High-Intensity Continuous Light from Red–Blue Light-Emitting Diodes Improved Yield, Nutritional Quality and Reactive Oxygen Species Accumulation in Two Leaf-Color Lettuces
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Light Treatments
2.3. Measurement Items and Methods
2.4. Statistical Analysis
3. Results
3.1. Effects of LED Red–Blue CL Intensity on Biomass and Leaf Area of Two Lettuce Varieties
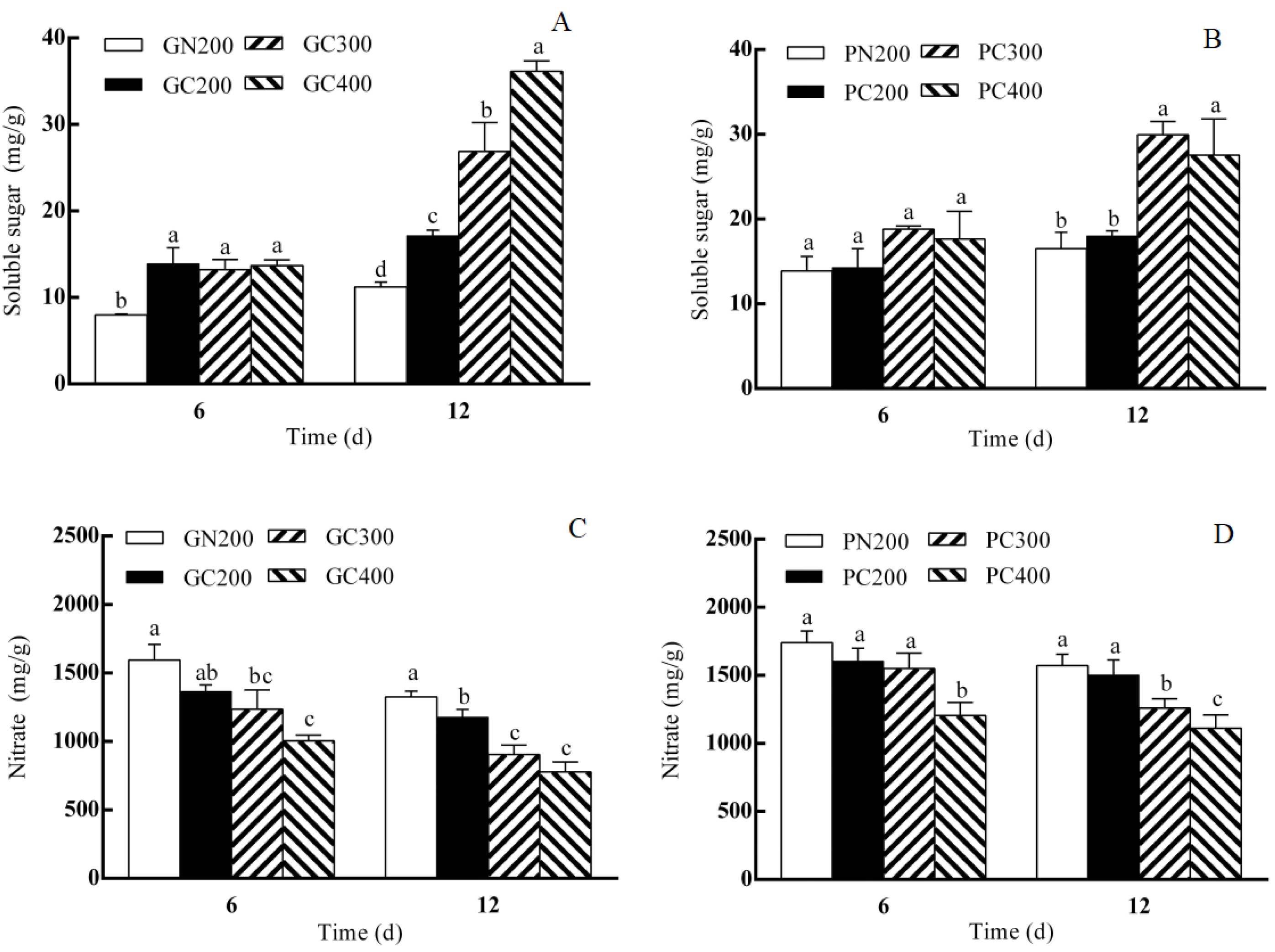
3.2. Effects of LED Red–Blue CL Intensity on Soluble Sugar and Nitrate Contents of Two Lettuce Varieties
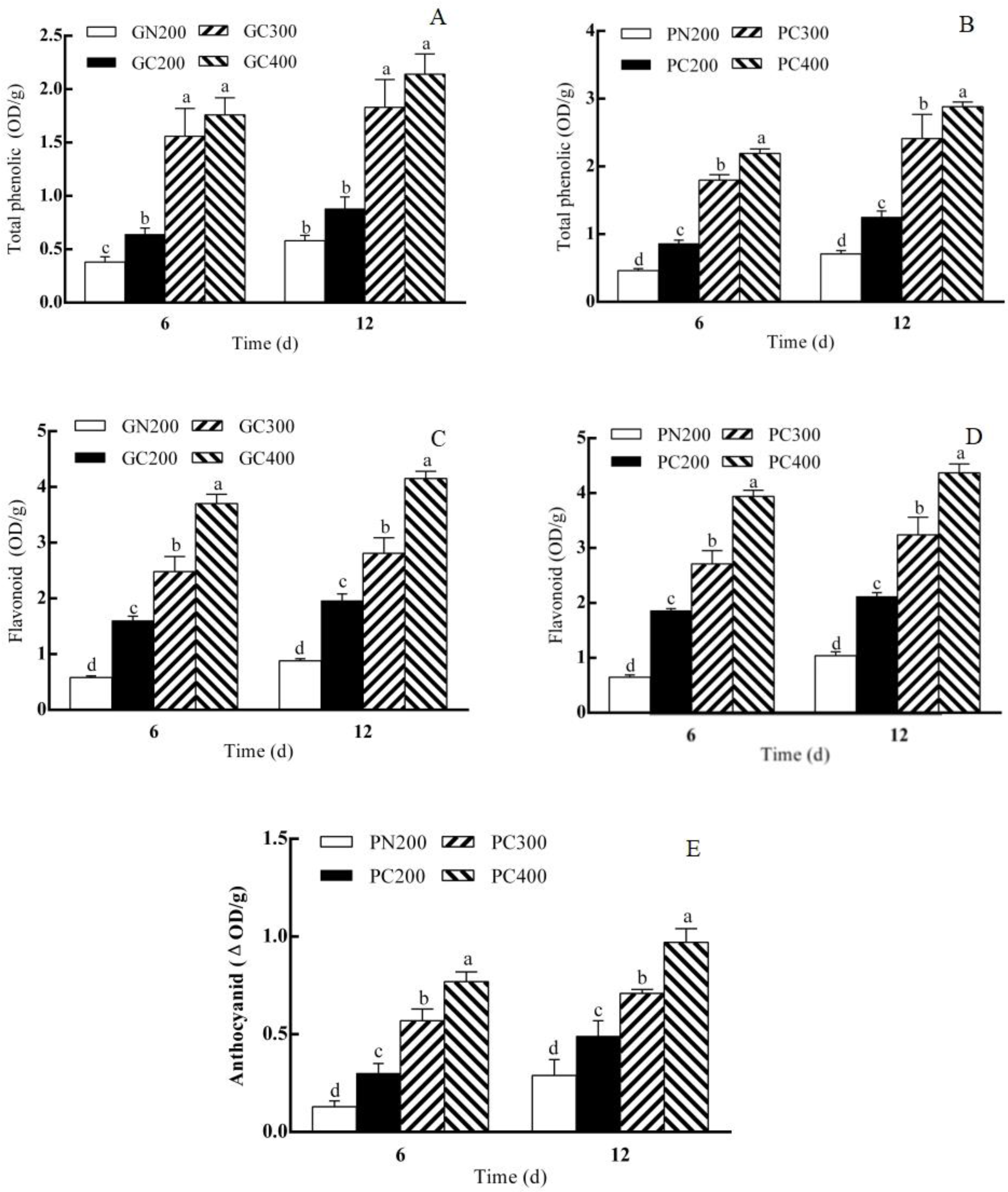
3.3. Effects of LED RED–BLUE CL Intensity on Anthocyanin, Total Phenolic and Flavonoid Contents in Two Lettuce Varieties
3.4. Effects of LED Red–Blue CL Intensity on the DPPH of Two Lettuce Varieties
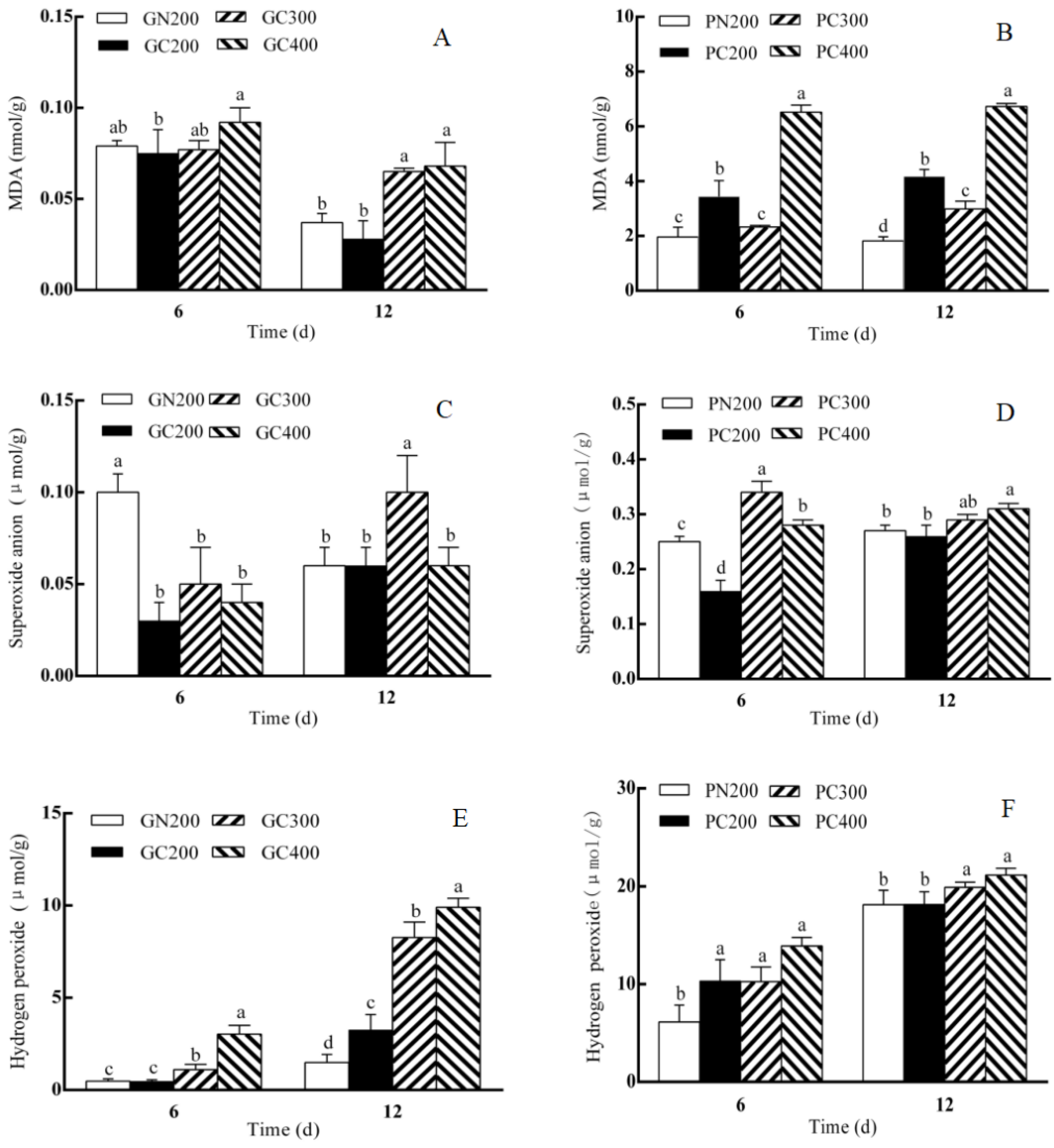
3.5. Effects of LED Red–Blue CL Intensities on the MDA, H2O2 and Superoxide Anion Contents of Two Lettuce Varieties
4. Discussion
4.1. Effects of CL Intensity of LED Red–Blue Light on Yield of Two Lettuce Cultivars
4.2. Effects of CL Intensity of LED Red–Blue Light on the Soluble Sugar and Nitrate Contents of Two Lettuce Cultivars
4.3. Effects of CL Intensity of LED Red–Blue Light on the DPPH, and MDA, Hydrogen Peroxide and Superoxide Anion Contents of Two Lettuce Varieties
4.4. Effects of CL Intensity of LED Red–Blue Light on the Contents of Antioxidant Substances in Two Lettuce Cultivars
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Heuvelink, E.; Van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Continuous light as a way to increase greenhouse tomato production: Expected challenges. Acta Hortic. 2012, 956, 51–57. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Van Ieperen, W.; Vreugdenhil, D.; Van Poppel, P.; Heuvelink, E.; Millenaar, F.F. A single locus confers tolerance to continuous light and allows substantial yield increase in tomato. Nat. Commun. 2014, 5, 4549. [Google Scholar] [CrossRef]
- Zha, L.Y.; Liu, W.K.; Zhang, Y.B.; Zhou, C.B.; Shao, M.J. Morphological and physiological stress responses of lettuce to different intensities of continuous light. Front. Plant Sci. 2019, 10, 1440. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, N.; Nesbit, A.E.; Gordon, E.P.; Green, C.; Gordon, D.T. Continuous light may induce photosynthetic downregulation in onion-consequences for growth and biomass partitioning. Physiol. Plant. 2005, 125, 235–246. [Google Scholar] [CrossRef]
- Zha, L.Y.; Liu, W.K. Effects of continuous red and blue LED light on growth, photosynthesis and fluorescence characteristics of five lettuce species under the same DLI. Plant Physiol. J. 2017, 53, 1735–1741. [Google Scholar]
- Zha, L.Y.; Zhang, Y.B.; Liu, W.K. Dynamic responses of ascorbate pool and metabolism in lettuce to long-term continuous light provided by red and blue LEDs. Environ. Exp. Bot. 2019, 163, 15–23. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of ascorbate accumulation and metabolism in lettuce by the red:blue ratio of continuous light using LEDs. Front. Plant Sci. 2020, 11, 704. [Google Scholar] [CrossRef]
- Azad, M.O.K.; Kjaer, K.H.; Adnan, M.; Naznin, M.T.; Lim, J.D.; Sung, I.J.; Park, C.H.; Lim, Y.S. The evaluation of growth performance, photosynthetic capacity, and primary and secondary metabolite content of leaf lettuce grown under limited irradiation of blue and red LED light in an urban plant factory. Agriculture 2020, 10, 28. [Google Scholar] [CrossRef]
- Chen, X.-L.; Wang, L.-C.; Li, T.; Yang, Q.-C.; Guo, W.-Z. Sugar accumulation and growth of lettuce exposed to different lighting modes of red and blue LED light. Sci. Rep. 2019, 9, 6926. [Google Scholar] [CrossRef]
- Samuolienė, G.; Brazaitytė, A.; Sirtautas, R.; Viršilė, A.; Sakalauskaitė, J.; Sakalauskienė, S.; Duchovskis, P. LED illumination affects bioactive compounds in romaine baby leaf lettuce. J. Sci. Food Agric. 2013, 93, 3286–3291. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; De Pascale, S. Daily variation in leaf nitrate content of two cultivars of hydroponically grown basil. Acta Hortic. 2007, 747, 203–210. [Google Scholar] [CrossRef]
- Proietti, S.; Moscatello, S.; Colla, G.; Battistelli, Y. The effect of growing spinach (Spinacia oleracea L.) at two light intensities on the amounts of oxalate, ascorbate and nitrate in their leaves. J. Hortic. Sci. Biotechnol. 2004, 79, 606–609. [Google Scholar] [CrossRef]
- Zhou, W.L.; Liu, W.K.; Yang, Q.C. Quality changes of hydroponic lettuce under pre-harvest short-term continuous light by LEDs with different intensity. J. Hortic. Sci. Biotechnol. 2012, 87, 429–434. [Google Scholar] [CrossRef]
- Bian, Z.H.; Cheng, R.F.; Wang, Y.; Yang, Q.; Lu, C. Effect of green light on nitrate reduction and edible quality of hydroponically grown lettuce (Lactuca sativa L.) under short-term continuous light from red and blue light-emitting diodes. Environ. Exp. Bot. 2018, 153, 63–71. [Google Scholar] [CrossRef]
- Lanoue, J.; Zheng, J.M.; Little, C.; Thibodeau, A.; Grodzinski, B.; Hao, X.M. Alternating red and blue light-emitting diodes allows for injury-free tomato production with continuous lighting. Front. Plant Sci. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.M.; Chun, C. Growth and leaf injury in tomato plants under continuous light at different settings of constant and diurnally varied photosynthetic photon flux densities. Sci. Hortic. 2020, 269, 109347. [Google Scholar] [CrossRef]
- Huang, J.; Xu, Y.L.; Duan, F.M.; Du, X.; Yang, Q.C.; Zheng, Y.J. Improvement of the growth and nutritional quality of two-leaf-color pak choi by supplemental alternating red and blue light. HortScience 2021, 56, 118–125. [Google Scholar] [CrossRef]
- Liu, W.K.; Liu, J.Y. Alternating red-blue light improved lettuce yield and alleviated physiological injury by reducing oxidative stress rather than carbohydrate accumulation and circadian rhythm disorder under both high light and continuous light from red-blue LEDs. Hortic. Environ. Biotechnol. 2024, 65, 831–845. [Google Scholar] [CrossRef]
- Elstner, E.F. Oxygen activation and oxygen toxicity. Annu. Rev. Plant Physiol. 1982, 33, 73–96. [Google Scholar] [CrossRef]
- Edreva, A. The importance of non-photosynthetic pigments and cinnamic acid derivatives in photoprotection. Agric. Ecosyst. Environ. 2005, 106, 135–146. [Google Scholar] [CrossRef]
- Huang, J.; Hammerbacher, A.; Forkelová, L.; Hartmann, H. Release of resource constraints allows greater carbon allocation to secondary metabolites and storage in winter wheat. Plant Cell Environ. 2007, 40, 672–685. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.; Miao, L.F. Comparative physiological and proteomic responses to drought stress in two poplar species originating from different altitudes. Physiol. Plant. 2010, 139, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef]
- Hodges, D.M.; Delong, J.M.; Prange, F. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Cataldo, D.A.; Maeoon, M.; Schrader, L.E.; Youngs, V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolicic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Çoruh, N.; Sağdıçoğlu Celep, A.G.; Özgökçe, F. Antioxidant properties of Prangos ferulacea (L.) Lindl., Chaerophyllum macropodum Boiss. and Heracleum persicum Desf. from Apiaceae family used as food in Eastern Anatolia and their inhibitory effects on glutathione-S-transferase. Food Chem. 2007, 100, 1237–1242. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Lin, K.H.; Huang, M.Y.; Huang, W.D.; Hsu, M.H.; Yang, Z.W.; Yang, C.M. The effects of red, blue, and white light-emitting diodes on the growth, development, and edible quality of hydroponically grown lettuce (Lactuca sativa, L. var. capitata). Sci. Hortic. 2013, 150, 86–91. [Google Scholar] [CrossRef]
- Bian, Z.H.; Cheng, R.F.; Yang, Q.C.; Wang, J.; Lu, C. Continuous light from red, blue, and green light-emitting diodes reduces nitrate content and enhances phytochemical concentrations and antioxidant capacity in lettuce. J. Am. Soc. Hortic. Sci. 2016, 141, 186–195. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Li, T.; Cheng, R.F.; Barnett, Y.; Lu, C. Study of the beneficial effects of green light on lettuce grown under short-term continuous red and blue light-emitting diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef] [PubMed]
- Arve, L.E.; Terfa, M.T.; Gislerød, H.R.; Olsen, J.E.; Torre, S. High relative air humidity and continuous light reduce stomata functionality by affecting the ABA regulation in rose leaves. Plant Cell Environ. 2013, 36, 382–392. [Google Scholar] [CrossRef]
- Heyneke, E.; Luschin-Ebengreuth, N.; Krajcer, I.; Wolkinger, V.; Muller, M.; Zechmann, B. Dynamic compartment specific changes in glutathione and ascorbate levels in Arabidopsis plants exposed to different light intensities. BMC Plant Biol. 2013, 13, 104. [Google Scholar] [CrossRef]
- Cakmak, I.; Kirkby, E.A. Role of magnesium in carbon partitioning and alleviating photooxidative damage. Physiol. Plant. 2008, 133, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Danon, A.; Coll, N.; Apel, K. Cryptochrome-1-dependent execution of programmed cell death induced by singlet oxygen in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2006, 103, 17036–17041. [Google Scholar] [CrossRef]
- Liu, J.Y.; Liu, W.K. Regulation of accumulation and metabolism circadian rhythms of starch and sucrose in two leaf-color lettuces by red:blue ratios of LED continuous light. Environ. Exp. Bot. 2022, 196, 104811. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Dünner-Planella, G.; Vreugdenhil, D. On the induction of injury in tomato under continuous light: Circadian asynchrony as the main triggering factor. Funct. Plant Biol. 2017, 44, 597. [Google Scholar] [CrossRef] [PubMed]


| Sampling Times | Treatments | Shoot Fresh Weight (g) | Shoot Dry Weight (g) | Leaf Area (cm2) |
|---|---|---|---|---|
| 6 days | GN200 | 26.75 ± 2.37 b | 1.56 ± 0.13 b | 520 ± 32.85 a |
| GC200 | 29.33 ± 1.69 b | 1.84 ± 0.09 b | 474 ± 11.66 a | |
| GC300 | 37.89 ± 3.38 a | 2.69 ± 0.20 a | 499 ± 48.51 a | |
| GC400 | 32.25 ± 3.14 ab | 2.67 ± 0.12 a | 394 ± 14.73 b | |
| PN200 | 10.50 ± 1.39 b | 0.97 ± 0.13 b | 280 ± 22.00 a | |
| PC200 | 12.17 ± 0.75 b | 1.16 ± 0.04 ab | 286 ± 17.05 a | |
| PC300 | 15.61 ± 2.09 a | 1.42 ± 0.32 a | 246 ± 20.73 ab | |
| PC400 | 11.09 ± 0.41 b | 1.19 ± 0.05 ab | 228 ± 13.49 b | |
| 12 days | GN200 | 52.37 ± 6.08 b | 3.88 ± 0.09 b | 1082 ± 31.03 a |
| GC200 | 57.29 ± 8.74 ab | 4.20 ± 0.26 b | 950 ± 111.46 a | |
| GC300 | 63.02 ± 3.43 ab | 5.00 ± 0.20 a | 673 ± 28.45 b | |
| GC400 | 69.28 ± 8.97 a | 5.19 ± 0.70 a | 653 ± 58.51 b | |
| PN200 | 28.06 ± 0.29 c | 3.33 ± 0.35 b | 675 ± 93.65 a | |
| PC200 | 31.12 ± 0.83 bc | 3.58 ± 0.30 b | 678 ± 35.32 a | |
| PC300 | 35.21 ± 0.31 ab | 3.95 ± 0.10 b | 622 ± 22.38 a | |
| PC400 | 40.05 ± 6.71 a | 4.82 ± 0.39 a | 623 ± 37.64 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Liu, B.; Wu, Q. High-Intensity Continuous Light from Red–Blue Light-Emitting Diodes Improved Yield, Nutritional Quality and Reactive Oxygen Species Accumulation in Two Leaf-Color Lettuces. Biology 2024, 13, 1077. https://doi.org/10.3390/biology13121077
Liu W, Liu B, Wu Q. High-Intensity Continuous Light from Red–Blue Light-Emitting Diodes Improved Yield, Nutritional Quality and Reactive Oxygen Species Accumulation in Two Leaf-Color Lettuces. Biology. 2024; 13(12):1077. https://doi.org/10.3390/biology13121077
Chicago/Turabian StyleLiu, Wenke, Bing Liu, and Qibao Wu. 2024. "High-Intensity Continuous Light from Red–Blue Light-Emitting Diodes Improved Yield, Nutritional Quality and Reactive Oxygen Species Accumulation in Two Leaf-Color Lettuces" Biology 13, no. 12: 1077. https://doi.org/10.3390/biology13121077
APA StyleLiu, W., Liu, B., & Wu, Q. (2024). High-Intensity Continuous Light from Red–Blue Light-Emitting Diodes Improved Yield, Nutritional Quality and Reactive Oxygen Species Accumulation in Two Leaf-Color Lettuces. Biology, 13(12), 1077. https://doi.org/10.3390/biology13121077








