Impact of Kalanchoe (Kalanchoe daigremontiana) Supplementation in Goat Maternal Diet on Hepatic and Renal Function and Reproductive Performance †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
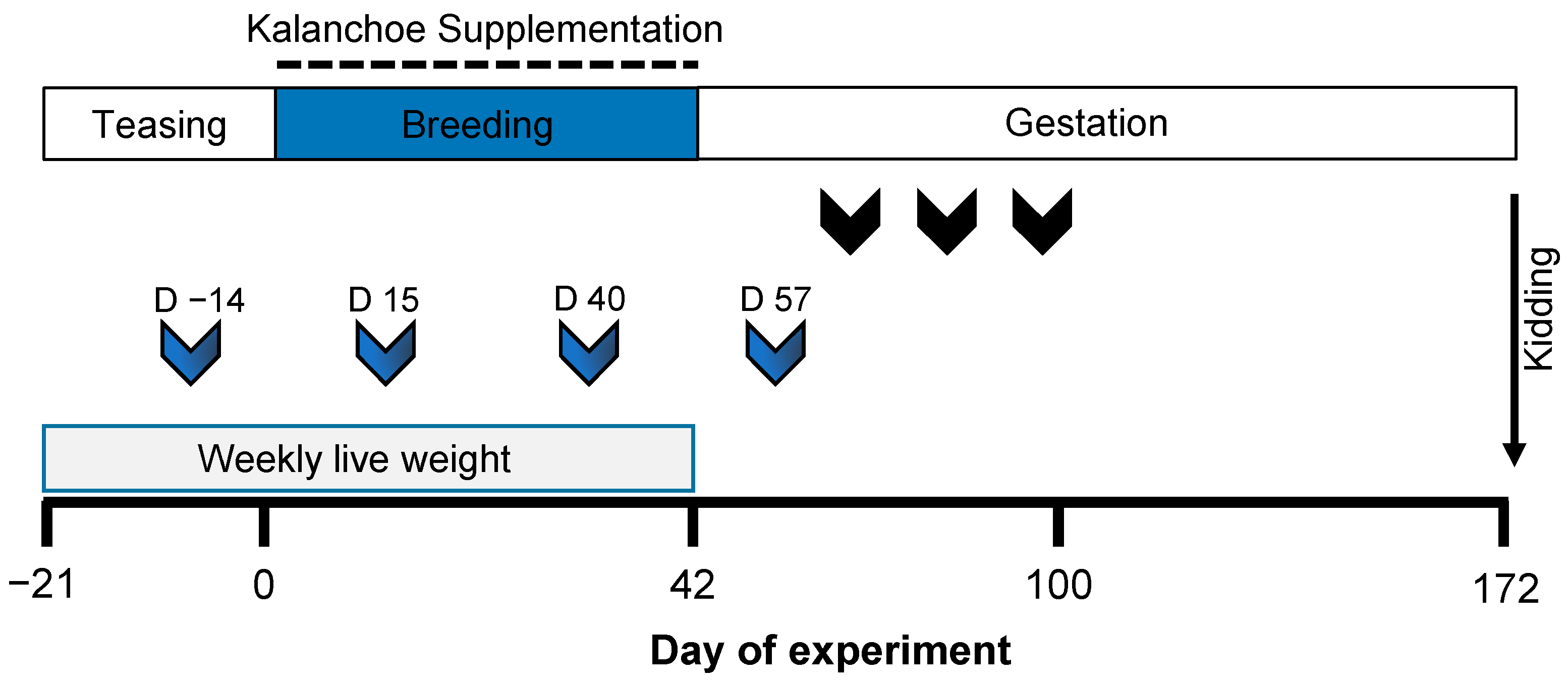
2.1. Experimental Design
2.2. Kalanchoe daigremontiana and Nutritional Diet
2.3. Reproductive Management and Variables
2.4. Plasma Metabolite Profiles
2.5. Statistical Analysis
3. Results
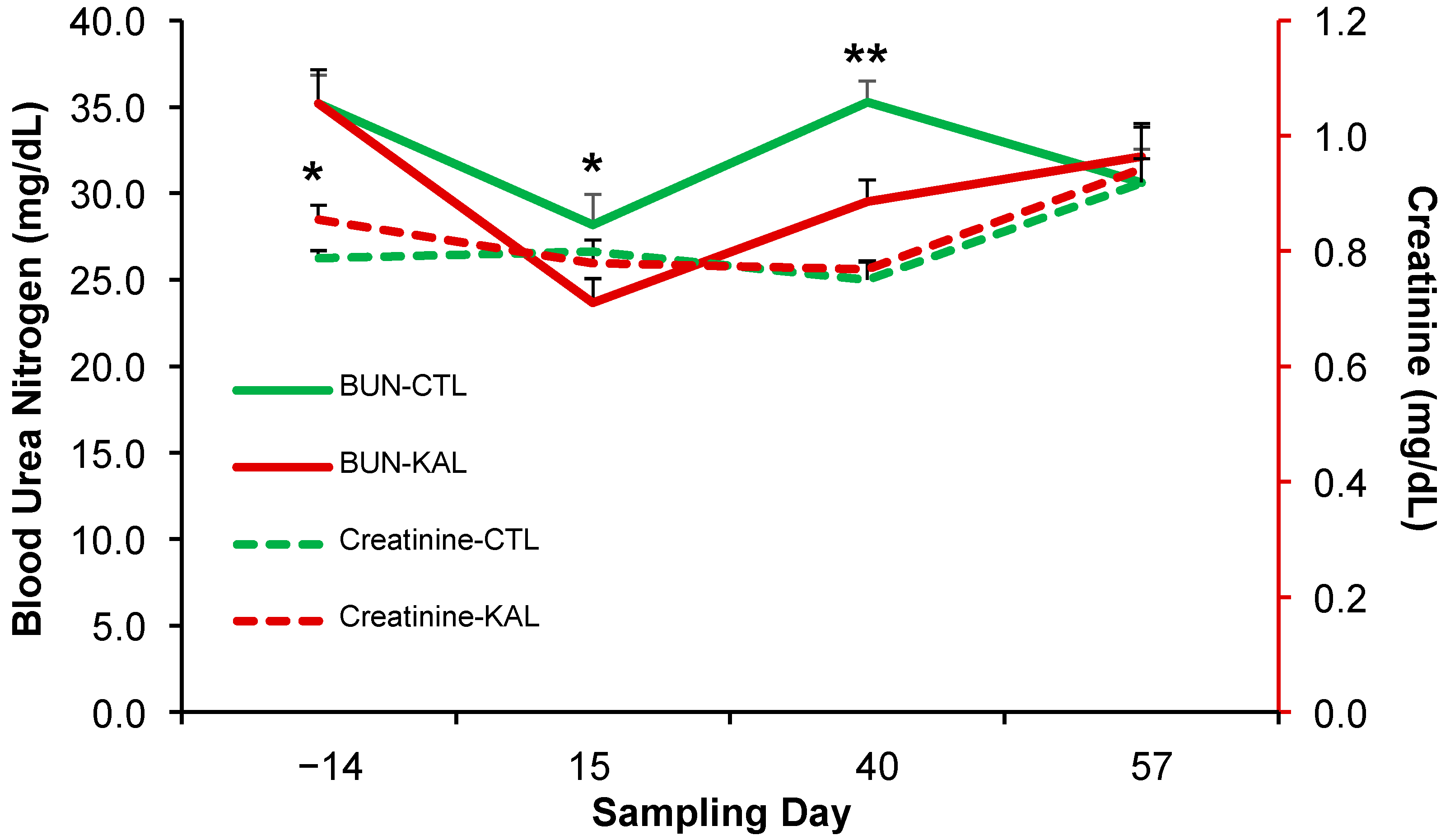
3.1. Kalanchoe daigremontiana and Kidney Function
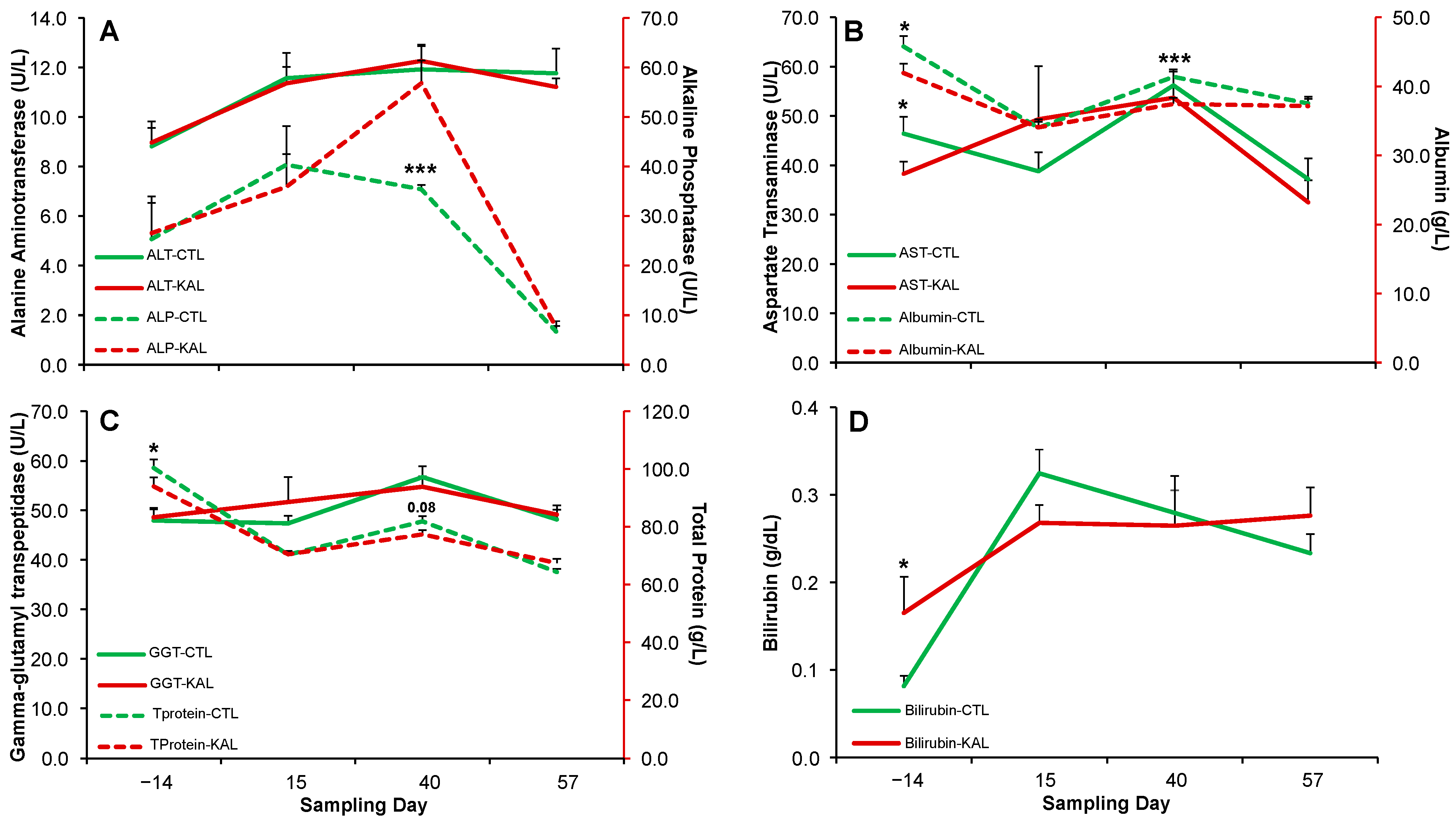
3.2. Kalanchoe daigremontiana and Livcr Function
3.3. Kalanchoe daigremontiana and Metabolites and Minerals
3.4. Kalanchoe daigremontiana and Body Weight Variables
3.5. Kalanchoe daigremontiana and Reproductive Performance
4. Discussion
4.1. Kalanchoe daigremontiana and Kidney Function
4.2. Kalanchoe daigremontiana and Liver Function
4.3. Kalanchoe daigremontiana and Metabolites and Minerals
4.4. Kalanchoe daigremontiana Body Weight and Reproductive Performance
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pattanayak, S.; Maity, D.; Mitra, S.; Debnath, P.; Mandal, T.; Bandyopadhyay, S. Use of Fresh Parts of Medicinal Plants for Health and Production in Livestock—A New Concept of Farming. Explor. Anim. Med. Res. 2013, 3, 7–16. [Google Scholar]
- Kuralkar, P.; Kuralkar, S.V. Role of herbal products in animal production—An updated review. J. Ethnopharmacol. 2021, 278, 114246. [Google Scholar] [CrossRef]
- Domingues Passero, L.F.; Laurenti, M.D.; Santos-Gomes, G.; Soares Campos, B.L.; Sartorelli, P.; Lago, J.H.G. Chapter 7—In Vivo Antileishmanial Activity of Plant-Based Secondary Metabolites. In Fighting Multidrug Resistance with Herbal Extracts, Essential Oils and Their Components; Rai, M.K., Kon, K.V., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 95–107. [Google Scholar]
- Abat, J.K.; Mattoo, A.K.; Deswal, R. S-nitrosylated proteins of a medicinal CAM plant Kalanchoe pinnata–ribulose-1,5-bisphosphate carboxylase/oxygenase activity targeted for inhibition. FEBS J. 2008, 275, 2862–2872. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Brulfert, J. Crassulacean acid metabolism in the genus Kalanchoë: Ecological, physiological and biochemical aspects. In Crassulacean Acid Metabolism: Biochemistry, Ecophysiology and Evolution; Winter, K., Smith, J.A.C., Eds.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 324–335. [Google Scholar]
- Pattewar, S.V. Kalanchoe daigremontiana: Phytochemical and pharmacological profile. Int. J. Pharm. Sci. Res. 2012, 3, 993–1000. [Google Scholar] [CrossRef]
- Burrows, G.; Tyrl, R.J. Cucurbitaceae Juss. In Toxic Plants of North America; Wiley: Hoboken, NJ, USA, 2012; pp. 387–394. [Google Scholar]
- Vargas, A.; Herrera, I.; Nualart, N.; Guézou, A.; Gómez-Bellver, C.; Freire, E.; Jaramillo Díaz, P.; López-Pujol, J. The Genus Kalanchoe (Crassulaceae) in Ecuador: From Gardens to the Wild. Plants 2022, 11, 1746. [Google Scholar] [CrossRef]
- Medina-Tenorio, M.A.; Hernández-Arteaga, L.E.S.; Ojeda-Galván, H.J.; Álvarez-Fuentes, G.; López-Aguirre, S.; Rosales-Nieto, C.A. Caracterización Fisicoquímica del Kalanchoe (Bryophyllium daigremontiana) para su uso Como Aditivo Nutricional para cabras. Memorias del XX Congreso Internacional Recursos Bióticos de Zonas Áridas. Available online: https://congresorebiza.mx/ (accessed on 1 December 2024).
- Milad, R. Genus Kalanchoe (Crassulaceae): A Review of Its Ethnomedicinal, Botanical, Chemical and Pharmacological Properties. Eur. J. Med. Phys. 2014, 4, 86–104. [Google Scholar]
- Stefanowicz-Hajduk, J.; Hering, A.; Gucwa, M.; Sztormowska-Achranowicz, K.; Kowalczyk, M.; Soluch, A.; Ochocka, J.R. An In Vitro Anticancer, Antioxidant, and Phytochemical Study on Water Extract of Kalanchoe daigremontiana Raym.-Hamet and H. Perrier. Molecules 2022, 27, 2280. [Google Scholar] [CrossRef]
- Nascimento, L.B.d.S.; Casanova, L.M.; Costa, S.S. Bioactive Compounds from Kalanchoe Genus Potentially Useful for the Development of New Drugs. Life 2023, 13, 646. [Google Scholar] [CrossRef]
- Menon, N.; Sparks, J.; Omoruyi, F.O. Oxidative Stress Parameters and Erythrocyte Membrane Adenosine Triphosphatase Activities in Streptozotocin-induced Diabetic Rats Administered Aqueous Preparation of Kalanchoe Pinnata Leaves. Pharmacognosy Res 2016, 8, 85–88. [Google Scholar] [CrossRef]
- Anandan, J.; Shanmugam, R. Antioxidant, Anti-inflammatory, and Antimicrobial Activity of the Kalanchoe pinnata and Piper longum Formulation Against Oral Pathogens. Cureus 2024, 16, e57824. [Google Scholar] [CrossRef]
- Rosales-Nieto, C.A.; Ehrhardt, R.; Mantey, A.; Makela, B.; Byrem; Veiga-Lopez, A. Preconceptional diet manipulation and fetus number can influence placenta endocrine function in sheep. Dom. Anim. Endocrinol. 2021, 74, 106577. [Google Scholar] [CrossRef]
- Banchero, G.E.; Clariget, R.P.; Bencini, R.; Lindsay, D.R.; Milton, J.T.B.; Martin, G.B. Endocrine and metabolic factors involved in the effect of nutrition on the production of colostrum in female sheep. Reprod. Nutr. Dev. 2006, 46, 447–460. [Google Scholar] [CrossRef]
- Rosales-Nieto, C.A.; Rodríguez-Aguilar, M.; Santiago-Hernandez, F.; Cuevas-Reyes, V.; Flores-Najera, M.J.; Vázquez-García, J.M.; Urrutia-Morales, J.; Ghaffari, M.H.; Meza-Herrera, C.A.; González-Bulnes, A.; et al. Periconceptional nutrition with spineless cactus (Opuntia ficusindica) improves metabolomic profiles and pregnancy outcomes in sheep. Sci. Rep. 2021, 11, 7214. [Google Scholar] [CrossRef]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nut. 2015, 99, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Rosales Nieto, C.A.; Meza-Herrera, C.A.; Morón Cedillo, F.J.; Flores Najera, M.J.; Gámez Vázquez, H.G.; Cuevas Reyes, V.; Liu, S.M. Effects of vitamin E supply during late gestation and early lactation upon colostrum composition, milk production and quality in nutritional restricted ewes. Small Rum. Res. 2015, 133, 77–81. [Google Scholar] [CrossRef]
- Gwaltney-Brant, S.M. Chapter 39—Christmastime Plants. In Small Animal Toxicology, 3rd ed.; Peterson, M.E., Talcott, P.A., Eds.; W.B. Saunders: Saint Louis, MI, USA, 2013; pp. 499–511. [Google Scholar]
- Bhalla, A.; Thirumalaikolundusubramanian, P.; Fung, J.; Cordero-Schmidt, G.; Soghoian, S.; Sikka, V.K.; Dhindsa, H.S.; Singh, S. Chapter 6—Native Medicines and Cardiovascular Toxicity. In Heart and Toxins; Ramachandran, M., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 175–202. [Google Scholar]
- Silanikove, N.; Gilboa, N.; Perevolotsky, A.; Nitsan, Z. Goats fed tannin-containing leaves do not exhibit toxic syndromes. Small Rum. Res. 1996, 21, 195–201. [Google Scholar] [CrossRef]
- García-Monjaras, S.; Santos-Díaz, R.E.; Flores-Najera, M.J.; Cuevas-Reyes, V.; Meza-Herrera, C.A.; Mellado, M.; Chay-Canul, A.J.; Rosales-Nieto, C.A. Diet selected by goats on xerophytic shrubland with different milk yield potential. J. Arid Environ. 2021, 186, 104429. [Google Scholar] [CrossRef]
- IPoC Change. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems. 2019. Available online: https://www.ipcc.ch/srccl/ (accessed on 1 December 2024).
- Cheng, M.; McCarl, B.; Fei, C. Climate Change and Livestock Production: A Literature Review. Atmosphere 2022, 13, 140. [Google Scholar] [CrossRef]
- González, F.H.D.; Hernández, F.; Madrid, J.; Martínez-Subiela, S.; Tvarijonaviciute, A.; Cerón, J.J.; Tecles, F. Acute phase proteins in experimentally induced pregnancy toxemia in goats. J. Vet. Diagn. Investig. 2011, 23, 57–62. [Google Scholar] [CrossRef]
- Mellado, M.; Rodríguez, I.J.; Alvarado-Espino, A.; Véliz, F.G.; Mellado, J.; García, J.E. Short communication: Reproductive response to concentrate supplementation of mixed-breed goats on rangeland. Trop. Anim. Health Prod. 2020, 52, 2737–2741. [Google Scholar] [CrossRef]
- Mellado, M.; Véliz, F.G.; Macías-Cruz, U.; Avendaño-Reyes, L.; García, J.E.; Rosales-Nieto, C.A. Effect of breed and management practices on reproductive and milking performance of rangeland goats. Trop. Anim. Health Prod. 2022, 54, 193. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Arteaga, L.E.S.; Vázquez-García, J.M.; Flores-Najera, M.J.; Cuevas-Reyes, V.; Mellado, M.; Sims, R.; Bruner, B.; Cavazos-Galindo, J.M.; Rosales-Nieto, C.A. Evaluating reproductive outcomes in Saanen and Alpine doelings with suboptimal live weight and performance of their progeny. Span. J. Agric. Res. 2025, 23, 21200. [Google Scholar] [CrossRef]
- López-Aguirre, S.; Pinos Rodríguez, J.M.; Devezé-Murillo, P.; Estrada-Coates, A.T.; Rosales-Nieto, C.A.; Torres-Martínez, C.J. Effect of Bryophyllum daigremontiana as an additive on productive parameters, concentration of liver enzymes and troponin T in broilers. Rev. Mex. Cienc. Pecu. 2025. accepted for publication. [Google Scholar]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLOS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- FASS. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- NAM-National Academy of Medicine. Guide for the Care and Use of Laboratory Animals. In Co-Produced by the National Academy of Medicine–Mexico and the Association for Assessment and Accreditation of Laboratory Animal Care International, 1st ed.; Harlan: Mexico City, Mexico, 2010. [Google Scholar]
- Urrutia Morales, J.; Rosales Nieto, C.A.; Ávila, H.R.V.; Manjarres, E.V.A. Resumption of ovarian activity is modified by non-photoperiodic environmental cues in Criollo goats in tropical latitudes. Small Rumin. Res. 2016, 137, 9–16. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids; National Academies Press: Washington, DC, USA, 2007; 292p. [Google Scholar]
- Kolodziejczyk-Czepas, J.; Nowak, P.; Wachowicz, B.; Piechocka, J.; Głowacki, R.; Moniuszko-Szajwaj, B.; Stochmal, A. Antioxidant efficacy of Kalanchoe daigremontiana bufadienolide-rich fraction in blood plasma in vitro. Pharm. Biol. 2016, 54, 3182–3188. [Google Scholar] [CrossRef]
- Cuevas-Reyes, V.; Flores-Sánchez, J.; Ramírez de la Cruz, E.; Vázquez-García, J.M.; Hernández-Arteaga, L.E.S.; Sims, R.; Cavazos-Galindo, J.M.; Mellado, M.; Rosales-Nieto, C.A. Effects of prenatal herbal methionine supplementation on growth indices, onset of puberty, blood metabolites, and fertility of Alpine doelings. Biology 2025, 14, 237. [Google Scholar] [CrossRef]
- Rosales-Nieto, C.A.; Thompson, A.N.; Cuevas-Reyes, V.; Hérnandez-Arteaga, L.E.S.; Greeff, J.C.; Ehrhardt, R.; Veiga-Lopez, A.; Martin, G.B. Utilising male stimulus to improve the reproductive efficiency of 8-month-old nulliparous ewes and adult parous ewes. Theriogenology 2024, 217, 143–150. [Google Scholar] [CrossRef]
- SAS Institute. SAS/Stat User’s Guide, Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2010. [Google Scholar]
- Salazar, J.H. Overview of Urea and Creatinine. Lab. Med. 2014, 45, e19–e20. [Google Scholar] [CrossRef]
- Kohn, R.A.; Dinneen, M.M.; Russek-Cohen, E. Using blood urea nitrogen to predict nitrogen excretion and efficiency of nitrogen utilization in cattle, sheep, goats, horses, pigs, and rats. J. Anim. Sci. 2005, 83, 879–889. [Google Scholar] [CrossRef]
- Omidi, A.; Nik, H.A.; Nazifi, S. Biochemical reference values for healthy captive Persian wild goat (Capra aegagrus). Comp. Clin. Path 2018, 27, 483–491. [Google Scholar] [CrossRef]
- Liang, Z.; Jin, C.; Bai, H.; Liang, G.; Su, X.; Wang, D.; Yao, J. Low rumen degradable starch promotes the growth performance of goats by increasing protein synthesis in skeletal muscle via the AMPK-mTOR pathway. Anim. Nut. 2023, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- So-In, C.; Sunthamala, N. Influence of goat management systems on hematological, oxidative stress profiles, and parasitic gastrointestinal infection. Vet. World 2023, 16, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Tharwat, M.; Ali, A.; Al-Sobayil, F. Hematological and biochemical profiles in goats during the transition period. Comp. Clin. Path 2015, 24, 1–7. [Google Scholar] [CrossRef]
- Santos, S.A.; Prates, L.L.; Carvalho, G.G.P.d.; Santos, A.C.S.d.; Valadares Filho, S.d.C.; Tosto, M.S.L.; Mariz, L.D.S.; Neri, F.d.S.; Sampaio, M.d.Q. Creatinine as a metabolic marker to estimate urinary volume in growing goats. Small Rum. Res. 2017, 154, 105–109. [Google Scholar] [CrossRef]
- Puschett, J.B.; Agunanne, E.; Uddin, M.N. Emerging role of the bufadienolides in cardiovascular and kidney diseases. Am. J. Kidney Dis. 2010, 56, 359–370. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Hering, A.; Kowalczyk, M.; Hałasa, R.; Gucwa, M.; Ochocka, J.R. Kalanchoe sp. Extracts—Phytochemistry, Cytotoxic, and Antimicrobial Activities. Plants 2023, 12, 2268. [Google Scholar] [CrossRef]
- Marutsova, V.J.; Binev, R.G. Changes in blood enzyme activities and some liver parameters in goats with subclinical ketosis. Bulg. J Vet. Med. 2020, 23, 70–79. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, N.; Anamika, S. Significance of hepatic enzymes: A review. Int. J. Adv. Biochem. Res. 2023, 7, 95–100. [Google Scholar] [CrossRef]
- Djuricic, D.; Dobranic, T.; Grizelj, J.; Gracner, D.; Harapin, I.; Stanin, D.; Folnozic, I.; Getz, I.; Cvitkovic, D.; Samardzija, M. Concentrations of Total Proteins and Albumins, and AST, AP, CK and GGT Activities in the Blood Serum Boer and Saanen Goats During Puerperium. Rep. Dom. Anim. 2011, 46, 674–677. [Google Scholar] [CrossRef]
- McGill, M.R. The past and present of serum aminotransferases and the future of liver injury biomarkers. Excli. J. 2016, 15, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Ruiz, D.R.; Molina-Alcaide, E. A comparative study of nutrients utilization, alkaline phosphatase activity and creatinine concentration in the serum of sheep and goats fed diets based on olive leaves. J. Anim. Physiol. Anim. Nut 2008, 92, 141–148. [Google Scholar] [CrossRef]
- Tibbo, M.; Jibril, Y.; Woldemeskel, M.; Dawo, F.; Aragaw, K.; Rege, J.E.O. Serum enzymes levels and influencing factors in three indigenous Ethiopian goat breeds. Trop. Anim. Health Prod. 2008, 40, 657–666. [Google Scholar] [CrossRef]
- Karaşahin, T.; Aksoy, N.H.; Dursun, Ş.; Bulut, G.; Haydardedeoğlu, A.E.; Çamkerten, G.; Çamkerten, İ.; İlgün, R. Effects of age and sex on some hematological and biochemical parameters in Hair goats. Vet. Res. Forum. 2022, 13, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.B.; Sun, L.L.; Cong, R.H.; Tao, S.Y.; DuanMu, Y.Q.; Tian, J.; Ni, Y.D.; Zhao, R.Q. Changes in milk performance and hepatic metabolism in mid-lactating dairy goats after being fed a high concentrate diet for 10 weeks. Animal 2017, 11, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Ghavipanje, N.; Fathi Nasri, M.H.; Farhangfar, S.H.; Ghiasi, S.E.; Vargas-Bello-Pérez, E. Regulation of Nutritional Metabolism in Transition Dairy Goats: Energy Balance, Liver Activity, and Insulin Resistance in Response to Berberine Supplementation. Animals 2021, 11, 2236. [Google Scholar] [CrossRef]
- Bazer, F.W.; Wang, X.; Johnson, G.A.; Wu, G. Select nutrients and their effects on conceptus development in mammals. Animal Nutrition 2015, 1, 85–95. [Google Scholar] [CrossRef]
- Chung, J.Y.; Kim, J.H.; Ko, Y.H.; Jang, I.S. Effects of Dietary Supplemented Inorganic and Organic Selenium on Antioxidant Defense Systems in the Intestine, Serum, Liver and Muscle of Korean Native Goats. Asian-Australas J. Anim. Sci. 2007, 20, 52–59. [Google Scholar] [CrossRef]
- York, M.J. Chapter 14—Clinical Pathology. In A Comprehensive Guide to Toxicology in Nonclinical Drug Development, 2nd ed.; Faqi, A.S., Ed.; Academic Press: Boston, MA, USA, 2017; pp. 325–374. [Google Scholar]
- Mbassa, G.K.; Poulsen, J.S.D. Reference ranges for clinical chemical values in Landrace goats. Small Rum. Res. 1993, 10, 133–142. [Google Scholar] [CrossRef]
- Brobst, M.N.; Abi-Nader, B.A.; Blasczynski, S.J.; Chigerwe, M. Evaluation of a continuous glucose monitoring system in healthy dairy calves and adult goats. Am. J. Vet Res. 2024, 85, ajvr.24.03.0076. [Google Scholar] [CrossRef]
- Patil, S.B.; Dongare, V.R.; Kulkarni, C.R.; Joglekar, M.M.; Arvindekar, A.U. Antidiabetic activity of Kalanchoe pinnata in streptozotocin-induced diabetic rats by glucose independent insulin secretagogue action. Pharm. Biol. 2013, 51, 1411–1418. [Google Scholar] [CrossRef] [PubMed]
- Menon, N.; Sparks, J.; Omoruyi, F. Hypoglycemic and hypocholesterolemic activities of the aqueous preparation of Kalanchoe pinnata leaves in streptozotocin-induced diabetic rats. Asian Pac. J. Trop. Biomed. 2015, 5, 3–9. [Google Scholar] [CrossRef]
- Agüero-Hernández, A.L.; Rosales-López, C.; Herrera, C.; Vargas-Picado, A.; Muñoz, R.; Abdelnour-Esquivel, A. Hypoglycemic Effect of Kalanchoe pinnata (Lam) Pers. Leaf Extract. Pharmacogn. J. 2020, 12, 557–561. [Google Scholar] [CrossRef]
- Plakkot, B.; Mohanan, A.; Kanakkaparambil, R. Prolificacy in small ruminants. JDVAR 2020, 9, 85–90. [Google Scholar] [CrossRef]


| K. daigremontiana (kg/ton) | ||
|---|---|---|
| Ingredient | 0 | 2 |
| Alfalfa (%) | 65 | 64.9 |
| Oat hay (%) | 15 | 15 |
| Maize silage (%) | 20 | 19.9 |
| K. daigremontiana (%) | 0 | 0.2 |
| Chemical composition | ||
| Dry matter (%) | 80.3 | 80.4 |
| Crude protein (%) | 13.4 | 13.4 |
| ME (Mcal/kg/DM) | 2.42 | 2.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-García, J.M.; Ballesteros-Rodea, G.; Cuevas-Reyes, V.; Hernández-Arteaga, L.E.S.; Peña-Avelino, L.Y.; López-Aguirre, S.; Sims, R.; Cavazos-Galindo, J.M.; Rosales-Nieto, C.A. Impact of Kalanchoe (Kalanchoe daigremontiana) Supplementation in Goat Maternal Diet on Hepatic and Renal Function and Reproductive Performance. Biology 2025, 14, 376. https://doi.org/10.3390/biology14040376
Vázquez-García JM, Ballesteros-Rodea G, Cuevas-Reyes V, Hernández-Arteaga LES, Peña-Avelino LY, López-Aguirre S, Sims R, Cavazos-Galindo JM, Rosales-Nieto CA. Impact of Kalanchoe (Kalanchoe daigremontiana) Supplementation in Goat Maternal Diet on Hepatic and Renal Function and Reproductive Performance. Biology. 2025; 14(4):376. https://doi.org/10.3390/biology14040376
Chicago/Turabian StyleVázquez-García, Juan M., Gilberto Ballesteros-Rodea, Venancio Cuevas-Reyes, Luisa E. S. Hernández-Arteaga, Luz Y. Peña-Avelino, Samuel López-Aguirre, Reagan Sims, Jaime M. Cavazos-Galindo, and Cesar A. Rosales-Nieto. 2025. "Impact of Kalanchoe (Kalanchoe daigremontiana) Supplementation in Goat Maternal Diet on Hepatic and Renal Function and Reproductive Performance" Biology 14, no. 4: 376. https://doi.org/10.3390/biology14040376
APA StyleVázquez-García, J. M., Ballesteros-Rodea, G., Cuevas-Reyes, V., Hernández-Arteaga, L. E. S., Peña-Avelino, L. Y., López-Aguirre, S., Sims, R., Cavazos-Galindo, J. M., & Rosales-Nieto, C. A. (2025). Impact of Kalanchoe (Kalanchoe daigremontiana) Supplementation in Goat Maternal Diet on Hepatic and Renal Function and Reproductive Performance. Biology, 14(4), 376. https://doi.org/10.3390/biology14040376







