Changes in Seasonal Spatial Distribution Patterns of Euprymna berryi and Euprymna morsei: The Current and Predictions Under Climate Change Scenarios
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
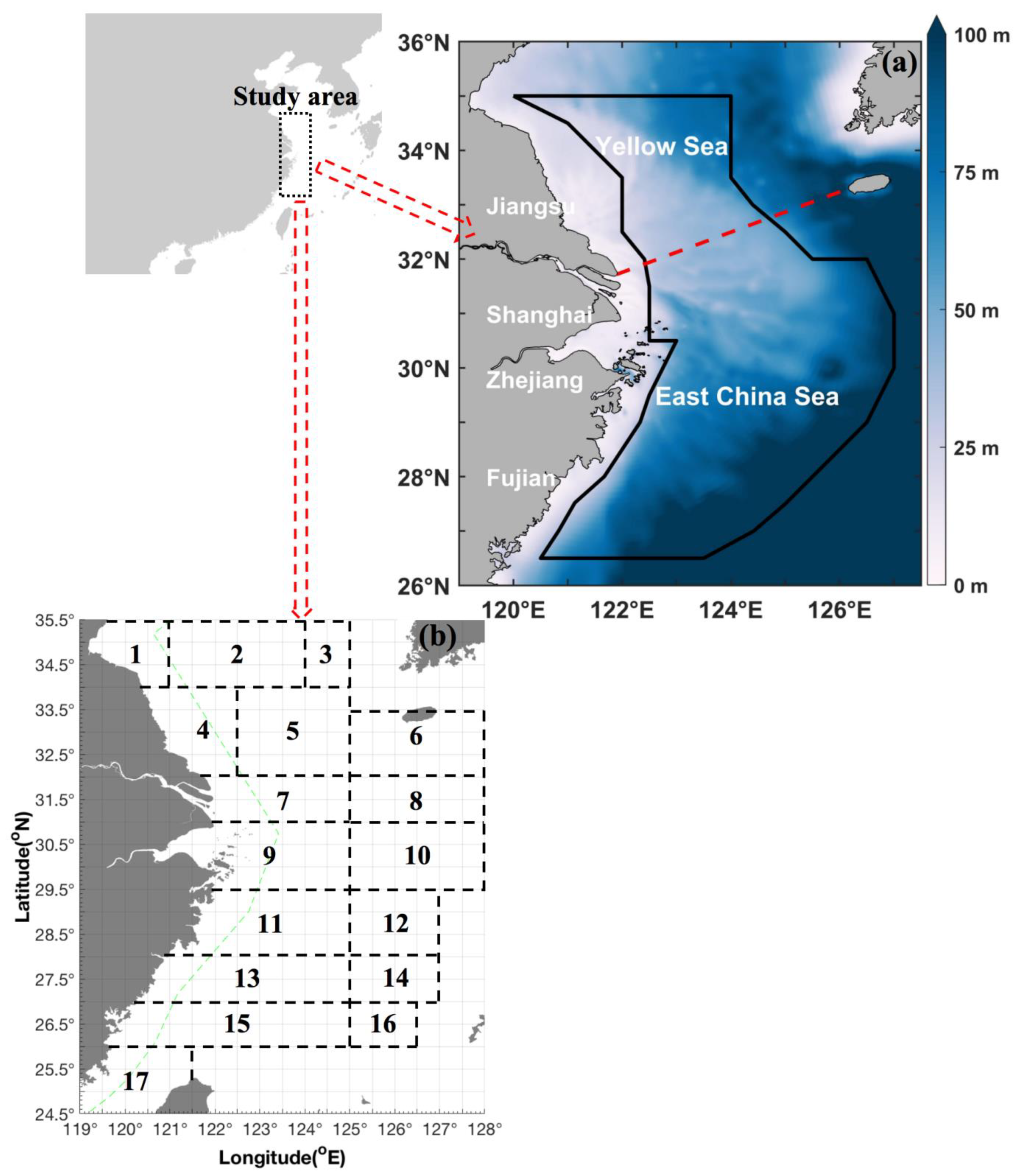
2.1. Sampling and Survey Procedures
2.2. Ensemble Model, Selection of Environmental Variables, and Evaluations
3. Results and Discussion
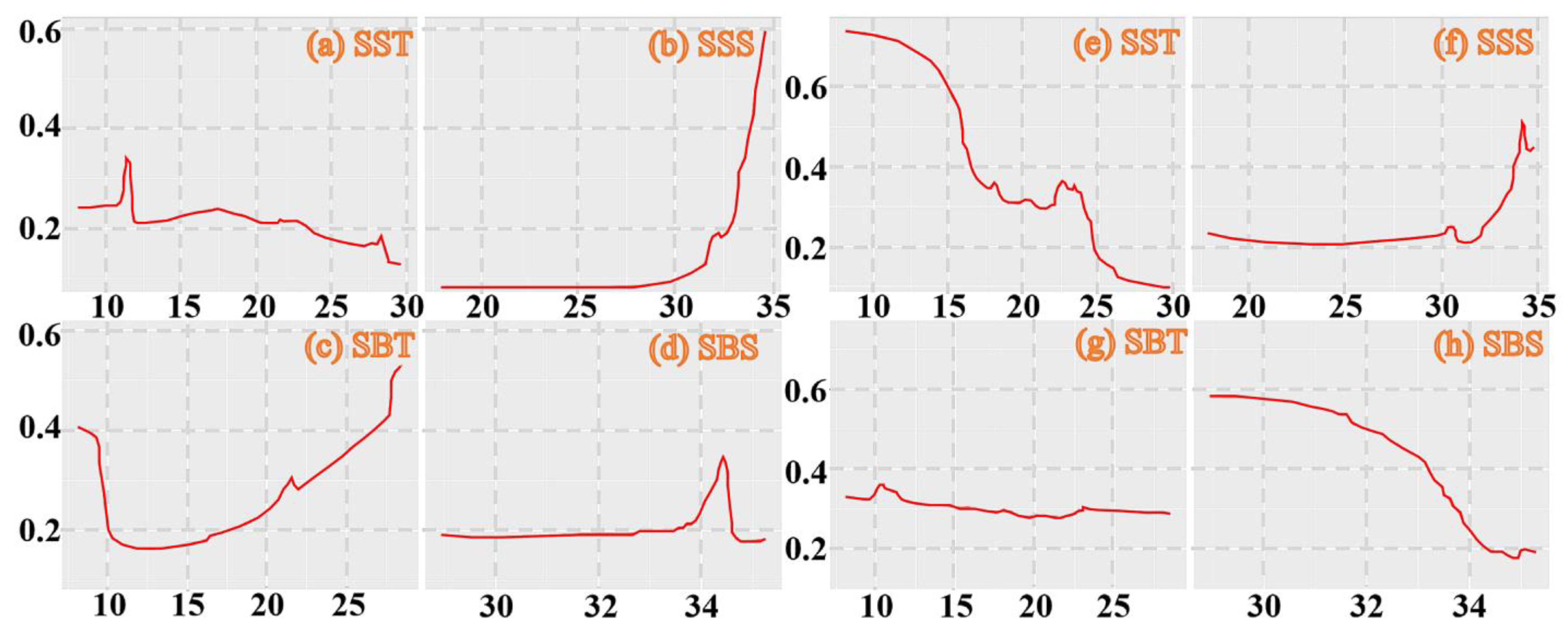
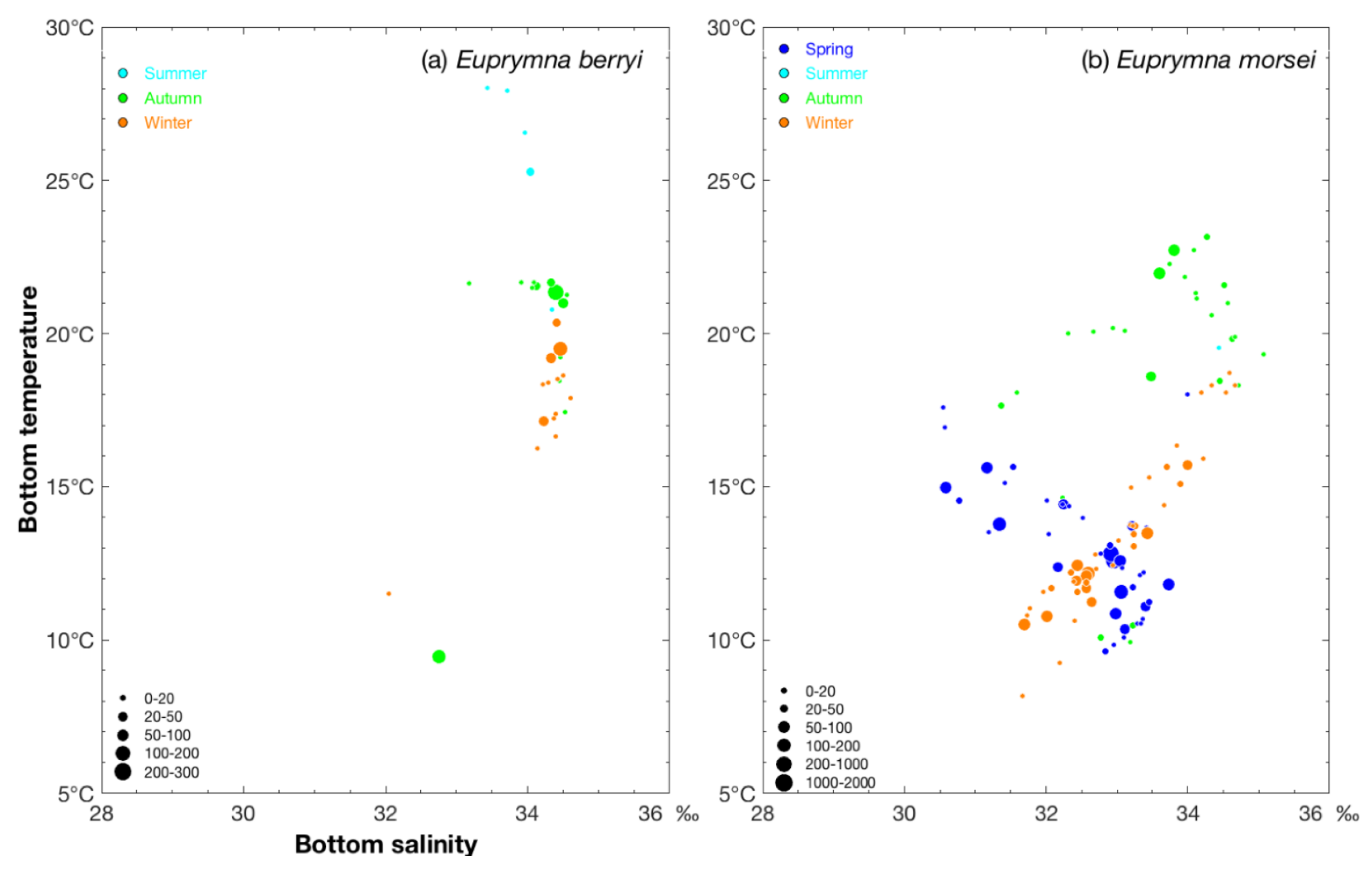
3.1. Seasonal Variations in Environmental Variables of Both Species
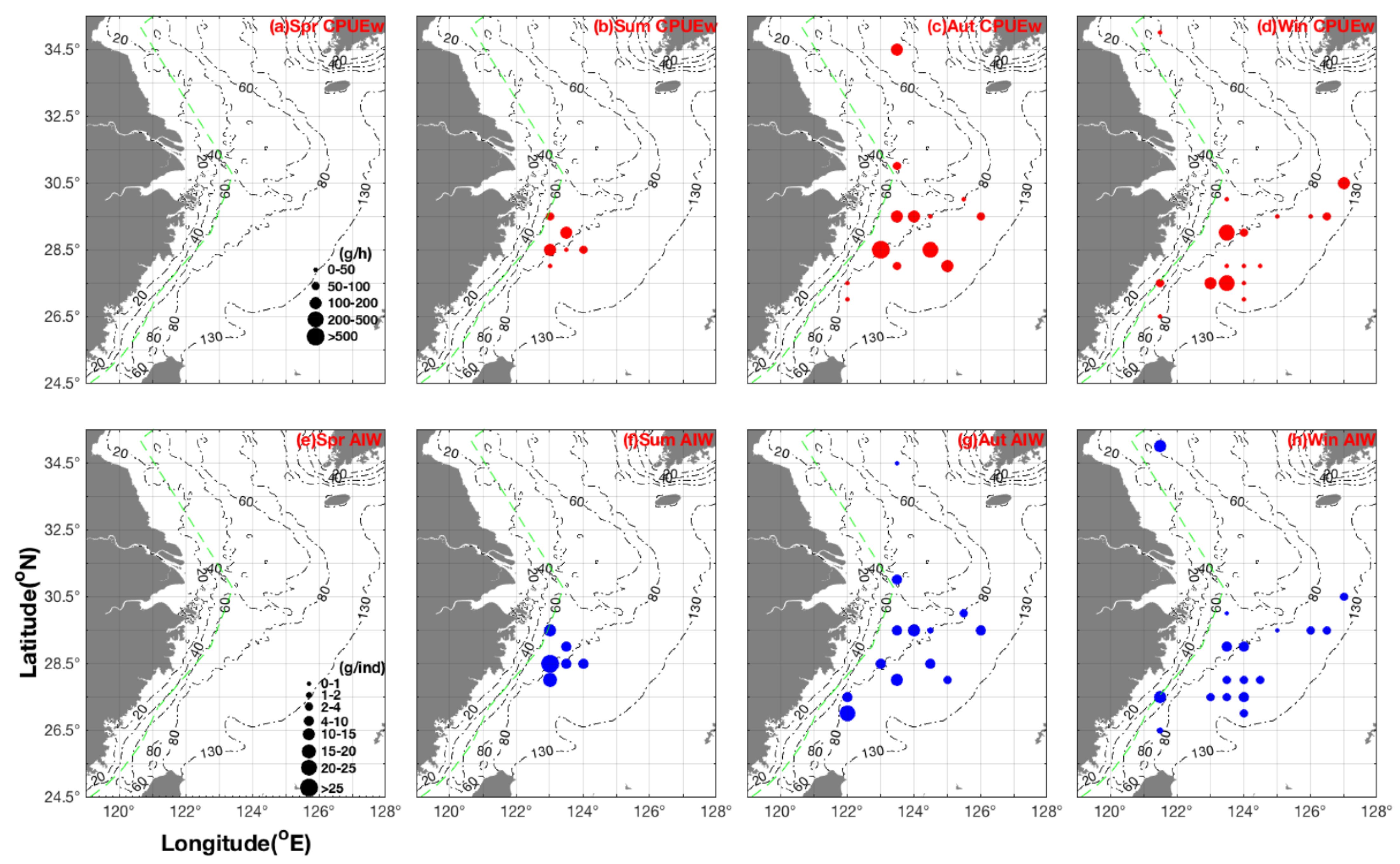
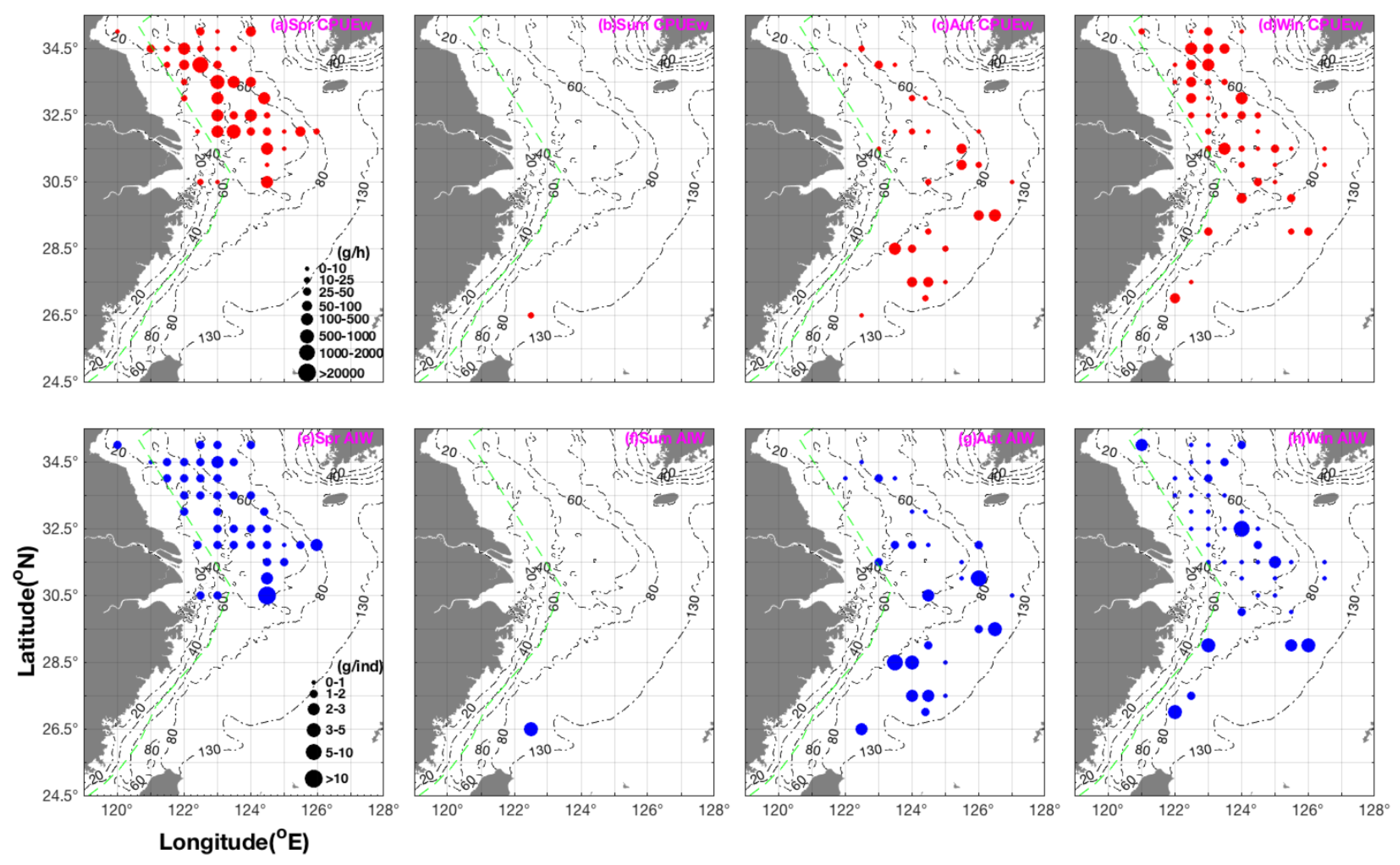
3.2. Seasonal Spatial Distribution Patterns and Characteristics of CPUEw and AIW
3.3. Model Evaluation and Suitable Habitat and Environmental Factors
3.4. Habitat Predictions Under Different Climate Projections
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Gao, W.; Han, Y.; Zhou, T. Predicting the Impact of Climate Change on the Distribution of North China Leopards (Panthera pardus japonensis) in Gansu Province Using MaxEnt Modeling. Biology 2025, 14, 126. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; De Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [PubMed]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar]
- Chen, I.C.; Hill, J.K.; Ohlemuller, R.; Roy, D.B.; Tomas, C.D. Rapid range shifts of species associated with high levels of climate warming. Science 2011, 333, 1024–1026. [Google Scholar]
- Berg, M.P.; Kiers, E.T.; Driessen, G.; Van Der HeijdenJ, M.; Kooi, B.W.; Kuenen, F.; Liefting, M.; Verhoef, H.A.; Ellers, J. Adapt or disperse: Understanding species persistence in a changing world. Glob. Change Biol. 2010, 16, 587–598. [Google Scholar]
- Xue, K.M.; Yuan, M.J.; Shi, Z.; Qin, Y.; Deng, Y.E.; Cheng, L.; Wu, L.; He, Z.; Van Nostrand, J.D.; Bracho, R.; et al. Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Change 2016, 6, 595–600. [Google Scholar]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar]
- Miller, T.E.; Gornish, E.S.; Buckley, H.L. Climate and coastal dune vegetation: Disturbance, recovery, and succession. Plant Ecol. 2010, 206, 97–104. [Google Scholar]
- Guo, X.; Feng, J.; Shi, Z.; Zhou, X.; Yuan, M.; Tao, X.; Hale, L.; Yuan, T.; Wang, J.; Qin, Y.; et al. Climate warming leads to divergent succession of grassland microbial communities. Nat. Clim. Change 2018, 8, 813–818. [Google Scholar]
- Wang, X.; Qiu, Y.; Du, F.; Liu, W.; Sun, D.; Chen, X.; Yuan, W.; Chen, Y. Roles of fishing and climate change in long-term fish species succession and population dynamics in the outer Beibu Gulf, South China Sea. Acta Oceanol. Sinica 2019, 38, 1–8. [Google Scholar]
- Ma, S.; Liu, Y.; Li, J.; Fu, C.; Ye, Z.; Sun, P.; Yu, H.; Cheng, J.; Tian, Y. Climate-induced long-term variations in ecosystem structure and atmosphere-ocean-ecosystem processes in the Yellow Sea and East China Sea. Prog. Oceanogr. 2019, 175, 183–197. [Google Scholar]
- Cai, R.; Tan, H.; Qi, Q. Impacts of and adaptation to inter-decadal marine climate change in coastal China seas. Int. J. Climatol. 2016, 36, 3770–3780. [Google Scholar]
- Pisano, A.; Marullo, S.; Artale, V.; Falcini, F.; Yang, C.; Leonelli, F.E.; Santoleri, R.; Buongiorno Nardelli, B. New Evidence of Mediterranean Climate Change and Variability from Sea Surface Temperature Observations. Remote Sens. 2020, 12, 132. [Google Scholar] [CrossRef]
- Darmaraki, S.; Denaxa, D.; Theodorou, I.; Livanou, E.; Rigatou, D.; Raitsos, E.D.; Stavrakidis-Zachou, O.; Dimarchopoulou, D.; Bonino, G.; McAdam, R.; et al. Marine Heatwaves in the Mediterranean Sea: A Literature Review. Mediterr. Mar. Sci. 2024, 25, 586–620. [Google Scholar]
- Reid, A.; Jereb, P. Family Sepiolidae. In Cephalopods of the World; FAO: Rome, Italy, 2005; Available online: http://www.fao.org/3/ac479e/ac479e00.htm (accessed on 8 February 2025).
- McFall-Ngai, M.; Montgomery, M.K. The anatomy and morphology of the adult bacterial light organ of Euprymna scolopes Berry (Cephalopoda: Sepiolidae). Biol. Bull. 1990, 179, 332–339. [Google Scholar] [PubMed]
- Nabhitabhata, J.; Nishiguchi, M.K. Euprymna hyllebergi and Euprymna tasmanica. In Cephalopod Culture; Springer: Dordrecht, The Netherlands, 2014; pp. 253–269. [Google Scholar]
- Jolly, J.; Hasegawa, Y.; Sugimoto, C.; Zhang, L.; Kawaura, R.; Sanchez, G.; Gavriouchkina, D.; Marlétaz, F.; Rokhsar, D. Lifecycle, culture, and maintenance of the emerging cephalopod models Euprymna berryi and Euprymna morsei. Front. Mar. Sci. 2022, 9, 1039775. [Google Scholar]
- Sundaram, S.; Sreeram, M.P. First record of sepiolid squid, Euprymna berryi Sasaki, 1929 from the west coast of India. J. Mar. Biol. Assoc. India 2008, 50, 221–223. [Google Scholar]
- Singley, C. Euprymna scolopes. In Cephalopod Life Cycles; Academic Press: London, UK, 1983; Volume 1, pp. 69–74. [Google Scholar]
- Norman, M.D. Cephalopods, A World Guide; ConchBooks: Hackenheim, Germany, 2000; pp. 1–320. [Google Scholar]
- Ottersen, G.; Kim, S.; Huse, G.; Polovina, J.J.; Stenseth, N.C. Major pathways by which climate may force marine fish populations. J. Mar. Syst. 2010, 79, 343–360. [Google Scholar]
- Jakeman, A.J.; Elsawah, S.; Wang, H.H.; Hamilton, S.H.; Melsen, L.; Grimm, V. Towards normalizing good practice across the whole modeling cycle: Its instrumentation and future research topics. Socio-Environ. Syst. Model. 2024, 6, 18755. [Google Scholar]
- Araujo, M.; New, M. Ensemble forecasting of species distributions. Trends. Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [PubMed]
- Liu, S.; Liu, Y.; Alabia, I.D.; Tian, Y.; Ye, Z.; Yu, H.; Li, J.; Cheng, J. Impact of Climate Change on Wintering Ground of Japanese Anchovy (Engraulis japonicus) Using Marine Geospatial Statistics. Front. Mar. Sci. 2020, 7, 604. [Google Scholar]
- Liu, S.; Liu, Y.; Teschke, K.; Hindell, M.A.; Downey, R.; Woods, B.; Kang, B.; Ma, S.; Zhang, C.; Li, J.; et al. Incorporating mesopelagic fish into the evaluation of conservation areas for marine living resources under climate change scenarios. Mar. Life Sci. Technol. 2024, 6, 68–83. [Google Scholar]
- Liu, S.; Tian, Y.; Liu, Y.; Alabia, I.D.; Cheng, J.; Ito, S. Development of a prey-predator species distribution model for a large piscivorous fish: A case study for Japanese Spanish mackerel Scomberomorus niphonius and Japanese anchovy Engraulis japonicus. Deep Sea Res. Part II 2023, 207, 105227. [Google Scholar]
- Liu, S.; Liu, Y.; Xing, Q.; Li, Y.; Tian, H.; Luo, Y.; Ito, S.; Tian, Y. Climate change drives fish communities: Changing multiple facets of fish biodiversity in the Northwest Pacific Ocean. Sci. Total Environ. 2024, 955, 176854. [Google Scholar]
- Silva, C.; Yáñez, E.; Barbieri, M.A.; Bernal, C.; Aranis, A. Forecasts of swordfish (Xiphias gladius) and common sardine (Strangomera bentincki) off Chile under the A2 IPCC climate change scenario. Prog. Oceanogr. 2015, 134, 343–355. [Google Scholar]
- Cheung, W.W.L.; Brodeur, R.D.; Okey, T.A.; Pauly, D. Projecting future changes in distributions of pelagic fish species of Northeast Pacific shelf seas. Prog. Oceanogr. 2015, 130, 19–31. [Google Scholar]
- Choe, S. On the eggs, rearing, habits of the fry, and growth of some Cephalopoda. Bull. Mar. Sci. 1966, 16, 330–348. [Google Scholar]
- Huang, C.P. Species Diversity of the Genus Euprymna Steenstrup, 1887 in Taiwan and Reproductive Cycle of Euprymna berryi Sasaki, 1929. Master’s Thesis, National Chung Hsing University, Taiwan, China, 2006. [Google Scholar]
- Naeem, M.; Salam, A.; Ashraf, M.; Imran, M.; Ahmed, M.; Khan, M.J.; Ayaz, M.M.; Ali, M.; Qayyum, M.; Ishtiaq, A. Studies on seasonal variations of physic-chemical parameters of fish farm at Govt. nursery unit, Muzaffargarh, Punjab, Pakistan. Proc. World Acad. Sci. Technol. 2011, 5, 60. [Google Scholar]
- Watanabe, K. Comparative Developmental Biology of the Embryonic and Early Life Stages of Decapod Cephalopods. Ph.D. Thesis, Tokyo University of Fisheries, Tokyo, Japan, 1997. (In Japanese). [Google Scholar]
- Xu, M.; Yang, L.; Liu, Z.; Zhang, Y.; Zhang, H. Seasonal and spatial distribution characteristics of Sepia esculenta in the East China Sea Region: Transfer of the central distribution from 29° N to 28° N. Animals 2024, 14, 1412. [Google Scholar] [CrossRef]
- Xu, M.; Feng, W.J.; Liu, Z.; Li, Z.G.; Song, X.J.; Zhang, H.; Zhang, C.L.; Yang, L.L. Seasonal-spatial distribution variations and predictions of Loliolus beka and Loliolus uyii in the East China Sea region: Implications from climate change scenarios. Animals 2024, 14, 2070. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, S.; Zhang, H.; Li, Z.; Song, X.; Yang, L.; Tang, B. Seasonal analysis of spatial distribution patterns and characteristics of Sepiella maindroni and Sepia kobiensis in the East China Sea Region. Animals 2024, 14, 2716. [Google Scholar] [CrossRef]
- Yang, L.; Xu, M.; Cui, Y.; Liu, S. Seasonal spatial distribution patterns of AmphiOctopus ovulum in the East China Sea: Current status future projections under various climate change scenarios. Front. Mar. Sci. 2025. accepted. [Google Scholar]
- Yang, L.L.; Xu, M.; Liu, Z.L.; Zhang, Y.; Cui, Y.; Li, S.F. Seasonal and spatial distribution of AmphiOctopus fangsiao and Octopus variabilis in the southern Yellow and East China Seas: Fisheries management implications based on climate scenario predictions. Reg. Stud. Mar. Sci. 2025, 83, 104072. [Google Scholar] [CrossRef]
- Xu, M.; Liu, S.; Yang, C.; Yang, L. Seasonal spatial distribution patterns of Abralia multihamata in the East China Sea Region: Predictions under various climate scenarios. Animals 2025, 15, 903. [Google Scholar] [CrossRef]
- Torrejón-Magallanes, J.; Ángeles-González, L.E.; Csirke, J.; Bouchon, M.; Morales-Bojórquez, E.; Arreguín-Sánchez, F. Modeling the Pacific chub mackerel (Scomber japonicus) ecological niche and future scenarios in the northern Peruvian Current System. Prog. Oceanogr. 2021, 197, 102672. [Google Scholar] [CrossRef]
| Season | Euprymna berryi | Euprymna morsei | ||
|---|---|---|---|---|
| CPUEw (g·h−1) | CPUEn (ind·h−1) | CPUEw (g·h−1) | CPUEn (ind·h−1) | |
| Spring | - | - | 8555.88 | 5243 |
| Summer | 483.12 | 48 | 11.1 | 3 |
| Autumn | 3328.73 | 636 | 986.4 | 662 |
| Winter | 1612.86 | 431 | 1664.7 | 1732 |
| Factor | Euprymna berryi | Euprymna morsei | |||||
|---|---|---|---|---|---|---|---|
| Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | |
| Depth (m) | 55–84 | 58–107 | 38–126 | 13–82 | 104 | 16–112 | 16–114 |
| SST (°C) | 26.39–28.56 | 18.92–23.66 | 11.51–20.42 | 12.85–17.35 | 26.11 | 18–24.56 | 8.21–20.52 |
| SBT (°C) | 20.78–28.02 | 9.47–21.69 | 11.52–20.36 | 9.64–17.99 | 19.54 | 9.92–23.15 | 8.17–18.73 |
| SSS (‰) | 31.92–34.02 | 31.7–34.23 | 32.06–34.52 | 30.21–33.49 | 34.07 | 30.49–34.29 | 31.5–34.51 |
| SBS (‰) | 33.43–34.34 | 32.75–34.55 | 32.04–34.61 | 30.55–33.99 | 34.43 | 31.37–35.07 | 31.67–34.66 |
| SSDO (mg/L) | 5.22–6.44 | / | 7.35–8 | 7.93–8.63 | 6.06 | / | 7.33–9.08 |
| SBDO (mg/L) | 4.25–6.62 | / | 7.36–7.97 | 7.71–9.23 | 5.3 | / | 7.58–9.06 |
| Mean CPUEw at collection stations (g/h) | 80.52 | 256.06 | 94.87 | 213.9 | 11.1 | 36.53 | 38.71 |
| CPUEw range (g/h) | 25.2–154.96 | 1.35–2181.6 | 3.2–494.6 | 1.34–3203.2 | 11.1 | 2.4–178.82 | 0.5–297.6 |
| Mean CPUEn at collection stations (ind/h) | 8 | 48.92 | 25.35 | 131.08 | 3 | 24.52 | 40.28 |
| CPUEn range (ind/h) | 3–21 | 1–288 | 1–161 | 1–1824 | 3 | 1–124 | 1–272 |
| Mean AIW (g/ind) | 12.77 | 7.33 | 4.35 | 1.7 | 3.7 | 1.74 | 1.33 |
| AIW range (g/ind) | 6.3–26.33 | 0.75–20.9 | 0.8–13 | 0.71–11.29 | 3.7 | 0.53–5.56 | 0.16–8.47 |
| Suitable habitat range | 26.55°–29.35° N, 121.55°–126.95° E | 26.55°–32.65° N, 121.55°–126.95° E | 26.55°–32.25° N, 120.05°–126.95° E | 28.45°–34.95° N, 120.05°–126.45° E | 26.55°–34.95° N, 120.05°–125.65° E | 26.55°–34.95° N, 120.05°–126.95° E | 29.65°–34.95° N, 120.05°–126.25° E |
| Climate Scenario | Loss | Gain | Total | Suitable Habitat Range | |
|---|---|---|---|---|---|
| Current | E. berryi | / | / | / | 26.55° N–29.35° N, 121.45° E–126.95° E |
| E. morsei | / | / | / | 26.55° N–34.95° N, 120.05° E–126.95° E | |
| SSP126-2050 | E. berryi | −0.681% | 5.917% | 5.236% | 26.55° N–29.55° N, 121.55° E–126.95° E |
| E. morsei | −61.571% | 18.09% | −43.481% | 26.55° N–34.95° N, 120.05° E–126.95° E | |
| SSP126-2100 | E. berryi | −9.751% | 7.649% | −2.102% | 26.55° N–29.35° N, 121.55° E–126.95° E |
| E. morsei | −51.547% | 4.734% | −46.813% | 26.55° N–34.95° N, 120.05° E–126.95° E | |
| SSP245-2050 | E. berryi | −0.584% | 4.885% | 4.301% | 26.55° N–29.55° N, 121.45° E–126.95° E |
| E. morsei | −61.412% | 11.267% | −50.145% | 26.55° N–34.95° N, 120.05° E–126.95° E | |
| SSP245-2100 | E. berryi | −19.015% | 10.568% | −8.447% | 26.55° N–29.35° N, 121.55° E–126.95° E |
| E. morsei | −91.775% | 0.344% | −91.431% | 27.05° N–34.95° N, 120.05° E–126.25° E | |
| SSP370-2050 | E. berryi | −5.605% | 3.503% | −2.102% | 26.55° N–29.45° N, 121.45° E–126.95° E |
| E. morsei | −56.043% | 0.45% | −55.594% | 26.55° N–34.95° N, 120.05° E–126.95° E | |
| SSP370-2100 | E. berryi | −24.912% | 18.276% | −6.637% | 26.55° N–29.35° N, 121.55° E–126.95° E |
| E. morsei | −70.114% | 0% | −70.114% | 29.55° N–34.95° N, 120.05° E–126.25° E | |
| SSP585-2050 | E. berryi | −1.012% | 4.301% | 3.289% | 26.55° N–30.15° N, 121.55° E–126.95° E |
| E. morsei | −68.659% | 3.227% | −65.432% | 26.55° N–34.95° N, 120.05° E–126.95° E | |
| SSP585-2100 | E. berryi | −35.5% | 18.606% | −16.894% | 26.55° N–29.45° N, 121.75° E–126.95° E |
| E. morsei | −90.743% | 0% | −90.743% | 29.65° N–34.95° N, 120.05° E–126.25° E | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Liu, Y.; Song, X.; Yang, L. Changes in Seasonal Spatial Distribution Patterns of Euprymna berryi and Euprymna morsei: The Current and Predictions Under Climate Change Scenarios. Biology 2025, 14, 327. https://doi.org/10.3390/biology14040327
Xu M, Liu Y, Song X, Yang L. Changes in Seasonal Spatial Distribution Patterns of Euprymna berryi and Euprymna morsei: The Current and Predictions Under Climate Change Scenarios. Biology. 2025; 14(4):327. https://doi.org/10.3390/biology14040327
Chicago/Turabian StyleXu, Min, Yong Liu, Xiaojing Song, and Linlin Yang. 2025. "Changes in Seasonal Spatial Distribution Patterns of Euprymna berryi and Euprymna morsei: The Current and Predictions Under Climate Change Scenarios" Biology 14, no. 4: 327. https://doi.org/10.3390/biology14040327
APA StyleXu, M., Liu, Y., Song, X., & Yang, L. (2025). Changes in Seasonal Spatial Distribution Patterns of Euprymna berryi and Euprymna morsei: The Current and Predictions Under Climate Change Scenarios. Biology, 14(4), 327. https://doi.org/10.3390/biology14040327







