Simple Summary
Research indicates that seminal plasma is essential for creating an optimal environment for spermatozoa, thereby enhancing sperm function and fertility. Effective communication among sperm cells, developing embryos, and maternal tissues is critical for successful pregnancies. Small extracellular vesicles play a significant role in these interactions, contributing to reproductive processes and supporting embryo development. This review underscores the importance of small extracellular vesicles in improving gamete quality in both males and females across various species, highlighting their crucial role in reproductive success.
Abstract
Extracellular vesicles (EVs) are gaining recognition for their essential role in enhancing gamete quality and improving outcomes in assisted reproductive technologies. These nanosized particles, released by cells, carry proteins, lipids, and RNAs, facilitating critical cell communication and offering the potential to enhance gamete maturation and improve fertilization rates. Most research on males has concentrated on seminal plasma, a complex fluid produced by the testes and accessory glands vital in modulating sperm fertility potential. The components of seminal plasma significantly affect sperm functionality, embryo survival, and placental development, making this a prominent area of interest in reproductive biology. The EVs within seminal plasma contribute to maintaining sperm membrane stability, enhancing motility, and promoting capacitation, which may influence the female reproductive tract following mating. In females, EVs have been identified in both the follicular and uterine environments, where effective embryo–maternal communication is crucial. The oviduct epithelium supports gamete transport and early embryonic development, with EVs found in oviductal fluid playing a key role in reproductive processes. These EVs support the embryo’s growth in the nutrient-rich uterine environment. These important studies underscore the significant role of EVs in transporting essential molecular compounds to gametes and embryos, leading to an enhanced understanding and potential manipulation of reproductive processes. This review aims to summarize the current research on the benefits of EVs in gamete manipulation and embryo development, highlighting their promising implications for reproductive health.
1. Introduction
Gamete quality is a vital component driving the success of the livestock industry, particularly as global demand for animal protein continues to rise. The pig industry, a significant facet of international agriculture, primarily utilizes artificial insemination (AI) as its primary breeding method. By employing fresh, chilled semen, commercial pig centers not only propagate superior genetics but also significantly mitigate the risks of disease transmission [1]. The importance of high-quality semen for effective AI cannot be overstated; selecting boars with outstanding semen quality is crucial for minimizing costs associated with animals not used for semen production [2,3]. Despite notable advancements in precision assessment tools, such as computer-assisted sperm analysis (CASA), the lack of reliable predictive markers for semen quality remains a significant challenge. This gap emphasizes the pressing need for ongoing research and innovation in this field.
In males, most research has focused on seminal plasma (SP), the non-cellular component of semen, employing advanced omics approaches like transcriptomics, metabolomics, and proteomics. However, why concentrate on this fluid that serves to transport sperm? SP is a complex mixture of secretions from the testes, epididymis, and accessory sexual glands. It provides the essential living environment for sperm and is rich in biomolecules, including lipids, proteins, RNA, energy substrates, enzymes, and extracellular vesicles [4]. Importantly, SP is critical in regulating sperm functionality, supporting embryo survival, and facilitating proper placental development, thereby influencing fertility following natural mating and AI [4,5,6]. Notably, SP proteins are essential for maintaining sperm membrane stability, ensuring the sperm’s fertilizing capacity during transport, and modulating both motility and capacitation.
Research on the female reproductive tract has predominantly concentrated on the environments surrounding follicles and the uterus, explicitly examining follicular, oviductal, and uterine fluids. Establishing and maintaining pregnancy in mammals hinges upon effective communication between the embryo and maternal tissues. Oocyte maturation occurs within the preovulatory follicle, followed by oviduct fertilization and early embryonic development during the luteal phase [7]. The oviduct epithelium, comprising ciliated and secretory cells, plays a pivotal role in gamete transport, fertilization [8,9], and embryonic genome activation (EGA) [10].
Extracellular vesicles (EVs) serve as integral mediators of intercellular communication within both male and female reproductive tissues, regulating various physiological processes [8,11]. These vesicles are enriched with bioactive molecules, including microRNAs (miRNAs), messenger RNAs (mRNAs), proteins, and lipids [12,13], which influence several reproductive aspects, such as sperm motility and capacitation, follicular development, oocyte maturation, fertilization, and early embryo development [8,11,14,15,16]. The comprehensive review by De Ávila et al. [17] emphasizes the critical function of EVs as communication entities within follicular [11], oviductal [18], and uterine fluids [19]. Small EVs or exosomes in the oviductal fluid are essential for numerous reproductive processes [20]. In bovine species, the embryo typically reaches the uterus 4 to 5 days post-fertilization [21,22], subsequently developing into a blastocyst [23]. Uterine fluid, abundant in nutrients and EVs, provides vital support for embryonic development and placentation, facilitating crucial intercellular communication for implantation and the establishment of pregnancy [24,25]. These findings illustrate the indispensable role of EVs within the female reproductive tract. Recent research investigates the therapeutic applications of EVs, highlighting their potential roles in drug delivery, diagnostics, and enhancing gamete quality.
A thorough understanding of EVs’ influence on gamete and embryo qualities is crucial for strengthening reproductive health and advancing assisted reproductive technologies (ARTs). Insights into EV-mediated interactions between sperm, egg, and embryo development could lead to the optimization of IVF protocols and the identification of EV-based fertility biomarkers. This knowledge gain offers valuable resources for further genetic selection programs. This review emphasizes the physiological implications of EVs for the functionality of both male and female gametes and embryos.
2. Extracellular Vesicle (EV) Characteristics
EVs are membrane-bound substances released by maturing blood reticulocytes into the extracellular space [26]. They are crucial in cell-to-cell communication, facilitating local (autocrine and paracrine) and distant interactions between cells and tissues. These vesicles, encased by a phospholipid bilayer membrane, circulate in body fluids and serve as carriers for proteins, lipids, and RNAs [27].
EVs can originate from the endosomal system or be shed from the plasma membrane of parent cells and tissues [28]. Based on size, they are classified as exosomes (40–120 nm), microvesicles (50–1000 nm), and apoptotic bodies (1–5 μm) [29]. The overlapping size ranges can complicate the differentiation between these subtypes. Exosomes form through the inward budding of endosomal membranes, maturing into multivesicular bodies (MVBs) that release exosomes into the extracellular environment upon fusion with the plasma membrane [27,30]. In contrast, microvesicles are generated by the outward budding of the plasma membrane, while apoptotic bodies emerge from membrane blebbing during programmed cell death [31].
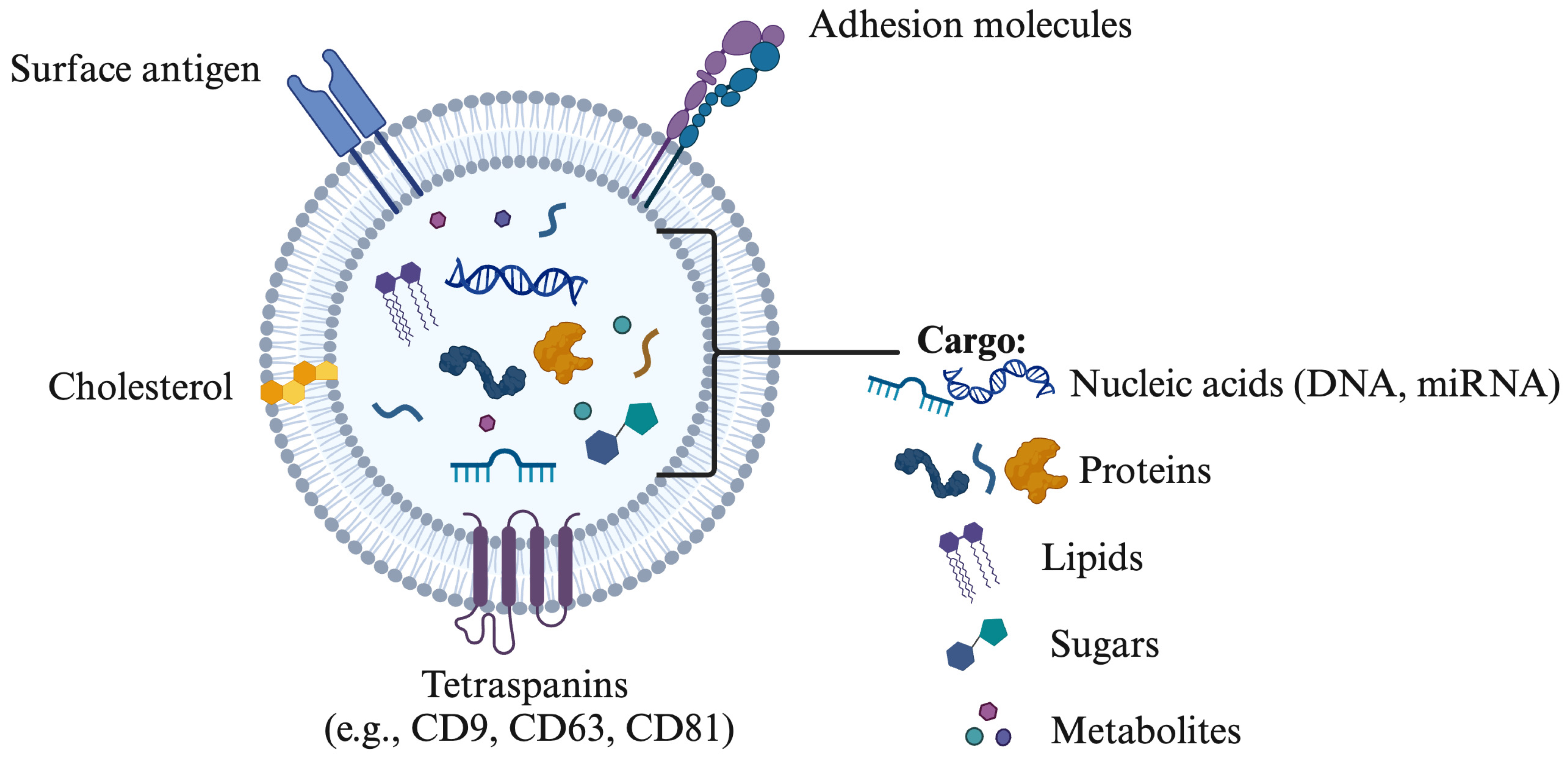
The terminology associated with EVs includes terms such as prostasomes, epididymosomes, oviductosomes, and uterosomes, each representing vesicles released by the prostate, epididymis, oviduct, and uterus, respectively [8,32,33,34]. In mammals, EVs play a crucial role in various physiological processes by transporting cell-specific cargo that facilitates communication with target cells while protecting against extracellular degradation [26,35]. The binding of EVs to target cells is influenced by their composition—such as proteins, sphingomyelin, and miRNAs—and the specific receptors on the target cells or tissues (Figure 1). Tetraspanins, including CD9, CD63, CD81, and CD82, are significant membrane proteins abundant in exosomes and serve as key markers for their characterization and isolation [36,37]. Additionally, exosomes contain a range of membrane proteins, signaling molecules, multivesicular body (MVB) proteins, and proteins vital for vesicle trafficking [14].
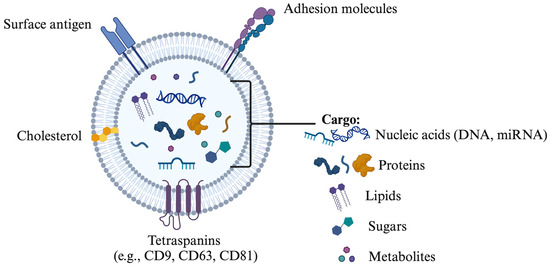
Figure 1.
Composition of extracellular vesicles including surface antigen, cholesterol, tetraspanins (CD9, CD63, CD81), nucleic acids (DNA, miRNA), proteins and lipids. The drawing was created using Biorender.com.
The investigation of EVs, particularly those from the male and female reproductive tracts, is progressing rapidly due to their potential in therapeutic strategies and biomarker development. This review will examine the roles of EVs derived from seminal plasma and follicular/oviductal fluids, emphasizing their unique populations and functions.
3. Extracellular Vesicles (EVs) in the Male Reproductive Tract
3.1. Detection
Sperm develop the essential ability to move and fertilize an oocyte during its passage through the epididymis, a process intricately regulated by interactions with seminal fluid during ejaculation [38]. The composition of seminal plasma (SP) is not only complex but also critical for facilitating intense communication through EVs [37]. These EVs are key components of SP, originating from various organs within the male reproductive system, including the testes, epididymis, vas deferens, and accessory glands, such as the prostate and seminal vesicles [6,37]. The existence of EVs in the male reproductive tract was first noted over 50 years ago, with initial observations of vesicle-like structures in rabbit semen [39].
3.1.1. Epididymosomes
In the mid-1980s, Yanagimachi et al. [40] revealed that these EVs, known as epididymosomes, are produced through apocrine secretion, whereby cytoplasmic blebs detach from the epithelial cells lining the epididymis. Epididymosomes constitute a heterogeneous population of vesicles, ranging from 50 to 250 nm, and have been extensively examined across different segments of the epididymis (caput, corpus, and cauda), where they play a vital role in sperm maturation [37].
Epididymosomes play a crucial role in the interaction between sperm and the epididymis by temporarily fusing with sperm to deliver essential proteins such as macrophage migration inhibitory factor (MIF) and P26h/P34H. These proteins are vital for sperm maturation and facilitate binding to the extracellular matrix and the zona pellucida [41,42]. MIF, aldose reductase, and other polyol pathway enzymes significantly enhance sperm motility during epididymal maturation, while type 5 glutathione peroxidase (GPX5) prevents premature acrosome reactions [43,44]. Additionally, the presence of Liprin α3 and the kinase c-Src—proteins associated with the acrosome reaction—within epididymosomes highlights their importance. This suggests that these vesicles are instrumental in the biochemical modifications of ejaculated sperm [45,46], preparing them for successful fertilization.
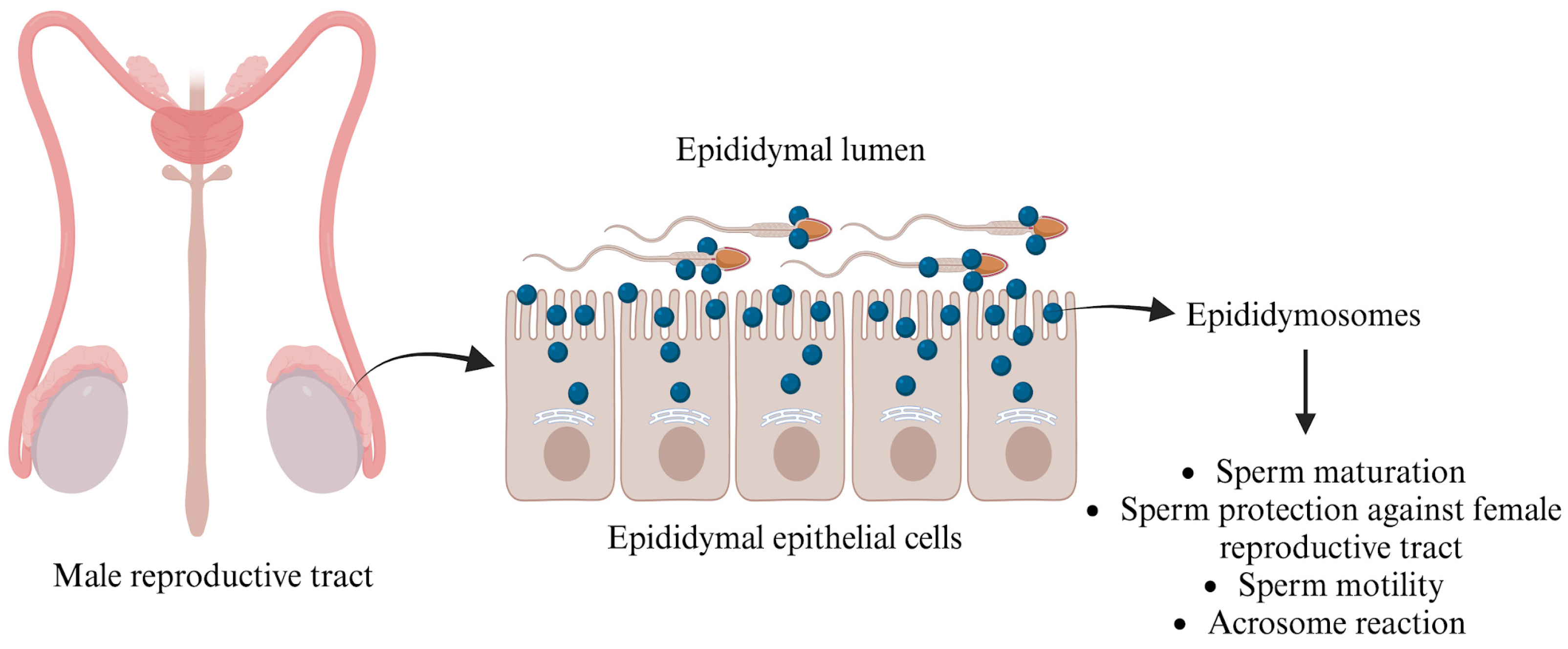
Numerous studies have emphasized the essential role that epididymal secreted proteins, particularly those anchored with glycosylphosphatidylinositol (GPI), play in sperm maturation. A notable protein in this context is P26h [47]. Research conducted on hamster spermatozoa indicates that the association of P26h with epididymosomes decreases as sperm travel through the excurrent duct, providing compelling evidence that epididymosomes are critical for acquiring this protein during sperm maturation [48]. In bulls, P25b, the ortholog of P26h, is similarly GPI-anchored to spermatozoa. Investigations have demonstrated that this protein is transferred to sperm collected from the bovine epididymis, enabling these sperm cells to effectively bind to the zona pellucida of oocytes [49]. The importance of epididymosomes goes beyond mere protein transfer; they also transport the transmembrane protein “a disintegrin and metalloproteinase” (ADAM7) to sperm cells (Figure 2), which is vital for successful sperm–oocyte interactions [50]. Moreover, epididymosomes encapsulate small non-coding RNAs (sncRNAs), which are crucial for transferring microRNAs (miRNAs) and transfer RNA-derived RNA fragments (tRFs) to sperm, thus influencing embryo development [51]. After leaving the testes, sperm engage with epididymal EVs, leading to significant changes in miRNA profiles that are important for maturation. For instance, studies reveal that mouse sperm collected from the caput and cauda segments of the epididymis lose 133 miRNAs while gaining 115 miRNAs, respectively [52]. Additionally, sperm incubated with epididymosomes show a significant increase in five specific miRNAs (miR-191, miR-375, miR-467a, miR-467d, and miR-467e), which are present in much higher amounts in epididymosomes compared to sperm that were not exposed to epididymosomes [51]. This evidence underscores the indispensable role of epididymosomes in enhancing sperm functionality and ultimately promoting successful fertilization.
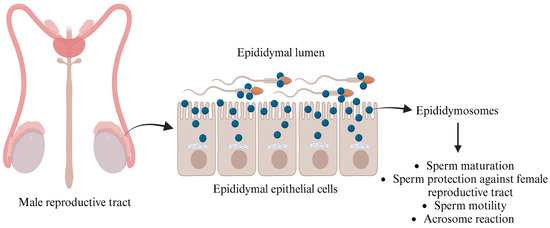
Figure 2.
Functions of epididymosomes in the male reproductive tract. The figure is created using BioRender.com and adapted from [53].
3.1.2. Prostasomes
Prostasomes are prostate-derived vesicles averaging 150 nm in diameter (with a range of 30 to 200 nm) and were first described in human semen in 1978 [54]. They protect spermatozoa from the female immune response within the reproductive tract. They effectively modulate the complement pathway and inhibit the phagocytosis of sperm by monocytes and neutrophils [32]. In addition, prostasomes transport a variety of important proteins [33,55], including signal transduction proteins, guanosine triphosphates, adenosine triphosphates, and prostate-specific markers, such as prostatic acid phosphatase and prostate stem-cell antigen. These proteins serve structural functions and exhibit antimicrobial, antioxidant, and immune-regulatory effects that enhance sperm health [56,57,58]. Furthermore, prostasomes play a significant role in influencing the physiological parameters of ejaculated spermatozoa. Studies have demonstrated their crucial involvement in processes such as capacitation and the acrosome reaction [59], which are vital for successful fertilization. Notably, prostasomes are high in cholesterol and sphingomyelin, which contribute to the fluidity and stability of sperm membranes, particularly in the acidic environment of the vagina [60]. Overall, the diverse functions of prostasomes are designed to ensure that spermatozoa remain in optimal condition and retain their full functional capabilities before encountering the oocyte.
Yet, the precise physiological functions of these vesicles are still under investigation, but numerous studies emphasize their importance in enhancing the recovery of hyperactive motile sperm and improving calcium signaling—both crucial factors in elevating sperm motility [61,62]. Understanding and leveraging these mechanisms could significantly advance our knowledge of male fertility and reproductive health.
3.2. Role of Seminal Plasma-EVs (SP-EVs) in Modulating Gamete Function
Most SP-EVs’ effects are reported in spermatozoa, known to be transcriptionally and translationally silent following spermatogenesis, underscoring EVs’ crucial role in the male reproductive tract concerning post-testicular sperm maturation and fertilizing capacity. SP-EVs significantly influence various sperm functions through their binding and fusion with ejaculated sperm. The epididymis facilitates sperm maturation via sequential interactions with intraluminal fluids that change sperm morphology [38]. These interactions prompt essential structural and compositional changes, including adjustments in phospholipid composition, cholesterol/phospholipid ratio modifications, increased total negative charges, and alterations to surface proteins. As a result, spermatozoa acquire critical functions, including progressive motility, zona pellucida binding, and fusion with the oocyte’s plasma membrane [37,38]. Seminal plasma contains the highest concentration of EVs among bodily fluids, with approximately ~1012 particles per ejaculate. SP-EVs interact with spermatozoa to support sperm development [63]. The specific binding sites of SP-EVs correlate directly with their functions: EVs engaging with the sperm head enhance capacitation, activate the acrosomal reaction, and improve oocyte binding capacity; conversely, those bound to the mid-piece and central piece of the tail regulate mitochondrial activity, energy metabolism, and overall motility [5].
A substantial body of research underscores the pivotal role of SP-EVs in promoting sperm functionality and enhancing reproductive success. For instance, studies conducted by Arienti et al. [64] have indicated that EVs derived from human seminal plasma markedly influence sperm function, with EVs from normozoospermic men significantly enhancing sperm motility and facilitating capacitation when co-cultured with ejaculated sperm [65]. In porcine models, Du et al. [14] noted that SP-EVs considerably improved sperm motility during the incubation of extended sperm. Additionally, boar sperm subjected to higher concentrations of exosomes demonstrated increased motility, prolonged survival, enhanced membrane integrity, and elevated total antioxidant capacity following thawing while simultaneously preventing premature capacitation [14]. In other species, such as sheep, spermatozoa in seminal plasma EVs are associated with proteins involved in vesicle biogenesis, metabolism, and membrane adhesion and remodeling functions [66]. Furthermore, analyses of boar SP-EVs have revealed that the predominant proteins comprise structural proteins (notably actin), enzymes, intracellular ion channels, and sperm adhesins [4], thereby emphasizing the multifaceted contributions of SP-EVs in optimizing sperm functionality and promoting reproductive success.
Extracellular vesicles are integral to transferring small non-coding RNAs, which carry various biological functions depending on their source [67]. This remarkable ability enables EVs to deliver specific microRNAs (miRNAs) to spermatozoa, playing a significant role in post-testicular sperm function. Notable miRNAs found in SP-EVs, such as ssc-miR-148a, ssc-miR-10a-5p, and ssc-miR-10b, are essential for spermatogenesis and fertility [68,69]. Interestingly, most small RNAs identified in SP-EVs from Yorkshire boars are miRNAs [70]. Analysis of expression levels shows that 83.71% of these miRNAs are at low levels, while 16 are highly expressed in SP-EVs [71], suggesting a selective mechanism for sorting into exosomes [72]. Additionally, studies indicate that boar SP-EVs contain differentially expressed miRNAs associated with low sperm cryotolerance [73] and divergent motility [74]. This suggests the potential of these miRNAs as biomarkers to aid in predicting sperm viability and quality during cryopreservation and AI dose preparation, respectively.
In mammals, miRNAs found in SP-EVs are essential for assessing semen quality and fertility [75,76]. A notable study by Barcelo et al. (2018) demonstrated that miR-31-5p is a highly sensitive and specific biomarker for azoospermia based on its expression in human seminal plasma exosomes derived from azoospermic individuals [77]. The study also identified miR-210-3p as highly expressed in the seminal exosomes of patients with varicocele, further emphasizing the diagnostic potential of these miRNAs.
In porcine studies, researchers [74] conducted a comparative analysis of SP-EVs from Duroc semen of varying quality, identifying key miRNAs such as ssc-miR-205, ssc-miR-493-5p, and ssc-miR-378b-3p, which are directly linked to boar sperm quality (good vs. poor). Furthermore, high-throughput sequencing was employed to examine the miRNA profiles of boars, revealing critical differences in low- and high-motility sperm across Landrace and Yorkshire breeds [78]. Six miRNAs—ssc-miR-122-5p, ssc-miR-486, ssc-miR-451, ssc-miR-345-3p, ssc-miR-362, and ssc-miR-500-5p—were identified as being differentially expressed in both breeds. These miRNAs play significant roles in essential biological processes, including gene expression regulation and critical signaling pathways such as PI3K-Akt, MAPK, and Ras, highlighting their potential as non-invasive markers for predicting boar semen motility. These signaling pathways regulate sperm motility by inhibiting PI3K expression in semen [78]. Specifically, ssc-miR-362, ssc-miR-486, and ssc-miR-122-5p enhance sperm motility by activating vital genes associated with the PI3K-Akt signaling pathway, including PIK3R1, PRKCA, PIK3CB, IGF1, LAMC1, and FOXO3. Additionally, ssc-miR-362 and ssc-miR-486 may influence the MAPK pathway through interactions with PRKCA, IGF1, and STK4 [78,79], underscoring their potential significance as biomarkers in reproductive science and as non-invasive biomarkers for predicting sperm motility in boars.
In avians, SP-EVs are found to be significantly more abundant in the semen of fertile roosters compared to their sub-fertile counterparts. This distinction is critical, as studies suggest that transferring SP-EVs from fertile roosters to sub-fertile ones can effectively enhance sperm motility [80]. Additionally, the differentially expressed miRNAs present in SP-EVs derived from duck semen are enriched in essential pathways related to sperm functions, such as the ErbB signaling pathway, glycometabolism, and ECM-receptor interactions, all of which are vital for regulating sperm motility [81]. Beyond their miRNA content, SP-EVs can synthesize ATP through glycolysis, further promoting mitochondrial metabolism and motility in sperm [82]. It is noteworthy that the interactions between sperm and EVs are pH-dependent [5]: The alkaline pH of prostatic secretions inhibits early EV/sperm fusion, while the acidic environment of the vagina facilitates it. These studies provide compelling evidence for SP-EVs’ role in enhancing sperm function; however, further research is needed to fully understand the specific contributions of their various components.
4. Extracellular Vesicles (EVs) in the Female Reproductive Tract
EVs play a crucial role in regulating female reproductive biology, being released from various regions within the female reproductive tract, including follicles, the oviductal and uterine epithelium, as well as the embryo/conceptus [8,15,83].
4.1. EVs in Follicular Fluid
More than 30 studies have explored these EVs’ biological functions and molecular cargo, which encompass both exosomes and microvesicles found within the follicular fluid (FF). The process of folliculogenesis, which involves the growth and maturation of ovarian follicles, relies significantly on effective cell-to-cell communication among theca, granulosa, and cumulus cells. This communication is facilitated by constituents in FF, including hormones, proteins, and anti-apoptotic factors. Notably, the cargo profile of EVs undergoes substantial changes throughout the stages of follicular development, as FF-EVs transport essential RNAs, miRNAs, and proteins that influence the transcriptional activity of recipient cells [11,83].
Research spanning equine, bovine, and human subjects has confirmed the presence and importance of EVs in FF. For example, studies in horses have identified specific miRNAs within exosomes and microvesicles in FF that regulate key pathways, including transforming growth factor beta (TGF-β), wingless (WNT), and mitogen-activated protein kinase (MAPK). These pathways are essential to folliculogenesis, encompassing processes such as follicular development, granulosa cell proliferation, cumulus expansion, steroidogenesis, and meiosis, as well as mammalian embryo mitosis [11,83]. In bovine research, a significant correlation was observed between elevated expression of EV-miRNAs around immature oocytes and increased transcriptional activity during oocyte growth [83]. This suggests that the uptake of exosomes by follicular cells is linked to higher levels of miRNAs. Additionally, EV-miRNAs found in small follicles, indicative of early antral follicle growth, are associated with genes involved in cell proliferation pathways. In contrast, those in larger follicles, representing later stages of growth, are connected to genes related to inflammatory response pathways [84]. In humans, the miRNAs present in FF-EVs primarily participate in pathways such as WNT, epidermal growth factor receptor (ErbB), MAPK, and TGF-β, which regulate crucial processes like follicular development, meiotic resumption, and ovulation [85].
Studies in bovines revealed that the stage of the estrous cycle markedly influences the composition of miRNA and proteins within FF-EVs, primarily attributed to fluctuations in progesterone (P4) levels in the FF, resulting from the corpus luteum. Interestingly, FF-EVs with low P4 concentrations display a unique miRNA profile that regulates essential biological pathways, including MAPK, RNA transport, Hippo signaling, cell cycle processes, FoxO signaling, oocyte meiosis, and TGF-beta signaling [17]. Additionally, a thorough study using an in vivo model demonstrated that the protein content of FF-EVs varies depending on the follicle’s proximity to the corpus luteum, specifically whether it is in the ovary ipsilateral or contralateral to the CL [86]. Notably, FF-EVs from the ovary ipsilateral to the CL, characterized by higher intrafollicular P4 levels, exhibit an increased abundance of proteins linked to the retraction of actin filament-based membrane projections, such as transzonal projections (TZPs). In contrast, FF-EVs from the contralateral ovary, showing lower P4 concentrations, have reduced protein abundance [86]. These findings highlight the crucial role of FF-EVs in regulating key molecular pathways essential for folliculogenesis, oocyte development, and cumulus cell function.
4.2. Extracellular Vesicles (EVs) in Embryo–Maternal Interactions
The endometrial epithelium significantly contributes to communication with the embryo during implantation by releasing EVs, responsible for transferring vital signaling miRNAs and adhesion molecules to the blastocyst [59]. Exosomal markers CD9 and CD63 located on the apical surfaces of human endometrial epithelial cells suggest that the endometrial epithelium is a source of EVs in the uterine cavity generated via the apocrine pathway. These EVs carry proteins essential for cell migration, adhesion, angiogenesis, and focal adhesion kinase (FAK) signaling, fostering a supportive embryo implantation microenvironment [87].
4.2.1. Evidence from the Maternal Side
Oviductal EVs: Protein analyses of bovine oviduct epithelial cells (BOECs) indicate that oviductal EVs contain proteins such as oviductal glycoprotein (OVGP), heat shock protein A8 (HSPA8), and myosin 9 (MYH9), which play essential roles in fertilization, early pregnancy development, and zona pellucida maturation [18,88]. However, the BOECs used in the in vitro system did not present OVGP [18], suggesting that the in vitro environment can alter the composition of oviductal EVs. An in vivo model has shown that EVs derived from oviductal fluid in pregnant cows 120 h after ovulation induction display a unique miRNA profile compared to those from non-pregnant cows. Specifically, eight miRNAs (bta-miR-126-5p, bta-miR-129, bta-miR-140, bta-miR-188, bta-miR-219, bta-miR-345-3p, bta-miR-4523, and bta-miR-760-3p) were found to be upregulated in the pregnant group and are predicted to influence crucial signaling pathways, such as PI3K-Akt, MAPK, and mTOR [89]. Furthermore, EVs derived from oviductal fluid in the isthmus are associated with higher embryo survival rates following vitrification in cattle, with increased expression of Aquaporin 3 (AQP3) contributing to enhanced membrane fluidity [90].
Uterine EVs: They are detected in the uterine lumen of various species, originating from both the luminal and glandular epithelium of the uterus [91]. EVs derived from uterine lumen fluid play a significant role in conceptus elongation [19] and communication between the embryo and the maternal environment, influencing various biological processes, such as the differentiation of trophoblast binucleate cells, the induction of apoptosis in immune cells, and the promotion of cellular proliferation, which are essential elements for establishing uterine receptivity to conceptus implantation [92]. These beneficial effects are attributed to the EVs content in interferon tau (IFNT) and prostaglandin contents [93], while a study demonstrated the participation of uterine EVs of pregnant sheep in RNA transfer, further emphasizing the uterine EVs role in embryo–endometrium interactions [94].
4.2.2. Evidence from the Embryo Side
A recent study utilizing an ex vivo model with endometrial and luteal explants has revealed that EVs secreted by embryos and endometrial cells exhibit varying miRNA content based on the origin of the embryo (in vivo vs. in vitro). These EVs play a role in modulating mRNA and miRNA profiles in luteal tissues, indicating their involvement in embryo–maternal interactions during the establishment of pregnancy [95]. Additionally, both day-7 to day-16 in vivo- and in vitro-derived embryos release EVs containing distinct miRNA that are predicted to influence pathways in the endometrium and corpus luteum (CL) associated with the maternal recognition of pregnancy [96]. When the bovine endometrium is exposed to these EVs, it undergoes global transcriptomic changes, underscoring the endometrium’s capability as a biosensor in cattle, which is partly mediated by its response to the unique miRNA cargo from conceptus-derived EVs during the establishment of pregnancy.
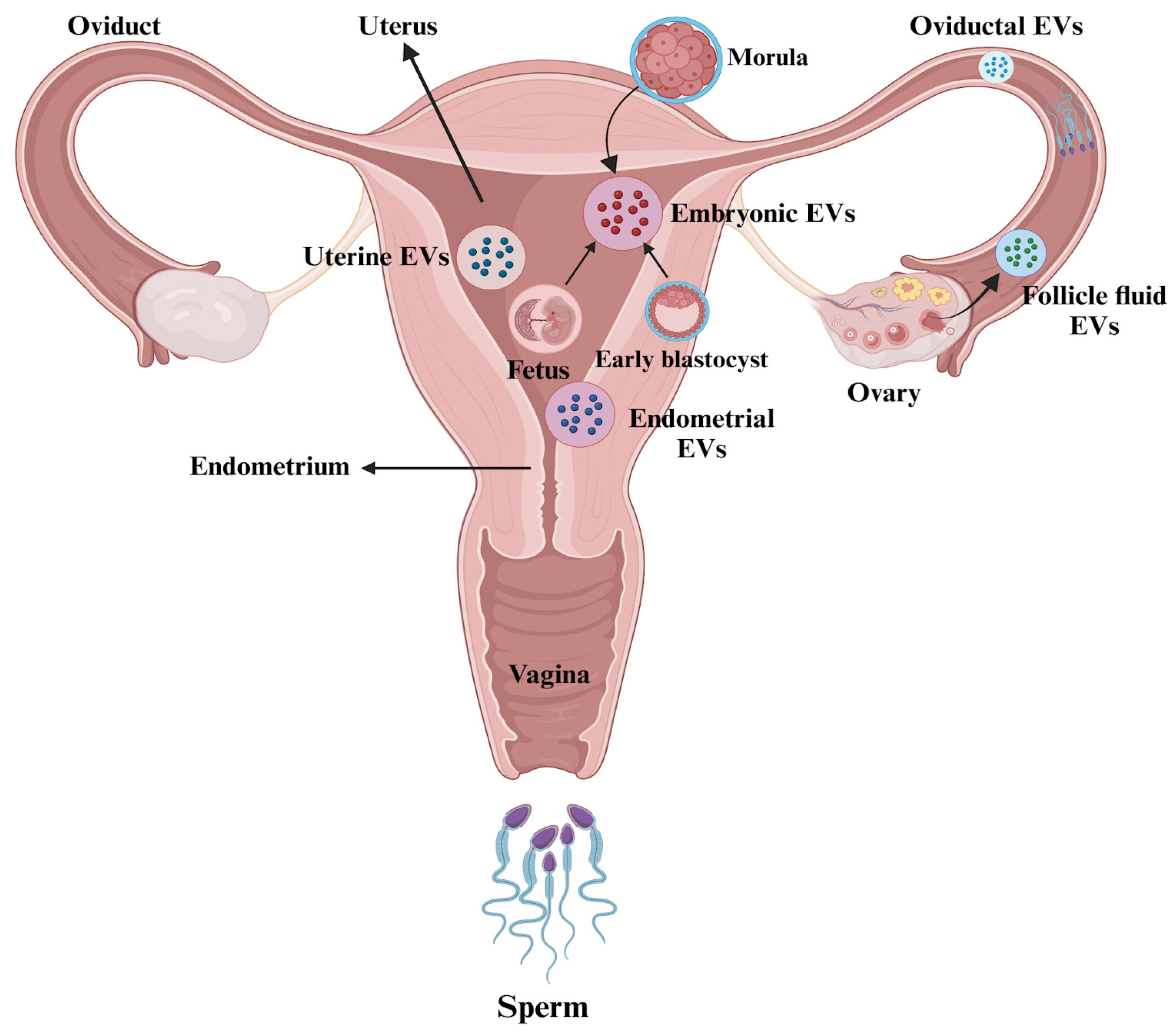
Overall, these findings highlight the significant functional roles of EVs in follicular growth, the modulation of embryo development, and the interactions between the embryo and the oviduct, all of which can enhance fertilization and embryo quality. It is critical to consider ways to improve the application of EVs in ART. Figure 3 illustrates the presence of EVs in the female reproductive tract.
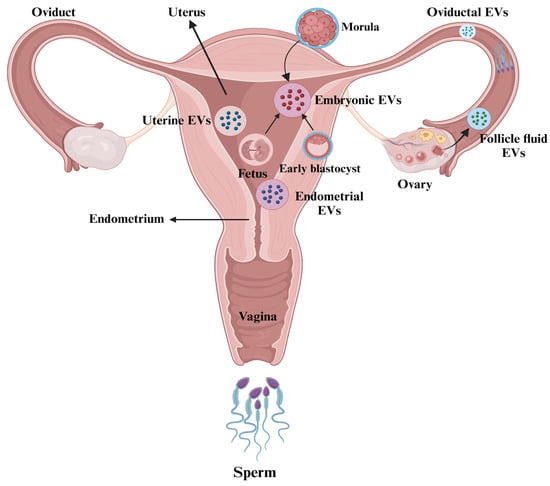
Figure 3.
Extracellular vesicles (EVs) in the female reproductive tract. The figure is created using BioRender.com and adapted from [97].
4.3. Impacts of EVs on Sperm Function
EVs in the female reproductive tract support sperm function, which is essential for various biological processes in female reproduction. Effective cell-to-cell communication within this microenvironment is crucial for developing oocytes and follicles, potentially facilitated by the secretion and uptake of EVs [83]. EVs in follicular fluid facilitate the transfer of miRNAs and proteins between cells. Sohel et al. [83] reported an upregulation of numerous miRNAs in the exosome fraction of follicular fluid, suggesting increased transcriptional activity during oocyte growth. These exosomes regulate several signaling pathways, including ubiquitin-mediated, neurotrophin, MAPK, and insulin signaling, important for ovarian follicular development and other developmental processes. Moreover, exosomes derived from follicular fluid influence the expression of ACVR1 and ID2 in cultured granulosa cells by transferring mRNAs, proteins, and miRNAs, thereby affecting follicular development [98].
In oviductal fluid across various species, EVs contain proteins and RNAs (mRNA, miRNA, rRNA, tRNA) critical for fertilization processes [34]. Research has shown that EVs from porcine oviductal fluid enhance the efficiency of in vitro fertilization by improving parameters such as sperm viability, motility, capacitation, and sperm binding while reducing the likelihood of polyspermy. These beneficial effects are attributed to proteins such as OVGP1, MYH9, ANXA1, 4, and 5 [99,100]. Additionally, oviductal EVs have been found to improve sperm viability when combined with frozen semen, with the degree of effectiveness varying according to the stage of the estrous cycle [100].
In domestic cats, it was found that oviductal EVs enhance sperm motility and acrosomal integrity when pre-incubated with fresh epididymal spermatozoa. This effect is mediated by proteins such as OVGP1, HSP70, HSP90, CD9, CCT8, and CCT7 [101]. Additionally, research demonstrated that adding oviductal EVs improves in vitro embryo development in pigs and influences the embryonic transcriptome, thereby highlighting the maternal impact at the molecular level [102]. Furthermore, EVs found in uterine fluid play crucial roles in somatic cell nuclear transfer (SCNT) embryo development during the early luteal phase [103]. Recent studies have also isolated EVs from human uterine fluid, showing their rapid interaction with sperm, which promotes capacitation, enhances motility, and induces the acrosome reaction [104].
During the early stages of pregnancy in pigs, EVs derived from uterine luminal fluid (ULF) have been shown to contain miRNAs that regulate EV transportation and conceptus development [105]. These ULF EVs regulate the proliferation and migration of embryonic trophoblast cells, which are essential for successful implantation. Overall, these studies underscore the functional roles of EVs in the female reproductive system and elucidate their significance in reproductive processes such as sperm function, gamete maturation, fertilization, implantation, and embryo development.
4.4. Impacts of EVs on Embryo–Oviduct–Endometrium Interactions
There is a growing interest in the role of EVs in gamete and embryo–maternal communication, particularly concerning their implications in embryo–oviduct interactions. The oviduct facilitates gamete maturation, sperm transport, capacitation, fertilization, and early embryo development. Oviductal EVs (oEVs) and embryo-secreted EVs (eEVs) have emerged as key participants in the bidirectional communication between the oviduct and the embryo [106]. Numerous studies have highlighted this interaction, demonstrating that oEVs are internalized by embryos at the blastocyst stage in vitro [18], as well as by oviductal epithelial cells [107], enhancing embryo implantation, development, and overall quality [18,20,108]. Analyses of these vesicles in mice have identified specific miRNAs such as miR-143-3p, miR-22-3p, and miR-34c-5p, which are crucial for the first cleavage and implantation of mouse embryos [109]. In bovine models, EVs are enriched with mRNAs that include genes for histone methyltransferases, histone demethylases, and the DNA methyltransferase gene (DNMT1), all of which are associated with embryo development, cell proliferation, and epigenetic regulation [34]. Furthermore, miR-449a, which is upregulated in oEVs during the post-ovulatory period, has been linked to various factors contributing to infertility in both men and women. Target analysis of miR-449a revealed a network of genes involved in embryo development, angiogenesis, and responses to oxidative stress, suggesting its significance in achieving a successful pregnancy [34].
4.4.1. Embryo-Secreted EVs
Exosomes derived from the ovine conceptus trophectoderm at 15 and 17 days of gestation contain several crucial proteins, including interferon tau (IFNT), macrophage-capping protein (CAPG), and aldo-keto reductase family 1, member B1 (AKR1B1) [110]. Treatment of endometrial epithelial cells with these exosomes during the pre-implantation period (P17 UFs) resulted in an upregulation of apoptosis-related genes, such as BCL2-associated X, apoptosis regulator (BAX), caspase 3 (CASP3), tumor necrosis factor (TNFA), and tumor protein P53 (TP53) transcripts [111]. Furthermore, exosomes from the post-implantation phase (P20 and P22 UFs) stimulated the expression of adhesion molecules, notably vascular cell adhesion molecule 1 (VCAM1) mRNA [112]. These findings highlight the critical role of exosome-mediated changes in the uterine environment for the attachment and development of the conceptus. Conversely, exosomes from porcine trophectoderm and aortic endothelial cells contain miRNAs that are predicted to influence angiogenesis and placental development pathways, indicating their significant role in communication between the conceptus and the maternal endometrium, ultimately impacting the establishment of pregnancy [113].
4.4.2. Embryo-Secreted EVs Influenced by Culture Conditions
The secretion of embryo-secreted extracellular vesicles (eEVs) varies according to the biotechnology utilized for embryo production. A study by [111] observed differences in the size and concentration of eEVs between parthenogenetic (PA) and in vitro fertilization (IVF) embryos. Recent research also suggests that the concentration of eEVs is influenced by the embryo’s developmental stage, sex, and the in vitro culture (IVC) conditions. Moreover, in vitro-produced bovine embryos were found to secrete more eEVs than their in vivo counterparts from day 7 to day 9 of development. However, the size of the vesicles remained comparable between the two groups [96]. Notably, fourteen miRNAs were upregulated, while two were downregulated in EVs from in vivo embryos compared to those from in vitro embryos. These miRNAs are predicted to regulate key pathways involved in pregnancy establishment in cattle, including oxytocin signaling, MAPK, Ras, glycerophospholipid metabolism, lysine degradation, HIF-1, and the WNT pathway [96]. Additionally, a higher concentration of eEVs was observed in day 7 bovine embryos compared to day 3 under 20% oxygen tension, indicating a positive correlation with successful pregnancy outcomes [114]. Conversely, a lower concentration of eEVs in culture media was associated with the failure to achieve pregnancies, in contrast to embryos with higher eEV concentrations [115].
Furthermore, a study utilizing a co-culture system to examine paracrine communication between porcine embryos—specifically parthenogenetic (PA) embryos and cloned embryos produced through somatic cell nuclear transfer (SCNT)—demonstrated that co-culturing PA embryos significantly enhanced the in vitro development of cloned embryos. This enhancement was linked to the upregulation of mRNA expression for key pluripotency markers, such as OCT4, KLF4, and NANOG, which are contained within EVs [116]. EVs derived from oviductal and uterine fluids closely mimic the physiological conditions present during the in vitro culture (IVC) of bovine embryos. A recent study found that incorporating these EVs into IVC systems did not significantly alter embryo development rates; however, it markedly improved blastocyst quality. Notable enhancements included higher survival rates following vitrification and warming, increased total cell numbers, and improved lipid metabolism [117]. These findings highlight the crucial role of EVs from embryos of different origins—oviductal fluid (OF) and uterine fluid (UF)—as key modulators of embryo quality in both swine and bovine IVC systems. These observations underscore the crucial roles of embryonic EVs in embryo development and inter-embryo communication. Table 1 illustrates the multifunctional roles of male and female EVs in mammalian female reproduction.

Table 1.
Examples of multifunctional roles of male and female extracellular vesicles in mammalian female reproduction.
5. Possible Therapeutic Applications of Extracellular Vesicles in Treating Infertility
Infertility is a widespread reproductive condition that increasingly affects both human health and the livestock industry on a global scale [136]. EVs have emerged as significant mediators of intercellular communication, playing essential roles in various physiological processes related to mammalian reproduction [137,138]. Numerous studies highlight the potential of EVs as therapeutic agents and diagnostic tools in ARTs, which could revolutionize reproductive health [139]. Recent investigations in mares have identified multiple miRNAs derived from FF-EVs associated with distinct stages of follicle development and variations between breeding and non-breeding seasons [140,141]. These specific miRNA profiles may be potential biomarkers to enhance fertility outcomes through improved ART practices. Research has also focused on the use of EVs as biomarkers for various fertility disorders, including polycystic ovary syndrome (PCOS), endometriosis, Asherman’s syndrome, and preeclampsia [142,143,144]. For example, EVs derived from human follicular fluid contain small RNAs that may play a role in the pathogenesis of PCOS, thus serving as molecular biomarkers for its diagnosis [145]. Furthermore, one study found that platelet-derived EVs were elevated in the plasma of women with PCOS, correlating with increased serum testosterone levels [146].
EVs have shown considerable potential in addressing primary ovarian insufficiency (POI). Research indicates that EV-derived miRNAs are associated with the progression and treatment of POI. For instance, a study highlighted that EVs from human adipose mesenchymal stem cells (hAMSCs) can mitigate ovarian function damage through the SMAD signaling pathway in mouse models of POI [147]. Similarly, EVs from human umbilical cord mesenchymal stem cells (hUMSCs), which encapsulate miR-17-5p, have demonstrated therapeutic potential by suppressing PARP1, γH2AX, and XRCC6 via SIRT7 inhibition [148]. Additionally, research has shown that endometriosis stromal cells can enhance angiogenesis in vitro through the EVs they secrete, further influencing angiogenic processes by regulating endothelial and stromal cells [149,150].
Regarding male fertility, numerous studies have identified exosome-related proteins, particularly exosomal annexin II, as potential biomarkers for male reproductive disorders [151,152]. Moreover, the prostaglandin D2 synthase (PTGDS) gene, which codes for an enzyme, was found to be significantly lower in patients with obstructive azoospermia compared to those with non-obstructive azoospermia (NOA), underlining its potential as a diagnostic biomarker for obstructive azoospermia [153]. Beyond the male reproductive tract, EVs derived from normozoospermic men, including those post-vasectomy, have been shown to significantly enhance sperm motility. In contrast, EVs from asthenozoospermic men appear to impair motility, highlighting their potential implications for male fertility and therapeutic applications [154].
Small EVs or exosomes have gained significant attention as therapeutic drug delivery systems due to their stability, low immunogenicity, and potential for bioengineering [155]. Recent advancements in nanotechnology have facilitated the encapsulation of therapeutic agents, such as miRNAs and small molecules, within EVs and their modification with various ligands for targeted delivery [155,156]. While research into the role of EVs in reproductive disorders is progressing rapidly, further investigation is essential, and these findings open up promising opportunities for clinical applications.
6. Conclusions
This review underscores the crucial role of extracellular vesicles (EVs) as emerging tools for identifying specific molecular markers linked to mammalian reproduction. EVs transport diverse biomolecules essential for cellular communication and gamete function. They are instrumental in various reproductive processes, including sperm motility, maturation, fertilization, embryo development, and implantation. The impact of EVs derived from in vivo, in vitro, or cloned embryos highlights their potential as biomarkers for evaluating embryo quality and pregnancy potential. However, despite their significance, further mechanistic studies are necessary to gain a comprehensive understanding of the physiological roles of EVs in both male and female reproductive systems, their origins, and the specific alterations in their cargo within target tissues. These insights could elucidate the molecular mechanisms governing EVs and pave the way for innovative therapeutic strategies to improve assisted reproductive techniques.
Author Contributions
Conceptualization, J.M.F. and J.C.d.S.; methodology, N.H.D. and A.B.; investigation, N.H.D.; resources, J.M.F. and J.C.d.S.; writing—original draft preparation, N.H.D.; writing—review and editing, N.H.D., A.B., J.C.d.S. and J.M.F.; supervision, J.M.F.; funding acquisition, J.M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch project accession #1016077 (Multistate #W4171 and NCERA-57), USDA-ARS Biophotonics (grant#6066-31000-015-00D)” and the authors would like to thank the National Council for Scientific and Technological Development—CNPq (grant number #420152/2018-0) and research fellowship of J.C.S (308101/2021-9); the São Paulo Research Foundation—FAPESP (grant number #2021/06645-0); this study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
There are no generated data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Vyt, P.; Maes, D.; Rijsselaere, T.; Dewulf, J.; de Kruif, A.; Van Soom, A. Semen handling in porcine AI centers: The Belgian situation. Vlaams Diergeneeskd. Tijdschr. 2007, 76, 195–200. [Google Scholar] [CrossRef]
- Maes, D.; Nauwynck, H.; Rijsselaere, T.; Mateusen, B.; Vyt, P.; de Kruif, A.; Van Soom, A. Diseases in swine transmitted by artificial insemination: An overview. Theriogenology 2008, 70, 1337–1345. [Google Scholar] [CrossRef]
- Schulze, M.; Buder, S.; Rüdiger, K.; Beyerbach, M.; Waberski, D. Influences on semen traits used for selection of young AI boars. Anim. Reprod. Sci. 2014, 148, 164–170. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef]
- Roca, J.; Rodriguez-Martinez, H.; Padilla, L.; Lucas, X.; Barranco, I. Extracellular vesicles in seminal fluid and effects on male reproduction. An overview in farm animals and pets. Anim. Reprod. Sci. 2021, 16, 106853. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Martinez, H.; Roca, J. Extracellular vesicles in seminal plasma: A safe and relevant tool to improve fertility in livestock? Anim. Reprod. Sci. 2022, 244, 107051. [Google Scholar] [CrossRef] [PubMed]
- Holm, P.; Callesen, H. In vivo versus in vitro produced bovine ova: Similarities and differences relevant for practical application. Reprod. Nutr. Dev. 1998, 38, 579–594. [Google Scholar] [CrossRef]
- Al-Dossary, A.A.; Strehler, E.E.; Martin-DeLeon, P.A. Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: Association with oviductal exosomes and uptake in sperm. PLoS ONE 2013, 8, e80181. [Google Scholar] [CrossRef]
- Li, S.; Winuthayanon, W. Oviduct: Roles in fertilization and early embryo development. J. Endocrinol. 2017, 232, R1–R26. [Google Scholar] [CrossRef]
- Memili, E.; Dominko, T.; First, N.L. Onset of transcription in bovine oocytes and preimplantation embryos. Mol. Reprod. Dev. Inc. Gamete Res. 1998, 51, 36–41. [Google Scholar] [CrossRef]
- da Silveira, J.C.; Veeramachaneni, D.N.R.; Winger, Q.A.; Carnevale, E.M.; Bouma, G.J. Cell-Secreted Vesicles in Equine Ovarian Follicular Fluid Contain miRNAs and Proteins: A Possible New Form of Cell Communication Within the Ovarian Follicle. Biol. Reprod. 2012, 86, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Simpson, R.J.; Jensen, S.S.; Lim, J.W. Proteomic profiling of exosomes: Current perspectives. Proteomics 2008, 8, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Shen, J.; Wang, Y.; Pan, C.; Pang, W.; Diao, H.; Dong, W. Boar seminal plasma exosomes maintain sperm function by infiltrating into the sperm membrane. Oncotarget 2016, 7, 58832. [Google Scholar] [CrossRef]
- Ruiz-González, I.; Xu, J.; Wang, X.; Burghardt, R.C.; Dunlap, K.A.; Bazer, F.W. Exosomes, endogenous retroviruses and toll-like receptors: Pregnancy recognition in ewes. Reproduction 2015, 149, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.T.; Hong, X.; Christenson, L.K.; McGinnis, L.K. Extracellular Vesicles from Bovine Follicular Fluid Support Cumulus Expansion. Biol. Reprod. 2015, 93, 117. [Google Scholar] [CrossRef] [PubMed]
- de Ávila, A.C.F.C.M.; Bridi, A.; Andrade, G.M.; del Collado, M.; Sangalli, J.R.; Nociti, R.P.; da Silva Junior, W.A.; Bastien, A.; Robert, C.; Meirelles, F.V. Estrous cycle impacts microRNA content in extracellular vesicles that modulate bovine cumulus cell transcripts during in vitro maturation. Biol. Reprod. 2020, 102, 362–375. [Google Scholar] [CrossRef]
- Almiñana, C.; Corbin, E.; Tsikis, G.; Alcântara-Neto, A.S.; Labas, V.; Reynaud, K.; Galio, L.; Uzbekov, R.; Garanina, A.S.; Druart, X.; et al. Oviduct extracellular vesicles protein content and their role during oviduct–embryo cross-talk. Reproduction 2017, 154, 153–168. [Google Scholar] [CrossRef]
- Burns, G.W.; Brooks, K.E.; O’Neil, E.V.; Hagen, D.E.; Behura, S.K.; Spencer, T.E. Progesterone effects on extracellular vesicles in the sheep uterus. Biol. Reprod. 2018, 98, 612–622. [Google Scholar] [CrossRef]
- Lopera-Vasquez, R.; Hamdi, M.; Fernandez-Fuertes, B.; Maillo, V.; Beltran-Brena, P.; Calle, A.; Redruello, A.; Lopez-Martin, S.; Gutierrez-Adan, A.; Yanez-Mo, M.; et al. Extracellular vesicles from BOEC in in vitro embryo development and quality. PLoS ONE 2016, 11, e0148083. [Google Scholar] [CrossRef]
- Betteridge, K.; Fléchon, J.-E. The anatomy and physiology of pre-attachment bovine embryos. Theriogenology 1988, 29, 155–187. [Google Scholar] [CrossRef]
- Spencer, T.E. Early pregnancy: Concepts, challenges, and potential solutions. Anim. Front. 2013, 3, 48–55. [Google Scholar] [CrossRef]
- Guillomot, M. Cellular interactions during implantation in domestic ruminants. J. Reprod. Fertil.-Suppl. Only 1995, 49, 39–52. [Google Scholar] [CrossRef]
- Forde, N.; Lonergan, P. Transcriptomic analysis of the bovine endometrium: What is required to establish uterine receptivity to implantation in cattle? J. Reprod. Dev. 2012, 58, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Oh, H.J.; Lee, B.C. Embryonic–maternal cross-talk via exosomes: Potential implications. Stem Cells Cloning Adv. Appl. 2015, 8, 103–107. [Google Scholar]
- Margolis, L.; Sadovsky, Y. The biology of extracellular vesicles: The known unknowns. PLoS Biol. 2019, 17, e3000363. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Stahl, P.D.; Raposo, G. Extracellular vesicles: Exosomes and microvesicles, integrators of homeostasis. Physiology 2019, 34, 169–177. [Google Scholar] [CrossRef]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Denzer, K.; Kleijmeer, M.J.; Heijnen, H.F.; Stoorvogel, W.; Geuze, H.J. Exosome: From internal vesicle of the multivesicular body to intercellular signaling device. J. Cell Sci. 2000, 113, 3365–3374. [Google Scholar] [CrossRef] [PubMed]
- Dickhout, A.; Koenen, R.R. Extracellular vesicles as biomarkers in cardiovascular disease; chances and risks. Front. Cardiovasc. Med. 2018, 5, 113. [Google Scholar] [CrossRef]
- Saez, F.; Frenette, G.; Sullivan, R. Epididymosomes and prostasomes: Their roles in posttesticular maturation of the sperm cells. J. Androl. 2003, 24, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, G.; Larsson, A.; Stavreus-Evers, A.; Ronquist, G. Prostasomes are heterogeneous regarding size and appearance but affiliated to one DNA-containing exosome family. Prostate 2012, 72, 1736–1745. [Google Scholar] [CrossRef] [PubMed]
- Almiñana, C.; Tsikis, G.; Labas, V.; Uzbekov, R.; da Silveira, J.C.; Bauersachs, S.; Mermillod, P. Deciphering the oviductal extracellular vesicles content across the estrous cycle: Implications for the gametes–oviduct interactions and the environment of the potential embryo. BMC Genom. 2018, 19, 622. [Google Scholar] [CrossRef] [PubMed]
- Tamessar, C.T.; Trigg, N.A.; Nixon, B.; Skerrett-Byrne, D.A.; Sharkey, D.J.; Robertson, S.A.; Bromfield, E.G.; Schjenken, J.E. Roles of male reproductive tract extracellular vesicles in reproduction. Am. J. Reprod. Immunol. 2021, 85, e13338. [Google Scholar] [CrossRef] [PubMed]
- Wubbolts, R.; Leckie, R.S.; Veenhuizen, P.T.; Schwarzmann, G.; Mobius, W.; Hoernschemeyer, J.; Slot, J.W.; Geuze, H.J.; Stoorvogel, W. Potential implications for their function and multivesicular body formation:Proteomic and biochemical analyses of human B cell-derived exosomes. J. Biol. Chem. 2003, 278, 10963–10972. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Saez, F. Epididymosomes, prostasomes, and liposomes: Their roles in mammalian male reproductive physiology. Reproduction 2013, 146, R21–R35. [Google Scholar] [CrossRef]
- Caballero, J.; Frenette, G.; Sullivan, R. Post testicular sperm maturational changes in the bull: Important role of the epididymosomes and prostasomes. Vet. Med. Int. 2011, 2011, 757194. [Google Scholar] [CrossRef] [PubMed]
- Metz, C.B.; Hinsch, G.W.; Anika, J.L. Ultrastructure and antigens of particles from rabbit semen. J. Reprod. Fertil. 1968, 17, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Yanagimachi, R.; Kamiguchi, Y.; Mikamo, K.; Suzuki, F.; Yanagimachi, H. Maturation of spermatozoa in the epididymis of the Chinese hamster. Am. J. Anat. 1985, 172, 317–330. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Saez, F.; Girouard, J.; Frenette, G. Role of exosomes in sperm maturation during the transit along the male reproductive tract. Blood Cells Mol. Dis. 2005, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Girouard, J.; Frenette, G.; Sullivan, R. Compartmentalization of proteins in epididymosomes coordinates the association of epididymal proteins with the different functional structures of bovine spermatozoa. Biol. Reprod. 2009, 80, 965–972. [Google Scholar] [CrossRef][Green Version]
- Rejraji, H.; Vernet, P.; Drevet, J. GPX5 is present in the mouse caput and cauda epididymidis lumen at three different locations. Mol. Reprod. Dev. 2002, 63, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Frenette, G.; Lessard, C.; Sullivan, R. Polyol pathway along the bovine epididymis. Mol. Reprod. Dev. 2004, 69, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Krapf, D.; Ruan, Y.C.; Wertheimer, E.V.; Battistone, M.A.; Pawlak, J.B.; Sanjay, A.; Pilder, S.H.; Cuasnicu, P.; Breton, S.; Visconti, P.E. cSrc is necessary for epididymal development and is incorporated into sperm during epididymal transit. Dev. Biol. 2012, 369, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Joshi, C.S.; Suryawanshi, A.R.; Khan, S.A.; Balasinor, N.H.; Khole, V.V. Liprin α3: A putative estrogen regulated acrosomal protein. Histochem. Cell Biol. 2013, 139, 535–548. [Google Scholar] [CrossRef]
- Sullivan, R. Interaction between sperm and epididymal secretory proteins. In The Male Gamete: From Basic to Clinical Applications; Cache River Pr: Vienna, IL, USA, 1999; pp. 130–136. [Google Scholar]
- Légaré, C.; Bérubé, B.; Boué, F.; Lefièvre, L.; Morales, C.R.; El-Alfy, M.; Sullivan, R. Hamster sperm antigen P26h is a phosphatidylinositol-anchored protein. Mol. Reprod. Dev. Inc. Gamete Res. 1999, 52, 225–233. [Google Scholar] [CrossRef]
- Frenette, G.; Sullivan, R. Prostasome-like particles are involved in the transfer of P25b from the bovine epididymal fluid to the sperm surface. Mol. Reprod. Dev. Inc. Gamete Res. 2001, 59, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.S.; Han, C.; Cho, C. ADAM7 is associated with epididymosomes and integrated into sperm plasma membrane. Mol. Cells 2009, 28, 441–446. [Google Scholar]
- Reilly, J.N.; Anderson, A.L.; Hutcheon, K.; Church, K.; Mihalas, B.P.; Tyagi, S.; Holt, J.E.; Eamens, A.L. Characterisation of mouse epididymosomes reveals a complex profile of microRNAs and a potential mechanism for modification of the sperm epigenome. Sci. Rep. 2016, 6, 31794. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Stanger, S.J.; Mihalas, B.P.; Reilly, J.N.; Anderson, A.L.; Tyagi, S.; Holt, J.E.; McLaughlin, E.A. The microRNA signature of mouse spermatozoa is substantially modified during epididymal maturation. Biol. Reprod. 2015, 93, 1–20. [Google Scholar] [CrossRef]
- Belleannée, C. Extracellular microRNAs from the epididymis as potential mediators of cell-to-cell communication. Asian J. Androl. 2015, 17, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, G.; Brody, I.; Gottfries, A.; Stegmayr, B. An Mg2+ and Ca2+-stimulated adenosine triphosphatase in human prostatic fluid: Part I. Andrologia 1978, 10, 261–272. [Google Scholar] [CrossRef]
- Utleg, A.; Yi, E.C.; Xie, T.; Shannon, P.; White, J.T.; Goodlett, D.R.; Hood, L.; Lin, B. Proteomic analysis of human prostasomes. Prostate 2003, 56, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Nilsson, O.; Larsson, A.; Stridsberg, M.; Sahlen, G.; Ronquist, G. Antibacterial activity of human prostasomes. Prostate 2000, 44, 279–286. [Google Scholar] [CrossRef]
- Pons-Rejraji, H.; Artonne, C.; Sion, B.; Brugnon, F.; Canis, M.; Janny, L.; Grizard, G. Prostasomes: Inhibitors of capacitation and modulators of cellular signalling in human sperm. Int. J. Androl. 2011, 34 Pt 1, 568–580. [Google Scholar] [CrossRef]
- Madison, M.N.; Roller, R.J.; Okeoma, C.M. Human semen contains exosomes with potent anti-HIV-1 activity. Retrovirology 2014, 11, 102. [Google Scholar] [CrossRef] [PubMed]
- Machtinger, R.; Laurent, L.C.; Baccarelli, A.A. Extracellular vesicles: Roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 2016, 22, 182–193. [Google Scholar] [CrossRef]
- Carlsson, L.; Nilsson, O.; Larsson, A.; Stridsberg, M.; Sahlen, G.; Ronquist, G. Characteristics of human prostasomes isolated from three different sources. Prostate 2003, 54, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; Ronquist, G. Association of some hydrolytic enzymes with the prostasome membrane and their differential responses to detergent and PIPLC treatment. Prostate 1995, 27, 95–101. [Google Scholar] [CrossRef]
- Park, K.H.; Kim, B.J.; Kang, J.; Nam, T.S.; Lim, J.M.; Kim, H.T.; Park, J.K.; Kim, Y.G.; Chae, S.W.; Kim, U.H. Ca2+ signaling tools acquired from prostasomes are required for progesterone-induced sperm motility. Sci. Signal. 2011, 4, 31. [Google Scholar] [CrossRef]
- Höög, J.L.; Lötvall, J.J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef] [PubMed]
- Arienti, G.; Carlini, E.; Nicolucci, A.; Cosmi, E.V.; Santi, F.; Palmerini, C.A. The motility of human spermatozoa as influenced by prostasomes at various pH levels. Biol. Cell 1999, 91, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Murdica, V.; Giacomini, E.; Alteri, A.; Bartolacci, A.; Cermisoni, G.C.; Zarovni, N.; Papaleo, E.; Montorsi, F.; Salonia, A.; Viganò, P. Seminal plasma of men with severe asthenozoospermia contain exosomes that affect spermatozoa motility and capacitation. Fertil. Steril. 2019, 111, 897–908.e2. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Rickard, J.P.; Pini, T.; Gadella, B.M.; de Graaf, S.P. Quantitative proteomic analysis of seminal plasma, sperm membrane proteins, and seminal extracellular vesicles suggests vesicular mechanisms aid in the removal and addition of proteins to the ram sperm membrane. Proteomics 2020, 20, 1900289. [Google Scholar] [CrossRef] [PubMed]
- Belleannée, C.; Calvo, É.; Caballero, J.; Sullivan, R. Epididymosomes convey different repertoires of microRNAs throughout the bovine epididymis. Biol. Reprod. 2013, 89, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rodriguez, M.; Ntzouni, M.; Wright, D.; Khan, K.I.; Lopez-B´ejar, M.; Martinez, C.A.; Rodriguez-Martinez, H. Chicken seminal fluid lacks CD9- and CD44-bearing extracellular vesicles. Reprod. Domest. Anim. 2020, 55, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xie, Y.; Zhou, C.; Hu, Q.; Gu, T.; Yang, J.; Zheng, E.; Huang, S.; Xu, Z.; Cai, G. Expression pattern of seminal plasma extracellular vesicle small RNAs in boar semen. Front. Vet. Sci. 2020, 7, 585276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, N.; Xie, S.; Ding, Y.; Huang, M.; Ding, X.; Jiang, L. Identification of crucial extracellular vesicle RNA molecules related to sperm motility and prostate cancer. Extracell. Vesicles Circ. Nucleic Acids 2021, 2, 104–126. [Google Scholar]
- Hessvik, N.P.; Phuyal, S.; Brech, A.; Sandvig, K.; Llorente, A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim. Biophys. Acta 2012, 1819, 1154–1163. [Google Scholar] [CrossRef]
- McKenzie, A.J.; Hoshino, D.; Hong, N.H. KRAS-MEK Signaling Controls Ago2 Sorting into Exosomes. Cell Rep. 2016, 15, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.C.; Torres, M.A.; Alkmin, D.V.; Pinzon, J.E.; Martins, S.M.M.K.; da Silveira, J.C.; de Andrade, A.F.C. Spermatozoa and seminal plasma small extracellular vesicles miRNAs as biomarkers of boar semen cryotolerance. Theriogenology 2021, 174, 60–72. [Google Scholar] [CrossRef]
- Dlamini, N.H.; Nguyen, T.; Gad, A.; Tesfaye, D.; Liao, S.F.; Willard, S.T.; Ryan, P.L.; Feugang, J.M. Characterization of extracellular vesicle-coupled miRNA profiles in seminal plasma of boars with divergent semen quality status. Int. J. Mol. Sci. 2023, 24, 3194. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Ding, N.; Zhang, Y.; Xie, S.; Huang, M.; Ding, X.; Dong, W.; Zhang, Q.; Jiang, L. MicroRNA-222 Transferred From Semen Extracellular Vesicles Inhibits Sperm Apoptosis by Targeting BCL2L11. Front. Cell Dev. Biol. 2021, 9, 736864. [Google Scholar] [CrossRef] [PubMed]
- Werry, N.; Russell, S.J.; Gillis, D.J.; Miller, S.; Hickey, K.; Larmer, S.; Lohuis, M.; Librach, C.; LaMarre, J. Characteristics of miRNAs Present in Bovine Sperm and Associations with Differences in Fertility. Front. Endocrinol. 2022, 13, 874371. [Google Scholar] [CrossRef] [PubMed]
- Barceló, M.; Mata, A.; Bassas, L.; Larriba, S. Exosomal microRNAs in seminal plasma are markers of the origin of azoospermia and can predict the presence of sperm in testicular tissue. Human Reproduction 2018, 33, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qin, J.; Sun, J.; He, J.; Sun, Y.; Yuan, R.; Li, Z. Motility-related microRNAs identified in pig seminal plasma exosomes by high-throughput small RNA sequencing. Theriogenology 2024, 215, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Almog, T.; Naor, Z. The role of Mitogen activated protein kinase (MAPK) in sperm functions. Mol. Cell. Endocrinol. 2010, 314, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.; Lin, H.L.H.; Carvalho, A.V.; Grasseau, I.; Uzbekov, R.; Blesbois, E. First insights on seminal extracellular vesicles in chickens of contrasted fertility. Reproduction 2021, 161, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Huang, L.; Guo, Y.; Yang, Y.; Gong, P.; Ye, S.; Wang, L.; Feng, Y. Identification of potential candidate miRNAs related to semen quality in seminal plasma extracellular vesicles and sperms of male duck (Anas Platyrhynchos). Poult. Sci. 2024, 103, 103928. [Google Scholar] [CrossRef]
- Guo, H.; Chang, Z.; Zhang, Z.; Zhao, Y.; Jiang, X.; Yu, H.; Zhang, Y.; Zhao, R.; He, B. Extracellular ATPs produced in seminal plasma exosomes regulate boar sperm motility and mitochondrial metabolism. Theriogenology 2019, 139, 113–120. [Google Scholar] [CrossRef]
- Sohel, M.M.H.; Hoelker, M.; Noferesti, S.S.; Salilew-Wondim, D.; Tholen, E.; Looft, C.; Rings, F.; Uddin, M.J.; Spencer, T.E.; Schellander, K.; et al. Exosomal and non-exosomal transport of extra-cellular microRNAs in follicular fluid: Implications for bovine oocyte developmental competence. PLoS ONE 2013, 8, e78505. [Google Scholar] [CrossRef] [PubMed]
- Navakanitworakul, R.; Hung, W.T.; Gunewardena, S.; Davis, J.S.; Chotigeat, W.; Christenson, L.K. Characterization and small RNA content of extracellular vesicles in follicular fluid of developing bovine antral follicles. Sci. Rep. 2016, 6, 25486. [Google Scholar] [CrossRef]
- Martinez, R.M.; Liang, L.; Racowsky, C.; Dioni, L.; Mansur, A.; Adir, M.; Bollati, V.; Baccarelli, A.A.; Hauser, R.; Machtinger, R. Extracellular microRNAs profile in human follicular fluid and IVF outcomes. Sci. Rep. 2018, 8, 17036. [Google Scholar] [CrossRef] [PubMed]
- da Silva Rosa, P.M.; Bridi, A.; de Ávila, F.G.; Nociti, R.P.; Dos Santos, A.C.; Cataldi, T.R.; Dos Santos, G.; Chiaratti, M.R.; Silva, L.A.; Pugliesi, G. Corpus luteum proximity alters molecular signature of the small extracellular vesicles and cumulus cells in the bovine ovarian follicle environment. Mol. Cell. Endocrinol. 2024, 592, 112347. [Google Scholar] [CrossRef] [PubMed]
- Greening, D.W.; Nguyen, H.P.T.; Elgass, K.; Simpson, R.J.; Salamonsen, L.A. Human Endometrial Exosomes Contain Hormone-Specific Cargo Modulating Trophoblast Adhesive Capacity: Insights into Endometrial-Embryo Interactions. Biol. Reprod. 2016, 94, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Avilés, M.; Gutiérrez-Adán, A.; Coy, P. Oviductal secretions: Will they be key factors for the future ARTs? Mol. Hum. Reprod. 2010, 16, 896–906. [Google Scholar] [CrossRef]
- Mazzarella, R.; Bastos, N.M.; Bridi, A.; Del Collado, M.; Andrade, G.M.; Pinzon, J.; Prado, C.M.; Silva, L.A.; Meirelles, F.V.; Pugliesi, G. Changes in oviductal cells and small extracellular vesicles miRNAs in pregnant cows. Front. Vet. Sci. 2021, 8, 639752. [Google Scholar] [CrossRef] [PubMed]
- Lopera-Vasquez, R.; Hamdi, M.; Maillo, V.; Gutierrez-Adan, A.; Bermejo-Alvarez, P.; Ramírez, M.Á.; Yáñez-Mó, M.; Rizos, D. Effect of bovine oviductal extracellular vesicles on embryo development and quality in vitro. Reproduction 2017, 153, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Racicot, K.; Schmitt, A.; Ott, T. The myxovirus-resistance protein, MX 1, is a component of exosomes secreted by uterine epithelial cells. Am. J. Reprod. Immunol. 2012, 67, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, A. Maternal communication with gametes and embryo: A personal opinion. Reprod. Domest. Anim. 2011, 46, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.W.; Brooks, K.E.; Spencer, T.E. Extracellular vesicles originate from the conceptus and uterus during early pregnancy in sheep. Biol. Reprod. 2016, 94, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Burns, G.; Brooks, K.; Wildung, M.; Navakanitworakul, R.; Christenson, L.K.; Spencer, T.E. Extracellular vesicles in luminal fluid of the ovine uterus. PLoS ONE 2014, 9, e90913. [Google Scholar] [CrossRef]
- Bridi, A.; Sangalli, J.R.; Nociti, R.P.; dos Santos, A.C.; Alves, L.; Bastos, N.M.; Ferronato, G.D.Á.; Rosa, P.M.D.S.; Fiorenza, M.F.; Pugliesi, G.; et al. Small extracellular vesicles derived from the crosstalk between early embryos and the endometrium potentially mediate corpus luteum function. Biol. Reprod. 2025, 112, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Bridi, A.; Andrade, G.M.; Del Collado, M.; Sangalli, J.R.; de Ávila, A.C.; Motta, I.G.; da Silva, J.C.; Pugliesi, G.; Silva, L.A.; Meirelles, F.V.; et al. Small extracellular vesicles derived from in vivo-or in vitro-produced bovine blastocysts have different miRNAs profiles—Implications for embryo-maternal recognition. Mol. Reprod. Dev. 2021, 88, 628–643. [Google Scholar] [CrossRef]
- Afzal, A.; Khan, M.; Gul, Z.; Asif, R.; Shahzaman, S.; Parveen, A.; Imran, M.; Khawar, M.B. Extracellular Vesicles: The Next Frontier in Pregnancy Research. Reprod. Sci. 2024, 31, 1204–1214. [Google Scholar] [CrossRef]
- da Silveira, J.; Carnevale, E.M.; Winger, Q.A.; Bouma, G. Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod. Biol. Endocrinol. 2014, 12, 44. [Google Scholar] [CrossRef] [PubMed]
- Coy, P.; Cánovas, S.; Mondéjar, I.; Saavedra, M.D.; Romar, R.; Grullón, L.; Matás, C.; Avilés, M. Oviduct specific glycoprotein and heparin modulate sperm-zona pellucida interaction during fertilization and contribute to the control of polyspermy. Proc. Natl. Acad. Sci. USA 2008, 105, 15809–15814. [Google Scholar] [CrossRef] [PubMed]
- Alcântara-Neto, A.S.; Schmaltz, L.; Caldas, E.; Blache, M.C.; Mermillod, P.; Almiñana, C. Porcine oviductal extracellular vesicles interact with gametes and regulate sperm motility and survival. Theriogenology 2020, 155, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, M.; Carothers, A.; Dahal, R.; Noonan, M.J.; Songsasen, N. Oviductal extracellular vesicles interact with the spermatozoon’s head and mid-piece and improves its motility and fertilizing ability in the domestic cat. Sci. Rep. 2019, 9, 9484. [Google Scholar] [CrossRef] [PubMed]
- Alcântara-Neto, A.S.; Cuello, C.; Uzbekov, R.; Bauersachs, S.; Mermillod, P.; Almiñana, C. Oviductal extracellular vesicles enhance porcine in vitro embryo development by modulating the embryonic transcriptome. Biomolecules 2022, 12, 1300. [Google Scholar] [CrossRef] [PubMed]
- Kolakowska, J.; Souchelnytskyi, S.; Saini, R.K.R.; Franczak, A. Proteomic analysis of the endometrium during early pregnancy in the domestic pig. Reprod. Fertil. Dev. 2017, 29, 2255–2268. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Wu, Z.; He, C.; Lu, C.; He, D.; Li, X.; Duan, Z.; Zhao, H. Exosomes from uterine fluid promote capacitation of human sperm. PeerJ 2024, 12, e16875. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zang, X.; Ding, Y.; Gu, T.; Shi, J.; Li, Z.; Cai, G.; Liu, D.; Wu, Z.; Hong, L. Porcine uterine luminal fluid-derived extracellular vesicles improve conceptus-endometrial interaction during implantation. Theriogenology 2022, 178, 8–17. [Google Scholar] [CrossRef]
- Almiñana, C.; Bauersachs, S. Extracellular vesicles: Multi-signal messengers in the gametes/embryo-oviduct cross-talk. Theriogenology 2020, 150, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Pavani, K.C.; Hendrix, A.; Van Den Broeck, W.; Couck, L.; Szymanska, K.; Lin, X.; De Koster, J.; Van Soom, A.; Leemans, B. Isolation and characterization of functionally active extracellular vesicles from culture medium conditioned by bovine embryos in vitro. Int. J. Mol. Sci. 2018, 20, 38. [Google Scholar] [CrossRef]
- Qu, P.; Zhao, Y.; Wang, R.; Zhang, Y.; Li, L.; Fan, J.; Liu, E. Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice. Reprod. Fertil. Dev. 2019, 31, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Fereshteh, Z.; Schmidt, S.A.; Al-Dossary, A.A.; Accerbi, M.; Arighi, C.; Cowart, J.; Song, J.L.; Green, P.J.; Choi, K.; Yoo, S.; et al. Murine Oviductosomes (OVS) microRNA profiling during the estrous cycle: Delivery of OVS-borne microRNAs to sperm where miR-34c-5p localizes at the centrosome. Sci. Rep. 2018, 8, 16094. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kusama, K.; Bai, R.; Sakurai, T.; Isuzugawa, K.; Godkin, J.D.; Suda, Y.; Imakawa, K. Induction of IFNT-stimulated genes by conceptus-derived exosomes during the attachment period. PLoS ONE 2016, 11, e0158278. [Google Scholar] [CrossRef]
- Mellisho, E.A.; Velásquez, A.E.; Nuñez, M.J.; Cabezas, J.G.; Cueto, J.A.; Fader, C.; Castro, F.O.; Rodríguez-Álvarez, L. Identification and characteristics of extracellular vesicles from bovine blastocysts produced in vitro. PLoS ONE 2017, 12, e178306. [Google Scholar] [CrossRef] [PubMed]
- Kusama, K.; Nakamura, K.; Bai, R.; Nagaoka, K.; Sakurai, T.; Imakawa, K. Intrauterine exosomes are required for bovine conceptus implantation. Biochem. Biophys. Res. Commun. 2018, 495, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Bidarimath, M.; Khalaj, K.; Kridli, R.T.; Kan, F.W.K.; Koti, M.; Tayade, C. Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: A new paradigm for conceptus-endometrial cross-talk. Sci. Rep. 2017, 7, 40476. [Google Scholar] [CrossRef] [PubMed]
- Andrade, G.M.; Bomfim, M.M.; Del Collado, M.; Meirelles, F.V.; Perecin, F.; da Silveira, J.C. Oxygen tension modulates extracellular vesicles and its miRNA contents in bovine embryo culture medium. Mol. Reprod. Dev. Inc. Gamete Res. 2019, 86, 1067–1080. [Google Scholar] [CrossRef] [PubMed]
- Abu-Halima, M.; Häusler, S.; Backes, C.; Fehlmann, T.; Staib, C.; Nestel, S.; Nazarenko, I.; Meese, E.; Keller, A. Micro-ribonucleic acids and extracellular vesicles repertoire in the spent culture media is altered in women undergoing in vitro fertilization. Sci. Rep. 2017, 7, 13525. [Google Scholar] [CrossRef] [PubMed]
- Saadeldin, I.M.; Kim, S.J.; Choi, Y.B.; Lee, B.C. Improvement of cloned embryos development by co-culturing with parthenotes: A possible role of exosomes/microvesicles for embryos paracrine communication. Cell. Reprogram. 2014, 16, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.L.V.; Cañón-Beltrán, K.; Cajas, Y.N.; Hamdi, M.; Yaryes, A.; Millán de la Blanca, M.G.; Beltrán-Breña, P.; Mazzarella, R.; Da Silveira, J.C.; Gutiérrez-Adán, A. Extracellular vesicles from oviductal and uterine fluids supplementation in sequential in vitro culture improves bovine embryo quality. J. Anim. Sci. Biotechnol. 2022, 13, 116. [Google Scholar] [CrossRef]
- Mei, S.; Chen, P.; Lee, C.L.; Zhao, W.; Wang, Y.; Lam, K.K.W.; Ho, P.C.; Yeung, W.S.B.; Fang, C.; Chiu, P.C.N. The role of galectin-3 in spermatozoa-zona pellucida binding and its association with fertilization in vitro. Mol. Hum. Reprod. 2019, 25, 458–470. [Google Scholar] [CrossRef]
- Jena, S.R.; Nayak, J.; Kumar, S.; Kar, S.; Dixit, A.; Samanta, L. Paternal contributors in recurrent pregnancy loss: Cues from comparative proteome profiling of seminal extracellular vesicles. Mol. Reprod. Dev. 2021, 88, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Foot, N.J.; Gonzalez, M.B.; Gembus, K.; Fonseka, P.; Sandow, J.J.; Nguyen, T.T.; Tran, D.; Webb, A.I.; Mathivanan, S.; Robker, R.L. Arrdc4-dependent extracellular vesicle biogenesis is required for sperm maturation. J. Extracell. Vesicles 2021, 10, e12113. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jueraitetibaike, K.; Tang, T.; Wang, Y.; Jing, J.; Xue, T.; Ma, J.; Cao, S.; Lin, Y.; Li, X.; et al. Seminal plasma and seminal plasma exosomes of aged male mice affect early embryo implantation via immunomodulation. Front. Immunol. 2021, 12, 723409. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, L.; Marcianò, V.; Carpino, A. Prostasome-like vesicles stimulate acrosome reaction of pig spermatozoa. Reprod. Biol. Endocrinol. 2008, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, K.G.; Ek, B.; Morrell, J.; Stavreus-Evers, A.; Holst, B.S.; Humblot, P.; Ronquist, G.; Larsson, A. Prostasomes from four different species are able to produce extracellular adenosine triphosphate (ATP). Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 4604–4610. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Luo, S.; Du, Y.; Zhang, Y.; Song, X.; Yuan, X.; Lin, Z.; Li, Y.; Liu, E. Extracellular vesicles and melatonin benefit embryonic develop by regulating reactive oxygen species and 5-methylcytosine. J. Pineal Res. 2020, 68, e12635. [Google Scholar] [CrossRef]
- Alcântara-Neto, A.S.M.; Fernandez-Rufete, E.; Corbin, G.; Tsikis, R.; Uzbekov, A.S.; Garanina, P.; Coy, B.C.; Almiñana, A.F.; Mermillod, P. Oviduct fluid extracellular vesicles regulate polyspermy during porcine in vitro fertilization. Reprod. Fertil. Dev. 2020, 32, 409–418. [Google Scholar] [CrossRef]
- Bauersachs, S.; Mermillod, P.; Almiñana, C. The oviductal extracellular vesicles’ RNA cargo regulates the bovine embryonic transcriptome. Int. J. Mol. Sci. 2020, 21, 1303. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, Y.; Kanke, T.; Maruyama, N.; Fujii, W.; Naito, K.; Sugiura, K. Characterization of mRNA profiles of the exosome-like vesicles in porcine follicular fluid. PLoS ONE 2019, 14, e0217760. [Google Scholar] [CrossRef] [PubMed]
- da Silveira, J.C.; Andrade, G.M.; Del Collado, M.; Sampaio, R.V.; Sangalli, J.R.; Silva, L.A.; Pinaffi, F.V.L.; Jardim, I.B.; Cesar, M.C.; Nogueira, M.F.G. Supplementation with small-extracellular vesicles from ovarian follicular fluid during in vitro production modulates bovine embryo development. PLoS ONE 2017, 12, e0179451. [Google Scholar] [CrossRef] [PubMed]
- Hung, W.-T.; Navakanitworakul, R.; Khan, T.; Zhang, P.; Davis, J.S.; McGinnis, L.K.; Christenson, L.K. Stage-specific follicular extracellular vesicle uptake and regulation of bovine granulosa cell proliferation. Biol. Reprod. 2017, 97, 644–655. [Google Scholar] [CrossRef]
- da Silva Rosa, P.M.; Bridi, A.; de Ávila Ferronato, G.; Prado, C.M.; Bastos, N.M.; Sangalli, J.R.; Meirelles, F.V.; Perecin, F.; da Silveira, J.C. Corpus luteum presence in the bovine ovary increase intrafollicular progesterone concentration: Consequences in follicular cells gene expression and follicular fluid small extracellular vesicles miRNA contents. J. Ovarian Res. 2024, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Shi, S.; Liang, J.; Zhang, X.; Cao, D.; Wang, Z. MicroRNAs in small extracellular vesicles indicate successful embryo implantation during early pregnancy. Cells 2020, 9, 645. [Google Scholar] [CrossRef]
- Lv, C.; Yu, W.X.; Wang, Y.; Yi, D.J.; Zeng, M.H.; Xiao, H.M. MiR-21 in extracellular vesicles contributes to the growth of fertilized eggs and embryo development in mice. Biosci. Rep. 2018, 38, BSR20180036. [Google Scholar] [CrossRef] [PubMed]
- Qu, P.; Qing, S.; Liu, R.; Qin, H.; Wang, W.; Qiao, F.; Ge, H.; Liu, J.; Zhang, Y.; Cui, W. Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS ONE 2017, 12, e0174535. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Ge, H.; Ma, X.; Zhang, Y.; Zuo, Z.; Wang, M.; Zhang, Y.; Wang, Y. Bovine uterus-derived exosomes improve developmental competence of somatic cell nuclear transfer embryos. Theriogenology 2018, 114, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Matzuk, M.M.; Lamb, D.J. The biology of infertility: Research advances and clinical challenges. Nat. Med. 2008, 14, 1197–1213. [Google Scholar] [CrossRef] [PubMed]
- Munoz, E.L.; Fuentes, F.B.; Felmer, R.N.; Yeste, M.; Arias, M.E. Extracellular vesicles in mammalian reproduction: A review. Zygote 2022, 30, 440–463. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.H.; Song, H.; Kim, N.H.; Kim, J.H. The role of extracellular vesicles in animal reproduction and diseases. J. Anim. Sci. Biotechnol. 2022, 13, 62. [Google Scholar] [CrossRef]
- Menjivar, N.G.; Oropallo, J.; Gebremedhn, S.; Souza, L.A.; Gad, A.; Puttlitz, C.M.; Tesfaye, D. MicroRNA Nano-Shuttles: Engineering Extracellular Vesicles as a Cutting-Edge Biotechnology Platform for Clinical Use in Therapeutics. Biol. Proced. Online 2024, 26, 14. [Google Scholar] [CrossRef]
- Feugang, J.M.; Gad, A.; Menjivar, N.G.; Ishak, G.M.; Gebremedhn, S.; Gastal, M.O.; Dlamini, N.H.; Prochazka, R.; Gastal, E.L.; Tesfaye, D. Seasonal influence on miRNA expression dynamics of extracellular vesicles in equine follicular fluid. J. Anim. Sci. Biotechnol. 2024, 15, 137. [Google Scholar] [CrossRef] [PubMed]
- Gebremedhn, S.; Gad, A.; Ishak, G.M.; Menjivar, N.G.; Gastal, M.O.; Feugang, J.M.; Prochazka, R.; Tesfaye, D.; Gastal, E.L. Dynamics of extracellular vesicle-coupled microRNAs in equine follicular fluid associated with follicle selection and ovulation. Mol. Hum. Reprod. 2023, 29, gaad009. [Google Scholar] [CrossRef] [PubMed]
- Esfandyari, S.; Elkafas, H.; Chugh, R.M.; Park, H.; Navarro, A.; Al-Hendy, A. Exosomes as biomarkers for female reproductive diseases diagnosis and therapy. Int. J. Mol. Sci. 2021, 22, 2165. [Google Scholar] [CrossRef] [PubMed]
- Candenas, L.; Chianese, R. Exosome composition and seminal plasma proteome: A promising source of biomarkers of male infertility. Int. J. Mol. Sci. 2020, 21, 7022. [Google Scholar] [CrossRef] [PubMed]
- Vickram, A.; Srikumar, P.S.; Srinivasan, S.; Jeyanthi, P.; Anbarasu, K.; Thanigaivel, S.; Nibedita, D.; Rani, D.J.; Rohini, K. Seminal exosomes–an important biological marker for various disorders and syndrome in human reproduction. Saudi J. Biol. Sci. 2021, 28, 3607–3615. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tang, T.; Zeng, Z.; Wu, J.; Tan, X.; Yan, J. The expression of small RNAs in exosomes of follicular fluid altered in human polycystic ovarian syndrome. PeerJ 2020, 8, e8640. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fan, H.; Zou, Y.; Yuan, Q.; Hu, X.; Chen, X.; Zhu, C.; Zhang, X.; Cui, H. Aberrant expression of long non-coding RNAs in exosomes in follicle fluid from PCOS patients. Front. Genet. 2021, 11, 608178. [Google Scholar] [CrossRef]
- Huang, B.; Lu, J.; Ding, C.; Zou, Q.; Wang, W.; Li, H. Exosomes derived from human adipose mesenchymal stem cells improve ovary function of premature ovarian insufficiency by targeting SMAD. Stem Cell Res. Ther. 2018, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Zhu, L.; Shen, H.; Lu, J.; Zou, Q.; Huang, C.; Li, H.; Huang, B. Exosomal miRNA-17-5p derived from human umbilical cord mesenchymal stem cells improves ovarian function in premature ovarian insufficiency by regulating SIRT7. Stem Cells 2020, 38, 1137–1148. [Google Scholar] [CrossRef]
- Zhang, A.; Wang, G.; Jia, L.; Su, T.; Zhang, L. Exosome-mediated microRNA-138 and vascular endothelial growth factor in endometriosis through inflammation and apoptosis via the nuclear factor-κB signaling pathway. Int. J. Mol. Med. 2019, 43, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Lian, Y.; Jiang, J.; Wang, L.; Ren, L.; Li, Y.; Yan, X.; Chen, Q. Differential expression of microRNA in exosomes derived from endometrial stromal cells of women with endometriosis-associated infertility. Reprod. Biomed. Online 2020, 41, 170–181. [Google Scholar] [CrossRef]
- Baskaran, S.; Selvam, M.K.P.; Agarwal, A. Exosomes of male reproduction. Adv. Clin. Chem. 2020, 95, 149–163. [Google Scholar] [PubMed]
- Ghafourian, M.; Mahdavi, R.; Akbari Jonoush, Z.; Sadeghi, M.; Ghadiri, N.; Farzaneh, M.; Mousavi Salehi, A. The implications of exosomes in pregnancy: Emerging as new diagnostic markers and therapeutics targets. Cell Commun. Signal. 2022, 20, 51. [Google Scholar] [CrossRef] [PubMed]
- Heshmat, S.M.; Mullen, J.B.; Jarvi, K.A.; Soosaipillai, A.; Diamandis, E.P.; Hamilton, R.J.; Lo, K.C. Seminal plasma lipocalin-type prostaglandin D synthase: A potential new marker for the diagnosis of obstructive azoospermia. J. Urol. 2008, 179, 1077–1080. [Google Scholar] [CrossRef]
- Ranjbaran, A.; Latifi, Z.; Nejabati, H.R.; Abroon, S.; Mihanfar, A.; Sadigh, A.R. Fattahi, A.; Nouri, M.; Raffel, N. Exosome-based intercellular communication in female reproductive microenvironments. J. Cell. Physiol. 2019, 234, 19212–19222. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.; Tehrani, F.R.; Tahmasebi, S.; Shafiee, A.; Hashemi, S.M. Exosome engineering in cell therapy and drug delivery. Inflammopharmacology 2023, 31, 145–169. [Google Scholar] [CrossRef] [PubMed]
- Jafari, D.; Shajari, S.; Jafari, R.; Mardi, N.; Gomari, H.; Ganji, F.; Forouzandeh Moghadam, M.; Samadikuchaksaraei, A. Designer exosomes: A new platform for biotechnology therapeutics. BioDrugs 2020, 34, 567–586. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).