Coating Seeds with Paenibacillus polymyxa ZF129 Microcapsule Suspension Enhanced Control Effect on Fusarium Root Rot and Promoted Seedling Growth in Cucumber
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Bacterial Strains
2.2. Plant Materials and Bacterial Strains
2.3. Preparation of P. polymyxa ZF129 Microcapsule Powder via Spray-Drying Method
2.4. Field Emission Scanning Electron Microscopy (FESEM)
2.5. Preparation of CS-ZF129 and Seed Coating
2.6. Storage Stability
2.7. Effects of CS-ZF129 on Growth Promotion of Cucumbers and Control of Cucumber Root Rot
2.8. Colonization Ability of P. polymyxa ZF129 in Cucumber Rhizosphere and Its Relationship with Population Changes in F. solani
2.9. Determination of Rhizosphere Soil Enzyme Activity and Root Defense Enzyme Activity
2.10. Statistical Analysis
3. Results
3.1. The Morphology of the Microcapsules
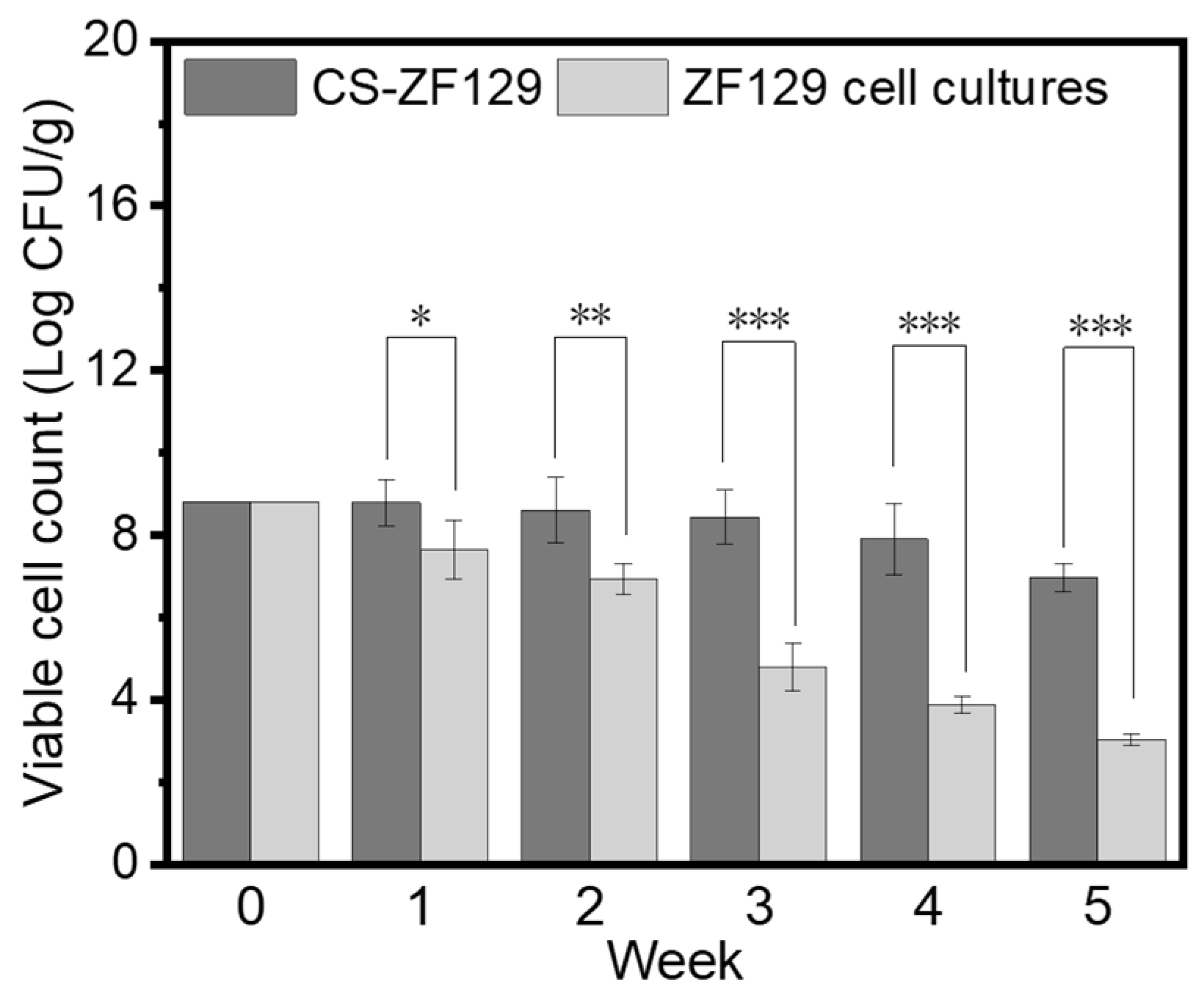
3.2. The Storage Stability of the CS-ZF129 Microcapsules
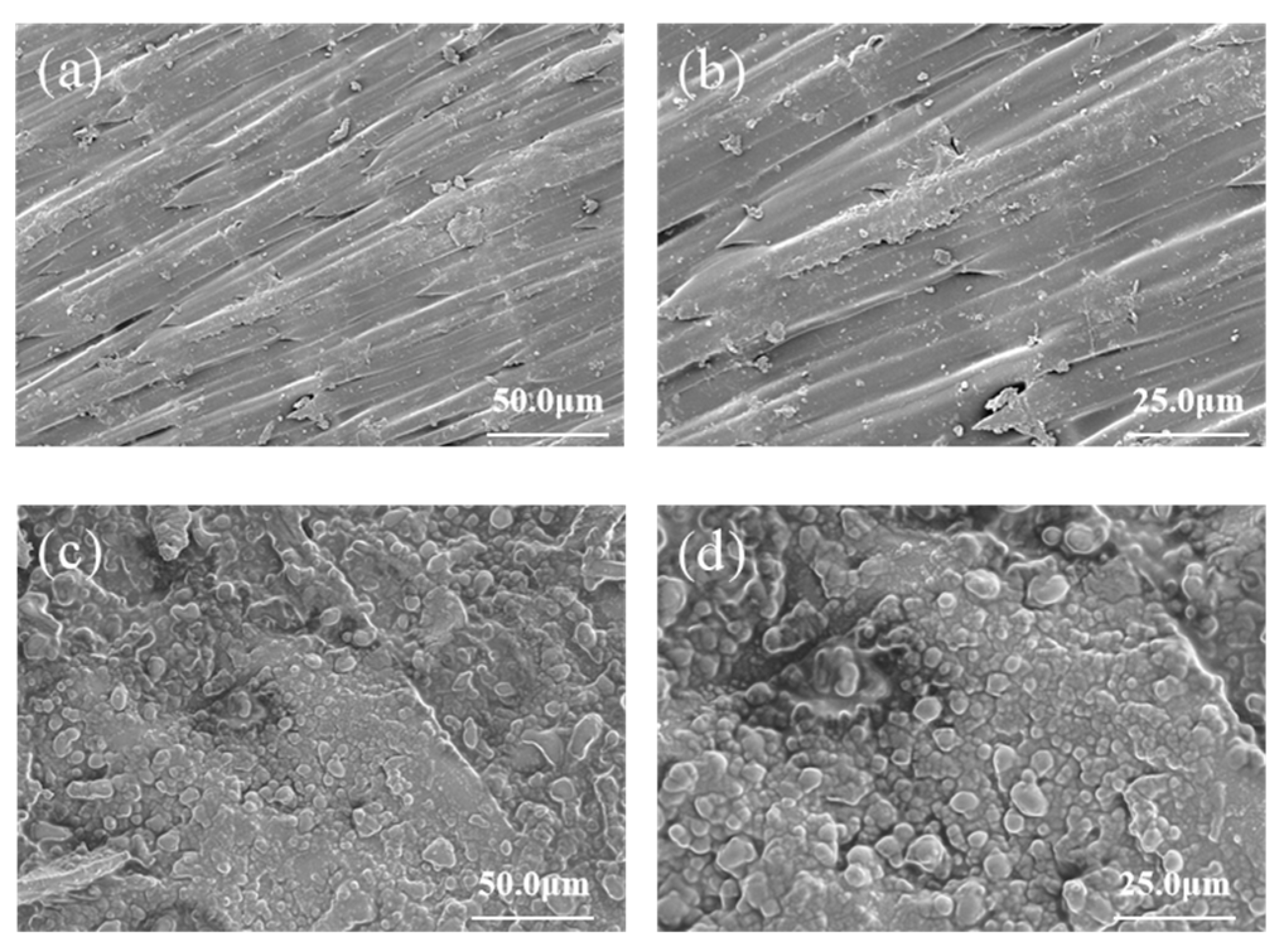
3.3. Surface Morphology of CS-ZF129-Coated Cucumber Seeds (ZF129m-Seeds)
3.4. Growth Response of ZF129m-Seeds in Pot Experiments
3.5. Control Effect of CS-ZF129 Against Cucumber Fusarium Root Rot
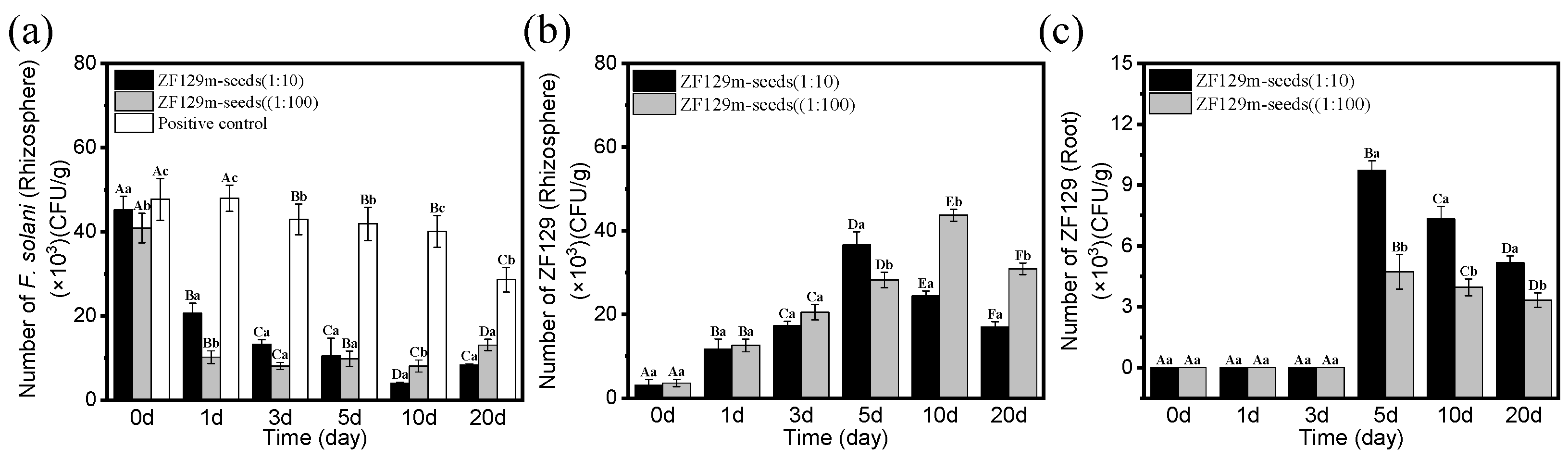
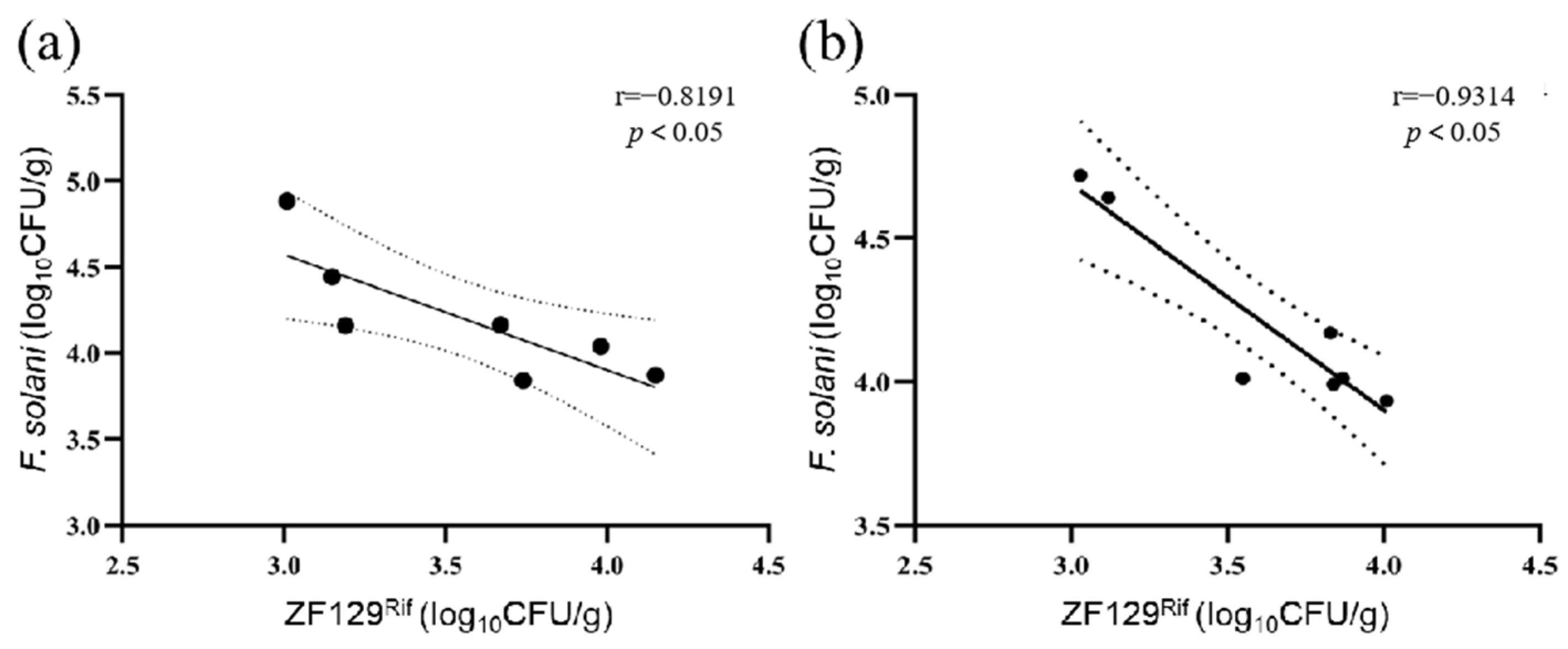
3.6. Dynamics of Populations of P. polymyxa ZF129Rif and F. solani in Rhizosphere
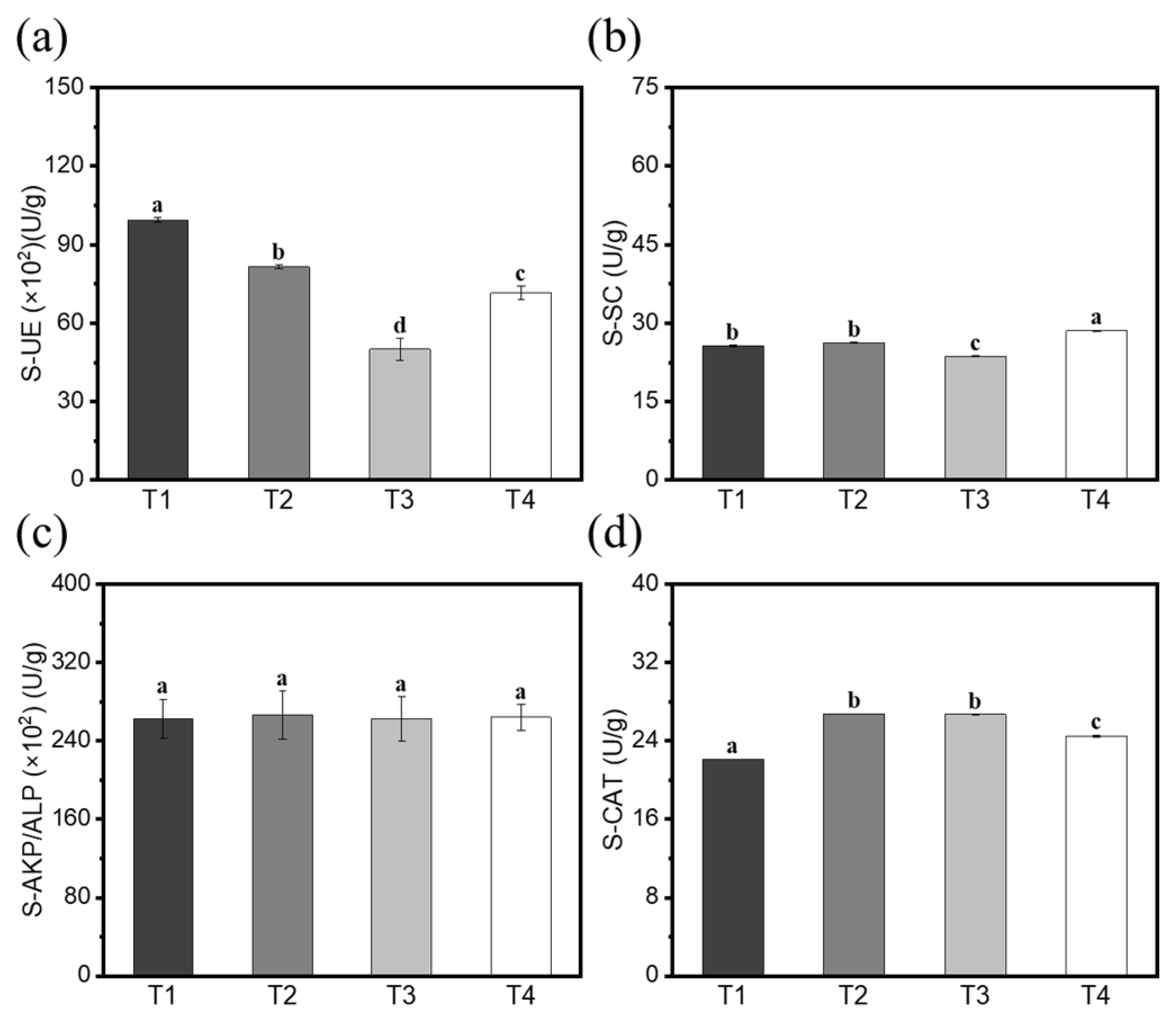
3.7. Changes in Rhizosphere Soil Enzyme Activity and Root Defense Enzyme Activity of Cucumbers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamel, S.M.; Elgobashy, S.F.; Omara, R.I.; Derbalah, A.S.; Abdelfatah, M.; El-Shaer, A.; Al-Askar, A.A.; Abdelkhalek, A.; Abd-Elsalam, K.A.; Essa, T.; et al. Antifungal activity of copper oxide nanoparticles against root rot disease in cucumber. J. Fungi 2022, 8, 911. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Nelson, B. Effects of soil type, temperature, and moisture on development of Fusarium root rot of soybean by Fusarium Solani (FSSC 11) and Fusarium Tricinctum. Plant Dis. 2022, 106, 2974–2983. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Skorik, Y.A.; Thakur, V.K.; Moradi Pour, M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of plant biocontrol bacteria with alginate as a main polymer material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Pethybridge, S.J.; Murphy, S.; Hay, F.; Branch, E.; Sharma, P.; Kikkert, J.R. Control of phoma leaf spot and root decay of table beet in New York. Plant Dis. 2022, 106, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulos, D.G.; Fiddaman, P.; Leifert, C.; Malathrakis, N.E. Biological control of cucumber and sugar beet damping-off caused by Pythium ultimum with bacterial and fungal antagonists. J. Appl. Microbiol. 2002, 92, 1078–1086. [Google Scholar] [CrossRef]
- Głodowska, M.; Husk, B.; Schwinghamer, T.; Smith, D. Biochar is a growth-promoting alternative to peat moss for the inoculation of corn with a pseudomonad. Agron. Sustain. Dev. 2016, 36, 1–10. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Bonini, P.; Cardarelli, M. Coating seeds with endophytic fungi enhances growth, nutrient uptake, yield and grain quality of winter wheat. Int. J. Plant Prod. 2015, 9, 171–190. [Google Scholar] [CrossRef]
- Angelopoulou, D.J.; Naska, E.J.; Paplomatas, E.J.; Tjamos, S.E. Biological control agents (BCAs) of verticillium wilt: Influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Plant Pathol. 2014, 63, 1062–1069. [Google Scholar] [CrossRef]
- Katz, E.; Demain, A. The peptide antibiotics of Bacillus: Chemistry, biogenesis, and possible functions. Bacteriol. Rev. 1977, 41, 449–474. [Google Scholar] [CrossRef]
- Ito, M.; Koyama, Y. Jolipeptin, a new peptide antibiotic. II. The mode of action of Jolipeptin. J. Antibiot. 1972, 25, 309–314. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Ma, S.; Meng, Y.; Wei, W.; Peng, C.; Ling, C.; Fan, S.; Liu, Z. Characterization of antagonistic bacteria Paenibacillus polymyxa ZYPP18 and the effects on plant growth. Plants 2023, 12, 2504. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Du, C.; Xu, L.; Yue, C.; Liu, X.; Fan, H. Endophytic bacteria from diseased plant leaves as potential biocontrol agents of cucumber Fusarium wilt. J. Plant Pathol. 2024, 106, 553–563. [Google Scholar] [CrossRef]
- Lin, S.; Chen, X.; Xie, L.; Zhang, Y.; Zeng, F.; Long, Y.; Ren, L.; Qi, X.; Wei, J. Biocontrol potential of lipopeptides produced by Paenibacillus polymyxa AF01 against Neoscytalidium dimidiatum in Pitaya. Front. Microbiol. 2023, 14, 1188722. [Google Scholar] [CrossRef] [PubMed]
- Langendries, S.; Goormachtig, S. Paenibacillus polymyxa, a Jack of all trades. Environ. Microbiol. 2021, 23, 5659–5669. [Google Scholar] [CrossRef]
- Xu, F.; Liao, H.; Yang, J.; Zhang, Y.; Yu, P.; Cao, Y.; Fang, J.; Chen, S.; Li, L.; Sun, L. Auxin-producing bacteria promote barley rhizosheath formation. Nat. Commun. 2023, 14, 1–12. [Google Scholar] [CrossRef]
- Yuan, H.; Yuan, M.; Shi, B.; Wang, Z.; Huang, T.; Qin, G.; Hou, H.; Wang, L.; Tu, H. Biocontrol activity and action mechanism of Paenibacillus polymyxa strain Nl4 against pear Valsa canker caused by Valsa pyri. Front. Microbiol. 2022, 13, 950742. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, J.; Fu, Q.; Hao, G.; Liang, C.; Duan, F.; Zhao, H.; Song, W. Biocontrol efficacy and induced resistance of Paenibacillus polymyxa J2-4 against Meloidogyne incognita infection in cucumber. Phytopathology 2024, 114, 538–548. [Google Scholar] [CrossRef]
- Li, J.; Gao, T.; Wang, Q. Comparative and functional analyses of two sequenced Paenibacillus polymyxa genomes provides insights into their potential genes related to plant growth-promoting features and biocontrol mechanisms. Front. Genet. 2020, 11, 564939. [Google Scholar] [CrossRef]
- Abdukerim, R.; Xiang, S.; Shi, Y.; Xie, X.; Li, L.; Chai, A.; Li, B.; Fan, T. Seed pelleting with gum arabic-encapsulated biocontrol bacteria for effective control of clubroot disease in pak choi. Plants 2023, 12, 3702. [Google Scholar] [CrossRef]
- Huang, D.; Li, J.; Yao, J.; Cao, C.; Yang, Z. The biocontrol effect of Paenibacillus polymyxa strain NBF188 on cucumber rhizoctonia rot and its growth-promoting effect. J. Pure Appl. Microbiol. 2014, 8, 179–184. [Google Scholar]
- Vihol, J.B.; Patel, K.D.; Jaiman, R.K.; Patel, N.R. Efficacy of plant extracts, biological agents and fungicides against alternaria blight of cumin. J. Mycol. Plant Pathol. 2009, 39, 516. [Google Scholar]
- Adorada, D.L.; Stodart, B.J.; Pangga, I.B.; Ash, G.J. Implications of bacterial contaminated seed lots and endophytic colonization by Pseudomonas fuscovaginae on rice establishment. Plant Pathol. 2015, 64, 43–50. [Google Scholar] [CrossRef]
- Chen, L.; Li, L.; Xie, X.; Chai, A.; Shi, Y.; Fan, T.; Xie, J.; Li, B. An improved method for quantification of viable fusarium cells in infected soil products by propidium monoazide coupled with real-time PCR. Microorganisms 2022, 10, 1037. [Google Scholar] [CrossRef]
- Freitas, S.; Merkle, H.P.; Gander, B. Microencapsulation by solvent extraction/evaporation: Reviewing the state of the art of microsphere preparation process technology. J. Control. Release 2005, 102, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Naik, S. Natural gums and its pharmaceutical application. J. Sci. Innov. Res. 2014, 3, 112–121. [Google Scholar] [CrossRef]
- Jaiswal, D.K.; Gawande, S.J.; Soumia, P.S.; Krishna, R.; Vaishnav, A.; Ade, A.B. Biocontrol strategies: An eco-smart tool for integrated pest and diseases management. BMC Microbiol. 2022, 22, 324. [Google Scholar] [CrossRef]
- Paravar, A.; Piri, R.; Balouchi, H.; Ma, Y. Microbial Seed Coating: An attractive tool for sustainable agriculture. Biotechnol. Rep. 2023, 37, e00781. [Google Scholar] [CrossRef]
- Yuan, Z.-S.; Liu, F.; Xie, B.-G.; Zhang, G.-F. The growth-promoting effects of endophytic bacteria on Phyllostachys edulis. Arch. Microbiol. 2018, 200, 921–927. [Google Scholar] [CrossRef]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Vendan, R.T.; Yu, Y.J.; Lee, S.H.; Rhee, Y.H. Diversity of endophytic bacteria in ginseng and their potential for plant growth promotion. J. Microbiol. 2010, 48, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Khabbaz, S.E.; Abbasi, P.A. Isolation, characterization, and formulation of antagonistic bacteria for the management of seedlings damping-off and root rot disease of cucumber. Can. J. Microbiol. 2014, 60, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Khalid, A.; Singh, U.S.; Sharma, A.K. Evaluation of arbuscular mycorrhizal fungus, fluorescent Pseudomonas and Trichoderma harzianum formulation against Fusarium oxysporum f. sp. lycopersici for the management of tomato Wilt. Biol. Control 2010, 53, 24–31. [Google Scholar] [CrossRef]
- Sivakumar, P.K.; Parthasarthi, R.; Lakshmipriya, V.P. Encapsulation of plant growth promoting inoculant in bacterial alginate beads enriched with humic acid. Excell. Publ. 2014, 3, 415–422. [Google Scholar]
- Kharkwal, A.C.; Joshi, H.; Shandilya, C.; Dabral, S.; Kumar, N.; Varma, A. Isolation and characterization of a newly discovered plant growth-promoting endophytic fungal strain from the genus Talaromyces. Sci. Rep. 2024, 14, 6022. [Google Scholar] [CrossRef]
- Moradipour, M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Nano-encapsulation of plant growth-promoting rhizobacteria and their metabolites using alginate-silica nanoparticles and carbon nanotube improves UCB1 pistachio micropropagation. J. Microbiol. Biotechnol. 2019, 29, 1096–1103. [Google Scholar] [CrossRef]
- Arbaugh, B.M.; Rezaei, F.; Mohiti-Asli, M.; Pena, S.; Scher, H.B.; Jeoh, T. A strategy for stable, on-seed application of a nitrogen-fixing microbial inoculant by microencapsulation in spray-dried cross-linked alginates. ACS Agric. Sci. Technol. 2022, 2, 950–959. [Google Scholar] [CrossRef]
- Morcuende, J.; Martín-García, J.; Velasco, P.; Sánchez-Gómez, T.; Santamaría, Ó.; Rodríguez, V.M.; Poveda, J. Effective biological control of chickpea rabies (Ascochyta rabiei) through systemic phytochemical defenses activation by Trichoderma roots colonization: From strain characterization to seed coating. Biol. Control 2024, 193, 105530. [Google Scholar] [CrossRef]
- Daud, N.S.; Mohd Din, A.R.J.; Rosli, M.A.; Azam, Z.M.; Othman, N.Z.; Sarmidi, M.R. Paenibacillus polymyxa bioactive compounds for agricultural and biotechnological applications. Biocatal. Agric. Biotechnol. 2019, 18, 101092. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhu, J.; Tan, T.; Xu, J.; Shen, A.; Yang, X.; Li, J.; Zeng, L.; Wei, L. Isolation and characterization of antagonistic Paenibacillus polymyxa HX-140 and its biocontrol potential against fusarium wilt of cucumber seedlings. BMC Microbiol. 2021, 21, 75. [Google Scholar] [CrossRef]
- Chen, B.; Han, H.; Hou, J.; Bao, F.; Tan, H.; Lou, X.; Wang, G.; Zhao, F. Control of maize sheath blight and elicit induced systemic resistance using Paenibacillus polymyxa Strain SF05. Microorganisms 2022, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Kuchlan, P.; Kuchlan, M.K.; Ansari, M.M. Efficient application of Trichoderma viride on soybean [Glycine max (L.) Merrill] seed using thin layer polymer coating. Legume Res. 2018, 42, 260–264. [Google Scholar] [CrossRef]




| Group | Treatment | Leaf Area (cm2) | Chlorophyll (SPDA) | Shoot Height (cm) | Root Length (cm) | Shoot Fresh Weight (g) | Root Fresh Weight (g) |
|---|---|---|---|---|---|---|---|
| T1 | ZF129m-seeds (coating agent/seed ratio = 1:10) | 44.57 ± 7.15 a | 39.33 ± 2.07 b | 14.52 ± 2.44 ab | 12.38 ± 0.10 a | 24.93 ± 0.23 b | 16.39 ± 0.61 a |
| T2 | ZF129m-seeds (coating agent/seed ratio = 1:100) | 46.22 ± 2.64 a | 38.37 ± 1.30 b | 11.90 ± 0.88 b | 12.75 ± 0.64 a | 24.35 ± 0.65 b | 9.48 ± 0.21 d |
| CK | Bare seeds (Non-treated control) | 41.00 ± 7.06 a | 38.60 ± 1.92 b | 11.82 ± 0.84 b | 8.90 ± 1.08 b | 19.67 ± 0.28 d | 8.66 ± 0.56 d |
| Group | Treatment | Emergence Rate (%) | Disease Index | Control Efficacy (%) |
|---|---|---|---|---|
| T1 | ZF129m-seeds (coating agent/seed ratio = 1:10) | 89.63 ± 1.35 a | 49.98 ± 6.30 a | 29.01 ± 0.09 a |
| T2 | ZF129m-seeds (coating agent/seed ratio = 1:100) | 91.42 ± 1.12 a | 36.30 ± 1.25 b | 46.30 ± 0.02 b |
| T3 | Bare seeds (Non-treated control) | 93.55 ± 2.84 a | — | — |
| T4 | Bare seeds (Pathogen control) | 83.55 ± 1.64 b | 68.00 ± 1.91 c | — |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Liu, J.; Shi, Y.; Xie, X.; Chai, A.; Xiang, S.; Sun, X.; Li, L.; Li, B.; Fan, T. Coating Seeds with Paenibacillus polymyxa ZF129 Microcapsule Suspension Enhanced Control Effect on Fusarium Root Rot and Promoted Seedling Growth in Cucumber. Biology 2025, 14, 375. https://doi.org/10.3390/biology14040375
Ma J, Liu J, Shi Y, Xie X, Chai A, Xiang S, Sun X, Li L, Li B, Fan T. Coating Seeds with Paenibacillus polymyxa ZF129 Microcapsule Suspension Enhanced Control Effect on Fusarium Root Rot and Promoted Seedling Growth in Cucumber. Biology. 2025; 14(4):375. https://doi.org/10.3390/biology14040375
Chicago/Turabian StyleMa, Jiayi, Jialin Liu, Yanxia Shi, Xuewen Xie, Ali Chai, Sheng Xiang, Xianhua Sun, Lei Li, Baoju Li, and Tengfei Fan. 2025. "Coating Seeds with Paenibacillus polymyxa ZF129 Microcapsule Suspension Enhanced Control Effect on Fusarium Root Rot and Promoted Seedling Growth in Cucumber" Biology 14, no. 4: 375. https://doi.org/10.3390/biology14040375
APA StyleMa, J., Liu, J., Shi, Y., Xie, X., Chai, A., Xiang, S., Sun, X., Li, L., Li, B., & Fan, T. (2025). Coating Seeds with Paenibacillus polymyxa ZF129 Microcapsule Suspension Enhanced Control Effect on Fusarium Root Rot and Promoted Seedling Growth in Cucumber. Biology, 14(4), 375. https://doi.org/10.3390/biology14040375








