Imbalance of Bile Acids Metabolism Mediated by Gut Microbiota Contributed to Metabolic Disorders in Diabetic Model Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Animals and Experimental Design
2.3. Animal Sample Collection and Processing
2.4. Serum Biochemical Analysis
2.5. Quantitation of Bile Acids’ Composition in Colon Contents
2.6. Real-Time Quantitative PCR to Measure Level of BAs-Related Genes
2.7. Gut Microbiota Analysis
2.8. Correlation Analysis
2.9. Statistical Analysis
3. Results
3.1. Body Weight, Fasting Blood Glucose and Serum Lipid Indices
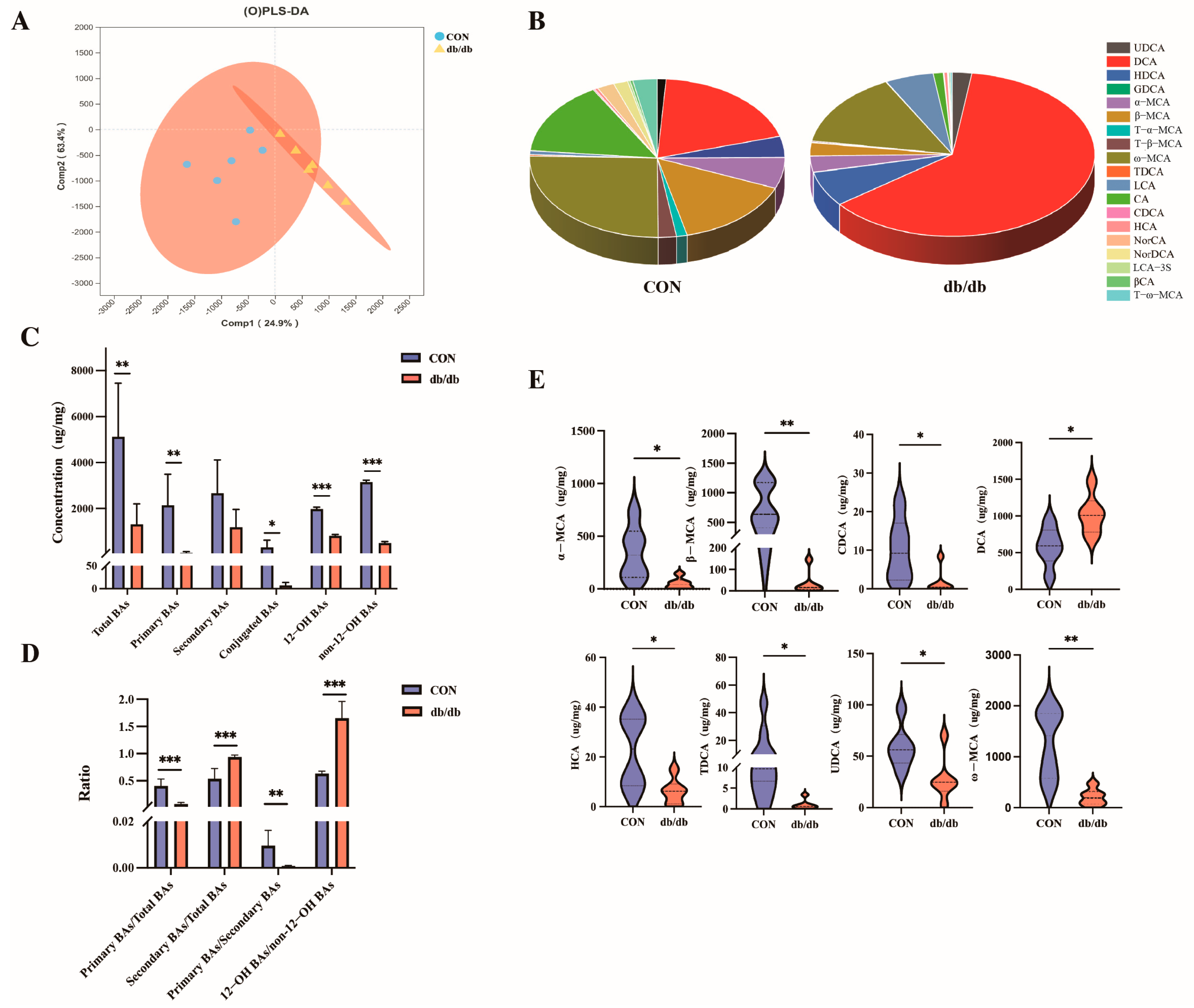
3.2. Alterations in Colonic Bile Acids Metabolism Between db/db and Wild-Type Mice
3.3. Changes in BAs-Related Genes Between db/db and Wild-Type Mice
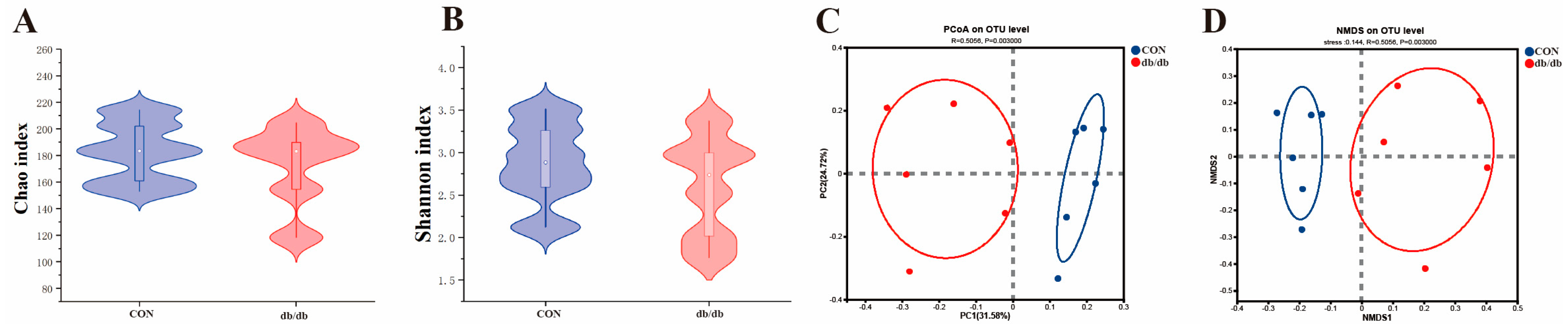
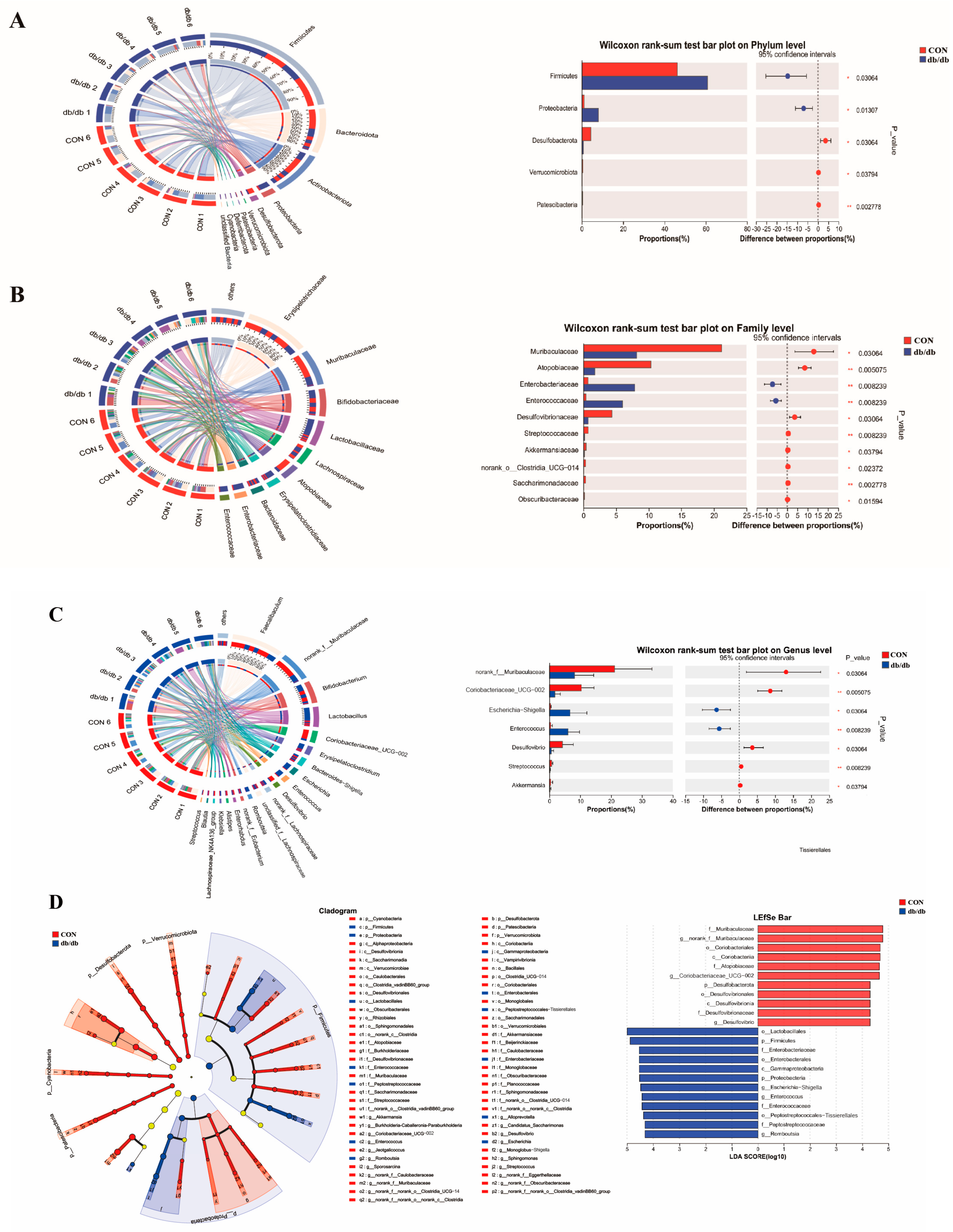
3.4. Microbiota Profile of the Colon Between db/db and Wild-Type Mice
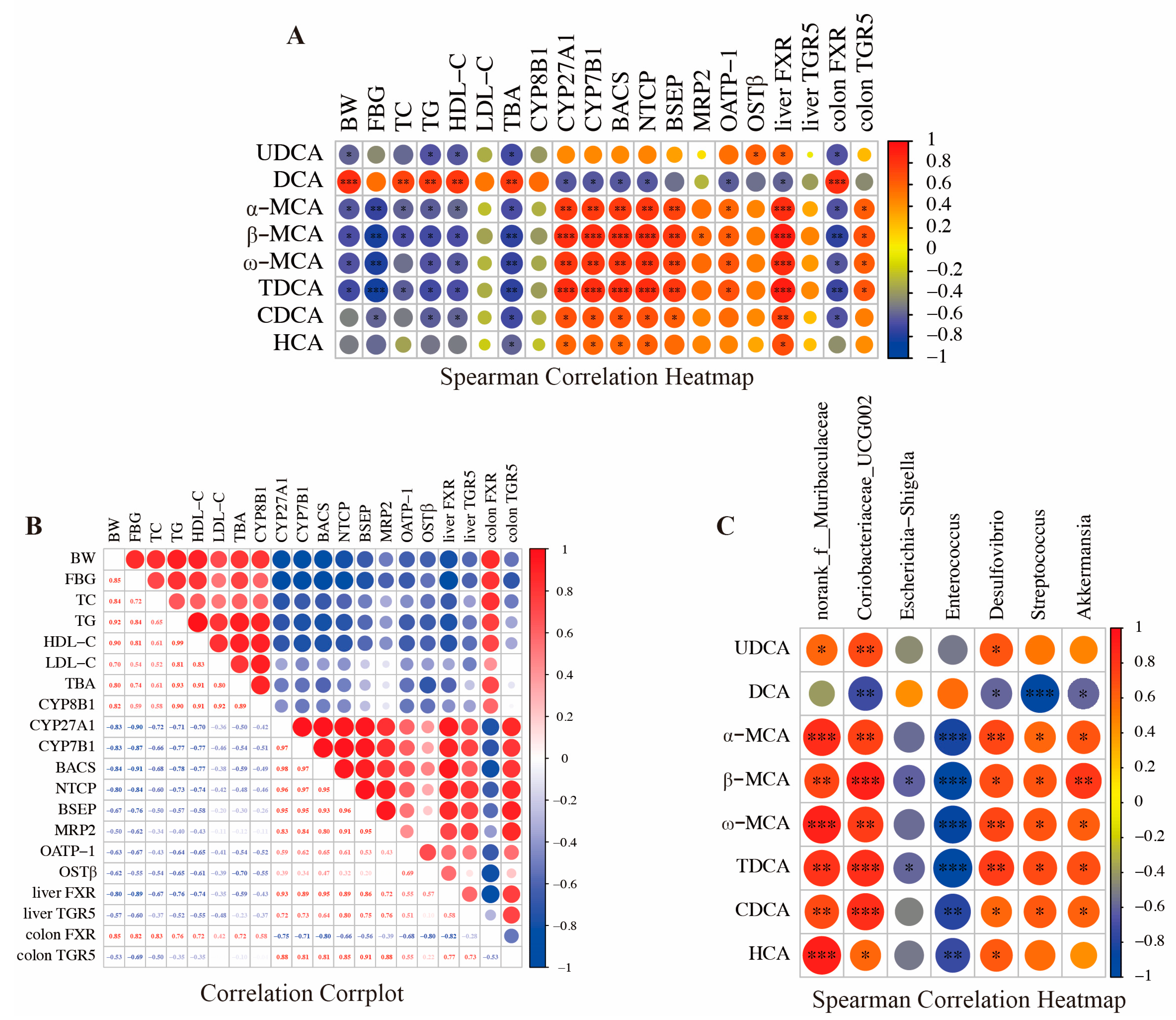
3.5. Association Analysis of Bile Acids, Gut Microbiota, and Metabolic Indicators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Roden, M.; Shulman, G.I. The integrative biology of type 2 diabetes. Nature 2019, 576, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Gauttam, V.K.; Munjal, K.; Mujwar, S.; Sawale, J.; Rohilla, M.; Gupta, S. Comparative Study of Developed Formulation and Market Formulation for Antidiabetic Potential in Alloxan Induced Diabetic Wistar Rats. J. Young Pharm. 2022, 14, 387–393. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Hou, Y.; Zhai, X.; Wang, X.; Wu, Y.; Wang, H.; Qin, Y.; Han, J.; Meng, Y. Research progress on the relationship between bile acid metabolism and type 2 diabetes mellitus. Diabetol. Metab. Syndr. 2023, 15, 235. [Google Scholar] [CrossRef]
- Jia, W.; Wei, M.; Rajani, C.; Zheng, X. Targeting the alternative bile acid synthetic pathway for metabolic diseases. Protein Cell 2021, 12, 411–425. [Google Scholar] [CrossRef]
- Yin, C.; Zhong, R.; Zhang, W.; Liu, L.; Chen, L.; Zhang, H. The potential of bile acids as biomarkers for metabolic disorders. Int. J. Mol. Sci. 2023, 24, 12123. [Google Scholar] [CrossRef]
- Jia, W.; Xie, G.; Jia, W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef]
- Song, Y.; Sun, L.; Ma, P.; Xu, L.; Xiao, P. Dihydromyricetin prevents obesity via regulating bile acid metabolism associated with the farnesoid X receptor in ob/ob mice. Food Funct. 2022, 13, 2491–2503. [Google Scholar] [CrossRef]
- Lu, Z.N.; He, H.W.; Zhang, N. Advances in understanding the regulatory mechanism of organic solute transporter α–β. Life Sci. 2022, 310, 121109. [Google Scholar] [CrossRef]
- Chiang, J.Y.; Ferrell, J.M. Bile acid receptors FXR and TGR5 signaling in fatty liver diseases and therapy. Am. J. Physiol.-Gastrointest. Liver Physiol. 2020, 318, G554–G573. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.J.; Wang, X.; Wang, Y.M.; Wang, B.M. Interplay between gut microbiota and bile acids in diarrhoea-predominant irritable bowel syndrome: A review. Crit. Rev. Microbiol. 2022, 48, 696–713. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Zheng, X.; Huang, F.; Wang, S.; Li, M.; Zhao, M.; Sang, C.; Ge, K.; Li, Y.; Li, J. Anti-adipogenic effect of theabrownin is mediated by bile acid alternative synthesis via gut microbiota remodeling. Metabolites 2020, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Pérez, O.; Cruz-Ramón, V.; Chinchilla-López, P.; Méndez-Sánchez, N. The role of the gut microbiota in bile acid metabolism. Ann. Hepatol. 2018, 16, 21–26. [Google Scholar] [CrossRef]
- Chen, C.; Hu, B.; Wu, T.; Zhang, Y.; Xu, Y.; Feng, Y.; Jiang, H. Bile acid profiles in diabetic (db/db) mice and their wild type littermates. J. Pharm. Biomed. Anal. 2016, 131, 473–481. [Google Scholar] [CrossRef]
- Mantovani, A.; Dalbeni, A.; Peserico, D.; Cattazzo, F.; Bevilacqua, M.; Salvagno, G.L.; Lippi, G.; Targher, G.; Danese, E.; Fava, C. Plasma bile acid profile in patients with and without type 2 diabetes. Metabolites 2021, 11, 453. [Google Scholar] [CrossRef]
- Eid, S.A.; O’Brien, P.D.; Kretzler, K.H.; Jang, D.G.; Mendelson, F.E.; Hayes, J.M.; Carter, A.; Zhang, H.; Pennathur, S.; Brosius, F.C., III. Dietary interventions improve diabetic kidney disease, but not peripheral neuropathy, in a db/db mouse model of type 2 diabetes. FASEB J. 2023, 37, e23115. [Google Scholar] [CrossRef]
- Reeves, P.G.; Nielsen, F.H.; Fahey Jr, G.C. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, R.; Kang, J.; Zhong, Y.; Abudurexiti, A.; Tan, H.; Lei, Y.; Ma, X. Effect of Cichorium glandulosum on intestinal microbiota and bile acid metabolism in db/db mice. Food Sci. Nutr. 2023, 11, 7765–7778. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Meng, C.; Huang, C.; Liu, F.; Xia, C. Oleanolic acid alleviates ANIT-induced cholestatic liver injury by activating Fxr and Nrf2 pathways to ameliorate disordered bile acids homeostasis. Phytomedicine 2022, 102, 154173. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Baden, M.Y.; Guasch-Ferré, M.; Wittenbecher, C.; Li, J.; Li, Y.; Wan, Y.; Bhupathiraju, S.N.; Tobias, D.K.; Clish, C.B. Plasma metabolite profiles related to plant-based diets and the risk of type 2 diabetes. Diabetologia 2022, 65, 1119–1132. [Google Scholar] [CrossRef]
- Lakić, B.; Škrbić, R.; Uletilović, S.; Mandić-Kovačević, N.; Grabež, M.; Šarić, M.P.; Stojiljković, M.P.; Soldatović, I.; Janjetović, Z.; Stokanović, A.; et al. Beneficial Effects of Ursodeoxycholic Acid on Metabolic Parameters and Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind, Placebo-Controlled Clinical Study. J. Diabetes Res. 2024, 2024, 4187796. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Li, Q.; Shen, L.; Guo, K.; Zhou, X. Chlorogenic acid improves glucose tolerance, lipid metabolism, inflammation and microbiota composition in diabetic db/db mice. Front. Endocrinol. 2022, 13, 1042044. [Google Scholar] [CrossRef]
- Ruan, Y.; Liu, R.; Gong, L. Investigation of dysregulated lipid metabolism in diabetic mice via targeted metabolomics of bile acids in enterohepatic circulation. Rapid Commun. Mass. Spectrom. 2022, 36, e9236. [Google Scholar] [CrossRef]
- Haeusler, R.A.; Astiarraga, B.; Camastra, S.; Accili, D.; Ferrannini, E. Human insulin resistance is associated with increased plasma levels of 12α-hydroxylated bile acids. Diabetes 2013, 62, 4184–4191. [Google Scholar] [CrossRef]
- Pan, D.; Xia, H.; Wang, Y.; Wang, P.; Fu, B.; Yin, S.; Wang, Y.; Qu, X.; Zhang, Y.; Hu, J. Whole grain oat attenuates high-fat and high-cholesterol diet-induced dyslipidemia by modulating non-12OH bile acid ratio via the gut-liver axis. Res. Sq. 2024, preprint. [Google Scholar]
- Mooranian, A.; Zamani, N.; Takechi, R.; Luna, G.; Mikov, M.; Goločorbin-Kon, S.; Kovacevic, B.; Arfuso, F.; Al-Salami, H. Modulatory nano/micro effects of diabetes development on pharmacology of primary and secondary bile acids concentrations. Curr. Diabetes Rev. 2020, 16, 900–909. [Google Scholar] [CrossRef]
- Mayerhofer, C.C.K.; Ueland, T.; Broch, K.; Vincent, R.P.; Cross, G.F.; Dahl, C.P.; Aukrust, P.; Gullestad, L.; Hov, J.R.; Trøseid, M. Increased Secondary/Primary Bile Acid Ratio in Chronic Heart Failure. J. Card. Fail. 2017, 23, 666–671. [Google Scholar] [CrossRef]
- Hu, Y.; Wu, A.; Yan, H.; Pu, J.; Luo, J.; Zheng, P.; Luo, Y.; Yu, J.; He, J.; Yu, B.; et al. Secondary bile acids are associated with body lipid accumulation in obese pigs. Anim. Nutr. 2024, 18, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Wahlström, A.; Brumbaugh, A.; Sjöland, W.; Olsson, L.; Wu, H.; Henricsson, M.; Lundqvist, A.; Makki, K.; Hazen, S.L.; Bergström, G. Production of deoxycholic acid by low-abundant microbial species is associated with impaired glucose metabolism. Nat. Commun. 2024, 15, 4276. [Google Scholar] [CrossRef] [PubMed]
- Zaborska, K.E.; Lee, S.A.; Garribay, D.; Cha, E.; Cummings, B.P. Deoxycholic acid supplementation impairs glucose homeostasis in mice. PLoS ONE 2018, 13, e0200908. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, F.; Zhang, Y.; Xiong, H.; Zhang, Y.; Zhuang, P.; Zhang, Y. Diabetic cognitive dysfunction is associated with increased bile acids in liver and activation of bile acid signaling in intestine. Toxicol. Lett. 2018, 287, 10–22. [Google Scholar] [CrossRef]
- Li, M.; Hu, X.; Xu, Y.; Hu, X.; Zhang, C.; Pang, S. A possible mechanism of metformin in improving insulin resistance in diabetic rat models. Int. J. Endocrinol. 2019, 2019, 3248527. [Google Scholar] [CrossRef]
- Pathak, P.; Chiang, J.Y. Sterol 12α-hydroxylase aggravates dyslipidemia by activating the ceramide/mTORC1/SREBP-1C pathway via FGF21 and FGF15. Gene Expr. 2019, 19, 161. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ueda, H.; Ikegami, T.; Honda, A. Upregulation of Taurine Biosynthesis and Bile Acid Conjugation with Taurine through FXR in a Mouse Model with Human-like Bile Acid Composition. Metabolites 2023, 13, 824. [Google Scholar] [CrossRef]
- Sun, L.; Xie, C.; Wang, G.; Wu, Y.; Wu, Q.; Wang, X.; Liu, J.; Deng, Y.; Xia, J.; Chen, B. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018, 24, 1919–1929. [Google Scholar] [CrossRef]
- Guo, Q.; Hou, X.; Cui, Q.; Li, S.; Shen, G.; Luo, Q.; Wu, H.; Chen, H.; Liu, Y.; Chen, A. Pectin mediates the mechanism of host blood glucose regulation through intestinal flora. Crit. Rev. Food Sci. Nutr. 2024, 64, 6714–6736. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, K.; Li, F.; Gu, Z.; Liu, Q.; He, L.; Shao, T.; Song, Q.; Zhu, F.; Zhang, L. Probiotic Lactobacillus rhamnosus GG prevents liver fibrosis through inhibiting hepatic bile acid synthesis and enhancing bile acid excretion in mice. Hepatology 2020, 71, 2050–2066. [Google Scholar] [CrossRef]
- Trabelsi, M.-S.; Daoudi, M.; Prawitt, J.; Ducastel, S.; Touche, V.; Sayin, S.I.; Perino, A.; Brighton, C.A.; Sebti, Y.; Kluza, J. Farnesoid X receptor inhibits glucagon-like peptide-1 production by enteroendocrine L cells. Nat. Commun. 2015, 6, 7629. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Okpara, E.S.; Hu, W.; Yan, C.; Wang, Y.; Liang, Q.; Chiang, J.Y.; Han, S. Interactive relationships between intestinal flora and bile acids. Int. J. Mol. Sci. 2022, 23, 8343. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Yang, H.; Tian, Z.; Zhu, M.; Sha, X.; Ran, J.; Li, L. Uygur type 2 diabetes patient fecal microbiota transplantation disrupts blood glucose and bile acid levels by changing the ability of the intestinal flora to metabolize bile acids in C57BL/6 mice. BMC Endocr. Disord. 2022, 22, 236. [Google Scholar] [CrossRef]
- Wang, M.; Li, K.; Jiao, H.; Zhao, J.; Li, H.; Zhou, Y.; Cao, A.; Wang, J.; Wang, X.; Lin, H. Dietary bile acids supplementation decreases hepatic fat deposition with the involvement of altered gut microbiota and liver bile acids profile in broiler chickens. J. Anim. Sci. Biotechnol. 2024, 15, 113. [Google Scholar]
- Mancera-Hurtado, Y.; Lopez-Contreras, B.E.; Flores-Lopez, R.; Villamil-Ramirez, H.; Del Rio-Navarro, B.E.; Canizales-Quinteros, S.; Moran-Ramos, S. A dysregulated bile acids pool is associated with metabolic syndrome and gut microbial dysbiosis in early adolescence. Obesity 2023, 31, 2129–2138. [Google Scholar] [CrossRef]
- Li, M.; Wang, S.; Li, Y.; Zhao, M.; Kuang, J.; Liang, D.; Wang, J.; Wei, M.; Rajani, C.; Ma, X. Gut microbiota-bile acid crosstalk contributes to the rebound weight gain after calorie restriction in mice. Nat. Commun. 2022, 13, 2060. [Google Scholar] [CrossRef]
- Pi, Y.; Wu, Y.; Zhang, X.; Lu, D.; Han, D.; Zhao, J.; Zheng, X.; Zhang, S.; Ye, H.; Lian, S. Gut microbiota-derived ursodeoxycholic acid alleviates low birth weight-induced colonic inflammation by enhancing M2 macrophage polarization. Microbiome 2023, 11, 19. [Google Scholar] [CrossRef]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Bile acids synthesis | ||
| CYP7A1 | GGGATGTATGCCTTCTGCT | AGTGCCGGAAATACTTGGT |
| CYP8B1 | AGCTCCTGGTGTGAAGATG | GTCCTTGGTGTAGCCGAA |
| CYP27A1 | ATCGGCACCTTTCCTGA | TTTTAGCAGAGGCATGTGG |
| CYP7B1 | CTTTGCCGCCACCTTAC | TCCCCACAAGGAAGACAG |
| BAAT | TGCAGAGCAAGCACAGAACATC | CTGTTTCAGGTGACCCACTCCA |
| BACS | TCCCGAAGCCAGCCATCCTC | GATCCCAACGACAAGTCCCATCAC |
| Bile acids transports | ||
| NTCP | TCCTCCCTGATGCCTTT | AATCCTGTTTCCATGCTGA- |
| BSEP | CCGCCAGCATCTTTGAGACA | CAATTTCACCCT TAATTCGATCCAG |
| MRP2 | GCACCGACTATCCAGCATCT | AACCAAAGGCACTCCAGAAA |
| MRP3 | CACACCACAACCACCTTCAC | TCAGGGTAGAGTCCAATGAGC |
| OATP-1 | TCCCCGCAGTCTTCATTCTAA | TGGATGTCGCCAGGGAAAT |
| OSTα | GTTCGCCTCCCTATTCCTCT | ACCTTGTGGTCTTTCCTTCG |
| OSTβ | GTATTTTCGTGCAGAAGATGCG | TTTCTGTTTGCCAGGATGCTC |
| Bile acids receptors | ||
| FXR | ACCAGGGAGAGACTGAGGT | AGGTGAGCGCGTTGTAGT |
| TGR5 | GCATGGGGAACGCTACA | AGGCTGGCAAAGAACAGG |
| Week | Body Weight (g) | Fasting Blood Glucose (mmol/L) | ||||
|---|---|---|---|---|---|---|
| CON | db/db | p Value | CON | db/db | p Value | |
| 1 | 20.51 ± 1.00 | 49.58 ± 3.73 | <0.001 | 7.93 ± 0.72 | 21.01 ± 4.47 | <0.001 |
| 2 | 20.89 ± 1.14 | 49.72 ± 4.93 | <0.001 | 6.11 ± 0.73 | 22.17 ± 5.60 | <0.001 |
| 3 | 20.86 ± 0.69 | 49.33 ± 5.79 | <0.001 | 6.75 ± 0.68 | 24.80 ± 5.72 | <0.001 |
| 4 | 21.48 ± 1.03 | 50.22 ± 5.01 | <0.001 | 7.15 ± 0.62 | 23.76 ± 4.03 | <0.001 |
| 5 | 21.39 ± 0.95 | 48.97 ± 5.59 | <0.001 | 7.18 ± 0.59 | 22.37 ± 6.06 | <0.001 |
| 6 | 22.80 ± 1.18 | 48.10 ± 5.73 | <0.001 | 7.35 ± 0.47 | 23.81 ± 5.26 | <0.001 |
| 7 | 23.25 ± 1.93 | 48.56 ± 5.71 | <0.001 | 7.54 ± 0.63 | 25.76 ± 4.82 | <0.001 |
| 8 | 22.12 ± 1.28 | 47.7 ± 5.30 | <0.001 | 7.08 ± 0.51 | 22.11 ± 3.92 | <0.001 |
| CON | db/db | p Value | |
|---|---|---|---|
| TC | 6.75 ± 0.87 | 12.87 ± 3.27 | 0.0018 |
| TG | 0.81 ± 0.098 | 1.41 ± 0.22 | <0.001 |
| LDL–C | 2.08 ± 0.63 | 3.60 ± 1.24 | 0.026 |
| HDL–C | 1.03 ± 0.15 | 2.71 ± 0.77 | <0.001 |
| TBA | 10.31 ± 2.32 | 15.06 ± 2.59 | 0.0014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, H.; Liu, X.; Song, G.; Peng, W.; Sun, X.; Fang, W.; Qi, W. Imbalance of Bile Acids Metabolism Mediated by Gut Microbiota Contributed to Metabolic Disorders in Diabetic Model Mice. Biology 2025, 14, 291. https://doi.org/10.3390/biology14030291
Dong H, Liu X, Song G, Peng W, Sun X, Fang W, Qi W. Imbalance of Bile Acids Metabolism Mediated by Gut Microbiota Contributed to Metabolic Disorders in Diabetic Model Mice. Biology. 2025; 14(3):291. https://doi.org/10.3390/biology14030291
Chicago/Turabian StyleDong, Hongwang, Xinguo Liu, Ge Song, Wenting Peng, Xihan Sun, Wei Fang, and Wentao Qi. 2025. "Imbalance of Bile Acids Metabolism Mediated by Gut Microbiota Contributed to Metabolic Disorders in Diabetic Model Mice" Biology 14, no. 3: 291. https://doi.org/10.3390/biology14030291
APA StyleDong, H., Liu, X., Song, G., Peng, W., Sun, X., Fang, W., & Qi, W. (2025). Imbalance of Bile Acids Metabolism Mediated by Gut Microbiota Contributed to Metabolic Disorders in Diabetic Model Mice. Biology, 14(3), 291. https://doi.org/10.3390/biology14030291







