The Impact of LED Light Spectra on the Growth, Yield, Physiology, and Sweetness Compound of Stevia rebaudiana
Simple Summary
Abstract
1. Introduction
2. Methods and Materials
2.1. Source of Stevia rebaudiana, Plants and Cuttings
2.2. Transplantation and Light Treatments
2.3. Physiological and Growth Parameters
2.3.1. Chlorophyll Content Analysis
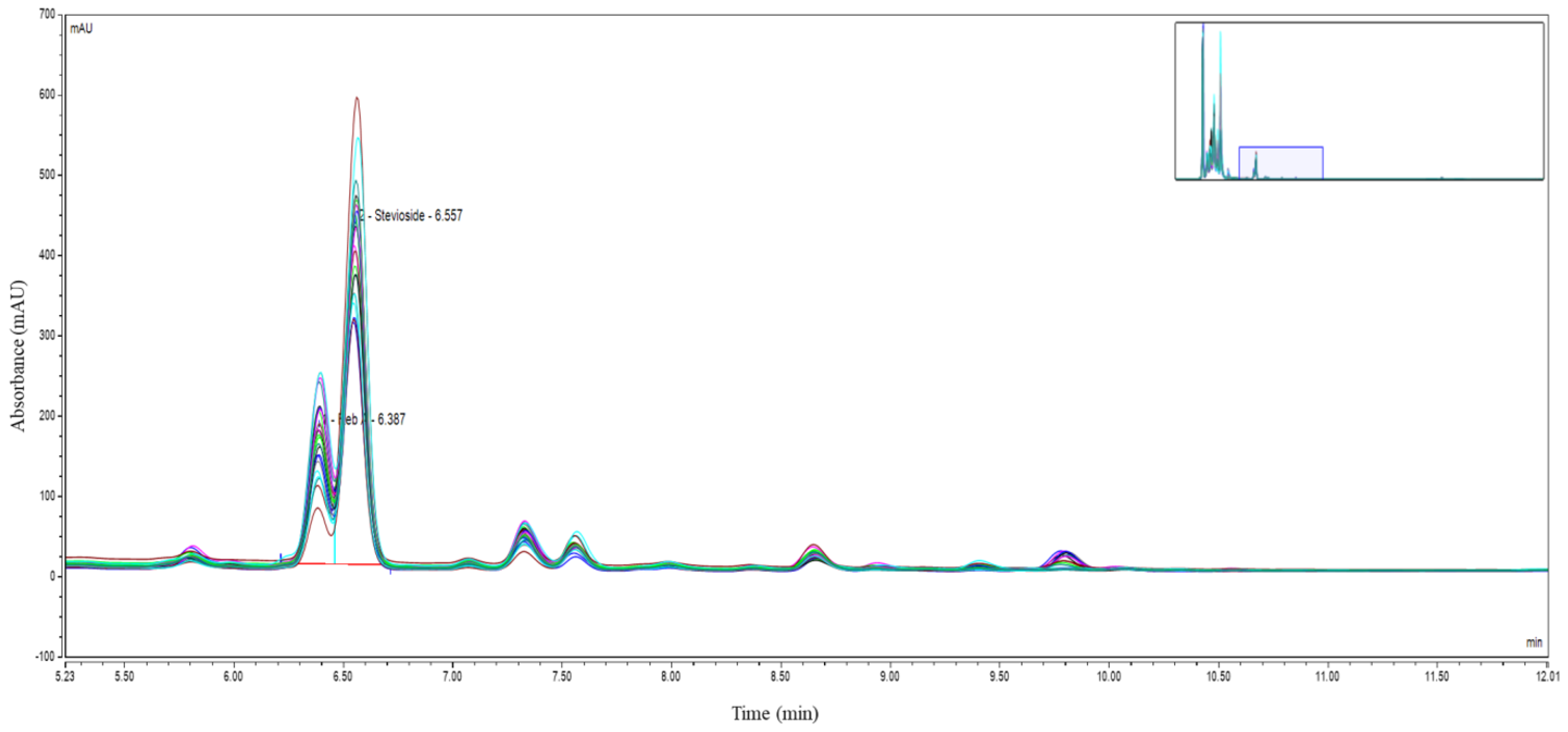
2.3.2. High-Performance Liquid Chromatography Analysis
2.4. Statistical Analysis
3. Results
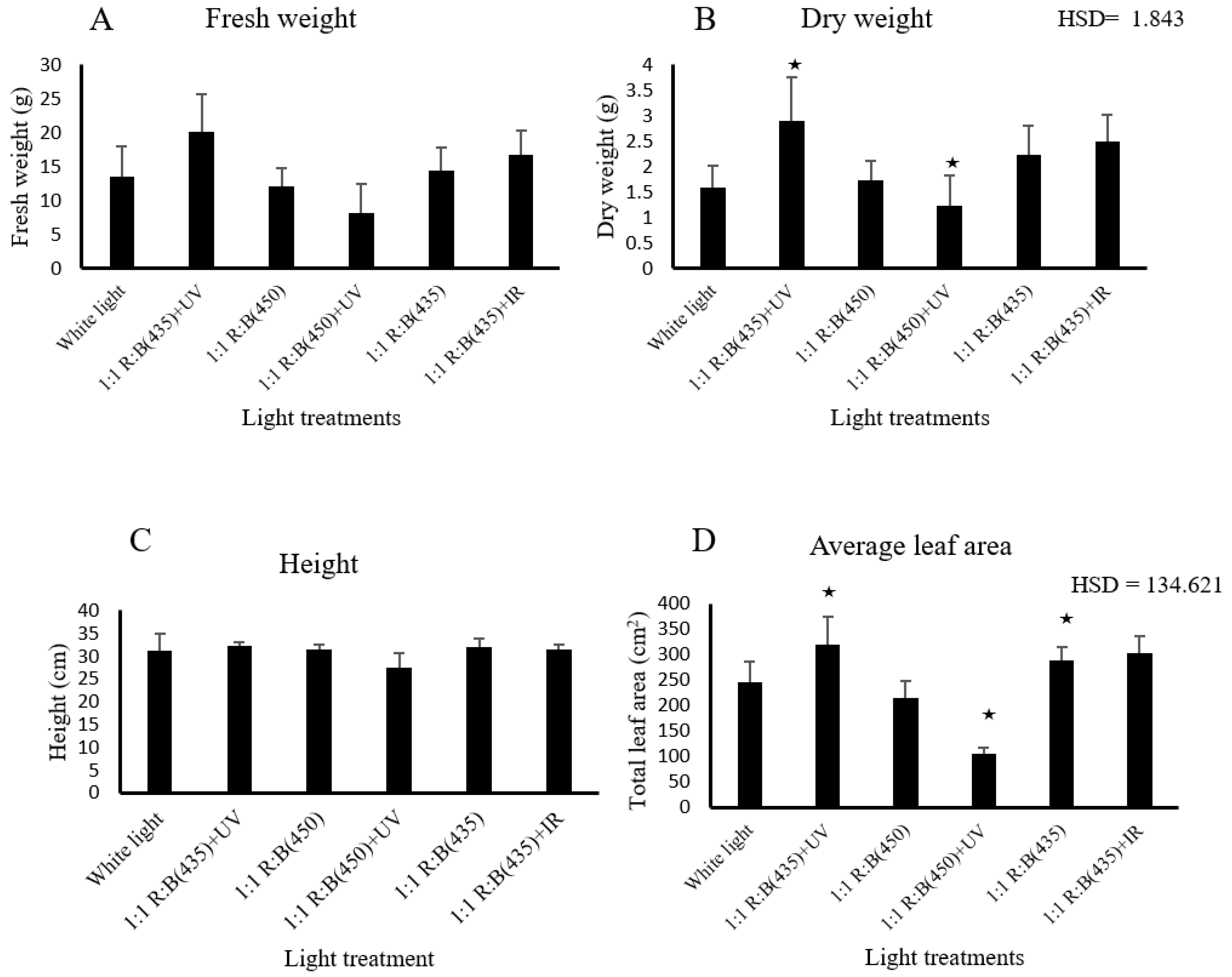
3.1. Morphological Parameters
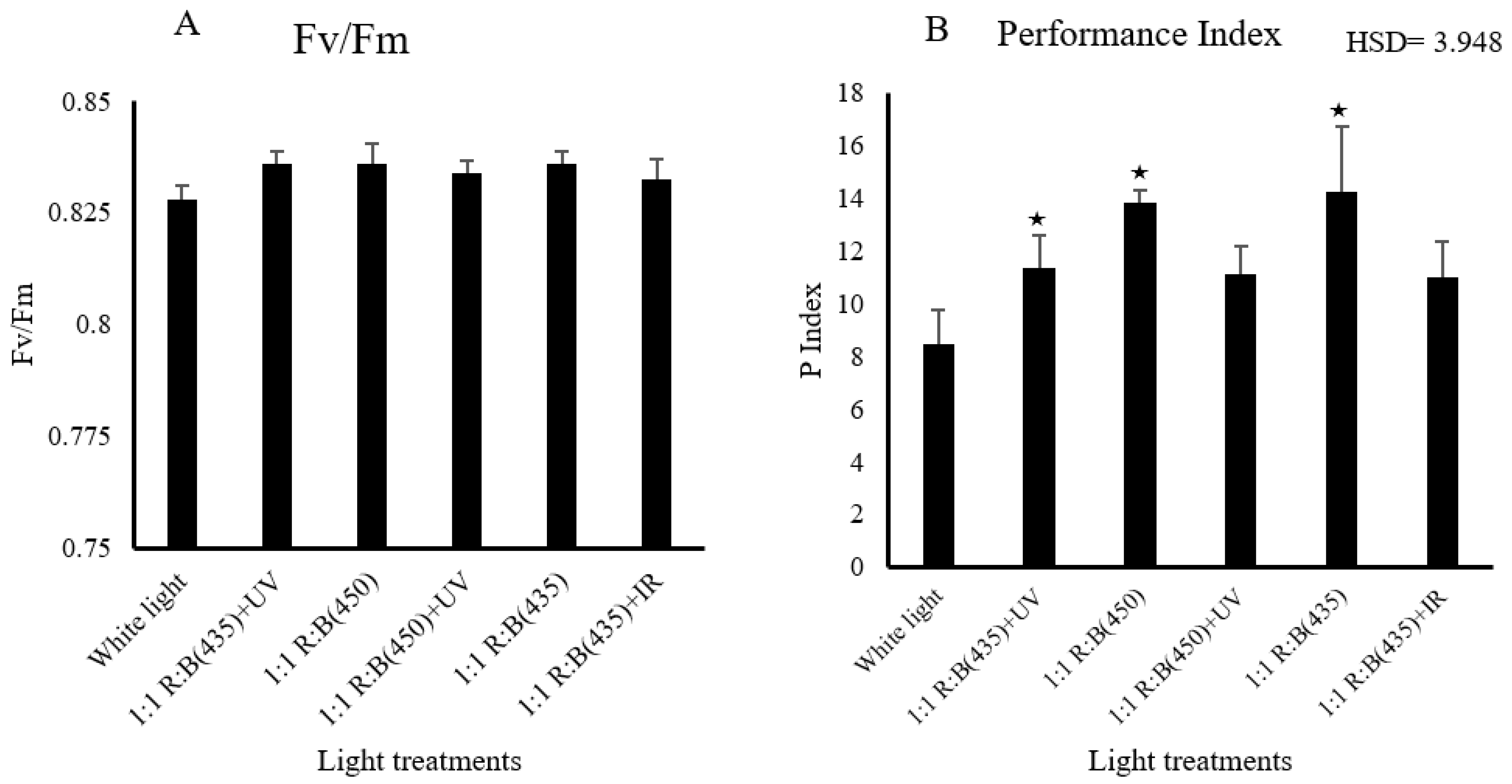
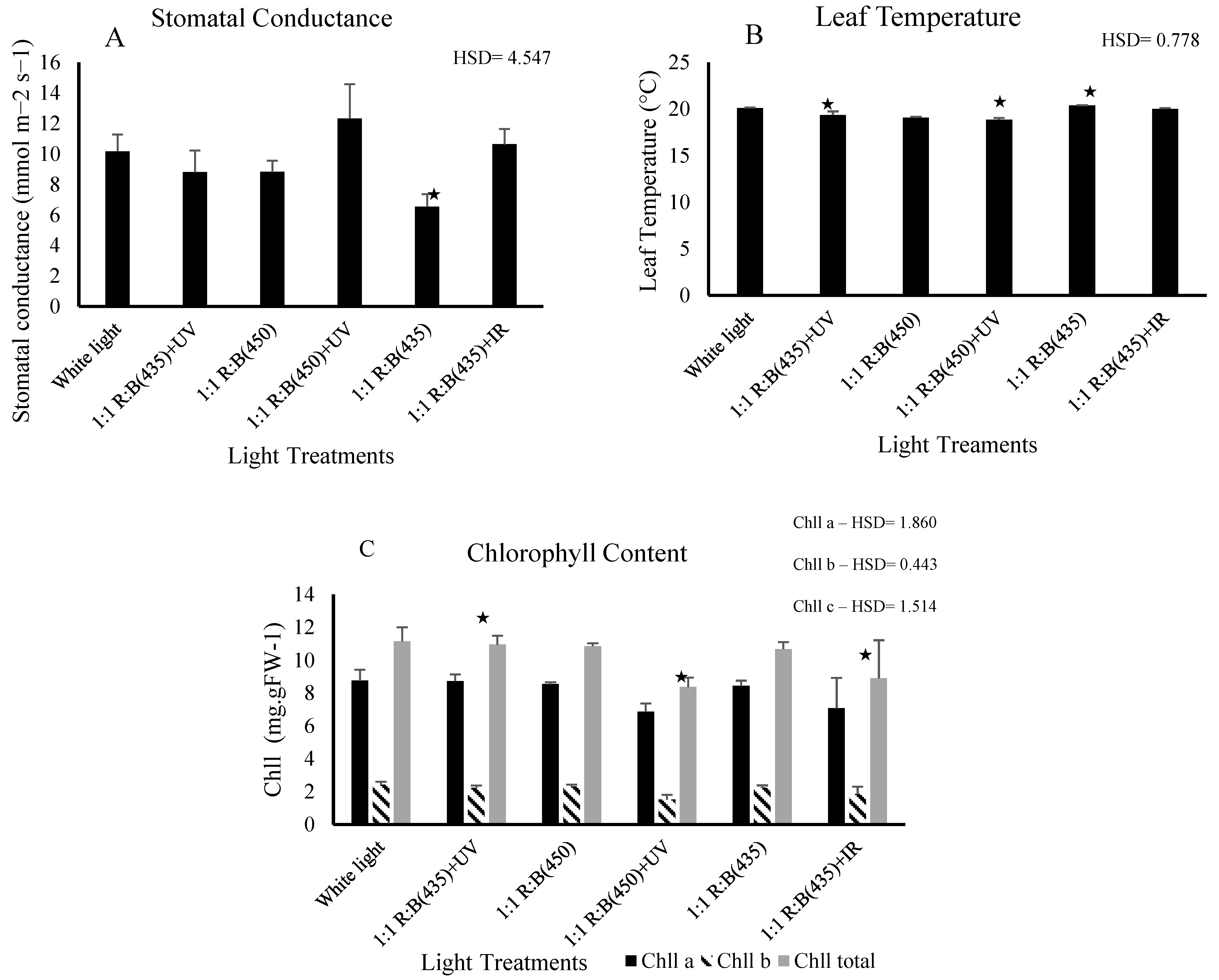
3.2. Physiological Parameters
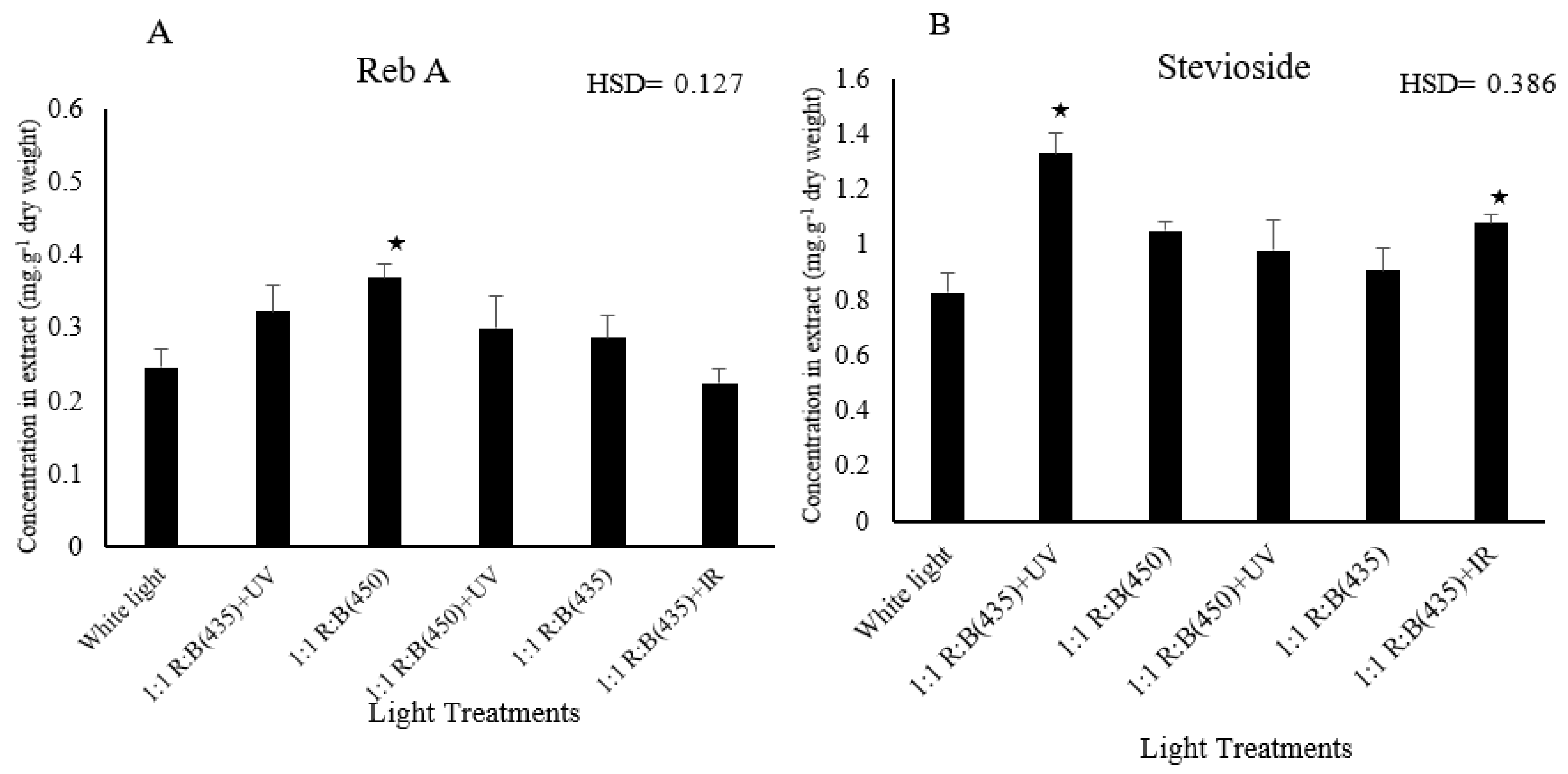
3.3. Sweetening Compound Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Attaya, A.S. LED Light Technology as a Source of Illumination and a Promising Method for Stevia rebaudiana Elite Propagation. Egypt. J. Agron. 2021, 43, 123–132. [Google Scholar] [CrossRef]
- Libik-Konieczny, M.; Capecka, E.; Kąkol, E.; Dziurka, M.; Grabowska-Joachimiak, A.; Sliwinska, E.; Pistelli, L. Growth, development and steviol glycosides content in the relation to the photosynthetic activity of several Stevia rebaudiana Bertoni strains cultivated under temperate climate conditions. Sci. Hortic. 2018, 234, 10–18. [Google Scholar] [CrossRef]
- Wojewoda, A.; Wodyk, T.; Stępniowski, D.; Cholewińska, E.; Stępniowska, A. Analysis of content of steviosides as biologically active compounds in stevia (Stevia rebaudiana) and products manufactured on the basis of this plant. World Sci. News 2018, 93, 132–141. [Google Scholar]
- Ciriminna, R.; Meneguzzo, F.; Pecoraino, M.; Pagliaro, M. A bioeconomy perspective for natural sweetener Stevia. Biofuels Bioprod. Biorefin. 2019, 13, 445–452. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, S.; Dhyani, D.; Ahuja, P.S. A review on the improvement of stevia [Stevia rebaudiana (Bertoni)]. Can. J. Plant Sci. 2011, 91, 1–27. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Zhou, C.; Fan, X.; Sun, Q.; Han, J.; Hua, C.; Li, Y.; Niu, Y.; Okonkwo, C.E. Properties, extraction and purification technologies of Stevia rebaudiana steviol glycosides: A review. Food Chem. 2024, 453, 139622. [Google Scholar] [CrossRef]
- Ahmad, A.; Haneef, M.; Ahmad, N.; Kamal, A.; Jaswani, S.; Khan, F. Application of natural sweetener Stevia rebaudiana in the medical field. Acta Sci. Biol. Sci. 2024, 46, e69877. [Google Scholar] [CrossRef]
- Abdulameer, D.A.; Osman, M.B.; Sulaiman, Z.; Yusop, M.R.; Abdullah, S.; Azizi, P.; Muttaleb, Q.A. Assessment of Stevia rebaudiana Bertoni genotypes via morpho-agronomic traits under two light conditions. Am. J. Plant Sci. 2018, 9, 1403. [Google Scholar] [CrossRef][Green Version]
- Saharudin AM, I.B.; Nazri NB, M.; Roslee, H.B.; Hawi MH, B.; Mar, S.O. Acceptance of stevia as a sugar substitute and its determinants among health educated individuals. Curr. Res. Nutr. Food Sci. J. 2020, 8, 226–237. [Google Scholar] [CrossRef]
- Rengasamy, N.; Othman, R.Y.; Che, H.S.; Harikrishna, J.A. Artificial lighting photoperiod manipulation approach to improve productivity and energy use efficacies of plant factory cultivated Stevia rebaudiana. Agronomy 2022, 12, 1787. [Google Scholar] [CrossRef]
- Rihan, H.Z.; Aljafer, N.; Jbara, M.; McCallum, L.; Lengger, S.; Fuller, M.P. The impact of LED lighting spectra in a plant factory on the growth, physiological traits and essential oil content of lemon balm (Melissa officinalis). Plants 2022, 11, 342. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.J.; Lopez, R.G.; Behe, B.K. Leveraging controlled-environment agriculture to increase key basil terpenoid and phenylpropanoid concentrations: The effects of radiation intensity and CO2 concentration on consumer preference. Front. Plant Sci. 2021, 11, 598519. [Google Scholar] [CrossRef]
- An, S.; Hwang, H.; Chun, C.; Jang, Y.; Lee, H.J.; Wi, S.H.; Yeo, K.-H.; Yu, I.-h.; Kwack, Y. Evaluation of air temperature, photoperiod and light intensity conditions to produce cucumber scions and rootstocks in a plant factory with artificial lighting. Horticulturae 2021, 7, 102. [Google Scholar] [CrossRef]
- Friesen, P. How Far-Red Photons Affect Plant Growth and Development; BioChambers: Winnipeg, MB, Canada, 2024. [Google Scholar]
- Palma, C.F.F.; Castro-Alves, V.; Morales, L.O.; Rosenqvist, E.; Ottosen, C.-O.; Strid, Å. Spectral composition of light affects sensitivity to UV-B and photoinhibition in cucumber. Front. Plant Sci. 2021, 11, 610011. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Eguchi, T.; Deveci, M.; Kubota, C. Tomato seedling physiological responses under different percentages of blue and red photon flux ratios using LEDs and cool white fluorescent lamps. Sci. Hortic. 2016, 213, 270–280. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Srivastava, V.; Chaturvedi, R. An interdisciplinary approach towards sustainable and higher steviol glycoside production from in vitro cultures of Stevia rebaudiana. J. Biotechnol. 2022, 358, 76–91. [Google Scholar] [CrossRef]
- Sipos, L.; Balázs, L.; Székely, G.; Jung, A.; Sárosi, S.; Radácsi, P.; Csambalik, L. Optimization of basil (Ocimum basilicum L.) production in LED light environments–a review. Sci. Hortic. 2021, 289, 110486. [Google Scholar] [CrossRef]
- Libik-Konieczny, M.; Capecka, E.; Tuleja, M.; Konieczny, R. Synthesis and production of steviol glycosides: Recent research trends and perspectives. Appl. Microbiol. Biotechnol. 2021, 105, 3883–3900. [Google Scholar] [CrossRef]
- Verma, P. Study of Medicinal Plant Stevia rebaudiana (Bert.) and Biosynthesis of its Chemical Compound as Well as Medicinal Uses and Pharmological Properties. Bioscene 2024, 21, 2. [Google Scholar]
- Yu, L.; Wang, W.; Zhang, X.; Zheng, W. A review on leaf temperature sensor: Measurement methods and application. In Proceedings of the Computer and Computing Technologies in Agriculture IX: 9th IFIP WG 5.14 International Conference, CCTA 2015, Beijing, China, 27–30 September 2015; Revised Selected Papers; Part I 9. pp. 216–230. [Google Scholar]
- Kim, B. Assessing accuracy over warm-up time of Lepton 3.5 thermal imaging for measuring leaf temperature of crops. J. Appl. Hortic. 2023, 25, 39–42. [Google Scholar] [CrossRef]
- Pappalardo, S.; Consoli, S.; Longo-Minnolo, G.; Vanella, D.; Longo, D.; Guarrera, S.; D’Emilio, A.; Ramírez-Cuesta, J. Performance evaluation of a low-cost thermal camera for citrus water status estimation. Agric. Water Manag. 2023, 288, 108489. [Google Scholar] [CrossRef]
- Mohamed, S.J.; Rihan, H.Z.; Aljafer, N.; Fuller, M.P. The Impact of Light Spectrum and Intensity on the Growth, Physiology, and Antioxidant Activity of Lettuce (Lactuca sativa L.). Plants 2021, 10, 2162. [Google Scholar] [CrossRef] [PubMed]
- Wouters Kuhn, A.; Lunardi Remuzzi, S.; Augusti Boligon, A.; Dail Laughinghouse IV, H.; Anraku de Campos, M.M.; Dal-Souto Frescura, V.; Andriolo, J.L.; de Bona da Silva, C.; Bosio Tedesco, S. Phytomass and essential oil of basil (cv. Basilicão) under periods of induced salt stress. J. Plant Nutr. 2023, 46, 4710–4719. [Google Scholar] [CrossRef]
- Porra, R.J. The chequered history of the development and use of simultaneous equations for the accurate determination of chlorophylls a and b. Photosynth. Res. 2002, 73, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Naz, B.; Afzal, A.; Ali, H.; Ahmad, N.; Fazal, H.; Ullah, R.; Ali, E.A.; Ali, M.; Ullah, Z.; Ali, A. Melatonin-Induced Stress Enhanced Biomass and Production of High-Value Secondary Cell Products in Submerged Adventitious Root Cultures of Stevia rebaudiana (Bert.). ACS Omega 2024, 9, 5548–5562. [Google Scholar] [CrossRef] [PubMed]
- Omidi, H.; Keshavarzi, M.H.B.; Mousavi, S.M.R. The effect of natural salinity on seed germination, seedling establishment growth and selected biochemical properties of Stevia rebaudiana Bertoni. Preprint 2024. [Google Scholar]
- Semenova, N.A.; Ivanitskikh, A.S.; Uyutova, N.I.; Smirnov, A.A.; Proshkin, Y.A.; Burynin, D.A.; Kachan, S.A.; Sokolov, A.V.; Dorokhov, A.S.; Chilingaryan, N.O. Effect of UV Stress on the Antioxidant Capacity, Photosynthetic Activity, Flavonoid and Steviol Glycoside Accumulation of Stevia rebaudiana Bertoni. Horticulturae 2024, 10, 210. [Google Scholar] [CrossRef]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G.; Bautista-Aguilar, J.R. The effect of light quality on growth and development of in vitro plantlet of Stevia rebaudiana Bertoni. Sugar Tech 2017, 19, 331–336. [Google Scholar] [CrossRef]
- Nakonechnaya, O.; Gafitskaya, I.; Burkovskaya, E.; Khrolenko, Y.A.; Grishchenko, O.; Zhuravlev, Y.N.; Subbotin, E.; Kulchin, Y.N. Effect of light intensity on the morphogenesis of Stevia rebaudiana under in vitro conditions. Russ. J. Plant Physiol. 2019, 66, 656–663. [Google Scholar] [CrossRef]
- Shulgina, A.A.; Kalashnikova, E.A.; Tarakanov, I.G.; Kirakosyan, R.N.; Cherednichenko, M.Y.; Polivanova, O.B.; Baranova, E.N.; Khaliluev, M.R. Influence of light conditions and medium composition on morphophysiological characteristics of Stevia rebaudiana Bertoni in vitro and in vivo. Horticulturae 2021, 7, 195. [Google Scholar] [CrossRef]
- Song, P.; Yang, Z.; Wang, H.; Wan, F.; Kang, D.; Zheng, W.; Gong, Z.; Li, J. Regulation of cryptochrome-mediated blue light signaling by the ABI4–PIF4 module. J. Integr. Plant Biol. 2024, 66, 2412–2430. [Google Scholar] [CrossRef] [PubMed]
- Lena, A.; Marino, M.; Manzano, M.; Comuzzi, C.; Maifreni, M. An overview of the application of blue light-emitting diodes as a non-thermic green technology for microbial inactivation in the food sector. Food Eng. Rev. 2024, 16, 59–84. [Google Scholar] [CrossRef]
- Lingwan, M.; Pradhan, A.A.; Kushwaha, A.K.; Dar, M.A.; Bhagavatula, L.; Datta, S. Photoprotective role of plant secondary metabolites: Biosynthesis, photoregulation, and prospects of metabolic engineering for enhanced protection under excessive light. Environ. Exp. Bot. 2023, 209, 105300. [Google Scholar] [CrossRef]
- Cavallaro, V.; Pellegrino, A.; Muleo, R.; Forgione, I. Light and plant growth regulators on in vitro proliferation. Plants 2022, 11, 844. [Google Scholar] [CrossRef]
- Lin, C.; Todo, T. The cryptochromes. Genome Biol. 2005, 6, 220. [Google Scholar] [CrossRef][Green Version]
- Esra, U.; Caglayan, N.; Turgut, K. The effects of various LED light wavelengths to the physiological and morphological parameters of stevia (Stevia rebaudiana) bertoni. Not. Sci. Biol. 2016, 8, 354–359. [Google Scholar]
- Verma, S.K.; Subrahmanyeswari, T.; Gantait, S. Ameliorated Morpho-Physiological Response of Stevia Under the Influence of Variable LEDs Inside Plant Factory System. Sugar Tech 2024, 26, 1601–1610. [Google Scholar] [CrossRef]
- Rengasamy, N.; Othman, R.Y.; Che, H.S.; Harikrishna, J.A. Beyond the PAR spectra: Impact of light quality on the germination, flowering, and metabolite content of Stevia rebaudiana (Bertoni). J. Sci. Food Agric. 2022, 102, 299–311. [Google Scholar] [CrossRef]
- Rashid, M.A.; Ding, P.; Hassan, S.A. Effects of Light Sources and Drying Methods on Plant Growth and Steviol Glycoside Content of Stevia rebaudiana Bertoni. J. Trop. Plant Physiol. 2021, 13, 14. [Google Scholar] [CrossRef]
- Aghighi Shahverdi, M.; Omidi, H.; Mosanaiey, H.; Pessarakli, M.; Mousavi, S.; Ghasemzadeh, M. Effects of light and temperature treatments on germination and physiological traits of stevia seedling (Stevia rebuadiana Bertoni). J. Plant Nutr. 2019, 42, 1125–1132. [Google Scholar] [CrossRef]
- Simlat, M.; Ślęzak, P.; Moś, M.; Warchoł, M.; Skrzypek, E.; Ptak, A. The effect of light quality on seed germination, seedling growth and selected biochemical properties of Stevia rebaudiana Bertoni. Sci. Hortic. 2016, 211, 295–304. [Google Scholar] [CrossRef]
- Rodriguez-Paez, L.A.; Jimenez-Ramirez, A.M.; Pompelli, M.F.; Pineda-Rodriguez, Y.Y.; Jarma-Orozco, A.; de Dios Jaraba-Navas, J.; Aramendiz-Tatis, H.; Combatt-Caballero, E.; Oloriz-Ortega, M.I.; Rodríguez, N.V. Physiological and enzymatic evaluation of selected genotypes of Stevia rebaudiana Bertoni. Agronomy 2023, 13, 403. [Google Scholar] [CrossRef]
- Ventura Zapata, E.; Brito Uribe, G.; Sánchez Rivera, M.M.; Nava Juárez, R.A.; Méndez Tinajero, M.; Sánchez Muñoz, J.; Tapia Barrera, N.P. Effect of Level of Pfd on Photosynthetic Parameters And Production of Steviol Glycosides By Hydroponic Culture from Stevia rebaudiana Bertoni. Int. J. Agric. Environ. Res. 2023, 9, 387–405. [Google Scholar]
- Liu, X.; Jing, Y.; Li, Z.; Wang, X.; Song, Y.; Zeng, J.; Lin, Q. Effects of dietary stevia extract supplementation on growth performance, serum biochemical indices, and intestinal health of yellow-feathered broilers. J. Anim. Sci. 2024, 102, skae245. [Google Scholar] [CrossRef]
- Ma, L.; Shi, Y. Effects of potassium fertilizer on physiological and biochemical index of Stevia rebaudiana Bertoni. Energy Procedia 2011, 5, 581–586. [Google Scholar] [CrossRef]
- Hastoy, C.; Cosson, P.; Cavaignac, S.; Boutie, P.; Waffo-Teguo, P.; Rolin, D.; Schurdi-Levraud, V. Deciphering performances of fifteen genotypes of Stevia rebaudiana in southwestern France through dry biomass and steviol glycoside evaluation. Ind. Crops Prod. 2019, 128, 607–619. [Google Scholar] [CrossRef]
- Nasution, A.M.; Muslihatin, W.; Patrialoka, S.N.; Pratama, I.P.E.W.; Aisyah, P.Y.; Jadid, N.; Antika, T.R.; Shovitri, M. The synergistic application of sucrose and various LED light exposures to enhance the in vitro growth of Stevia rebaudiana (Bertoni). Open Agric. 2024, 9, 20220305. [Google Scholar] [CrossRef]
- Aranda-González, I.; Moguel-Ordoñez, Y.; Betancur-Ancona, D. Determination of rebaudioside A and Stevioside in leaves of S. rebaudiana Bertoni grown in México by a validated HPLC method. Am. J. Anal. Chem. 2015, 6, 878. [Google Scholar] [CrossRef]
- Muthusamy, K.; Munaim, M.S.A. Determination of Factors Affecting Extraction of Rebaudioside A & Stevioside from Stevia Leaves. Int. J. Eng. Technol. Sci. 2019, 6, 120–130. [Google Scholar]
- Biswas, P.; Kumari, A.; Modi, A.; Kumar, N. Improvement and Regulation of Steviol Glycoside Biosynthesis in Stevia rebaudiana Bertoni. Gene 2023, 891, 147809. [Google Scholar] [CrossRef] [PubMed]
- Gunasena, M.; Senarath, W. Comparison of Phytochemicals in naturally grown plants, tissue cultured plants and calli of Stevia rebaudiana using HPLC and GC-MS. Preprint 2024. [Google Scholar]
- Martono, Y.; Rohman, A.; Riyanto, S.; Martono, S. Analysis study of stevioside and rebaudioside a from Stevia rebaudiana bertoni by normal phase SPE and RP-HPLC. IOP Conf. Ser. Mater. Sci. Eng. 2018, 394, 012071. [Google Scholar] [CrossRef]
- Hernández, K.V.; Moreno-Romero, J.; de la Torre, M.H.; Manríquez, C.P.; Leal, D.R.; Martínez-Garcia, J.F. Effect of light intensity on steviol glycosides production in leaves of Stevia rebaudiana plants. Phytochemistry 2022, 194, 113027. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.M.; Vallejo, V.A.; Beaudry, R.M.; Warner, R.M. Daily light integral influences steviol glycoside biosynthesis and relative abundance of specific glycosides in stevia. HortScience 2015, 50, 1479–1485. [Google Scholar] [CrossRef]
- Yoneda, Y.; Shimizu, H.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Effects of light intensity and photoperiod on improving steviol glycosides content in Stevia rebaudiana (Bertoni) Bertoni while conserving light energy consumption. J. Appl. Res. Med. Aromat. Plants 2017, 7, 64–73. [Google Scholar] [CrossRef]
- Calapardo, M.J.M.; Manigo, B.I. Enhancing herbage growth, yield and quality of stevia (Stevia rebaudiana Bertoni) using bio-organic nutrients in varied soil media. Sains Malays. 2024, 53, 533–547. [Google Scholar] [CrossRef]
- Le Bihan, Z.; Hastoy, C.; Cosson, P.; Boutié, P.; Rolin, D.; Schurdi-Levraud, V. To cut or not to cut? that is the question in first year harvest of Stevia rebaudiana bertoni production. Ind. Crops Prod. 2021, 162, 113209. [Google Scholar] [CrossRef]
- Samah, N.A.; Hisham, A.; Rahim, S. Determination of stevioside and rebaudioside A in Stevia rebaudiana leaves via preparative high performance liquid chromatography (prep-HPLC). Int. J. Chem. Environ. Eng. 2013, 4. [Google Scholar]
- Yoneda, Y.; Nakashima, H.; Miyasaka, J.; Ohdoi, K.; Shimizu, H. Impact of blue, red, and far-red light treatments on gene expression and steviol glycoside accumulation in Stevia rebaudiana. Phytochemistry 2017, 137, 57–65. [Google Scholar] [CrossRef]
- Ramezan, D.; Farrokhzad, Y.; Mokhtassi-Bidgoli, A.; Rasouli-Alamuti, M. Multi-walled carbon nanotubes interact with light intensity to affect morpho-biochemical, nutrient uptake, DNA damage, and secondary metabolism of Stevia rebaudiana. Environ. Sci. Pollut. Res. 2023, 30, 36915–36927. [Google Scholar] [CrossRef] [PubMed]
- Rihan, H.; Fuller, M. The Growth and Development of Sweet Basil (Ocimum basilicum) and Bush Basil (Ocimum minimum) Grown under Three Light Regimes in a Controlled Environment. Agronomy 2019, 9, 743. [Google Scholar] [CrossRef]
- Alrajhi, A.A.; Alsahli, A.S.; Alhelal, I.M.; Rihan, H.Z.; Fuller, M.P.; Alsadon, A.A.; Ibrahim, A.A. The effect of LED light spectra on the growth, yield and nutritional value of red and green lettuce (Lactuca sativa). Plants 2023, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- López, L.E.O.; Rodríguez-Fuentes, H.; Luna-Maldonado, A.I.; Márquez-Reyes, J.M.; Rojas, R. Effect of LED Light in Biomass Production and Presence of Metabolites in Medicinal Plants. In Biocontrol Systems and Plant Physiology in Modern Agriculture; Apple Academic Press: Palm Bay, FL, USA, 2022; pp. 201–220. [Google Scholar]
- Nawkar, G.M.; Khare, T.; Kumar, V.; Shelake, R.M. Light Signaling and Plant Secondary Metabolites. In Plant Secondary Metabolites and Abiotic Stress; Scrivener Publishing: Austin, TX, USA, 2024; pp. 623–643. [Google Scholar]
- Somanatham, S. Environmental Influence on Yield and Quality of Stevia (Stevia rebaudiana Bertoni); Department of Agronomy, College of Agriculture: Vellanikkara, India, 2022. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljafer, N.; Alrajhi, A.; Anderson von Trampe, T.; Vevers, W.; Fauset, S.; Rihan, H.Z. The Impact of LED Light Spectra on the Growth, Yield, Physiology, and Sweetness Compound of Stevia rebaudiana. Biology 2025, 14, 108. https://doi.org/10.3390/biology14020108
Aljafer N, Alrajhi A, Anderson von Trampe T, Vevers W, Fauset S, Rihan HZ. The Impact of LED Light Spectra on the Growth, Yield, Physiology, and Sweetness Compound of Stevia rebaudiana. Biology. 2025; 14(2):108. https://doi.org/10.3390/biology14020108
Chicago/Turabian StyleAljafer, Naofel, Abdullah Alrajhi, Toby Anderson von Trampe, William Vevers, Sophie Fauset, and Hail Zuhir Rihan. 2025. "The Impact of LED Light Spectra on the Growth, Yield, Physiology, and Sweetness Compound of Stevia rebaudiana" Biology 14, no. 2: 108. https://doi.org/10.3390/biology14020108
APA StyleAljafer, N., Alrajhi, A., Anderson von Trampe, T., Vevers, W., Fauset, S., & Rihan, H. Z. (2025). The Impact of LED Light Spectra on the Growth, Yield, Physiology, and Sweetness Compound of Stevia rebaudiana. Biology, 14(2), 108. https://doi.org/10.3390/biology14020108








