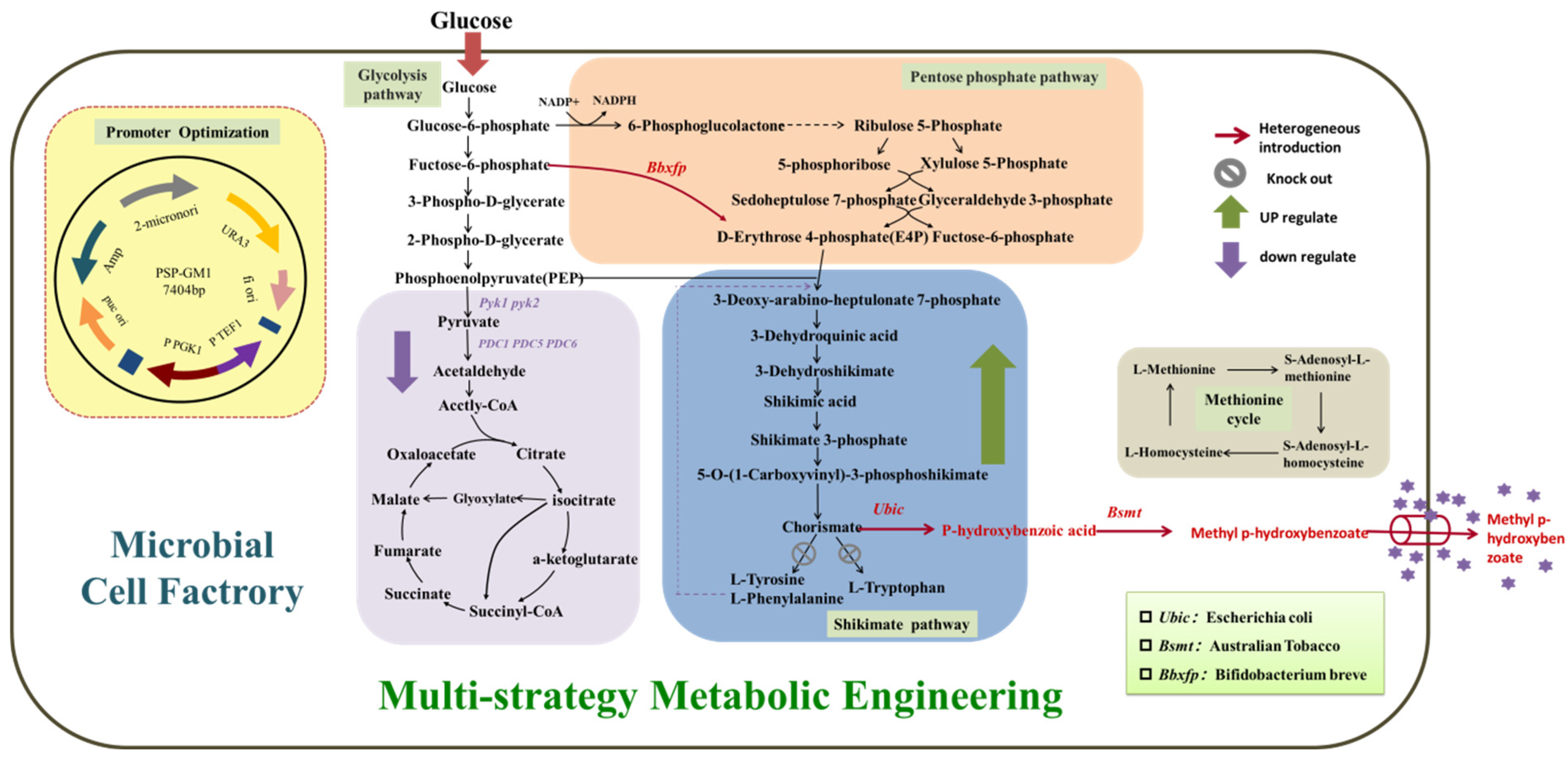
3.1. Construction of MP Synthesis Pathway
The key enzymes in the MP synthesis path that need to be constructed are chorismate lyase and benzoate carboxymethyltransferases. Ubic encoding the chorismate lyase from E. coli has been proven to be effective for the accumulation of P-HBA, which was expressed in S. cerevisiae BY4741 to construct the synthetic pathway. Bsmt encoding the benzoate carboxymethyltransferases from plants has been proven to be effective for the accumulation of MP. The engineered strains obtained by Bsmt from Australian tobacco, Arabidopsis, and Petunia co-overexpressed with Ubic were recorded as BYL-1, BYL-2, and BYL-3, respectively.
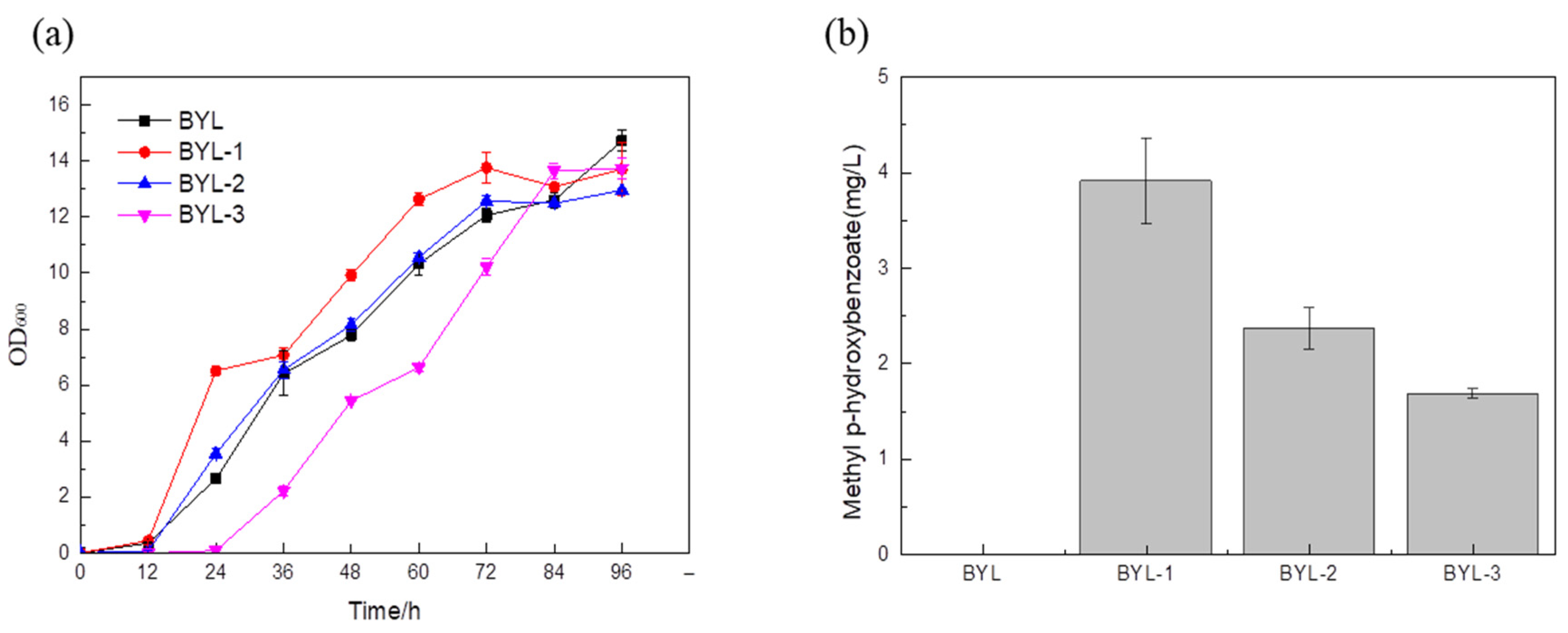
After fermentation for 96 h, the GC-MS detection of the recombinant BYL-1, BYL-2, and BYL-3 showed that a peak with a retention time of about 5.147 min was found in the fermentation broth, which was the same as the peak time of standard substances. Further, the NIST spectral library was used to retrieve the structural formula of the compounds at peak locations, as shown in the GC-MS detection results (
Supplementary data, Figure S1). The characteristic ion fragments 204 were found by comparing with the standard, and the results were searched through the NIST spectral library and compared with the structure of the standard to finally determine the retention time and mass spectrum of methylparaben and qualitatively analyze the target product.
Bsmt has been proven to be effective for the synthesis of MP, and three different sources encoding the benzoate carboxymethyltransferase gene were transformed into
S. cerevisiae BY4741 for expression to construct the MP biosynthetic pathway. The recombinant strain with the benzoate carboxymethyltransferase gene derived from
Australian tobacco was recorded as BYL-1, and an initial MP titer of 3.9 mg/L was obtained (
Figure 2).
3.2. Enhancement of the Carbon Flux Toward Shikimic Acid Pathway
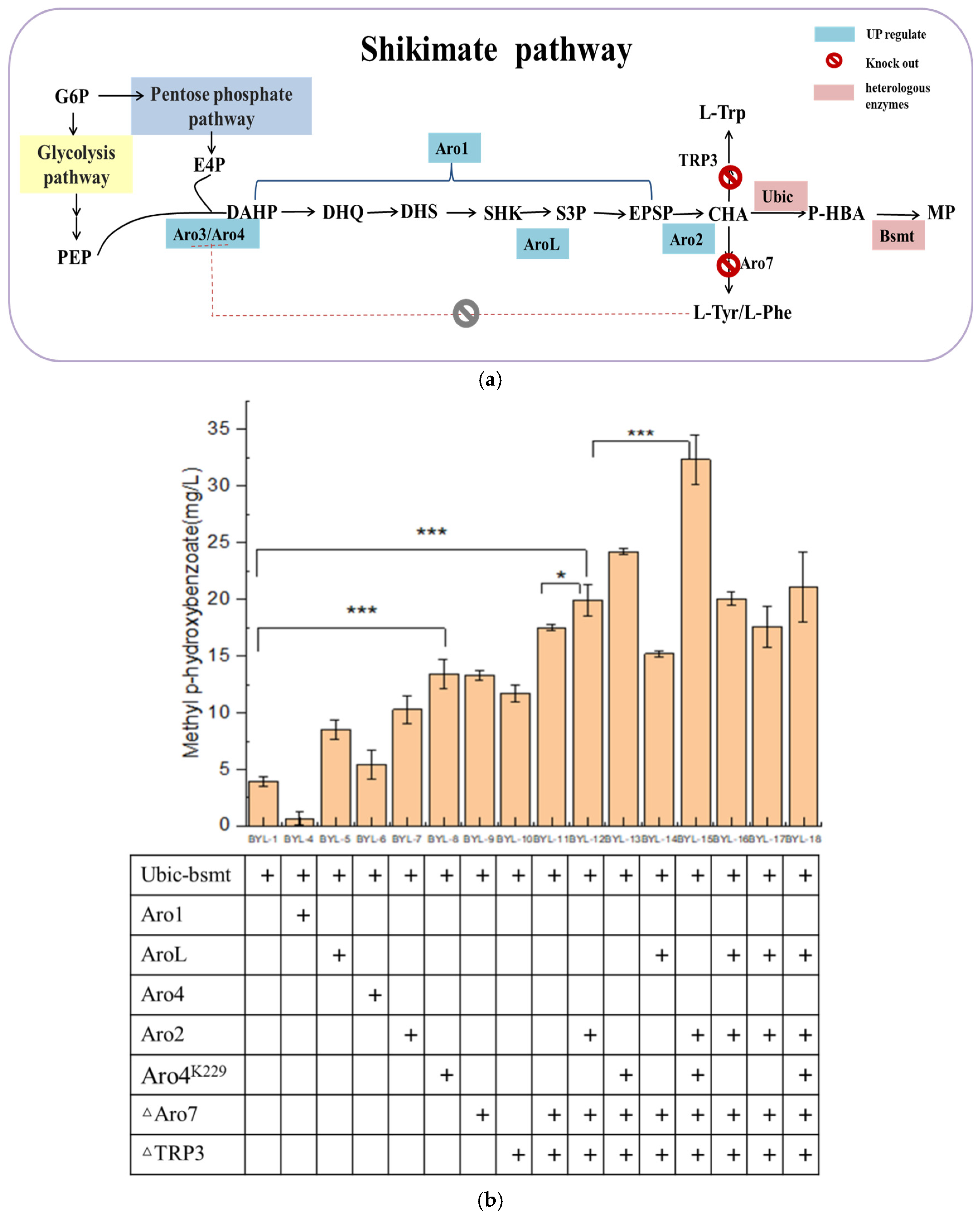
Since the carbon flux of the endogenous shikimate pathway in
Saccharomyces cerevisiae is low, it is necessary to increase the carbon flux of the shikimate pathway (
Figure 3a). Therefore, the key enzyme genes Aro4 and Aro2 (
Figure 3b) of the shikimate pathway were overexpressed to obtain strains BYL-6 and BYL-7, with MP titers of 5.4 mg/L and 10.29 mg/L, respectively. The L-Tyrosine and L-Phenylalanine produced downstream of the shikimate pathway give negative feedback on the DAHP synthase encoded by the Aro4 (
Figure 3b). It has been reported that the mutant Aro4(K229L) completely abolishes Tyr/Phe inhibition while maintaining >90% native activity in
S. cerevisiae. Therefore, the mutant Aro4(K229L) is thus overexpressed to explore its effect on the synthesis of MP. The MP titer is 13.4 mg/L by the strain BYL-8 expressing Aro4(K229L), which is about 2.43 times higher than that of the control strain [
15]. The introduction of shikimate kinase (encoded by AroL gene), which catalyzes shikimate (SHK) to shikimate-3-phosphate (S3P) in
E. coli, into
Saccharomyces cerevisiae has a certain effect on the production of aromatic amino acids. The AroL-overexpressed strain BYL-5 produced 8.509 mg/L of MP, which was about 1.06 times higher than that of the control strain BYL-1. The five-step reaction of 3-dehydroquinic acid (DHQ) to 5-enolpyruvate shikimate-3-phosphate (EPSP) is catalyzed by the pentafunctional enzyme Aro1 (
Figure 3a); thus, it is speculated that this enzyme plays an important role in the shikimate pathway. For the strain BYL-4 overexpressing Aro1 gene, the obtained MP titer was 0.67 mg/L, which was due to the fact that Aro1 encodes a five-functional polypeptide, which contains similar domains of five enzymes in
E. coli from DHQ to EPSP synthesis, whose structure is relatively large. Therefore, the Aro1 gene was constructed in the same plasmid with the Ubic and Bsmt genes at the same time, resulting in an increase in the vector burden. The gene transcription level is reduced, which affects the production of MP, or the Aro1 gene cannot be directly overexpressed in the plasmid; it may be necessary to further regulate the Aro1 gene at the genome level to increase its copy number to increase the carbon flux of the shikimate pathway.
Secondly, the biosynthetic pathway of the target product MP is based on CHA produced by the shikimate pathway as a precursor. MP is produced by introducing chorismate pyruvate lyase and benzoate carboxymethyltransferase at the same time, and the downstream pathway of chorismate mainly generates L- Aromatic amino acids such as Trp, L-Phe, and L-Tyr. To allow more carbon flux from CHA into the target product pathway, the chorismate dismutase encoded by the Aro7 gene and the anthranilate synthase encoded by the TRP3 gene were knocked out (
Figure 3a). According to the fermentation results, the simultaneous knockout of Aro7 and TRP3 has a better effect on the improvement of the target product, which is consistent with the reported results. The optimal
S. cerevisiae engineered strain BYL-15 for producing MP was determined by the optimal combination of overexpression of key enzyme genes of the shikimate pathway and the modification of the chassis cell. The titer of MP was 32.34 mg/L. Compared with BYL-11, it increased by about 84.7%, about 7.3 times higher than the original strain BYL-1, which completely proved that the enhancement of the shikimic acid pathway played an important role in the increase of the target product titer.
3.3. Improvement of Precursor Supply to Promote the Synthesis of MP
The production of MP is based on the endogenous shikimate pathway in
S. cerevisiae (
Figure 3a), while erythrose-4-phosphate (E4P) and phosphoenolpyruvate (PEP), as the direct precursors, significantly affect MP accumulation.
The precursor PEP of the shikimate pathway is mainly provided by the glycolytic pathway (
Figure 1). Due to the Crabtree effect of
S. cerevisiae, the pyruvate kinase converts most of the precursor PEP to pyruvate. Therefore, the main carbon flux of the PEP node is directed to pyruvate. It is then used for cell growth and ethanol fermentation, while the minor portion leads to the shikimate pathway. In the metabolic network, there are many enzymatic reactions around pyruvate and PEP, so controlling these reactions and maintaining cell growth will be an important step in the efficient synthesis of aromatic compounds [
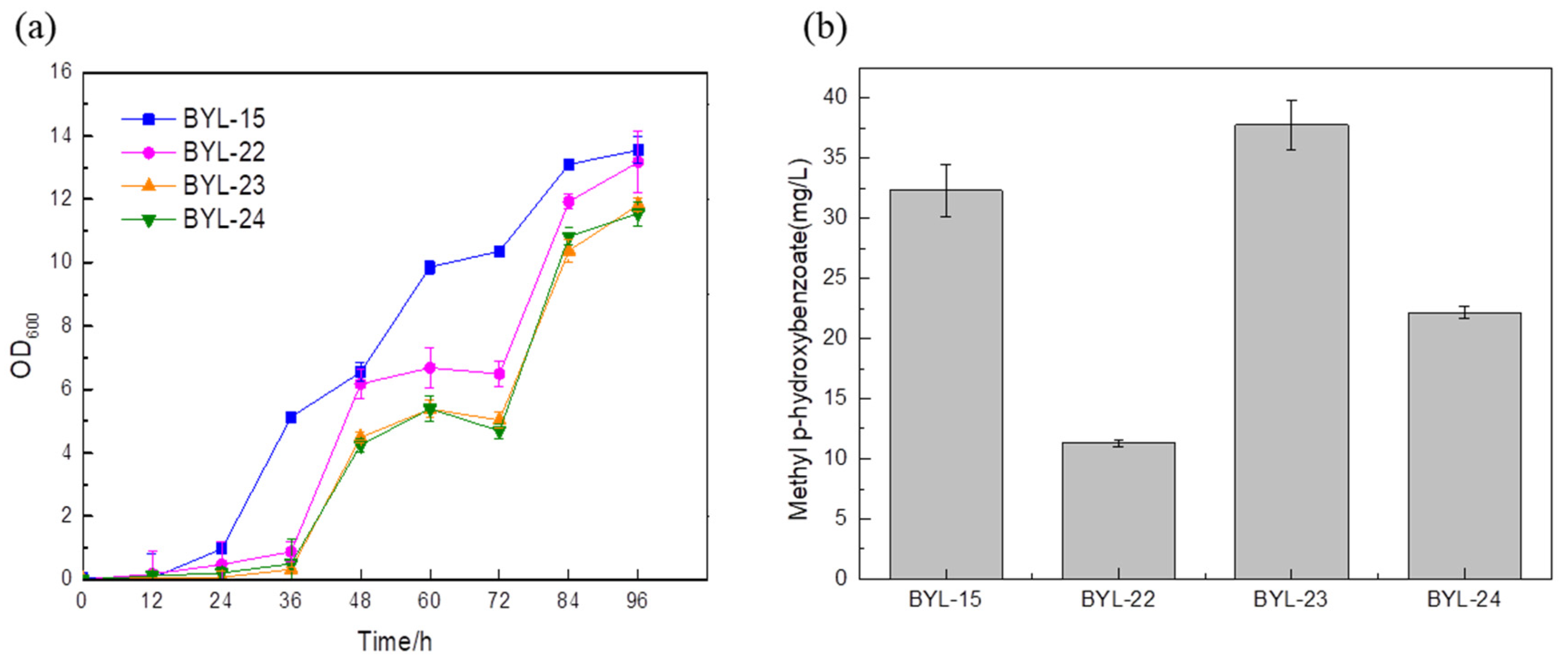
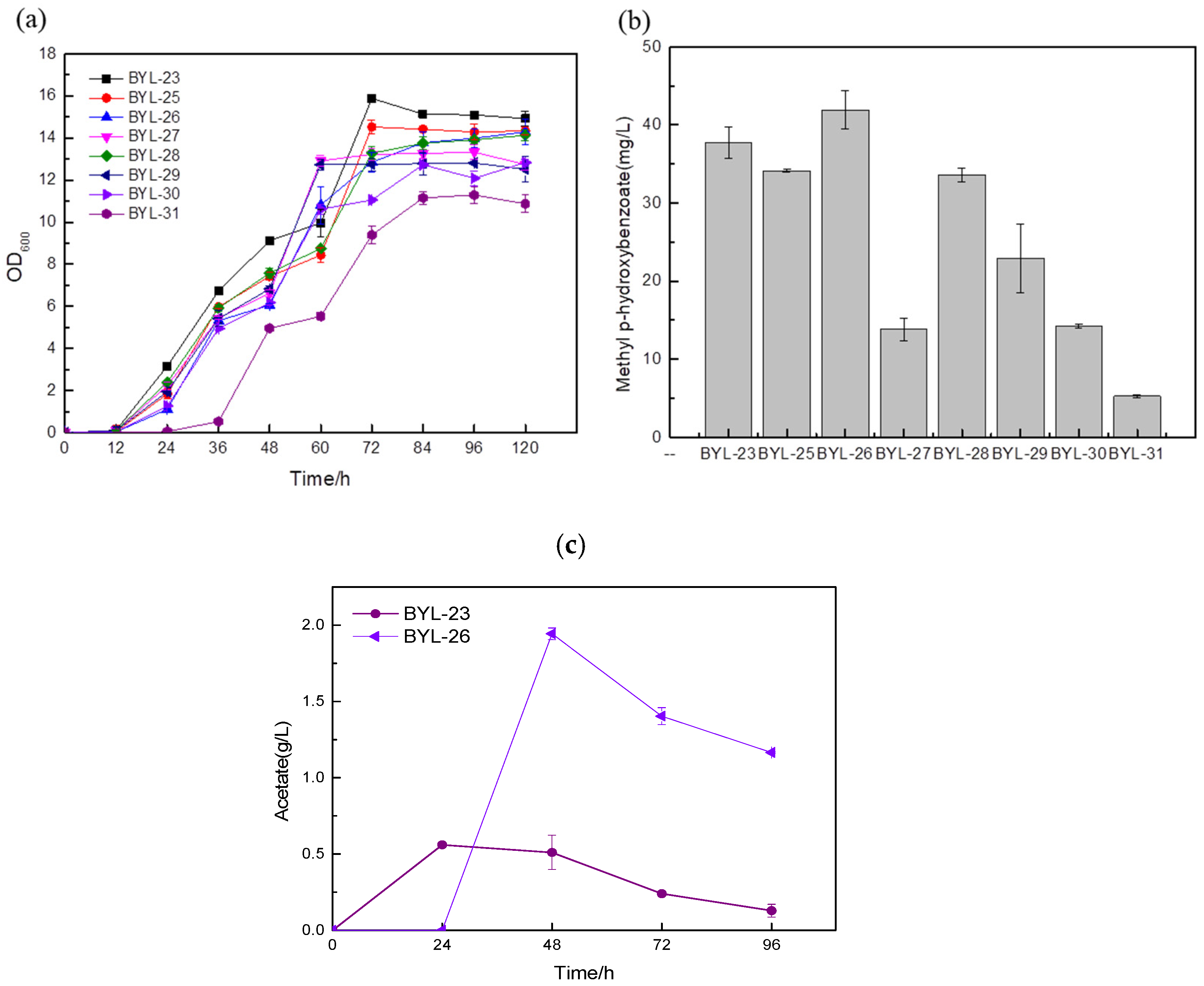
16]. In order to increase the carbon flux at the PEP node, efficiently make it lead to the shikimate pathway, and weaken the synthesis pathway from pyruvate to acetaldehyde, deficient strains were obtained by knocking out PDC1, PDC5, and PDC6 encoding pyruvate decarboxylase, respectively; these were BYL-22, BYL-23, and BYL-24. Taking strain BYL-15 as the control strain, it can be clearly observed that the biomass of the deficient strains was lower than that of the control strains, indicating that the activity of pyruvate decarboxylase was reduced. The weakened flux of the downstream energy supply module (TCA cycle) of glycolysis reduced the respiratory metabolism of cells and affected cell growth to some extent. The MP titers of strains BYL-22, BYL-23, and BYL-24 were calculated to be 11.3 mg/L, 37.75 mg/L, and 22.17 mg/L, respectively (
Figure 4b). Since pyruvate decarboxylase in
Saccharomyces cerevisiae is a protein encoded by three structural genes, PDC1, PDC5, and PDC6, in PDC1-deficient strains, in order to maintain normal cellular metabolism, the promoter on its nucleic acid sequence will regulate PDC6, and then express the fusion protein of PDC1-PDC6, but because PDC6 is a weakly expressed nucleic acid sequence, the activity of pyruvate kinase is greatly reduced [
17]; according to research, it was speculated that PDC6 has the ability to guide the transport of enzymes in the metabolic process. Therefore, knocking out PDC6 will affect the related metabolic regulatory system, which in turn affects other intracellular metabolic pathways and the balance of NAD+/NADH, because NADH and NAD+ play an important role in the biosynthetic pathway and metabolic process of aromatic compounds. Therefore, PDC1 and PDC6 gene-deficient strains not only exhibit certain effects on cell growth but also lead to a serious reduction in the titer of target compounds. In addition, it was clearly observed that the strain with only PDC5 knockout had a higher titer, which was about 16.73% higher than that of the control strain. The gradual increase in the biosynthetic pathway flux of aromatic compounds and their derivatives will lead to an increase in the content of by-products such as fuel compounds, affecting the specificity of benzoate carboxymethyltransferase binding. Since PDC5 has the same catalytic ability as phenylpyruvate decarboxylase Aro10 [
18], the deletion of PDC5 effectively blocked the aromatic amino acid degradation pathway, and the content of by-products was also significantly reduced, which was beneficial to the biosynthesis of methylparaben.
In
Saccharomyces cerevisiae, about 90% of the E4P produced by the endogenous PPP pathway enters the shikimate pathway, but it is only equivalent to 8% of the PEP content that enters the shikimate pathway; according to Guo W. et al. [
19], the enzyme that condenses PEP and E4P to form DAHP (DAHP synthase encoded by Aro3 and Aro4) shows a higher preference for the substrate PEP (
Figure 3a). Therefore, the severe imbalance of E4P and PEP carbon fluxes into the shikimate pathway and the availability of E4P are the main problems hindering the production of aromatic compounds. In this study, borrowing from the above phosphoketolase (PHK) pathway (
Figure 1), the phosphoketolase gene (
Bbxfp) derived from
Bifidobacterium breve was introduced, activated by seven constitutive promoters with different strengths (TEF1, TPI1, ADH1, ACS1, ALD5, CDC24, IDP1) and expressed to obtain highly expressed phosphoketolase. According to the biomass of the recombinant strains, it can be clearly observed that the growth status of the strains introduced with heterologous phosphoketolase is worse than that of the control strain BYL-23 because of a large amount of acetate produced (
Figure 5a). According to the research, acetyl-phosphate (Acp) is a by-product generated when Bbxfp catalyzes F6P in
Saccharomyces cerevisiae, which can be catalyzed to acetate by glycerophosphatase (GPP1) [
20]. This conclusion was also confirmed by the detection chart of acetic acid content (
Figure 5c); the accumulation of acetic acid seriously affects the growth of cells.
According to the comparative analysis of the fermentation results of the recombinant strains (b), the titers of strains BYL-30 and BYL-31 obtained by driving the Bbxfp with strong promoters TEF1p and IDP1p were 14.21 mg/L and 5.25 mg/L, respectively (
Figure 5b). The titers of the strains BYL-27, BYL-28, and BYL-29 obtained by the promoters ADH1p, ACS1p, and ALD5p driving the expression of Bbxfp were 13.84 mg/L, 33.61 mg/L, and 22.9 mg/L, respectively (
Figure 5b); the titer of the above five strains was significantly lower than that of the control strain BYL-23. The titer of BYL-25 and BYL-26 strains obtained by driving the expression of Bbxfp gene with weak promoters CDC24p and IDP1p were 34.11 mg/L and 41.91 mg/L, respectively (
Figure 5b), and the titer of BYL-25 was about 9.63% lower than that of the control strain. The decrease in the titer of the target product may be due to the reduction in biomass, which reduces the metabolites and energy required for the synthesis pathway, and cannot achieve the growth environment required for the target product; however, the titer of strain BYL-26 is 11% higher than that of the control strain. According to this analysis, the expression of Bbxfp driven by a weak promoter has a good effect on improving the titer of the target product. This is because strong promoters and medium-strength promoters drive gene transcription at a faster rate and because the glycolytic pathway also requires F6P to provide sufficient substrates for cell growth to maintain balance throughout the entire central carbon metabolism. A weak promoter drives the expression of heterologous phosphoketolase and slows its transcription rate, effectively increasing MP production.
3.4. Promoter Engineering to Regulate the Expression of MP Synthesis Pathway
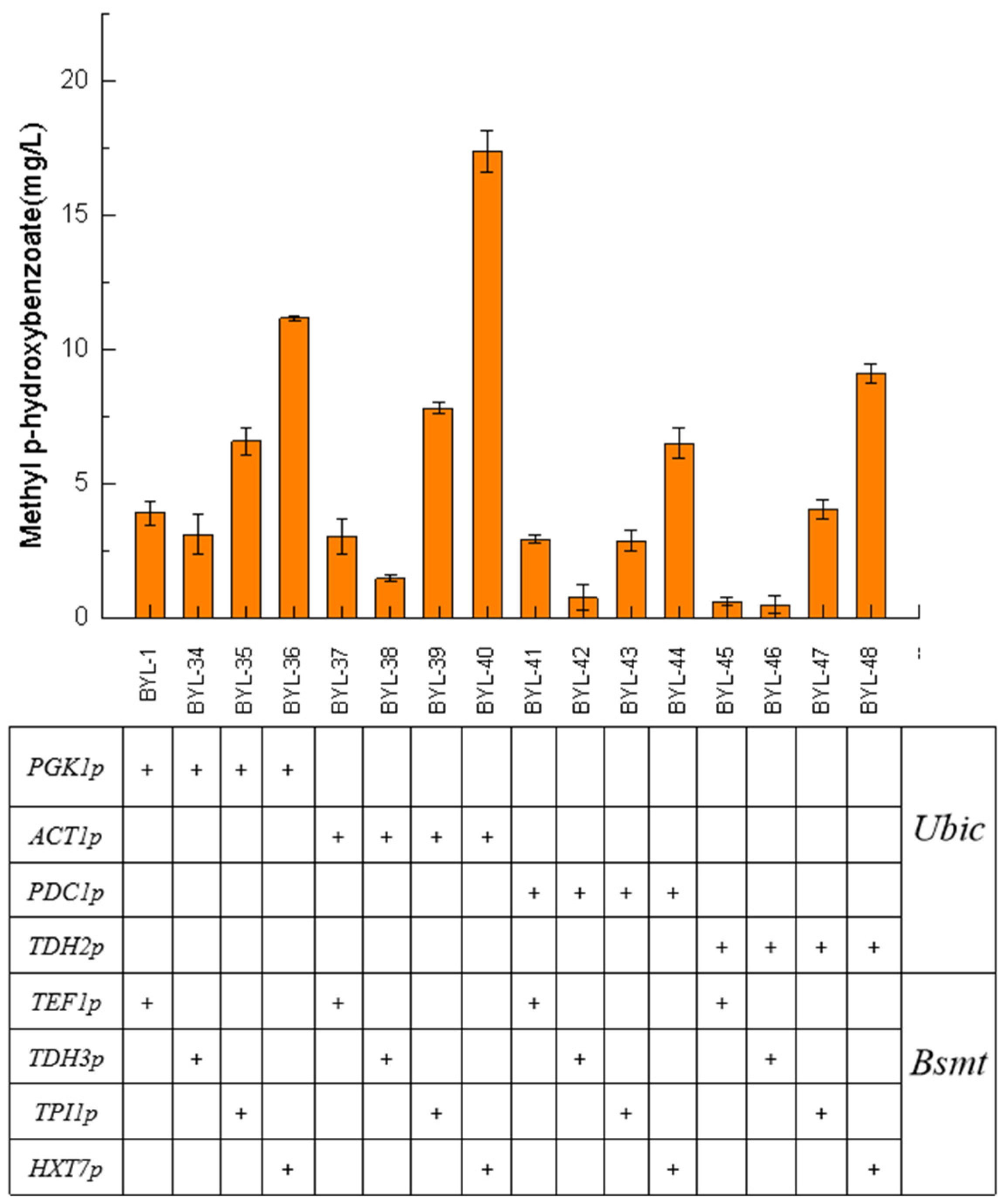
In synthetic biology research, promoters are the core biosynthetic elements that drive gene expression, regulate gene circuits, and obtain high enzymatic activity. Therefore, in order to achieve efficient and accurate expression of target genes, promoter engineering is one of the most critical strategies. The above experiments show that the accumulation of MP can be significantly promoted by enhancing the carbon flux of the shikimate pathway, knocking out the competitive pathway, and increasing the flux of the precursor. HBA had a high accumulation amount, but the content of the final product MP has not been greatly improved. It is speculated that Bsmt is the rate-limiting enzyme in this biosynthetic pathway. It was necessary to control the expression of Bsmt to match the supply of co-factor S-adenosyl-L-methionine (SAM). In this work, four constitutive promoters with different strengths were selected for the introduced Ubic and Bsmt, respectively, to screen for suitable promoter combination strategies.
Taking BYL-1 as the control strain, the strain BYL-40 obtained by combining the moderate-strength constitutive promoter ACT1p to activate the Ubic gene and the weak constitutive promoter HXT7p to activate the Bsmt-1 gene had the highest titer, about 17.39 mg/L, which was 3.46 times higher than that of the control strain (
Figure 6). From the titer comparison of the strains with different combinations of promoters, it was obvious that the driving of the Ubic gene required a strong promoter, and as the strength of the constitutive promoter gradually weakens, the titer of the target product gradually decreases; the driving of the Bsmt-1 gene required a weaker promoter, and as the strength of the constitutive promoter gradually weakened, the titer of the target product gradually increased. CHA was converted into P-HBA under the catalysis of the enzyme encoded by
Ubic, and the strong promoter drives the Ubic gene to make its transcription level reach a higher level. CHA was converted into P-HBA under the catalysis of the enzyme encoded by Ubic, and the strong promoter drives the Ubic gene to make its transcription level reach a high level. It can be inferred that the corresponding promoter did not drive the transcription of the Bsmt-1 gene until P-HBA reached a certain amount of accumulation. Since SAM was required to provide methyl groups in the pathway of MP synthesis, the rate of intracellular endogenous biosynthesis of SAM was slow, so the Bsmt-1 gene needed to be driven by a weak promoter to regulate the metabolic balance of intracellular substances and energy.
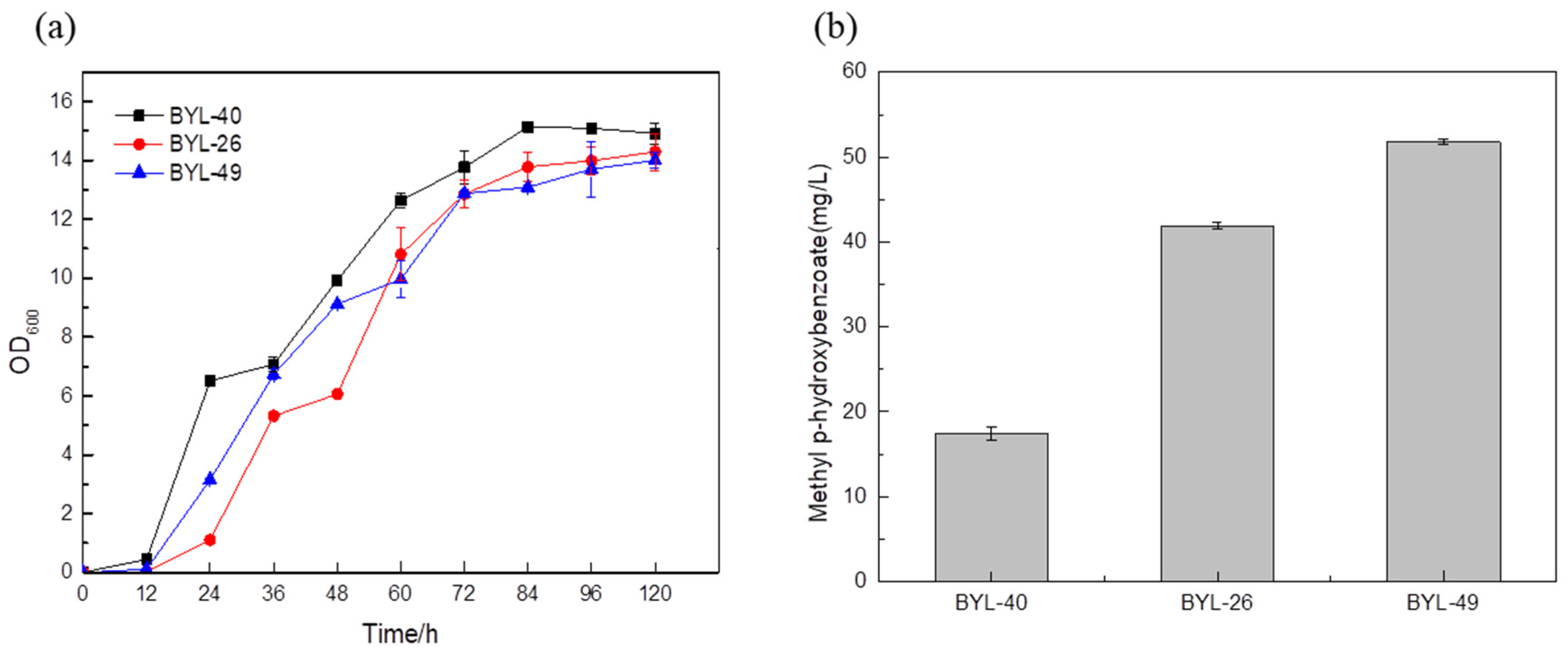
The MP titer reached 51.78 mg/L by the strain BYL-49 combining the optimized strain BYL-40 with the strain BYL-26 (
Figure 7b), which was about 23.56% higher than that of the control strain BYL-26, thus verifying that the promoter optimization had a good effect on the titer improvement.
3.5. Optimization of the Fermentation Process to Accelerate the Production of MP
The composition and content of the fermentation medium have an important impact on the titer of target products [
21,
22]. Therefore, it is necessary to optimize the conditions and medium during the fermentation process. In order to obtain more sufficient material conditions for the optimal strain, the composition and content of the fermentation medium were explored, and the main components of the fermentation medium were screened by designing orthogonal experiments and single-factor experiments. In this way, the best medium combination can be obtained to achieve the purpose of a high titer of methyl parahydroxybenzoate.
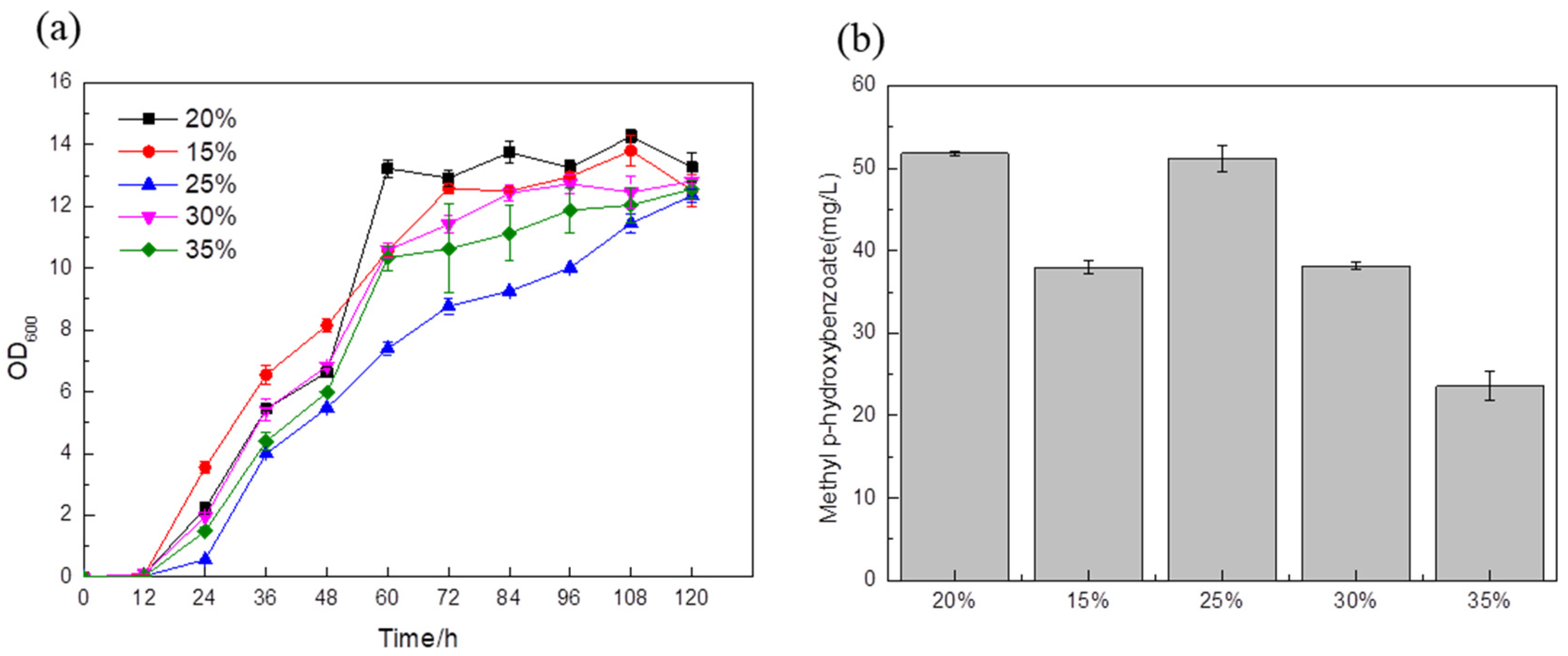
The optimal strain BYL-49 obtained above was used as the strain optimized for the medium. A 20% glucose supplementation in the medium was used as a blank control. A total of 15% glucose was added to the fermentation medium, and the titer was about 38 mg/L (
Figure 8b); between 0 and 48 h, the cells were in the exponential growth phase (
Figure 8a), and the biomass was highest. When the target substance begins to synthesize and accumulate, the strain begins to grow slowly, which also shows that in the early stage of fermentation, 15% glucose addition can fully provide the energy and substances required for cell growth, but the rapid growth of this process may cause more carbon flux to enter the downstream energy supply module of glycolysis, resulting in an unbalanced cellular metabolism and the inability to provide sufficient a carbon source for the synthesis of target products. With the addition of 25% glucose in the fermentation medium, the titer was about 51.18 mg/L (
Figure 8b), which had no obvious change with the blank control. The fermentation medium added 30% and 35% glucose while the titer was about 38.14 mg/L and 23.57 mg/L, respectively (
Figure 8b). It was clearly observed that no matter whether the amount of glucose added was too high or too low, the fermentation results showed adverse effects, and the glucose content of 20% and 25% was the most suitable for the cell growth and synthesis of target products.
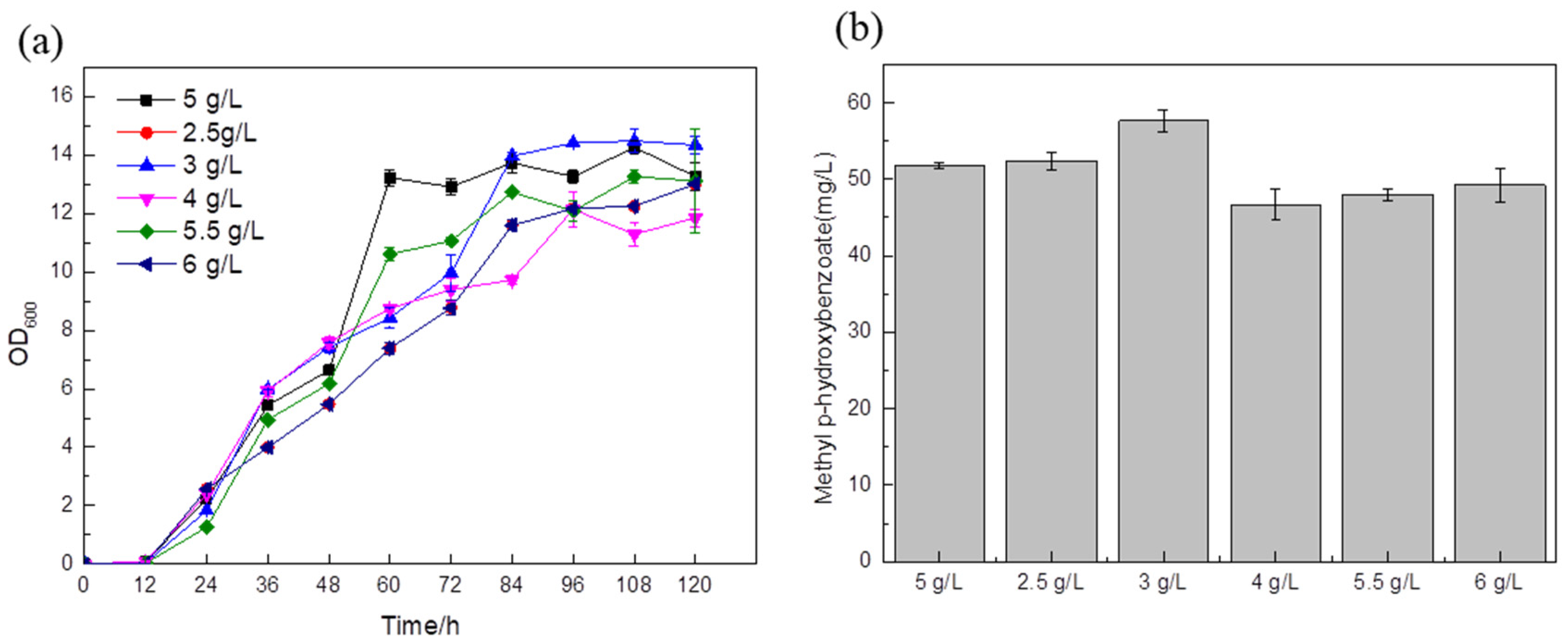
Taking the addition of 5 g/L (NH
4)
2SO
4 as the blank control, according to the fermentation results, when the amounts of (NH
4)
2SO
4 added were 4 g/L, 5.5 g/L, 6 g/L, the titer was slightly lower than that of the control strain (
Figure 9b). The titer of the strain with 3 g/L of ammonium sulfate in the medium was 57.63 mg/L (
Figure 9b), which was about 11.3% higher than that of the blank control. This shows that 3 g/L ammonium sulfate is the best condition for the growth of the
Saccharomyces cerevisiae strain. Since nitrogen sources can adjust the pH during the fermentation process, if there are too many nitrogen sources, the bacteria will grow vigorously and the pH of the fermentation broth will be high. Since
Saccharomyces cerevisiae prefers acidity, higher pH will affect cell growth and product accumulation; if the nitrogen source is too small, this will not only reduce the nutrients needed by the cells but may also make the medium acidic due to the fact that acidic metabolites produced during fermentation cannot reach the optimum pH for strain growth.
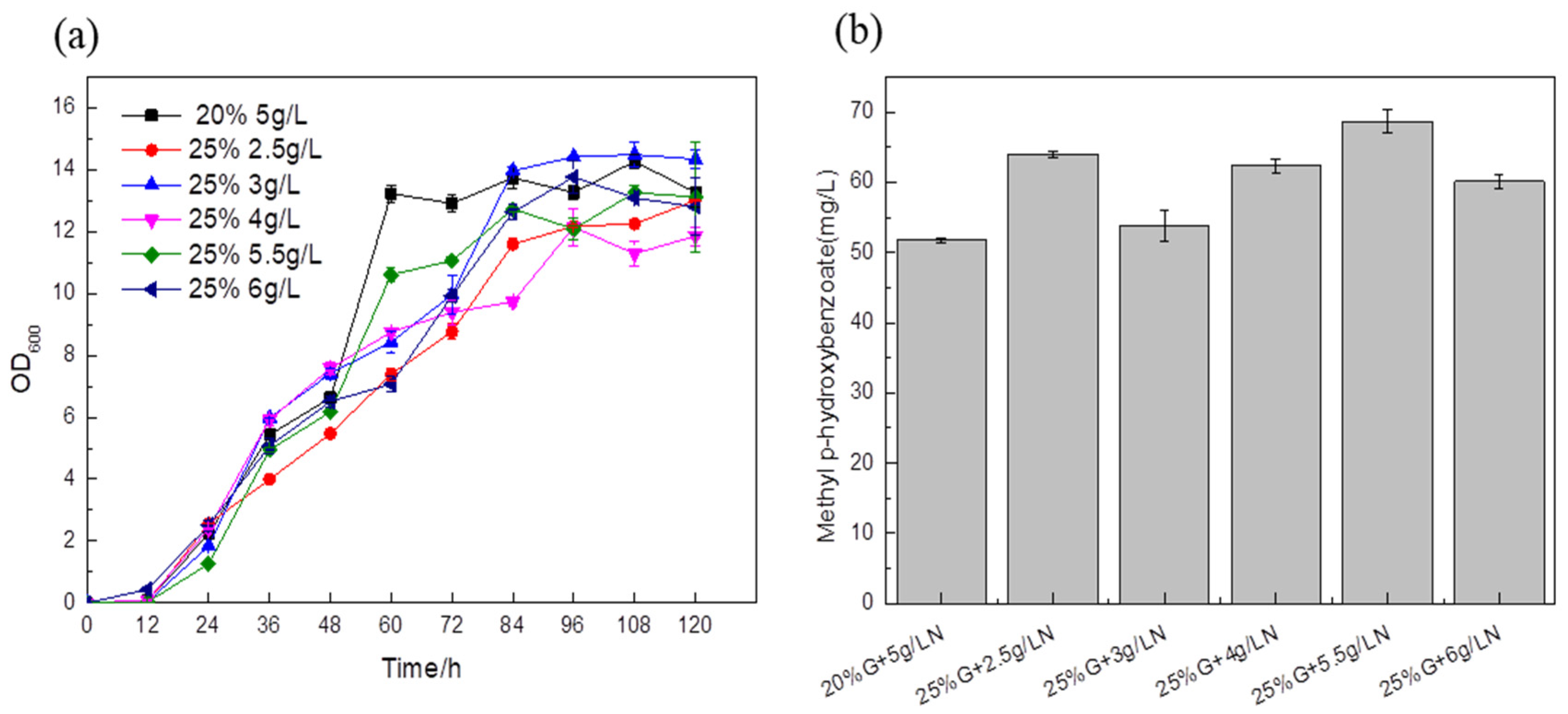
A total of 5 g/L ammonium sulfate and 20% glucose were added to the medium as a blank control to explore the effect of the carbon–nitrogen ratio on the titer. In the medium of the experimental group, the titers of the fermentation strains supplemented with 25% glucose and different ammonium sulfate were higher than those of the control strain, and the content of synthetic MP reached 68.59 mg/L, which was about 32.46% higher than that of the blank control (
Figure 10b). This shows that an inappropriate carbon–nitrogen ratio will affect the absorption of nutrients by
Saccharomyces cerevisiae, and directly affect the growth of
Saccharomyces cerevisiae and the formation of products. Therefore, the optimal carbon–nitrogen ratio in the fermentation medium is beneficial to the improvement of the target product titer.