Lohr, N.L. Wavelength-Dependence of Vasodilation and NO Release from S-Nitrosothiols and Dinitrosyl Iron Complexes by Far Red/near Infrared Light. Arch. Biochem. Biophys. 2018, 649, 47–52. [Google Scholar] [CrossRef]
- Cheng, Z.; Ristow, M. Mitochondria and Metabolic Homeostasis. Antioxid. Redox Signal. 2013, 19, 240–242. [Google Scholar] [CrossRef]
- Willems, P.H.G.M.; Rossignol, R.; Dieteren, C.E.J.; Murphy, M.P.; Koopman, W.J.H. Redox Homeostasis and Mitochondrial Dynamics. Cell Metab. 2015, 22, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research Involving Human Subjects: A Review of Seventh Revision. J. Nepal. Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef]
- WHO Laboratory Manual for the Examination and Processing of Human Semen. Available online: https://iris.who.int/handle/10665/343208 (accessed on 26 June 2024).
- Amaroli, A.; Arany, P.; Pasquale, C.; Benedicenti, S.; Bosco, A.; Ravera, S. Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity. Int. J. Mol. Sci. 2021, 22, 7788. [Google Scholar] [CrossRef]
- Amaroli, A.; Marcoli, M.; Venturini, A.; Passalacqua, M.; Agnati, L.F.; Signore, A.; Raffetto, M.; Maura, G.; Benedicenti, S.; Cervetto, C. Near-Infrared Laser Photons Induce Glutamate Release from Cerebrocortical Nerve Terminals. J. Biophotonics 2018, 11, e201800102. [Google Scholar] [CrossRef]
- Bârzu, O.; Michelson, S. Simple and Fast Purification of Escherichia coli Adenylate Kinase. FEBS Lett. 1983, 153, 280–284. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kornberg, A.; Pricer, W.E. Enzymatic phosphorylation of adenosine and 2,6-diaminopurine riboside. J. Biol. Chem. 1951, 193, 481–495. [Google Scholar] [CrossRef] [PubMed]
- El-Saadani, M.; Esterbauer, H.; El-Sayed, M.; Goher, M.; Nassar, A.Y.; Jurgens, G. A Spectrophotometric Assay for Lipid Peroxides in Serum Lipoproteins Using a Commercially Available Reagent. J. Lipid Res. 1989, 30, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Hwangbo, H.; Park, C.; Bang, E.; Kim, H.S.; Bae, S.J.; Kim, E.; Jung, Y.; Leem, S.H.; Seo, Y.R.; Hong, S.H.; et al. Morroniside Protects C2C12 Myoblasts from Oxidative Damage Caused by ROS-Mediated Mitochondrial Damage and Induction of Endoplasmic Reticulum Stress. Biomol. Ther. 2024, 32, 349–360. [Google Scholar] [CrossRef]
- Sjövall, F.; Morota, S.; Frostner, E.Å.; Hansson, M.J.; Elmér, E. Cytokine and Nitric Oxide Levels in Patients with Sepsis–Temporal Evolvement and Relation to Platelet Mitochondrial Respiratory Function. PLoS ONE 2014, 9, e97673. [Google Scholar] [CrossRef]
- Rosner, B. Fundamentals of Biostatistics, 8th ed.; Cengage Learning; Brooks/Cole ISE: Totnes, UK, 2015; p. 927. ISBN 978-1305268920. [Google Scholar]
- Johnson, G.D.; Lalancette, C.; Linnemann, A.K.; Leduc, F.; Boissonneault, G.; Krawetz, S.A. The Sperm Nucleus: Chromatin, RNA and the Nuclear Matrix. Reproduction 2010, 141, 21. [Google Scholar] [CrossRef]
- Paronetto, M.P.; Sette, C. Role of RNA-Binding Proteins in Mammalian Spermatogenesis. Int. J. Androl. 2010, 33, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Brucker, C.; Lipford, G.B. The Human Sperm Acrosome Reaction: Physiology and Regulatory Mechanisms. An Update. Hum. Reprod. Update 1995, 1, 51–62. [Google Scholar] [CrossRef]
- Kumar, N. Sperm Mitochondria, the Driving Force Behind Human Spermatozoa Activities: Its Functions and Dysfunctions—A Narrative Review. Curr. Mol. Med. 2022, 23, 332–340. [Google Scholar] [CrossRef]
- Boguenet, M.; Bouet, P.E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their Role in Spermatozoa and in Male Infertility. Hum. Reprod. Update 2021, 27, 697–719. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. Effect of 808 Nm Diode Laser on Swimming Behavior, Food Vacuole Formation and Endogenous ATP Production of Paramecium Primaurelia (Protozoa). Photochem. Photobiol. 2015, 91, 1150–1155. [Google Scholar] [CrossRef]
- Cohen, N.; Lubart, R.; Rubinstein, S.; Breitbart, H. Light Irradiation of Mouse Spermatozoa: Stimulation of in Vitro Fertilization and Calcium Signals. Photochem. Photobiol. 1998, 68, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Corral-Baqués, M.I.; Rigau, T.; Rivera, M.; Rodríguez, J.E.; Rigau, J. Effect of 655-Nm Diode Laser on Dog Sperm Motility. Lasers Med. Sci. 2005, 20, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Hasani, A.; Khosravi, A.; Rahimi, K.; Afshar, A.; Fadaei-Fathabadi, F.; Raoofi, A.; Raee, P.; Aghajanpour, F.; Aliaghaei, A.; Abdi, S.; et al. Photobiomodulation Restores Spermatogenesis in the Transient Scrotal Hyperthermia-Induced Mice. Life Sci. 2020, 254, 117767. [Google Scholar] [CrossRef]
- Preece, D.; Chow, K.W.; Gomez-Godinez, V.; Gustafson, K.; Esener, S.; Ravida, N.; Durrant, B.; Berns, M.W. Red Light Improves Spermatozoa Motility and Does Not Induce Oxidative DNA Damage. Sci. Rep. 2017, 7, srep46480. [Google Scholar] [CrossRef]
- Rezaei, F.; Bayat, M.; Nazarian, H.; Aliaghaei, A.; Abaszadeh, H.A.; Naserzadeh, P.; Amini, A.; Ebrahimi, V.; Abdi, S.; Abdollahifar, M.A. Photobiomodulation Therapy Improves Spermatogenesis in Busulfan-Induced Infertile Mouse. Reprod. Sci. 2021, 28, 2789–2798. [Google Scholar] [CrossRef]
- Yeste, M.; Castillo-Martín, M.; Bonet, S.; Rodríguez-Gil, J.E. Impact of Light Irradiation on Preservation and Function of Mammalian Spermatozoa. Anim. Reprod. Sci. 2018, 194, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Ziaeipour, S.; Norouzian, M.; Abbaszadeh, H.A.; Aliaghaei, A.; Nazarian, H.; Karamian, A.; Tabeie, F.; Naserzadeh, P.; Abdi, S.; Abdollahifar, M.A.; et al. Photobiomodulation Therapy Reverses Spermatogenesis Arrest in Hyperthermia-Induced Azoospermia Mouse Model. Lasers Med. Sci. 2023, 38, 1–9. [Google Scholar] [CrossRef]
- Peralta-Arias, R.D.; Vívenes, C.Y.; Camejo, M.I.; Piñero, S.; Proverbio, T.; Martínez, E.; Marín, R.; Proverbio, F. ATPases, Ion Exchangers and Human Sperm Motility. Reproduction 2015, 149, 475–484. [Google Scholar] [CrossRef]
- Thundathil, J.C.; Rajamanickam, G.D.; Kastelic, J.P. Na/K-ATPase and Regulation of Sperm Function. Anim. Reprod. 2018, 15, 711. [Google Scholar] [CrossRef]
- De Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 348–364. [Google Scholar] [CrossRef]
- Jansen, K.; Wu, M.; Van der Steen, A.F.W.; Van Soest, G. Photoacoustic Imaging of Human Coronary Atherosclerosis in Two Spectral Bands. Photoacoustics 2014, 2, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Santana-Blank, L.; Rodríguez-Santana, E.; Santana-Rodríguez, K. Theoretic, Experimental, Clinical Bases of the Water Oscillator Hypothesis in near-Infrared Photobiomodulation. Photomed. Laser Surg. 2010, 28 (Suppl. 1), S41–S52. [Google Scholar] [CrossRef] [PubMed]
- Santana-Blank, L.; Rodríguez-Santana, E. The Interaction of Light with Nanoscopic Layers of Water May Be Essential to the Future of Photobiomodulation. Photomed. Laser Surg. 2010, 28 (Suppl. 1), S173–S174. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Zekiy, A.; Benedicenti, S.; Pasquale, C. A Narrative Review on Oral and Periodontal Bacteria Microbiota Photobiomodulation, through Visible and Near-Infrared Light: From the Origins to Modern Therapies. Int. J. Mol. Sci. 2022, 23, 1372. [Google Scholar] [CrossRef]
- Buravlev, E.A.; Zhidkova, T.V.; Vladimirov, Y.A.; Osipov, A.N. Effects of Low-Level Laser Therapy on Mitochondrial Respiration and Nitrosyl Complex Content. Lasers Med. Sci. 2014, 29, 1861–1866. [Google Scholar] [CrossRef] [PubMed]
- Savelieff, M.G.; Lu, Y. CuA Centers and Their Biosynthetic Models in Azurin. J. Biol. Inorg. Chem. 2010, 15, 461–483. [Google Scholar] [CrossRef]
- Sommer, A.P.; Schemmer, P.; Pavláth, A.E.; Försterling, H.-D.; Mester, Á.R.; Trelles, M.A. Quantum Biology in Low Level Light Therapy: Death of a Dogma. Ann. Transl. Med. 2020, 8, 440. [Google Scholar] [CrossRef]
- Sommer, A.P.; Haddad, M.K.; Fecht, H.J. Light Effect on Water Viscosity: Implication for ATP Biosynthesis. Sci. Rep. 2015, 5, 12029. [Google Scholar] [CrossRef]
- Foresta, C.; Rossato, M.; Chiozzi, P.; Di Virgilio, F. Mechanism of Human Sperm Activation by Extracellular ATP. Am. J. Physiol. Physiol. 1996, 270, C1709–C1714. [Google Scholar] [CrossRef]
- Irvine, D.S.; Aitken, R.J. The Value of Adenosine Triphosphate (ATP) Measurements in Assessing the Fertilizing Ability of Human Spermatozoa. Fertil. Steril. 1985, 44, 806–813. [Google Scholar] [CrossRef]
- Kupitz, Y.; Atlas, D. A Putative ATP-Activated Na+ Channel Involved in Sperm-Induced Fertilization. Science 1993, 261, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Pang, M.G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef]
- Herrero, M.; Lamirande, E.; Gagnon, C. Nitric Oxide Is a Signaling Molecule in Spermatozoa. Curr. Pharm. Des. 2005, 9, 419–425. [Google Scholar] [CrossRef]
- Rosselli, M.; Dubey, R.K.; Imthurn, B.; Macas, E.; Keller, P.J. Andrology: Effects of Nitric Oxide on Human Spermatozoa: Evidence That Nitric Oxide Decreases Sperm Motility and Induces Sperm Toxicity. Hum. Reprod. 1995, 10, 1786–1790. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, R.L. Possible Role of Nitric Oxide on Fertile and Asthenozoospermic Infertile Human Sperm Functions. Free Radic. Res. 1996, 25, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Cassina, A.; Silveira, P.; Cantu, L.; Montes, J.M.; Radi, R.; Sapiro, R. Defective Human Sperm Cells Are Associated with Mitochondrial Dysfunction and Oxidant Production. Biol. Reprod. 2015, 93, 119–120. [Google Scholar] [CrossRef]
- Bagnato, V.S.; Kurachi, C.; Blanco, K.C.; Inada, N.M. Molecular Basis for Photobiomodulation: Light-Induced Nitric Oxide Synthesis by Cytochrome c Oxidase in Low-Level Laser Therapy. Handb. Low-Level Laser Ther. 2016, 201–220. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, Z.; Ma, X.; Zong, X.; Tesic, V.; Ding, B.; Wu, C.Y.C.; Lee, R.H.C.; Zhang, Q. Photobiomodulation Inhibits Ischemia-Induced Brain Endothelial Senescence via Endothelial Nitric Oxide Synthase. Antioxidants 2024, 13, 633. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K. A Therapeutic Photobiomodulation: Nitric Oxide and a Novel Function of Mitochondrial Cytochrome c Oxidase. Discov. Med. 2011, 11, 154–159. [Google Scholar]
- Rizzi, M.; Migliario, M.; Tonello, S.; Rocchetti, V.; Renò, F. Photobiomodulation Induces in Vitro Re-Epithelialization via Nitric Oxide Production. Lasers Med. Sci. 2018, 33, 1003–1008. [Google Scholar] [CrossRef]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible Nitric Oxide Synthase: Regulation, Structure, and Inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C. Nitric Oxide and Mitochondrial Respiration. Biochim. Et. Biophys. Acta (BBA)-Bioenerg. 1999, 1411, 351–369. [Google Scholar] [CrossRef]
- Sarti, P.; Arese, M.; Bacchi, A.; Barone, M.C.; Forte, E.; Mastronicola, D.; Brunori, M.; Giuffrè, A. Nitric Oxide and Mitochondrial Complex IV. IUBMB Life 2003, 55, 605–611. [Google Scholar] [CrossRef]
- Donnelly, E.T.; Lewis, S.E.M.; Thompson, W.; Chakravarthy, U. Sperm Nitric Oxide and Motility: The Effects of Nitric Oxide Synthase Stimulation and Inhibition. Mol. Hum. Reprod. 1997, 3, 755–762. [Google Scholar] [CrossRef]
- Balercia, G.; Moretti, S.; Vignini, A.; Magagnini, M.; Mantero, F.; Boscaro, M.; Ricciardo-Lamonica, G.; Mazzanti, L. Role of Nitric Oxide Concentrations on Human Sperm Motility. J. Androl. 2004, 25, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Aquila, S.; Sisci, D.; Gentile, M.; Middea, E.; Siciliano, L.; Andò, S. Human Ejaculated Spermatozoa Contain Active P450 Aromatase. J. Clin. Endocrinol. Metab. 2002, 87, 3385–3390. [Google Scholar] [CrossRef]
- TWEED, D.C. Evaluation of the Spermatozoa and Seminal Plasma of the Infertile Male. Cleve Clin. Q. 1963, 30, 30–38. [Google Scholar] [CrossRef]
- Gu, N.H.; Zhao, W.L.; Wang, G.S.; Sun, F. Comparative Analysis of Mammalian Sperm Ultrastructure Reveals Relationships between Sperm Morphology, Mitochondrial Functions and Motility. Reprod. Biol. Endocrinol. 2019, 17. [Google Scholar] [CrossRef]
- Alahmar, A. Role of Oxidative Stress in Male Infertility: An Updated Review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative Stress and Male Infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Kowalczyk, A. The Role of the Natural Antioxidant Mechanism in Sperm Cells. Reprod. Sci. 2022, 29, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Shiva, M.; Gautam, A.K.; Verma, Y.; Shivgotra, V.; Doshi, H.; Kumar, S. Association between Sperm Quality, Oxidative Stress, and Seminal Antioxidant Activity. Clin. Biochem. 2011, 44, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Gala, B.; Badge, A.; Bawaskar, P.; Gajbe, U.; Singh, B.R.; Kohale, M. The Potential of Theophylline and Pentoxifylline in Sperm Optimization and Its Intracytoplasmic Sperm Injection Outcomes. Cureus 2023, 15, e48192. [Google Scholar] [CrossRef] [PubMed]
- Mahaldashtian, M.; Khalili, M.A.; Nottola, S.A.; Woodward, B.; Macchiarelli, G.; Miglietta, S. Does in Vitro Application of Pentoxifylline Have Beneficial Effects in Assisted Male Reproduction? Andrologia 2021, 53, e13722. [Google Scholar] [CrossRef]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef]

| Characteristics | Mean ± SEM |
|---|---|
| Age (years) | 35.74 ± 2.06 |
| Volume (mL) | 3.51 ± 0.26 |
| Sperm concentration (106/mL) | 34.74 ± 4.73 |
| Normal morphological sperm (%) | 3.32 ± 0.57 |
| Total motility (%) | 34.10 ± 1.31 |
| Progressive motility (%) | 28.10 ± 1.03 |
| Sperm vitality (%) | 54.26 ± 1.56 |
| Laser Model | ENEA Trio diode laser system (Garda Laser S.A.S., Verona, Italy) https://gardalaser.it/ (accessed on 30 April 2025) | |||||
| Irradiation device | Flat-profiled handpiece [58] | |||||
| Max. power in continuous wave mode | 15 Watts (W) | |||||
| Light pointer | 635 nm red light at negligible power output, <0.5 mW | |||||
| Irradiation parameters | ||||||
| Wavelengths | 450 nm ± 10 nm | 635 nm ± 10 nm | 810 nm ± 10 nm | 940 nm ± 10 nm | 1064 nm ± 10 nm | |
| Power | Time | Energy | Area | Power-Density | Fluence | |
| 0.25 W | 60 s | 15 J | 1 cm2 | 0.25 W/cm2 | 15 J/cm2 | |
| 0.50 W | 60 s | 30 J | 1 cm2 | 0.50 W/cm2 | 30 J/cm2 | |
| 1.00 W | 60 s | 60 J | 1 cm2 | 1.00 W/cm2 | 60 J/cm2 | |
| 2.00 W | 60 s | 120 J | 1 cm2 | 2.00 W/cm2 | 120 J/cm2 | |
| Irradiation performed in continuous wave mode | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balbi, M.; Lai, R.; Stigliani, S.; Massarotti, C.; Bozzo, M.; Scaruffi, P.; Ravera, S.; Amaroli, A. Efficacy and Safety of Visible and Near-Infrared Photobiomodulation Therapy on Astenospermic Human Sperm: Wavelength-Dependent Regulation of Nitric Oxide Levels and Mitochondrial Energetics. Biology 2025, 14, 491. https://doi.org/10.3390/biology14050491
Balbi M, Lai R, Stigliani S, Massarotti C, Bozzo M, Scaruffi P, Ravera S, Amaroli A. Efficacy and Safety of Visible and Near-Infrared Photobiomodulation Therapy on Astenospermic Human Sperm: Wavelength-Dependent Regulation of Nitric Oxide Levels and Mitochondrial Energetics. Biology. 2025; 14(5):491. https://doi.org/10.3390/biology14050491
Chicago/Turabian StyleBalbi, Matilde, Rachele Lai, Sara Stigliani, Claudia Massarotti, Matteo Bozzo, Paola Scaruffi, Silvia Ravera, and Andrea Amaroli. 2025. "Efficacy and Safety of Visible and Near-Infrared Photobiomodulation Therapy on Astenospermic Human Sperm: Wavelength-Dependent Regulation of Nitric Oxide Levels and Mitochondrial Energetics" Biology 14, no. 5: 491. https://doi.org/10.3390/biology14050491
APA StyleBalbi, M., Lai, R., Stigliani, S., Massarotti, C., Bozzo, M., Scaruffi, P., Ravera, S., & Amaroli, A. (2025). Efficacy and Safety of Visible and Near-Infrared Photobiomodulation Therapy on Astenospermic Human Sperm: Wavelength-Dependent Regulation of Nitric Oxide Levels and Mitochondrial Energetics. Biology, 14(5), 491. https://doi.org/10.3390/biology14050491









