Deciphering the Regulatory Potential of Antioxidant and Electron-Shuttling Bioactive Compounds in Oolong Tea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Phytochemical Content
2.2.1. Total Polyphenol Content
2.2.2. Total Flavonoid Content
2.2.3. Total Condensed Tannin Content
2.3. Antioxidant Activity
2.3.1. 2,2-Diphenyl-1-picrylhydrazil Free Radical Scavenging Capacity
2.3.2. Ferric Reducing Antioxidant Power Assay
2.4. Electrochemical Property
2.4.1. Power Density Through Double Chambered-Microbial Fuel Cell
2.4.2. Cyclic Voltammetry Assay
2.5. Network Pharmacology
2.5.1. Screening and Evaluation of Oolong Tea Bioactive Compounds
2.5.2. Identification of Target Gene Candidates
2.5.3. Differential Expression Analysis of Breast Cancer Subtypes
2.5.4. Construction of Protein–Protein Interaction Networks for Identifying Hub Genes
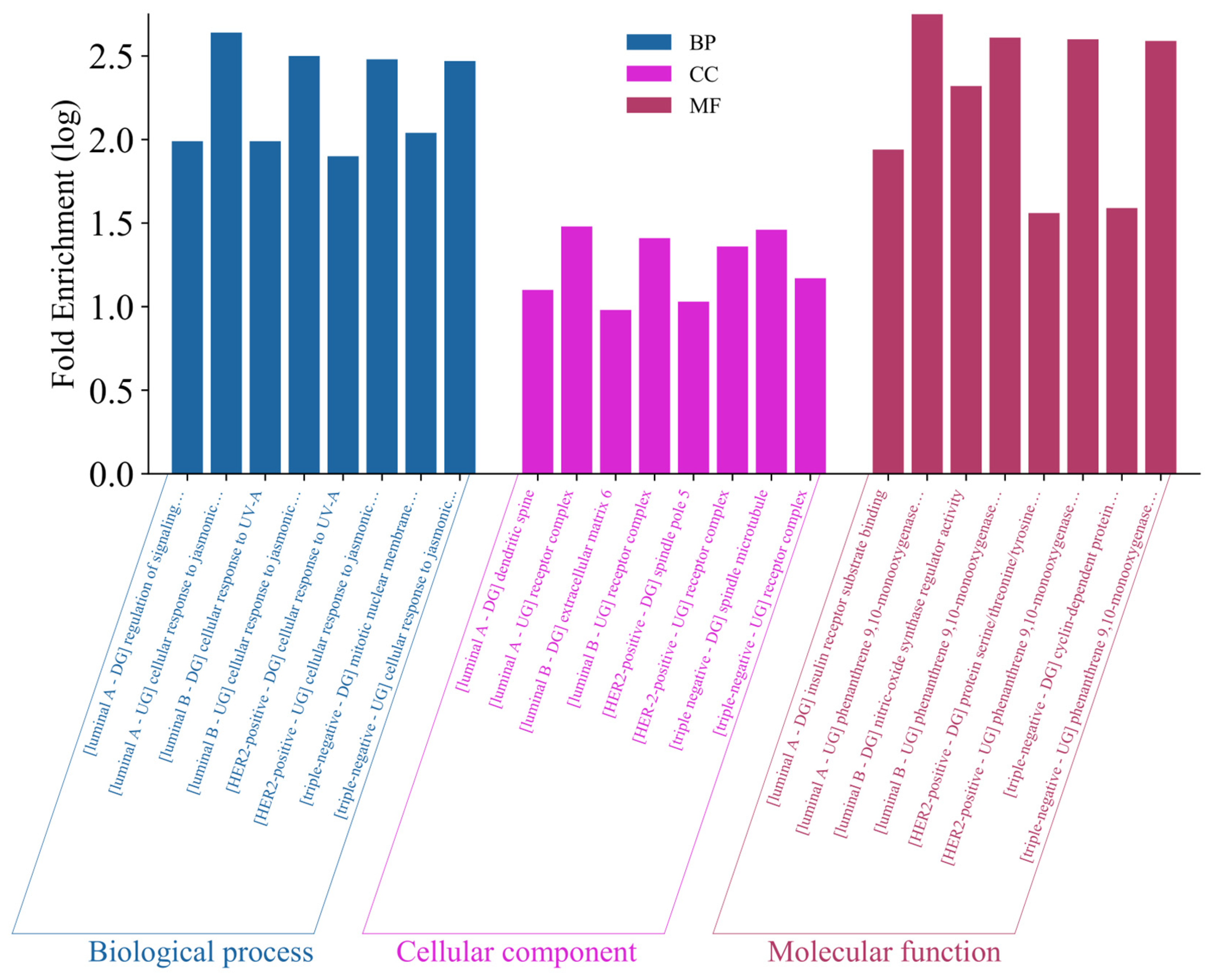
2.5.5. Gene Ontology Analysis
2.6. Molecular Simulations
2.6.1. Molecular Docking
2.6.2. Molecular Dynamics
2.6.3. Molecular Mechanics Poisson–Boltzmann Surface Area
2.6.4. Principal Component Analysis/Free Energy Landscape
3. Results
3.1. Phytochemical Analysis
3.2. Antioxidant Analysis
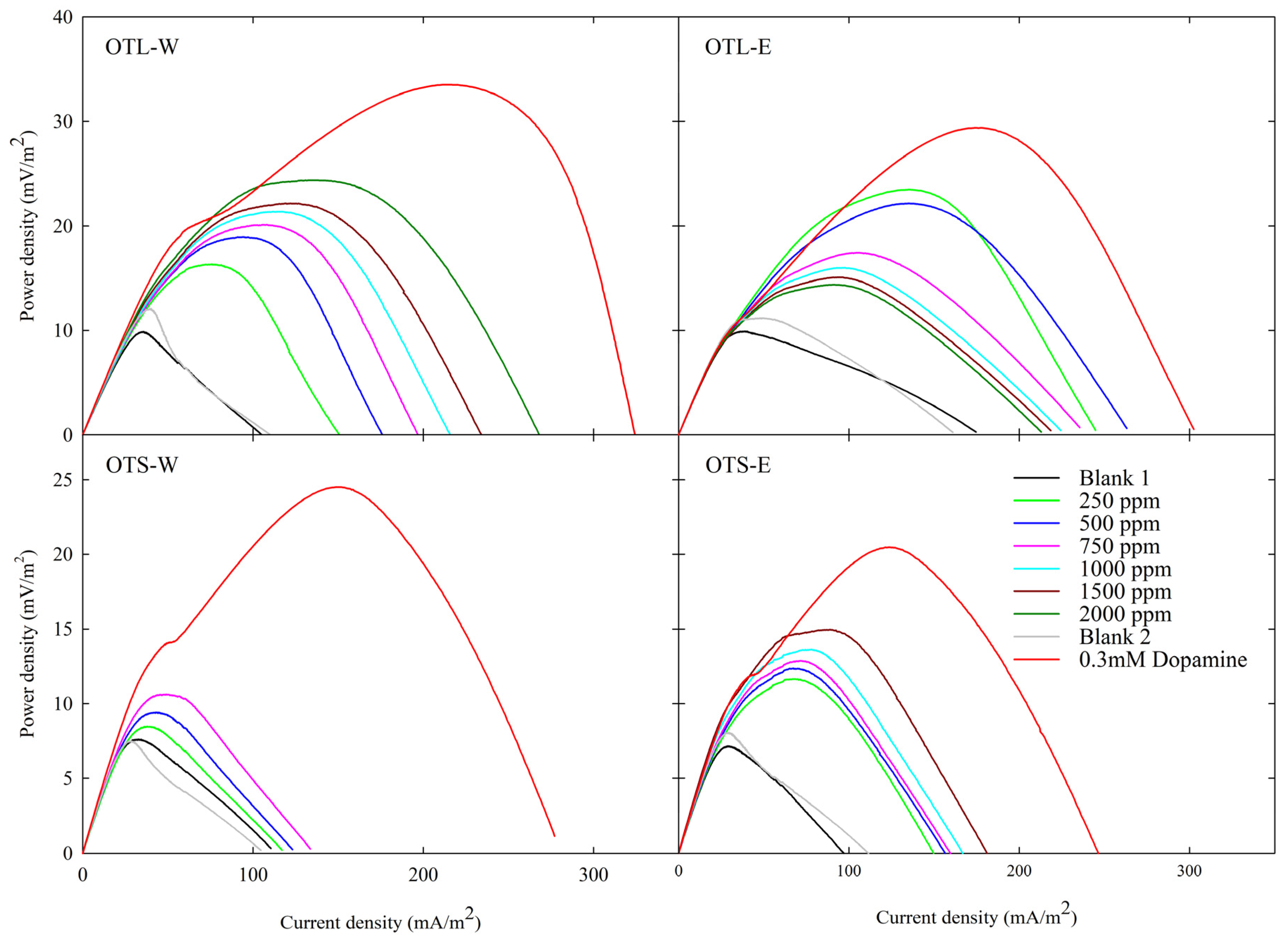
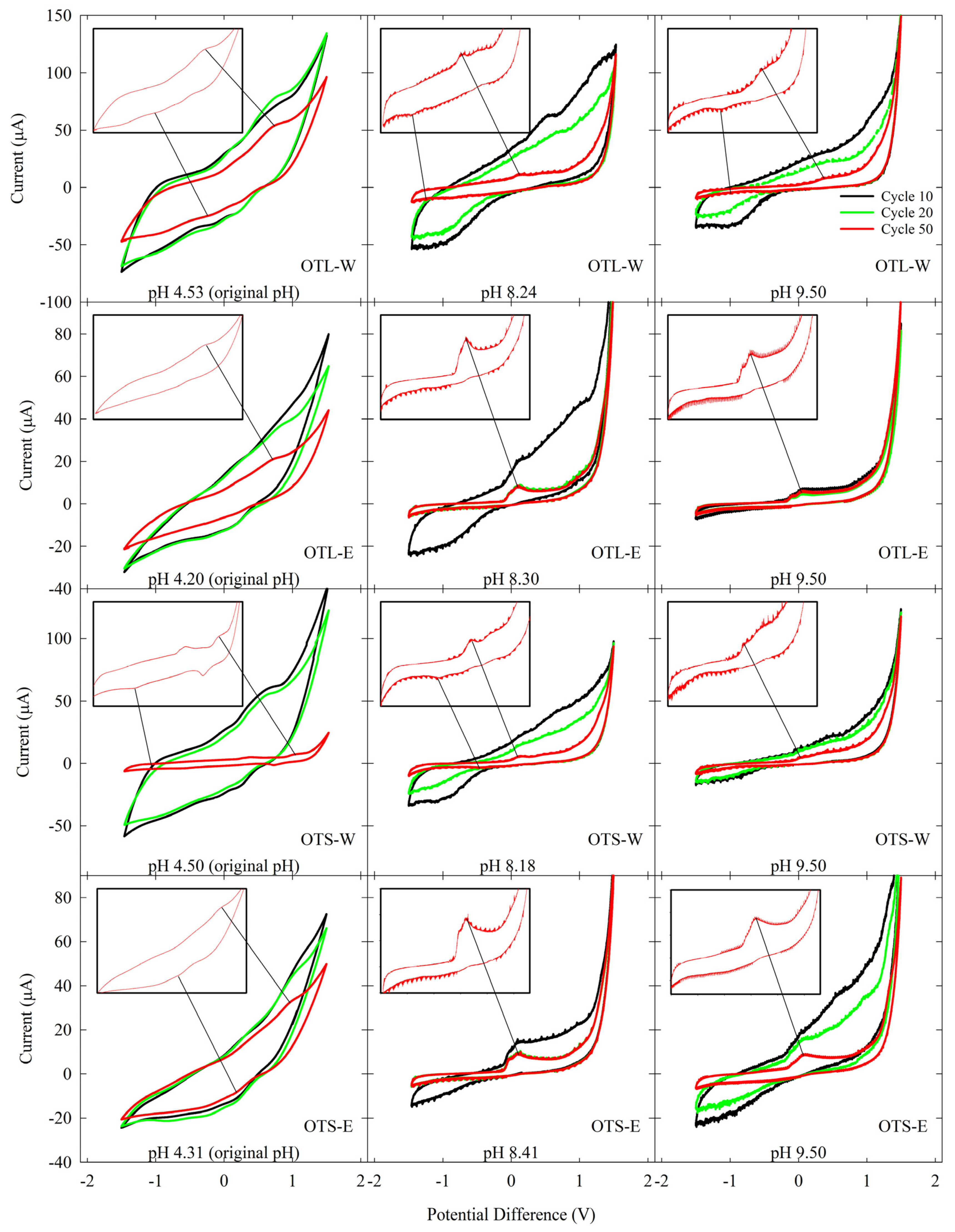
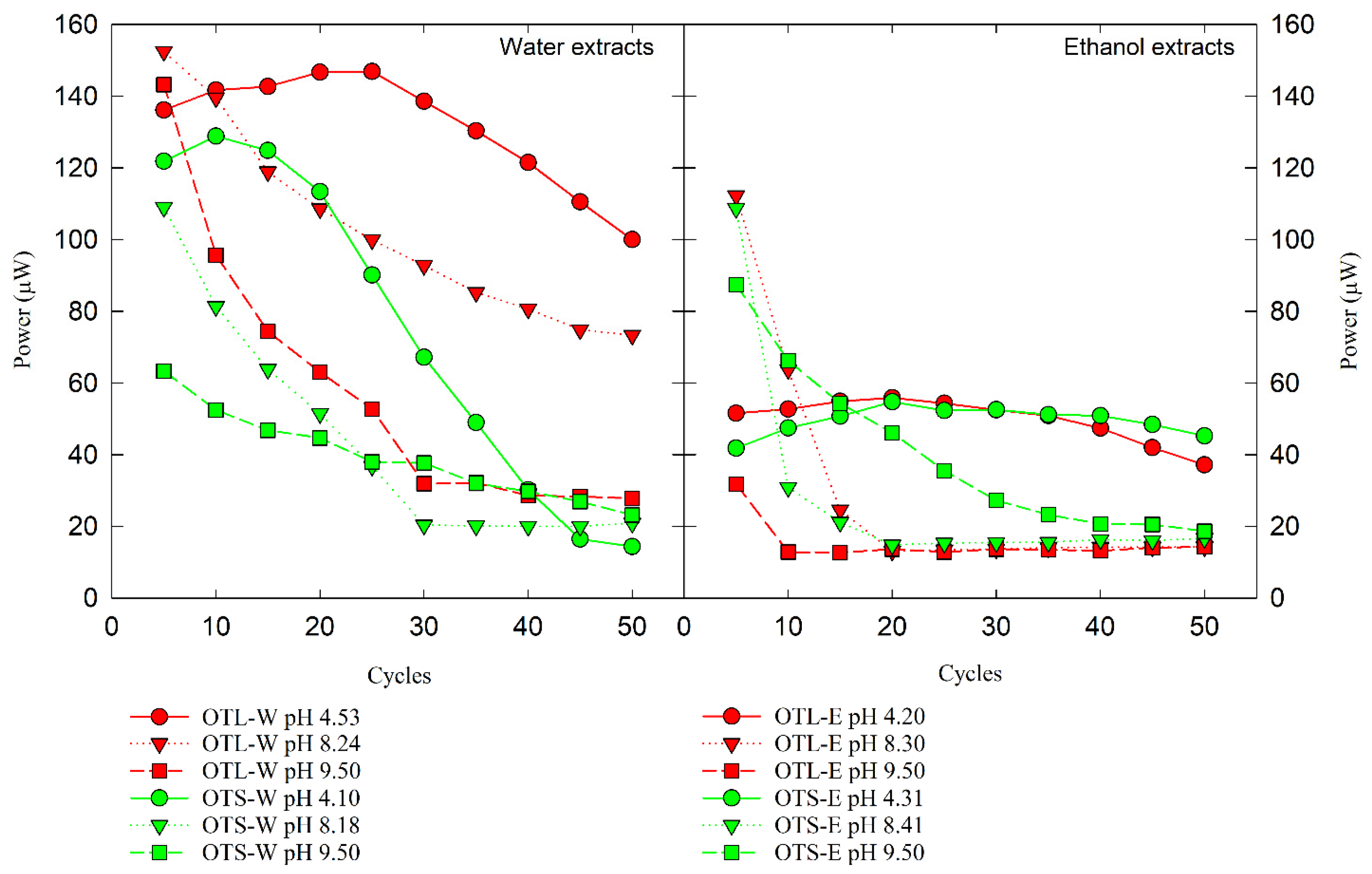
3.3. Bioenergy and Electrochemical Assessment via Microbial Fuel Cell and Cyclic Voltammetry
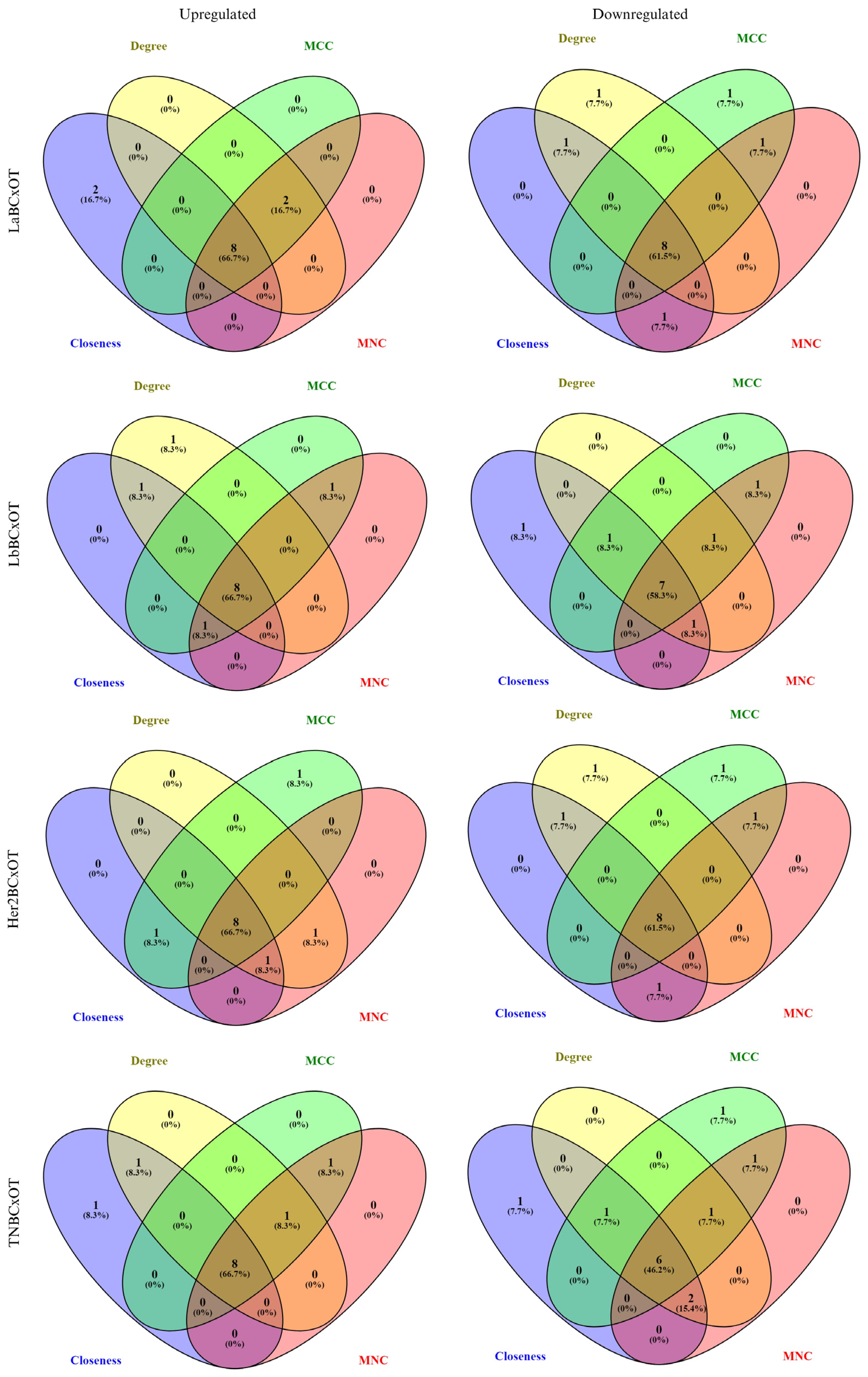
3.4. Network Pharmacology
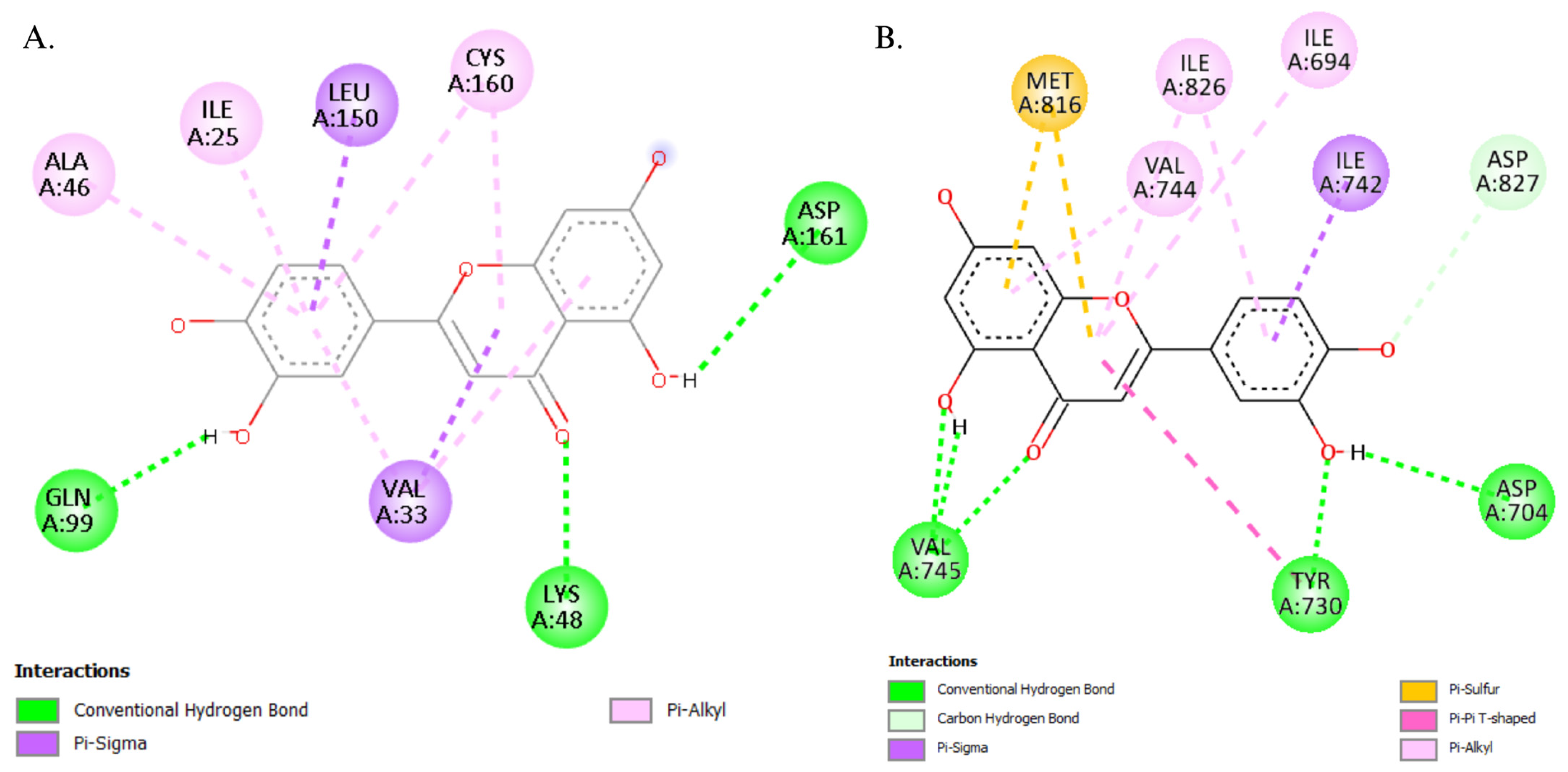
3.5. Molecular Docking
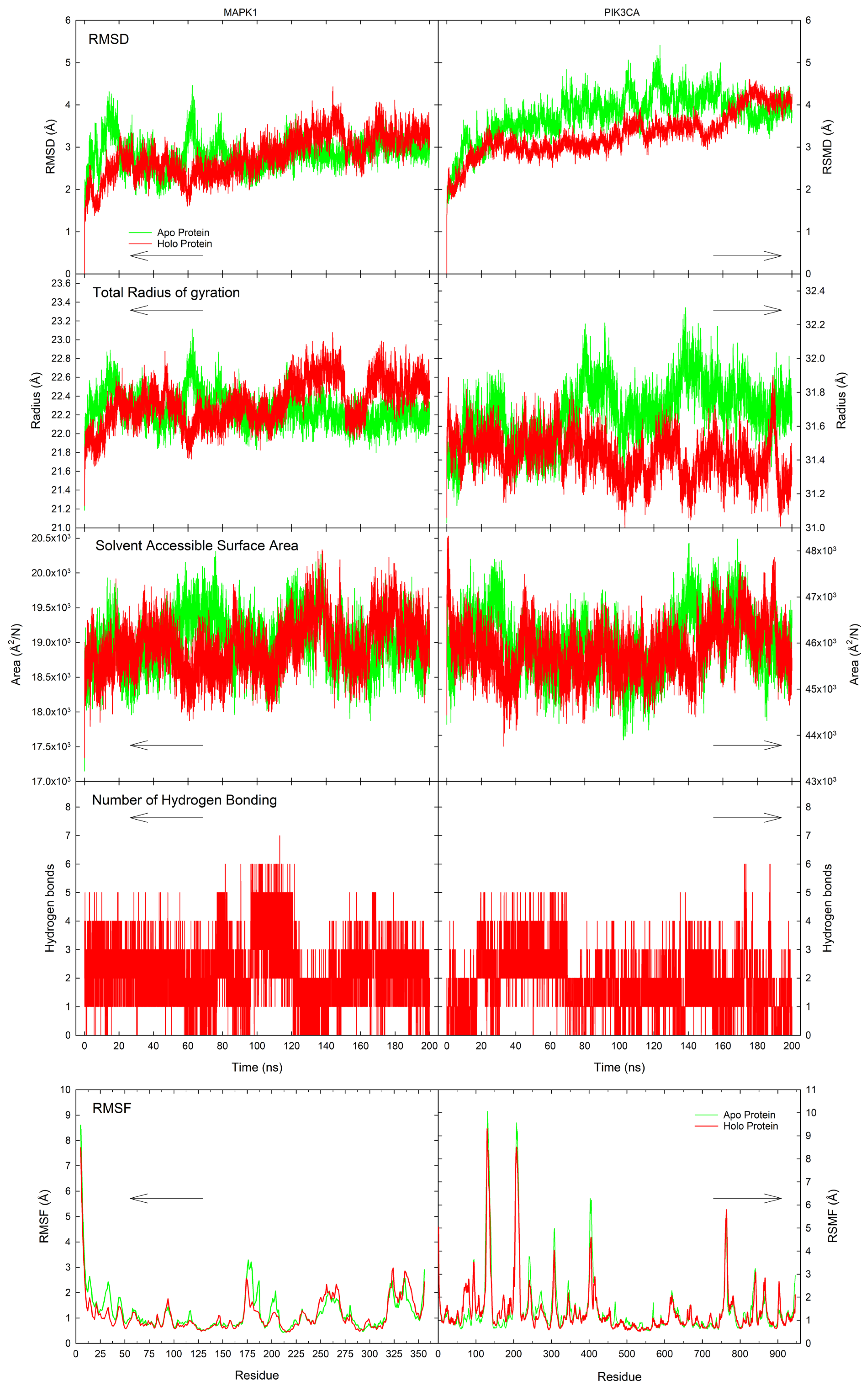
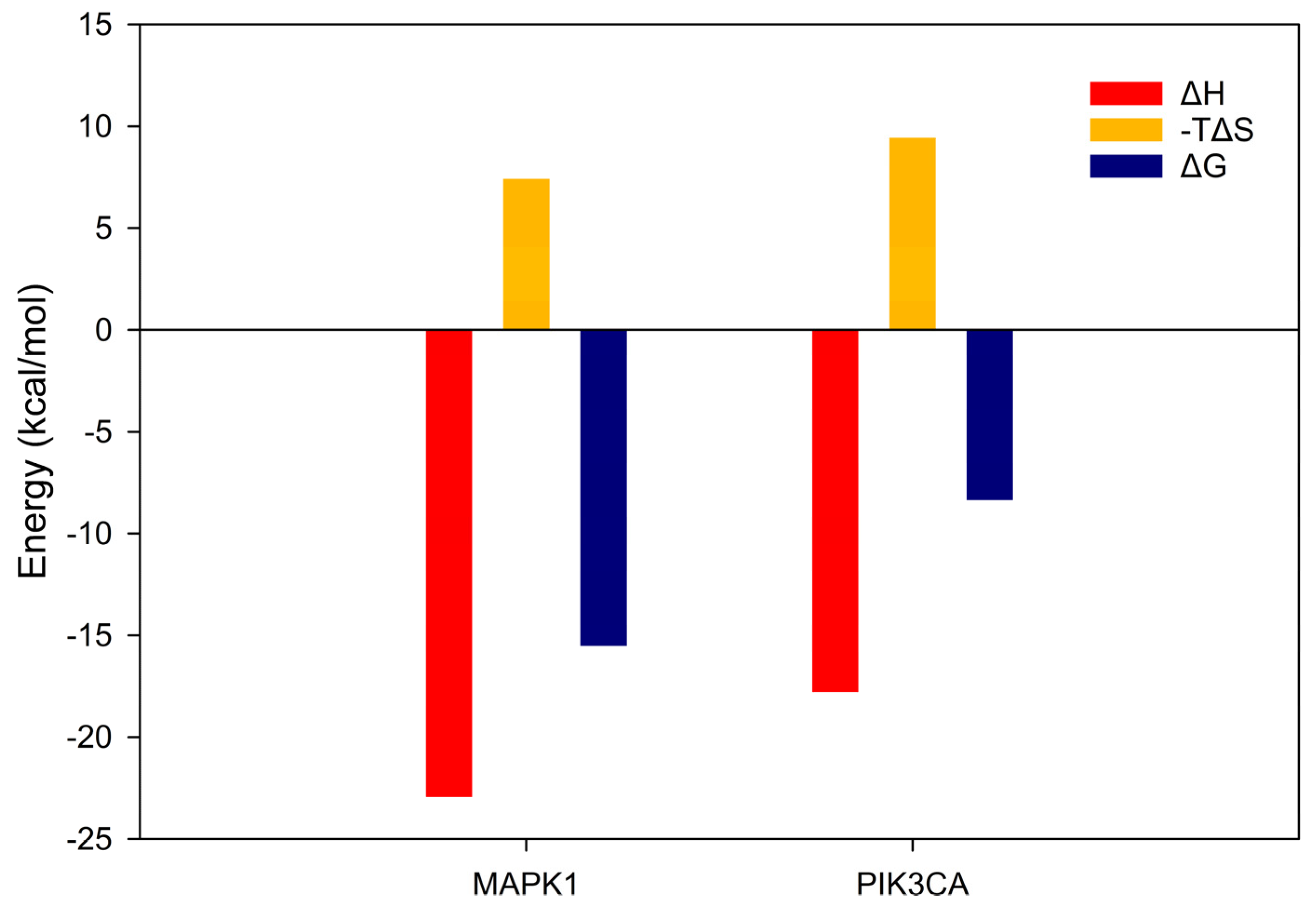
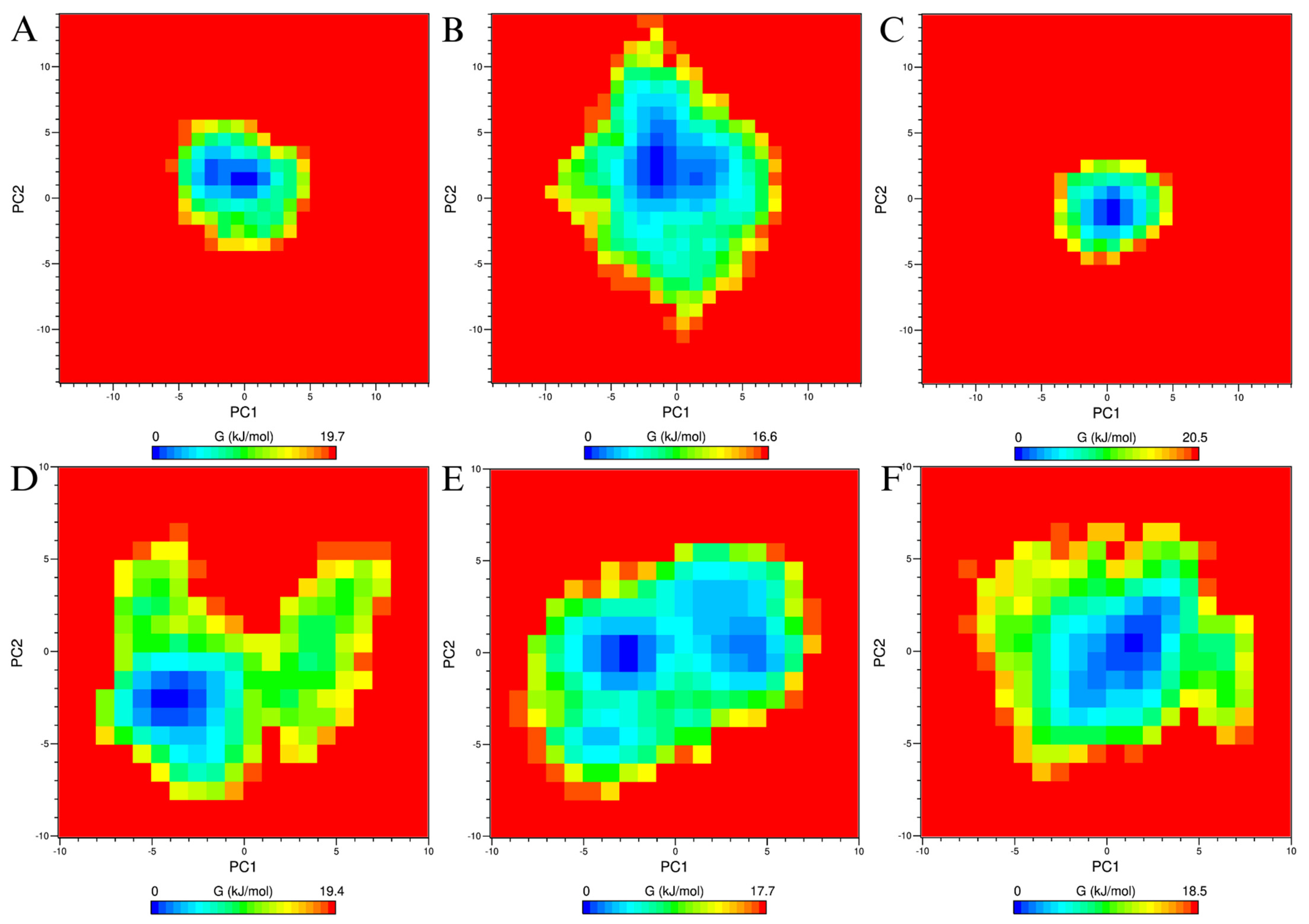
3.6. Molecular Dynamics
4. Discussion
4.1. Mechanism of Oolong Tea Against Breast Cancer
4.2. Phytochemical Content Assays
4.3. Antioxidant Activities
4.4. Electrochemical Analysis
4.5. Network Pharmacology
4.6. Molecular Simulations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Han, J.; Pu, Y.; Wang, X. Tea (Camellia sinensis): A Review of Nutritional Composition, Potential Applications, and Omics Research. Appl. Sci. 2022, 12, 5874. [Google Scholar] [CrossRef]
- Salman, S.; Yılmaz, C.; Gökmen, V.; Özdemir, F. Effects of Fermentation Time and Shooting Period on Amino Acid Derivatives and Free Amino Acid Profiles of Tea. LWT 2021, 137, 110481. [Google Scholar] [CrossRef]
- Ananingsih, V.K.; Sharma, A.; Zhou, W. Green Tea Catechins during Food Processing and Storage: A Review on Stability and Detection. Food Res. Int. 2013, 50, 469–479. [Google Scholar] [CrossRef]
- Chen, B.Y.; Liao, J.H.; Hsueh, C.C.; Qu, Z.; Hsu, A.W.; Chang, C.T.; Zhang, S. Deciphering Biostimulation Strategy of Using Medicinal Herbs and Tea Extracts for Bioelectricity Generation in Microbial Fuel Cells. Energy 2018, 161, 1042–1054. [Google Scholar] [CrossRef]
- Zeeb, D.J.; Nelson, B.C.; Albert, K.; Dallunge, J.J. Separation and Identification of Twelve Catechins in Tea Using Liquid Chromatography/Atmospheric Pressure Chemical Ionization-Mass Spectrometry. Anal. Chem. 2000, 72, 5020–5026. [Google Scholar] [CrossRef]
- Qin, L.; Guo, L.; Xu, B.; Hsueh, C.C.; Jiang, M.; Chen, B.Y. Exploring Community Evolutionary Characteristics of Microbial Populations with Supplementation of Camellia Green Tea Extracts in Microbial Fuel Cells. J. Taiwan. Inst. Chem. Eng. 2020, 113, 214–222. [Google Scholar] [CrossRef]
- Liu, P.P.; Yin, J.F.; Chen, G.S.; Wang, F.; Xu, Y.Q. Flavor Characteristics and Chemical Compositions of Oolong Tea Processed Using Different Semi-Fermentation Times. J. Food Sci. Technol. 2018, 55, 1185–1195. [Google Scholar] [CrossRef]
- Zeng, L.; Fu, Y.Q.; Liu, Y.Y.; Huang, J.S.; Chen, J.X.; Yin, J.F.; Jin, S.; Sun, W.J.; Xu, Y.Q. Comparative Analysis of Different Grades of Tieguanyin Oolong Tea Based on Metabolomics and Sensory Evaluation. LWT 2023, 174, 114423. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Wang, Q.; Li, Y.; Ling, J.; Liu, L.; Chen, X.; Bi, K. Simultaneous Determination of Seven Bioactive Components in Oolong Tea Camellia sinensis: Quality Control by Chemical Composition and HPLC Fingerprints. J. Agric. Food Chem. 2012, 60, 256–260. [Google Scholar] [CrossRef]
- Wang, S.; Zeng, T.; Zhao, S.; Zhu, Y.; Feng, C.; Zhan, J.; Li, S.; Ho, C.T.; Gosslau, A. Multifunctional Health-Promoting Effects of Oolong Tea and Its Products. Food Sci. Hum. Wellness 2022, 11, 512–523. [Google Scholar] [CrossRef]
- Weerawatanakorn, M.; Hung, W.L.; Pan, M.H.; Li, S.; Li, D.; Wan, X.; Ho, C.T. Chemistry and Health Beneficial Effects of Oolong Tea and Theasinensins. Food Sci. Hum. Wellness 2015, 4, 133–146. [Google Scholar] [CrossRef]
- Didier, A.J.; Stiene, J.; Fang, L.; Watkins, D.; Dworkin, L.D.; Creeden, J.F. Antioxidant and Anti-Tumor Effects of Dietary Vitamins A, C, and E. Antioxidants 2023, 12, 632. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.W.; Tayo, L.L.; Ting, J.U.; Hsieh, C.Y.; Lee, C.J.; Chen, C.L.; Yang, H.C.; Tsai, H.Y.; Hsueh, C.C.; Chen, B.Y. Interactive Deciphering Electron-Shuttling Characteristics of Coffea Arabica Leaves and Potential Bioenergy-Steered Anti-SARS-CoV-2 RdRp Inhibitor via Microbial Fuel Cells. Ind. Crops Prod. 2023, 191, 115944. [Google Scholar] [CrossRef]
- Tsai, P.W.; Aledia, M.R.G.G.; De Castro-Cruz, K.A.; Garcia, P.J.B.; Shen, C.J.; Hsueh, C.C.; Chen, C.Y.; Chen, B.Y. Exploring Promising Electron-Shuttling Characteristics of Perilla Frutescens and Potential Anti-Viral Activity via Bioenergy Generation in Microbial Fuel Cells. Sustain. Chem. Pharm. 2023, 34, 101141. [Google Scholar] [CrossRef]
- Hsueh, C.C.; Wu, C.C.; Chen, B.Y. Polyphenolic Compounds as Electron Shuttles for Sustainable Energy Utilization. Biotechnol. Biofuels 2019, 12, 271. [Google Scholar] [CrossRef]
- World Health Organization Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 30 October 2024).
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Simon, A.; Robb, K. Breast Cancer. In Cambridge Handbook of Psychology, Health and Medicine, 2nd ed.; Cambridge University Press: Cambridge, UK, 2024; pp. 577–580. [Google Scholar] [CrossRef]
- Cohen, S.Y.; Stoll, C.R.; Anandarajah, A.; Doering, M.; Colditz, G.A. Modifiable Risk Factors in Women at High Risk of Breast Cancer: A Systematic Review. Breast Cancer Res. 2023, 25, 45. [Google Scholar] [CrossRef]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. Breast Cancer 2022, 31–42. [Google Scholar] [CrossRef]
- Zardavas, D.; Phillips, W.A.; Loi, S. PIK3CA Mutations in Breast Cancer: Reconciling Findings from Preclinical and Clinical Data. Breast Cancer Res. 2014, 16, 201. [Google Scholar] [CrossRef]
- Opyrchal, M.; Salisbury, J.L.; Zhang, S.; McCubrey, J.; Hawse, J.; Goetz, M.P.; Lomberk, G.A.; Haddad, T.; Degnim, A.; Lange, C.; et al. Aurora-A Mitotic Kinase Induces Endocrine Resistance through Down-Regulation of ERα Expression in Initially ERα+ Breast Cancer Cells. PLoS ONE 2014, 9, e96995. [Google Scholar] [CrossRef]
- Yang, C.S.; Chung, J.Y.; Yang, G.Y.; Chhabra, S.K.; Lee, M.J. Tea and Tea Polyphenols in Cancer Prevention. J. Nutr. 2000, 130, 472S–478S. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Liu, Z.; Seril, D.N.; Liao, J.; Ding, W.; Kim, S.; Bondoc, F.; Yang, C.S. Black Tea Constituents, Theaflavins, Inhibit 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanone (NNK)-Induced Lung Tumorigenesis in A/J Mice. Carcinogenesis 1997, 18, 2361–2365. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hajiaghaalipour, F.; Kanthimathi, M.S.; Sanusi, J.; Rajarajeswaran, J. White Tea (Camellia sinensis) Inhibits Proliferation of the Colon Cancer Cell Line, HT-29, Activates Caspases and Protects DNA of Normal Cells against Oxidative Damage. Food Chem. 2015, 169, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Zhao, Q.; Chen, Q.; Xiao, W.; Jiang, Y.; Jiang, Y. The Synergic Inhibitory Effects of Dark Tea (Camellia sinensis) Extract and P38 Inhibition on the Growth of Pancreatic Cancer Cells. J. Cancer 2019, 10, 6557. [Google Scholar] [CrossRef]
- Sartippour, M.R.; Heber, D.; Ma, J.; Lu, Q.; Go, V.L.; Nguyen, M. Green Tea and Its Catechins Inhibit Breast Cancer Xenografts. Nutr. Cancer 2001, 40, 149–156. [Google Scholar] [CrossRef]
- Der Way, T.; Lee, H.H.; Kao, M.C.; Lin, J.K. Black Tea Polyphenol Theaflavins Inhibit Aromatase Activity and Attenuate Tamoxifen Resistance in HER2/Neu-Transfected Human Breast Cancer Cells through Tyrosine Kinase Suppression. Eur. J. Cancer 2004, 40, 2165–2174. [Google Scholar] [CrossRef]
- Saini, H.; Gupta, P.K.; Mahapatra, A.K.; Rajagopala, S.; Tripathi, R.; Nesari, T. Deciphering the Multi-Scale Mechanism of Herbal Phytoconstituents in Targeting Breast Cancer: A Computational Pharmacological Perspective. Sci. Rep. 2024, 14, 23795. [Google Scholar] [CrossRef]
- Kuban-Jankowska, A.; Kostrzewa, T.; Musial, C.; Barone, G.; Lo-Bosco, G.; Lo-Celso, F.; Gorska-Ponikowska, M. Green Tea Catechins Induce Inhibition of PTP1B Phosphatase in Breast Cancer Cells with Potent Anti-Cancer Properties: In vitro Assay, Molecular Docking, and Dynamics Studies. Antioxidants 2020, 9, 1208. [Google Scholar] [CrossRef]
- Ndacyayisenga, J.; Tolo, F.M.; Wamunyokoli, F.; Maina, E.N. Effects of Tea Catechin Extracts from BB35 and Purple (TRFK 306) Tea Clones on the Gene Expression of Egfr, App, Bcl2, Dnmt, Casp3, Hif1a, Gadd45b and Psmb5 Genes Involved in Triple Negative Breast Cancer Diseases: In silico and in vitro Study. Inform. Med. Unlocked 2024, 46, 101469. [Google Scholar] [CrossRef]
- Shi, H.; Liu, J.; Tu, Y.; Freter, C.E.; Huang, C. Oolong Tea Extract Induces DNA Damage and Cleavage and Inhibits Breast Cancer Cell Growth and Tumorigenesis. Anticancer. Res. 2018, 38, 6217–6223. [Google Scholar] [CrossRef]
- Tsai, P.-W.; Hsieh, C.-Y.; Ting, J.U.; Ciou, Y.-R.; Lee, C.-J.; Hsieh, C.-L.; Lien, T.-K.; Hsueh, C.-C.; Chen, B.-Y. Synergistic Deciphering of Bioenergy Production and Electron Transport Characteristics to Screen Traditional Chinese Medicine (TCM) for COVID-19 Drug Development. J. Taiwan. Inst. Chem. Eng. 2022, 135, 104365. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Kim, B.; Kim, S.; Kim, M.S.; Kim, H.; Hwang, S.R.; Kim, K.; Lee, J.H. Characterization of Metabolite Profiles from the Leaves of Green Perilla (Perilla Frutescens) by Ultra High Performance Liquid Chromatography Coupled with Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry and Screening for Their Antioxidant Properties. J. Food Drug Anal. 2017, 25, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Vivek-Ananth, R.P.; Mohanraj, K.; Sahoo, A.K.; Samal, A. IMPPAT 2.0: An Enhanced and Expanded Phytochemical Atlas of Indian Medicinal Plants. ACS Omega 2023, 8, 8827–8845. [Google Scholar] [CrossRef] [PubMed]
- Ali, M. PCIDB -PhytoChemical Interactions DataBase. In Proceedings of the Training Workshop: Metabolic Engineering for Primary and Secondary Compounds Production from Medicinal, Aromatic and Economical Plants, Cairo, Eqypt, 26 August 2019. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem 2023 Update. Nucleic Acids Res. 2023, 51, D1373–D1380. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An Integrated Online Platform for Accurate and Comprehensive Predictions of ADMET Properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-Approved Small Molecule Protein Kinase Inhibitors. Pharmacol. Res. 2019, 144, 19–50. [Google Scholar] [CrossRef]
- Eynde, V.; Barretta, R.; Kralj, S.; Jukič, M.; Bren, U. Molecular Filters in Medicinal Chemistry. Encyclopedia 2023, 3, 501–511. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Nickel, J.; Gohlke, B.O.; Erehman, J.; Banerjee, P.; Rong, W.W.; Goede, A.; Dunkel, M.; Preissner, R. SuperPred: Update on Drug Classification and Target Prediction. Nucleic Acids Res. 2014, 42, W26–W31. [Google Scholar] [CrossRef]
- Barrett, T.; Wilhite, S.E.; Ledoux, P.; Evangelista, C.; Kim, I.F.; Tomashevsky, M.; Marshall, K.A.; Phillippy, K.H.; Sherman, P.M.; Holko, M.; et al. NCBI GEO: Archive for Functional Genomics Data Sets—Update. Nucleic Acids Res. 2013, 41, D991–D995. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.P.T.; Tayo, L.L. Theoretical Studies of DNA Microarray Present Potential Molecular and Cellular Interconnectivity of Signaling Pathways in Immune System Dysregulation. Genes 2024, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Robinson, D.G.; Storey, J.D. The Functional False Discovery Rate with Applications to Genomics. Biostatistics 2021, 22, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision Weights Unlock Linear Model Analysis Tools for RNA-Seq Read Counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING Database in 2023: Protein-Protein Association Networks and Functional Enrichment Analyses for Any Sequenced Genome of Interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Garcia, J.P.T.; Tayo, L.L. Codes between Poles: Linking Transcriptomic Insights into the Neurobiology of Bipolar Disorder. Biology 2024, 13, 787. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and Integrative Analysis of Large Gene Lists Using DAVID Bioinformatics Resources. Nat. Protoc. 2008, 4, 44–57. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A Free Online Platform for Data Visualization and Graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform 2011, 3, 33. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Bhatt, R.; Bhikadiya, C.; Bi, C.; Biester, A.; Biswas, P.; Bittrich, S.; Blaumann, S.; Brown, R.; Chao, H.; et al. Updated Resources for Exploring Experimentally-Determined PDB Structures and Computed Structure Models at the RCSB Protein Data Bank. Nucleic Acids Res. 2025, 53, D564–D574. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Duarte, J.M.; Feng, Z.; Flatt, J.W.; Hudson, B.P.; Lowe, R.; Peisach, E.; Piehl, D.W.; Rose, Y.; et al. Protein Data Bank: A Comprehensive Review of 3D Structure Holdings and Worldwide Utilization by Researchers, Educators, and Students. Biomolecules 2022, 12, 1425. [Google Scholar] [CrossRef]
- Shao, C.; Bittrich, S.; Wang, S.; Burley, S.K. Assessing PDB Macromolecular Crystal Structure Confidence at the Individual Amino Acid Residue Level. Structure 2022, 30, 1385–1394.e3. [Google Scholar] [CrossRef]
- Kleywegt, G.J.; Jones, T.A. [11] Model Building and Refinement Practice. Methods Enzymol. 1997, 277, 208–230. [Google Scholar] [CrossRef]
- Krivák, R.; Hoksza, D. P2Rank: Machine Learning Based Tool for Rapid and Accurate Prediction of Ligand Binding Sites from Protein Structure. J. Cheminform 2018, 10, 39. [Google Scholar] [CrossRef]
- Jakubec, D.; Skoda, P.; Krivak, R.; Novotny, M.; Hoksza, D. PrankWeb 3: Accelerated Ligand-Binding Site Predictions for Experimental and Modelled Protein Structures. Nucleic Acids Res. 2022, 50, W593–W597. [Google Scholar] [CrossRef]
- Biovia, D.S. Biovia Discovery Studio Visualizer 2024, San Diego: Dassault Systèmes. 2025. Available online: https://discover.3ds.com/discovery-studio-visualizer-download (accessed on 9 December 2024).
- Sanner, M.F. A Component-Based Software Environment for Visualizing Large Macromolecular Assemblies. Structure 2005, 13, 447–462. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Sarkar, A.; Concilio, S.; Sessa, L.; Marrafino, F.; Piotto, S. Advancements and Novel Approaches in Modified AutoDock Vina Algorithms for Enhanced Molecular Docking. Results Chem. 2024, 7, 101319. [Google Scholar] [CrossRef]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
- Delfosse, V.; Grimaldi, M.; Cavaillès, V.; Balaguer, P.; Bourguet, W. Structural and Functional Profiling of Environmental Ligands for Estrogen Receptors. Environ. Health Perspect. 2015, 122, 1306–1313. [Google Scholar] [CrossRef]
- Stachowski, T.R.; Nithianantham, S.; Vanarotti, M.; Lopez, K.; Fischer, M. Pan-HSP90 Ligand Binding Reveals Isoform-Specific Differences in Plasticity and Water Networks. Protein Sci. 2023, 32, e4629. [Google Scholar] [CrossRef]
- Heinrich, T.; Grädler, U.; Böttcher, H.; Blaukat, A.; Shutes, A. Allosteric IGF-1R Inhibitors. ACS Med. Chem. Lett. 2010, 1, 199–203. [Google Scholar] [CrossRef]
- Anderson, J.W.; Vaisar, D.; Jones, D.N.; Pegram, L.M.; Vigers, G.P.; Chen, H.; Moffat, J.G.; Ahn, N.G. Conformation Selection by ATP-Competitive Inhibitors and Allosteric Communication in ERK2. eLife 2024, 12, RP91507. [Google Scholar] [CrossRef]
- Hanan, E.J.; Braun, M.G.; Heald, R.A.; Macleod, C.; Chan, C.; Clausen, S.; Edgar, K.A.; Eigenbrot, C.; Elliott, R.; Endres, N.; et al. Discovery of GDC-0077 (Inavolisib), a Highly Selective Inhibitor and Degrader of Mutant PI3Kα. J. Med. Chem. 2022, 65, 16589–16621. [Google Scholar] [CrossRef]
- Gong, G.Q.; Bilanges, B.; Allsop, B.; Masson, G.R.; Roberton, V.; Askwith, T.; Oxenford, S.; Madsen, R.R.; Conduit, S.E.; Bellini, D.; et al. A Small-Molecule PI3Kα Activator for Cardioprotection and Neuroregeneration. Nature 2023, 618, 159–168. [Google Scholar] [CrossRef]
- Abraham, M.; Alekseenko, A.; Basov, V.; Bergh, C.; Briand, E.; Brown, A.; Doijade, M.; Fiorin, G.; Fleischmann, S.; Gorelov, S.; et al. GROMACS 2024.0 Manual. Zenodo 2024. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field (CGenFF): A Force Field for Drug-like Molecules Compatible with the CHARMM All-Atom Additive Biological Force Fields. J. Comput. Chem. 2010, 31, 671. [Google Scholar] [CrossRef]
- Valdés-Tresanco, M.S.; Valdés-Tresanco, M.E.; Valiente, P.A.; Moreno, E. Gmx_MMPBSA: A New Tool to Perform End-State Free Energy Calculations with GROMACS. J. Chem. Theory Comput. 2021, 17, 6281–6291. [Google Scholar] [CrossRef] [PubMed]
- Kitao, A. Principal Component Analysis and Related Methods for Investigating the Dynamics of Biological Macromolecules. J 2022, 5, 298–317. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and Its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef]
- Tsao, R.; Li, H. Antioxidant Properties in vitro and in vivo: Realistic Assessments of Efficacy of Plant Extracts. CAB Rev. Perspect. Agric. Vet. Sci. Nutr. Nat. Resour. 2012, 7, 1–9. [Google Scholar] [CrossRef]
- Aykul, S.; Martinez-Hackert, E. Determination of Half-Maximal Inhibitory Concentration Using Biosensor-Based Protein Interaction Analysis. Anal. Biochem. 2016, 508, 97. [Google Scholar] [CrossRef]
- Odriozola-Serrano, I.; Bellí, G.; Puigpinós, J.; Herrero, E.; Martín-Belloso, O. Screening the Antioxidant Activity of Thermal or Non-Thermally Treated Fruit Juices by In vitro and In vivo Assays. Beverages 2022, 8, 36. [Google Scholar] [CrossRef]
- Gulcin, İ.; Alwasel, S.H. DPPH Radical Scavenging Assay. Processes 2023, 11, 2248. [Google Scholar] [CrossRef]
- Ganzon, M.A.D.; Tsai, P.W.; Tayo, L.L.; Hsueh, C.C.; Yang, Y.S.; Chen, B.Y. Electrochemical Analysis and in silico Molecular Docking with Tropomyosin-Related Kinase B Reveals Electron Shuttles-Mediated Neuroprotective Potential of Mandarin Orange Pu-Erh Tea Extracts. Food Biosci. 2025, 63, 105625. [Google Scholar] [CrossRef]
- Tsai, P.W.; Chen, B.Y.; Yang, L.L. Interactive Deciphering Electron-Mediating Characteristics of Rheum Species and Potential Bioenergy-Steered Anti-COVID-19 RdRp Inhibitor. J. Taiwan. Inst. Chem. Eng. 2023, 151, 105124. [Google Scholar] [CrossRef]
- Moreddu, R. Nanotechnology and Cancer Bioelectricity: Bridging the Gap Between Biology and Translational Medicine. Adv. Sci. 2023, 11, 2304110. [Google Scholar] [CrossRef] [PubMed]
- Chaurawal, N.; Misra, C.; Abul Barkat, H.; Jatyan, R.; Chitkara, D.; Barkat, M.A.; Sharma, T.; Singh, B.; Raza, K. Oral Sorafenib-Loaded Microemulsion for Breast Cancer: Evidences from the in-Vitro Evaluations and Pharmacokinetic Studies. Sci. Rep. 2022, 12, 13746. [Google Scholar] [CrossRef] [PubMed]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK Pathway for Cancer Therapy: From Mechanism to Clinical Studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- Adv, J.; Oncol, P.; Wilhoit, T.; Patrick, J.M.; May, M.B. Alpelisib: A Novel Therapy for Patients With PIK3CA-Mutated Metastatic Breast Cancer. J. Adv. Pract. Oncol. 2020, 11, 768. [Google Scholar] [CrossRef]
- Farrar, M.C.; Jacobs, T.F. Tamoxifen. In Encyclopedia of Toxicology, Fourth Edition: Volume 1–9; Wexler, P., Ed.; Academic Press: Cambridge, MA, USA, 2023; Volume 8, pp. V8-923–V8-927. [Google Scholar] [CrossRef]
- Youssef, M.E.; Cavalu, S.; Hasan, A.M.; Yahya, G.; Abd-Eldayem, M.A.; Saber, S. Role of Ganetespib, an HSP90 Inhibitor, in Cancer Therapy: From Molecular Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2023, 24, 5014. [Google Scholar] [CrossRef]
- Mulvihill, M.J.; Cooke, A.; Rosenfeld-Franklin, M.; Buck, E.; Foreman, K.; Landfair, D.; Oconnor, M.; Pirritt, C.; Sun, Y.; Yao, Y.; et al. Discovery of OSI-906: A Selective and Orally Efficacious Dual Inhibitor of the IGF-1 Receptor and Insulin Receptor. Future Med. Chem. 2009, 1, 1153–1171. [Google Scholar] [CrossRef]
- Aier, I.; Varadwaj, P.K.; Raj, U. Structural Insights into Conformational Stability of Both Wild-Type and Mutant EZH2 Receptor. Sci. Rep. 2016, 6, 34984. [Google Scholar] [CrossRef]
- Rampogu, S.; Lee, G.; Park, J.S.; Lee, K.W.; Kim, M.O. Molecular Docking and Molecular Dynamics Simulations Discover Curcumin Analogue as a Plausible Dual Inhibitor for SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 1771. [Google Scholar] [CrossRef]
- Borjian Boroujeni, M.; Shahbazi Dastjerdeh, M.; Shokrgozar, M.A.; Rahimi, H.; Omidinia, E. Computational Driven Molecular Dynamics Simulation of Keratinocyte Growth Factor Behavior at Different PH Conditions. Inform. Med. Unlocked 2021, 23, 100514. [Google Scholar] [CrossRef]
- Ali, S.; Hassan, M.; Islam, A.; Ahmad, F. A Review of Methods Available to Estimate Solvent-Accessible Surface Areas of Soluble Proteins in the Folded and Unfolded States. Curr. Protein Pept. Sci. 2014, 15, 456–476. [Google Scholar] [CrossRef]
- Scaletti, C.; Russell, P.P.S.; Hebel, K.J.; Rickard, M.M.; Boob, M.; Danksagmüller, F.; Taylor, S.A.; Pogorelov, T.V.; Gruebele, M. Hydrogen Bonding Heterogeneity Correlates with Protein Folding Transition State Passage Time as Revealed by Data Sonification. Proc. Natl. Acad. Sci. USA 2024, 121, e2319094121. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes. Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Smolarz, B.; Zadrożna Nowak, A.; Romanowicz, H. Breast Cancer—Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Moon, J.; Kitty, I.; Renata, K.; Qin, S.; Zhao, F.; Kim, W. DNA Damage and Its Role in Cancer Therapeutics. Int. J. Mol. Sci. 2023, 24, 4741. [Google Scholar] [CrossRef]
- Martins, S.G.; Zilhão, R.; Thorsteinsdóttir, S.; Carlos, A.R. Linking Oxidative Stress and DNA Damage to Changes in the Expression of Extracellular Matrix Components. Front. Genet. 2021, 12, 673002. [Google Scholar] [CrossRef]
- Lin, M.-W.; Lee, C.-C.; Bel’skaya, L.V.; Dyachenko, E.I. Oxidative Stress in Breast Cancer: A Biochemical Map of Reactive Oxygen Species Production. Curr. Issues Mol. Biol. 2024, 46, 4646–4687. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.G. Breast Cancer: An Overview of Current Therapeutic Strategies, Challenge, and Perspectives. Breast Cancer Targets Ther. 2023, 15, 721. [Google Scholar] [CrossRef]
- Tsai, P.W.; Mailem, R.C.; Tayo, L.L.; Hsueh, C.C.; Tseng, C.C.; Chen, B.Y. Interactive Network Pharmacology and Electrochemical Analysis Reveals Electron Transport-Mediating Characteristics of Chinese Medicine Formula Jing Guan Fang. J. Taiwan. Inst. Chem. Eng. 2023, 147, 104898. [Google Scholar] [CrossRef]
- Rejano, C.J.F.; Chen, B.Y.; Sobremisana, G.S.; Tayo, L.L.; Wang, K.T.; Tsai, P.W. Electrochemical Analysis via Microbial Fuel Cells Reveals Electron-Stimulating Characteristics, Immunomodulation and Antiviral Properties of Ji Qin Yin. J. Taiwan. Inst. Chem. Eng. 2023, 152, 105193. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, D.; Gao, Y.; Ying, L.; Huang, Q.; Xu, P. Chemical Characterization and Bioactivity of Phenolics from Tieguanyin Oolong Tea. J. Food Biochem. 2019, 43, e12894. [Google Scholar] [CrossRef]
- Mataraci Karakas, S.; Yilmaz, A.; Mercantepe, T.; Topcu, A.; Pinarbas, E. Positive Effects of White Tea on Breast Cancer: N-Methyl-N-Nitrozourea Intraductal Induced Breast Carcinoma Model. J. Funct. Foods 2024, 122, 106462. [Google Scholar] [CrossRef]
- Zhao, N.; Kong, H.; Liu, H.; Shi, Q.; Qi, X.; Chen, Q. A Network Pharmacology Approach to Evaluate the Synergistic Effect of Dihydromyricetin and Myricitrin in Vine Tea on the Proliferation of B16F10 Cells. Front. Nutr. 2022, 9, 993133. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mukhtar, H. Tea and Health: Studies in Humans. Curr. Pharm. Des. 2013, 19, 6141. [Google Scholar] [CrossRef] [PubMed]
- Atwi-Ghaddar, S.; Zerwette, L.; Destandau, E.; Lesellier, E. Supercritical Fluid Extraction (SFE) of Polar Compounds from Camellia sinensis Leaves: Use of Ethanol/Water as a Green Polarity Modifier. Molecules 2023, 28, 5485. [Google Scholar] [CrossRef]
- Rohadi; Lelita, D.I.; Putri, A.S. Antioxidant Capacity of White Tea (Camelia Sinensis) Extract: Compared to Green, Oolong and Black Tea. IOP Conf. Ser. Earth Environ. Sci. 2019, 292, 012018. [Google Scholar] [CrossRef]
- Šeremet, D.; Karlović, S.; Vojvodić Cebin, A.; Mandura, A.; Ježek, D.; Komes, D. Extraction of Bioactive Compounds from Different Types of Tea by High Hydrostatic Pressure. J. Food Process Preserv. 2021, 45, e15751. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of Extraction Solvent on Total Phenol Content, Total Flavonoid Content, and Antioxidant Activity of Limnophila Aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef]
- Nguyen, N.Q.; Nguyen, M.T.; Nguyen, V.T.; Le, V.M.; Trieu, L.H.; Le, X.T.; Khang, T.V.; Giang, N.T.L.; Thach, N.Q.; Hung, T.T. The Effects of Different Extraction Conditions on the Polyphenol, Flavonoids Components and Antioxidant Activity of Polyscias Fruticosa Roots. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 022067. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent Advances in Anticancer Activities and Drug Delivery Systems of Tannins. Med. Res. Rev. 2017, 37, 665–701. [Google Scholar] [CrossRef]
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed Tannins as Antioxidants in Ruminants—Effectiveness and Action Mechanisms to Improve Animal Antioxidant Status and Oxidative Stability of Products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef] [PubMed]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as Anticancer Agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Abotaleb, M.; Samuel, S.M.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in Cancer and Apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important Flavonoids and Their Role as a Therapeutic Agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The Molecular Basis of Working Mechanism of Natural Polyphenolic Antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-Antioxidant Activity Relationship of Methoxy, Phenolic Hydroxyl, and Carboxylic Acid Groups of Phenolic Acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.C.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A.M. Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Lin, C.C.; Li, C.W.; Shih, Y.T.; Chuang, L. Te Antioxidant and Anti-Inflammatory Properties of Lower-Polymerized Polyphenols in Oolong Tea. Int. J. Food Prop. 2014, 17, 752–764. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Mol. J. Synth. Chem. Nat. Prod. Chem. 2018, 23, 965. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Patanè, G.T.; Putaggio, S.; Tellone, E.; Barreca, D.; Ficarra, S.; Maffei, C.; Calderaro, A.; Laganà, G. Catechins and Proanthocyanidins Involvement in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 9228. [Google Scholar] [CrossRef] [PubMed]
- Al Qaisi, Y.; Alfarrayeh, I.; Alsarayreh, A.; Khleifat, K.; Abu-Nwas, N. Assessment of Antioxidant Potential, Cytotoxicity, and Anticancer Activity of Methanolic Extracts from Selected Wild Medicinal Plants. Phytomedicine Plus 2024, 4, 100534. [Google Scholar] [CrossRef]
- Mittal, A.; Pate, M.S.; Wylie, R.C.; Tollefsbol, T.O.; Katiyar, S.K. EGCG Down-Regulates Telomerase in Human Breast Carcinoma MCF-7 Cells, Leading to Suppression of Cell Viability and Induction of Apoptosis. Int. J. Oncol. 2004, 24, 703–710. [Google Scholar] [CrossRef]
- Toden, S.; Tran, H.-M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A.; Toden, S.; Tran, H.-M.; Tovar-Camargo, O.A.; Okugawa, Y.; Goel, A. Epigallocatechin-3-Gallate Targets Cancer Stem-like Cells and Enhances 5-Fluorouracil Chemosensitivity in Colorectal Cancer. Oncotarget 2016, 7, 16158–16171. [Google Scholar] [CrossRef]
- Afsar, T.; Trembley, J.H.; Salomon, C.E.; Razak, S.; Khan, M.R.; Ahmed, K. Growth Inhibition and Apoptosis in Cancer Cells Induced by Polyphenolic Compounds of Acacia Hydaspica: Involvement of Multiple Signal Transduction Pathways. Sci. Rep. 2016, 6, 23077. [Google Scholar] [CrossRef]
- Wang, T.K.; Xu, S.; Fan, Y.; Wu, J.; Wang, Z.; Chen, Y.; Zhang, Y. The Synergistic Effect of Proanthocyanidin and HDAC Inhibitor Inhibit Breast Cancer Cell Growth and Promote Apoptosis. Int. J. Mol. Sci. 2023, 24, 10476. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Wang, Y.M.; Ng, I.S.; Liu, S.Q.; Hung, J.Y. Deciphering Simultaneous Bioelectricity Generation and Dye Decolorization Using Proteus Hauseri. J. Biosci. Bioeng. 2012, 113, 502–507. [Google Scholar] [CrossRef]
- Ohgitani, E.; Shin-Ya, M.; Ichitani, M.; Kobayashi, M.; Takihara, T.; Kawamoto, M.; Kinugasa, H.; Mazda, O. Significant Inactivation of SARS-CoV-2 In vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins. Molecules 2021, 26, 3572. [Google Scholar] [CrossRef]
- Farías-Campomanes, A.M.; Rostagno, M.A.; Coaquira-Quispe, J.J.; Meireles, M.A.A. Supercritical Fluid Extraction of Polyphenols from Lees: Overall Extraction Curve, Kinetic Data and Composition of the Extracts. Bioresour. Bioprocess. 2015, 2, 45. [Google Scholar] [CrossRef]
- Ibrahim Alghamdi, A. Antibacterial Activity of Green Tea Leaves Extracts against Specific Bacterial Strains. J. King Saud. Univ. Sci. 2023, 35, 102650. [Google Scholar] [CrossRef]
- Debbarma, S.; Acharya, A.; Mangang, Y.A.; Monsang, S.J.; Choudhury, T.G.; Parhi, J.; Pandey, P.K. Immune-Biochemical Response and Immune Gene Expression Profiling of Labeo Rohita Fingerlings Fed with Ethanolic Tea Leaf Extracts and Its Survivability against Aeromonas Hydrophila Infection. Fish. Shellfish. Immunol. 2022, 130, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Kuo, I.P.; Liu, C.S.; Yang, S.D.; Hu, Y.F.; Chu, Y.T.; Nan, F.H. Anti-Discoloration Effect of Phytochemicals Mixture Extracted from Mango Leaf (Mangifera indica), Guava Leaf (Psidium guajava), and Green Tea Residue (Camellia sinensis Var. Sinensis Cv. Chin-Shin Dah-Pang) on Stored Nile Tilapia (Oreochromis niloticus) Fillets. Aquac. Rep. 2023, 33, 101818. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Q.; Li, H.; Qiu, Z.; Yu, Y. Comparative Assessment of the Antibacterial Efficacies and Mechanisms of Different Tea Extracts. Foods 2022, 11, 620. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Hsu, C.F. Characterisation of Polyphenols in Green, Oolong, and Black Teas, and in Coffee, Using Cyclic Voltammetry. Food Chem. 2003, 82, 501–512. [Google Scholar] [CrossRef]
- Guo, L.L.; Qin, L.J.; Xu, B.; Wang, X.Z.; Hsueh, C.C.; Chen, B.Y. Deciphering Electron-Shuttling Characteristics of Epinephrine and Dopamine for Bioenergy Extraction Using Microbial Fuel Cells. Biochem. Eng. J. 2019, 148, 57–64. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, P.; Liu, W.; Zhang, M.; Guan, J.; Qin, X.; Zhong, J.; Fang, J.; Cui, G.; Liu, X. Electrochemical Sensitive Detection of Cannabidiol Based on Carbon Black/Gold Nanoparticle Composite Modified Glassy Carbon Electrode. Int. J. Electrochem. Sci. 2023, 18, 100347. [Google Scholar] [CrossRef]
- Neffati, R.; Brokken-Zijp, J.M.C. Structure and Porosity of Conductive Carbon Blacks. Mater. Chem. Phys. 2021, 260, 124177. [Google Scholar] [CrossRef]
- Roginsky, V.; Barsukova, T.; Hsu, C.F.; Kilmartin, P.A. Chain-Breaking Antioxidant Activity and Cyclic Voltammetry Characterization of Polyphenols in a Range of Green, Oolong, and Black Teas. J. Agric. Food Chem. 2003, 51, 5798–5802. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Begines, E.; Heredia, F.J.; Escudero-Gilete, M.L.; Hernanz, D. Effectiveness of Cyclic Voltammetry in Evaluation of the Synergistic Effect of Phenolic and Amino Acids Compounds on Antioxidant Activity: Optimization of Electrochemical Parameters. Foods 2024, 13, 906. [Google Scholar] [CrossRef]
- Spiegel, M.; Cel, K.; Sroka, Z. The Mechanistic Insights into the Role of PH and Solvent on Antiradical and Prooxidant Properties of Polyphenols—Nine Compounds Case Study. Food Chem. 2023, 407, 134677. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Manefield, M.; Lee, M.; Kouzuma, A. Electron Shuttles in Biotechnology. Curr. Opin. Biotechnol. 2009, 20, 633–641. [Google Scholar] [CrossRef]
- Xu, B.; Lan, J.C.; Sun, Q.; Hsueh, C.; Chen, B.Y. Deciphering Optimal Biostimulation Strategy of Supplementing Anthocyanin-Abundant Plant Extracts for Bioelectricity Extraction in Microbial Fuel Cells. Biotechnol. Biofuels 2019, 12, 46. [Google Scholar] [CrossRef]
- Attal, R.; Bakkar, A.; Bouillaud, F.; Devin, A.; Henry, M.; Pontié, M.; Radman, M.; Schwartz, L. From Electrons to Cancer: Redox Shift as a Driving Force of Tumorigenesis. Adv. Redox Res. 2024, 10, 100087. [Google Scholar] [CrossRef]
- Cardenas, C.; Lovy, A.; Silva-Pavez, E.; Urra, F.; Mizzoni, C.; Ahumada-Castro, U.; Bustos, G.; Jaňa, F.; Cruz, P.; Farias, P.; et al. Cancer Cells with Defective Oxidative Phosphorylation Require Endoplasmic Reticulum-to-Mitochondria Ca2+ transfer for Survival. Sci. Signal 2020, 13, eaay1212. [Google Scholar] [CrossRef]
- Masatoshi, H.; Shigeo, I.; Toshio, T.; Kazuo, N.; Masaaki, I.; Hiroyoshi, H. Differential Effects of Flavonoids as Inhibitors of Tyrosine Protein Kinases and Serine/Threonine Protein Kinases. Biochem. Pharmacol. 1988, 37, 2987–2992. [Google Scholar] [CrossRef]
- Wright, B.; Watson, K.A.; McGuffin, L.J.; Lovegrove, J.A.; Gibbins, J.M. GRID and Docking Analyses Reveal a Molecular Basis for Flavonoid Inhibition of Src Family Kinase Activity. J. Nutr. Biochem. 2015, 26, 1156–1165. [Google Scholar] [CrossRef]
- Wright, B.; Moraes, L.A.; Kemp, C.F.; Mullen, W.; Crozier, A.; Lovegrove, J.A.; Gibbins, J.M. A Structural Basis for the Inhibition of Collagen-Stimulated Platelet Function by Quercetin and Structurally Related Flavonoids. Br. J. Pharmacol. 2010, 159, 1312–1325. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.S.; Kang, N.J.; Bode, A.M.; Dong, Z.; et al. Myricetin Suppresses UVB-Induced Wrinkle Formation and MMP-9 Expression by Inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.a. New Insights into the Mechanisms of Polyphenols beyond Antioxidant Properties; Lessons from the Green Tea Polyphenol, Epigallocatechin 3-Gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Lee, J.O.; Jeong, D.; Kim, M.Y.; Cho, J.Y. ATP-Binding Pocket-Targeted Suppression of Src and Syk by Luteolin Contributes to Its Anti-Inflammatory Action. Mediators Inflamm. 2015, 2015, 967053. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Liu, N.; Zeng, Y.; Xiao, Z.; Wu, K.; Yang, F.; Li, B.; Song, Q.; Xiao, Y.; Liu, Q. Luteolin Ameliorates Experimental Pulmonary Arterial Hypertension via Suppressing Hippo-YAP/PI3K/AKT Signaling Pathway. Front. Pharmacol. 2021, 12, 663551. [Google Scholar] [CrossRef] [PubMed]
- Singh Tuli, H.; Rath, P.; Chauhan, A.; Sak, K.; Aggarwal, D.; Choudhary, R.; Sharma, U.; Vashishth, K.; Sharma, S.; Kumar, M.; et al. Luteolin, a Potent Anticancer Compound: From Chemistry to Cellular Interactions and Synergetic Perspectives. Cancers 2022, 14, 5373. [Google Scholar] [CrossRef]
- Hayakawa, D.; Takahashi, F.; Mitsuishi, Y.; Tajima, K.; Hidayat, M.; Winardi, W.; Ihara, H.; Kanamori, K.; Matsumoto, N.; Asao, T.; et al. Activation of Insulin-like Growth Factor-1 Receptor Confers Acquired Resistance to Osimertinib in Non-Small Cell Lung Cancer with EGFR T790M Mutation. Thorac. Cancer 2020, 11, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Cianfruglia, L.; Laudadio, E.; Giovanna, M.; Galeazzi, R.; Armeni, T. Effect of Epigallocatechin-3-Gallate on Egfr Signaling and Migration in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2021, 22, 11833. [Google Scholar] [CrossRef]
- Sakata, R.; Ueno, T.; Nakamura, T.; Sakamoto, M.; Torimura, T.; Sata, M. Green Tea Polyphenol Epigallocatechin-3-Gallate Inhibits Platelet-Derived Growth Factor-Induced Proliferation of Human Hepatic Stellate Cell Line LI90. J. Hepatol. 2004, 40, 52–59. [Google Scholar] [CrossRef]
- Tudoran, O.; Soritau, O.; Balacescu, O.; Balacescu, L.; Braicu, C.; Rus, M.; Gherman, C.; Virag, P.; Irimie, F.; Berindan-Neagoe, I. Early Transcriptional Pattern of Angiogenesis Induced by EGCG Treatment in Cervical Tumour Cells. J. Cell Mol. Med. 2012, 16, 520. [Google Scholar] [CrossRef]
- Carrington, E.M.; Zhan, Y.; Brady, J.L.; Zhang, J.G.; Sutherland, R.M.; Anstee, N.S.; Schenk, R.L.; Vikstrom, I.B.; Delconte, R.B.; Segal, D.; et al. Anti-Apoptotic Proteins BCL-2, MCL-1 and A1 Summate Collectively to Maintain Survival of Immune Cell Populations Both in vitro and in vivo. Cell Death Differ. 2017, 24, 878–888. [Google Scholar] [CrossRef]
- Wu, X.; Chen, S.; Lu, C. Amyloid Precursor Protein Promotes the Migration and Invasion of Breast Cancer Cells by Regulating the MAPK Signaling Pathway. Int. J. Mol. Med. 2019, 45, 162. [Google Scholar] [CrossRef]
- Mortenson, M.M.; Galante, J.G.; Gilad, O.; Schlieman, M.G.; Virudachalam, S.; Kung, H.J.; Bold, R.J. BCL-2 Functions as an Activator of the AKT Signaling Pathway in Pancreatic Cancer. J. Cell Biochem. 2007, 102, 1171–1179. [Google Scholar] [CrossRef]
- Wang, S.H.; Wu, C.H.; Tsai, C.C.; Chen, T.Y.; Tsai, K.J.; Hung, C.M.; Hsu, C.Y.; Wu, C.W.; Hsieh, T.H. Effects of Luteolin on Human Breast Cancer Using Gene Expression Array: Inferring Novel Genes. Curr. Issues Mol. Biol. 2022, 44, 2107. [Google Scholar] [CrossRef] [PubMed]
- Rahman, N.; Khan, H.; Zia, A.; Khan, A.; Fakhri, S.; Aschner, M.; Gul, K.; Saso, L. Bcl-2 Modulation in P53 Signaling Pathway by Flavonoids: A Potential Strategy towards the Treatment of Cancer. Int. J. Mol. Sci. 2021, 22, 11315. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Song, T.; Li, C.; Mao, W. GSK-3β in DNA Repair, Apoptosis, and Resistance of Chemotherapy, Radiotherapy of Cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Research 2020, 1867, 118659. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Kise, Y.; Miki, H. GSK3β Positively Regulates Hedgehog Signaling through Sufu in Mammalian Cells. Biochem. Biophys. Res. Commun. 2007, 353, 501–508. [Google Scholar] [CrossRef]
- Papadopoli, D.; Pollak, M.; Topisirovic, I. The Role of GSK3 in Metabolic Pathway Perturbations in Cancer. Biochim. Biophys. Acta (BBA)-Mol. Cell Research 2021, 1868, 119059. [Google Scholar] [CrossRef]
- Predes, D.; Maia, L.A.; Matias, I.; Araujo, H.P.M.; Soares, C.; Barros-Aragão, F.G.Q.; Oliveira, L.F.S.; Reis, R.R.; Amado, N.G.; Simas, A.B.C.; et al. The Flavonol Quercitrin Hinders GSK3 Activity and Potentiates the Wnt/β-Catenin Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 12078. [Google Scholar] [CrossRef]
- Schmidt, S.; Lange, C.A. 8516 Role of ER, PR, IRS-1 Transcriptional Complexes in Endocrine Resistant Luminal Breast Cancer. J. Endocr. Soc. 2024, 8, bvae163-1824. [Google Scholar] [CrossRef]
- Murray, J.I.; West, N.R.; Murphy, L.C.; Watson, P.H. Intratumoural Inflammation and Endocrine Resistance in Breast Cancer. Endocr. Relat. Cancer 2015, 22, R51–R67. [Google Scholar] [CrossRef]
- Pegram, M.; Jackisch, C.; Johnston, S.R.D. Estrogen/HER2 Receptor Crosstalk in Breast Cancer: Combination Therapies to Improve Outcomes for Patients with Hormone Receptor-Positive/HER2-Positive Breast Cancer. npj Breast Cancer 2023, 9, 45. [Google Scholar] [CrossRef]
- Sulaiman, A.; Sulaiman, B.; Khouri, L.; McGarry, S.; Nessim, C.; Arnaout, A.; Li, X.; Addison, C.; Dimitroulakos, J.; Wang, L. Both Bulk and Cancer Stem Cell Subpopulations in Triple-Negative Breast Cancer Are Susceptible to Wnt, HDAC, and ERα Coinhibition. FEBS Lett. 2016, 590, 4606–4616. [Google Scholar] [CrossRef]
- Haase, M.; Fitze, G. HSP90AB1: Helping the Good and the Bad. Gene 2016, 575, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Oladapo, A.; Deshetty, U.M.; Callen, S.; Buch, S.; Periyasamy, P. Single-Cell RNA-Seq Uncovers Robust Glial Cell Transcriptional Changes in Methamphetamine-Administered Mice. Int. J. Mol. Sci. 2025, 26, 649. [Google Scholar] [CrossRef]
- Markaverich, B.M.; Shoulars, K.; Rodriguez, M.A. Luteolin Regulation of Estrogen Signaling and Cell Cycle Pathway Genes in MCF-7 Human Breast Cancer Cells. Int. J. Biomed. Sci. 2011, 7, 101. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Gong, X.; Wen, X.; Gu, X. Luteolin: Anti-Breast Cancer Effects and Mechanisms. J. Explor. Res. Pharmacol. 2018, 3, 85–90. Available online: http://www.xiahepublishing.com/ (accessed on 1 January 2025). [CrossRef]
- Shi, X.K.; Peng, T.; Azimova, B.; Li, X.L.; Li, S.S.; Cao, D.Y.; Fu, N.J.; Zhang, G.L.; Xiao, W.L.; Wang, F. Luteolin and Its Analog Luteolin-7-Methylether from Leonurus Japonicus Houtt Suppress Aromatase-Mediated Estrogen Biosynthesis to Alleviate Polycystic Ovary Syndrome by the Inhibition of Tumor Progression Locus 2. J. Ethnopharmacol. 2024, 331, 118279. [Google Scholar] [CrossRef]
- Van Der Woude, H.; Ter Veld, M.G.R.; Jacobs, N.; Van Der Saag, P.T.; Murk, A.J.; Rietjens, I.M.C.M. The Stimulation of Cell Proliferation by Quercetin Is Mediated by the Estrogen Receptor. Mol. Nutr. Food Res. 2005, 49, 763–771. [Google Scholar] [CrossRef]
- Cesari, I.M.; Carvalho, E.; Figueiredo Rodrigues, M.; Mendonça, B.D.S.; Amôedo, N.D.; Rumjanek, F.D. Methyl Jasmonate: Putative Mechanisms of Action on Cancer Cells Cycle, Metabolism, and Apoptosis. Int. J. Cell Biol. 2014, 2014, 572097. [Google Scholar] [CrossRef]
- Van Duursen, M.B.M. Modulation of Estrogen Synthesis and Metabolism by Phytoestrogens in vitro and the Implications for Women’s Health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef]
- Lee, C.C.; Huang, C.C.; Hsu, K. Sen Insulin Promotes Dendritic Spine and Synapse Formation by the PI3K/Akt/MTOR and Rac1 Signaling Pathways. Neuropharmacology 2011, 61, 867–879. [Google Scholar] [CrossRef]
- Poljsak, B. Strategies for Reducing or Preventing the Generation of Oxidative Stress. Oxid. Med. Cell Longev. 2011, 2011, 194586. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell Longev. 2015, 2016, 6475624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, L.; Wang, A.; Liu, J.; Huang, X.; Zan, T. Positive Feedback Loops between Fibroblasts and the Mechanical Environment Contribute to Dermal Fibrosis. Matrix Biol. 2023, 121, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a Versatile Component in Tissue Engineering. Acta Biomater. 2021, 119, 57–74. [Google Scholar] [CrossRef]
- Piras, A.R.; Ariu, F.; Maltana, A.; Leoni, G.G.; Martino, N.A.; Mastrorocco, A.; Dell’Aquila, M.E.; Bogliolo, L. Protective Effect of Resveratrol against Cadmium-Induced Toxicity on Ovine Oocyte in vitro Maturation and Fertilization. J. Anim. Sci. Biotechnol. 2022, 13, 83. [Google Scholar] [CrossRef]
- Huang, M.; Liu, C.; Shao, Y.; Zhou, S.; Hu, G.; Yin, S.; Pu, W.; Yu, H. Anti-Tumor Pharmacology of Natural Products Targeting Mitosis. Cancer Biol. Med. 2022, 19, 774. [Google Scholar] [CrossRef]
- Proença, C.; Freitas, M.; Ribeiro, D.; Oliveira, E.F.T.; Sousa, J.L.C.; Tomé, S.M.; Ramos, M.J.; Silva, A.M.S.; Fernandes, P.A.; Fernandes, E. α-Glucosidase Inhibition by Flavonoids: An in vitro and in silico Structure–Activity Relationship Study. J. Enzym. Inhib. Med. Chem. 2017, 32, 1216–1228. [Google Scholar] [CrossRef]
- Chen, D.; Oezguen, N.; Urvil, P.; Ferguson, C.; Dann, S.M.; Savidge, T.C. Regulation of Protein-Ligand Binding Affinity by Hydrogen Bond Pairing. Sci. Adv. 2016, 2, e1501240. [Google Scholar] [CrossRef]
- Itoh, Y.; Nakashima, Y.; Tsukamoto, S.; Kurohara, T.; Suzuki, M.; Sakae, Y.; Oda, M.; Okamoto, Y.; Suzuki, T. N+-C-H···O Hydrogen Bonds in Protein-Ligand Complexes. Sci. Rep. 2019, 9, 767. [Google Scholar] [CrossRef]
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure–Activity Relationship—A Quantum Chemical Study. Antioxidants 2020, 9, 461. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.; Gu, H.; Wei, D.; Xu, Y.C.; Fu, W.; Yu, Z. Conformational Preferences of π-π Stacking Between Ligand and Protein, Analysis Derived from Crystal Structure Data Geometric Preference of π-π Interaction. Interdiscip. Sci. 2015, 7, 211–220. [Google Scholar] [CrossRef]
- Arthur, D.E.; Uzairu, A. Molecular Docking Studies on the Interaction of NCI Anticancer Analogues with Human Phosphatidylinositol 4,5-Bisphosphate 3-Kinase Catalytic Subunit. J. King Saud. Univ. Sci. 2019, 31, 1151–1166. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Yu, R. Unraveling the Therapeutic Mechanism of Saussurea Involucrata against Rheumatoid Arthritis: A Network Pharmacology and Molecular Modeling-Based Investigation. Nutrients 2023, 15, 4294. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, C.; Xia, J.; Li, N.; Xiong, W. Luteolin Is a Potential Inhibitor of COVID-19: An in silico Analysis. Medicine 2023, 102, e35029. [Google Scholar] [CrossRef]
- Mallari, P.; Bhat, M.A. Amentoflavone from Selaginella Bryopteris Leaf Extracts Modulate MAPK Signaling—Molecular Modeling Study for Therapeutic Target Analysis. Phytomedicine Plus 2025, 5, 100759. [Google Scholar] [CrossRef]
- Sulaimani, M.N.; Ahmed, S.; Anjum, F.; Mohammad, T.; Shamsi, A.; Dohare, R.; Hassan, M.I. Structure-Guided Identification of Mitogen-Activated Protein Kinase-1 Inhibitors towards Anticancer Therapeutics. PLoS ONE 2025, 20, e0311954. [Google Scholar] [CrossRef]
- Sasidharan, S.; Radhakrishnan, K.; Lee, J.Y.; Saudagar, P.; Gosu, V.; Shin, D. Molecular Dynamics of the ERRγ Ligand-Binding Domain Bound with Agonist and Inverse Agonist. PLoS ONE 2023, 18, e0283364. [Google Scholar] [CrossRef]
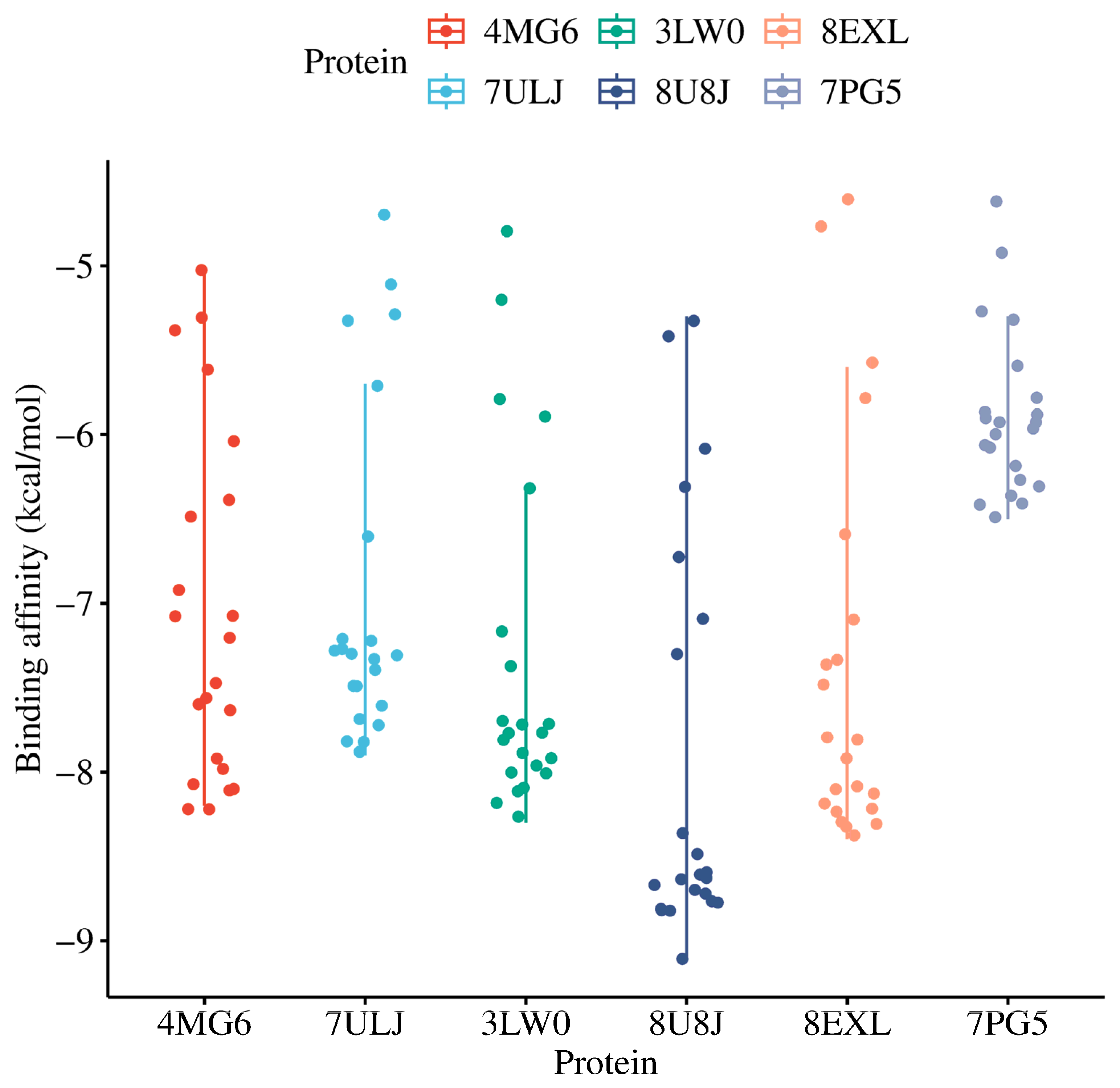
| Hub Gene | PDB ID | Coordinates | Resolution | Ref | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| ESR1 | 4MG6 | 22.3896 | 5.54 | 5.3394 | 2.10 Å | [67] |
| HSP90AB1 | 7ULJ | −22.3339 | 100.8248 | 4.0096 | 1.82 Å | [68] |
| IGF1R | 3LW0 | −5.6064 | −22.6792 | −53.4945 | 1.79 Å | [69] |
| MAPK1 | 8U8J | 10.5209 | 14.9872 | 16.18 | 2.10 Å | [70] |
| PIK3CA | 8EXL | −18.0216 | 13.4051 | 27.3344 | 1.99 Å | [71] |
| PIK3R1 | 7PG5 | −56.7456 | −21.2754 | 43.3115 | 2.20 Å | [72] |
| Extract | TPC Analysis (GAE mg/g) | TFC Analysis (RE mg/g) | TCTC Analysis (CE mg/g) |
|---|---|---|---|
| OTL-E | 352.226 ± 0.017 | 72.6407 ± 0.005 | 428.884 ± 0.012 |
| OTL-W | 249.497 ± 0.005 | 34.6878 ± 0.002 | 9.39799 ± 0.0002 |
| OTS-W | 273.433 ± 0.006 | 33.6675 ± 0.001 | 304.518 ± 0.001 |
| Sample | DPPH IC50 (mg/mL) | FRAP (Trolox mg/g) |
|---|---|---|
| OTL-E | 0.07282 ± 0.006 | 177.604 ± 0.590 |
| OTL-W | 0.10907 ± 0.006 | 142.21 ± 0.957 |
| OTS-W | 0.099131 ± 0.002 | 150.14 ± 1.978 |
| Ascorbic Acid | 0.0591 ± 0.002 |
| Test Sample | OTL-W | OTL-E | OTS-W | OTS-E |
|---|---|---|---|---|
| Blank 1 | 9.882 ± 0.4941.00±0.10 | 11.193 ± 0.7981.00±0.1411 | 7.604 ± 0.4711.00±0.12 | 7.150 ± 0.3651.00±0.10 |
| 250 ppm | 16.349 ± 1.1381.65±0.20 | 23.500 ± 4.7572.10±0.57 | 8.477 ± 1.5091.11±0.27 | 11.636 ± 1.5091.63±0.29 |
| 500 ppm | 18.946 ± 1.7411.92±0.27 | 22.158 ± 4.7171.98±0.56 | 9.404 ± 1.4141.24±0.26 | 12.363 ± 1.4141.73±0.29 |
| 750 ppm | 20.129 ± 1.5072.04±0.25 | 17.459 ± 2.4461.56±0.33 | 10.617 ± 1.4261.40±0.27 | 12.874 ± 1.4261.80±0.29 |
| 1000 ppm | 21.369 ± 1.8752.16±0.30 | 16.001 ± 2.3851.43±0.31 | - | 13.614 ± 1.4751.90±0.30 |
| 1500 ppm | 22.166 ± 2.0262.24±0.32 | 15.091 ± 2.0241.35±0.28 | - | 14.961 ± 1.4462.09±0.31 |
| 2000 ppm | 24.374 ± 4.1132.47±0.54 | 14.376 ± 1.9631.28±0.27 | - | - |
| Blank 2 | 12.070 ± 0.5061.22±0.11 | 9.929 ± 0.7620.89±0.13 | 7.488 ± 0.3090.98±0.10 | 8.051 ± 0.4301.13±0.12 |
| Dopamine | 33.531 ± 2.0893.39±0.38 | 29.407 ± 2.9752.63±0.45 | 24.496 ± 2.3233.22±0.51 | 20.473 ± 1.6612.86±0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer, R.A.; Chen, B.-Y.; Garcia, J.P.T.; Rejano, C.J.F.; Tsai, P.-W.; Hsueh, C.-C.; Tayo, L.L. Deciphering the Regulatory Potential of Antioxidant and Electron-Shuttling Bioactive Compounds in Oolong Tea. Biology 2025, 14, 487. https://doi.org/10.3390/biology14050487
Ferrer RA, Chen B-Y, Garcia JPT, Rejano CJF, Tsai P-W, Hsueh C-C, Tayo LL. Deciphering the Regulatory Potential of Antioxidant and Electron-Shuttling Bioactive Compounds in Oolong Tea. Biology. 2025; 14(5):487. https://doi.org/10.3390/biology14050487
Chicago/Turabian StyleFerrer, Regineil A., Bor-Yann Chen, Jon Patrick T. Garcia, Christine Joyce F. Rejano, Po-Wei Tsai, Chung-Chuan Hsueh, and Lemmuel L. Tayo. 2025. "Deciphering the Regulatory Potential of Antioxidant and Electron-Shuttling Bioactive Compounds in Oolong Tea" Biology 14, no. 5: 487. https://doi.org/10.3390/biology14050487
APA StyleFerrer, R. A., Chen, B.-Y., Garcia, J. P. T., Rejano, C. J. F., Tsai, P.-W., Hsueh, C.-C., & Tayo, L. L. (2025). Deciphering the Regulatory Potential of Antioxidant and Electron-Shuttling Bioactive Compounds in Oolong Tea. Biology, 14(5), 487. https://doi.org/10.3390/biology14050487






