Brain Gamma-Stimulation: Mechanisms and Optimization of Impact
Simple Summary
Abstract
1. Introduction
2. General Functions of γ-Rhythms, Mechanism of Regulations
3. Literature Data Analysis Protocol
3.1. Criteria for Including Publications in the Analysis
3.2. Data Processing
4. Dependence of the Effectiveness of γ-Stimulation on the Characteristics of the Stimulus and Responding Systems
4.1. Dependence of the Effectiveness of γ-Stimulation on Species
4.2. Dependence of the Efficiency of γ-Stimulation on the Type of Stimulus
4.3. Dependence of the Effectiveness of γ-Stimulation on Age
4.4. Dependence of the Efficiency of γ-Stimulation on the Frequency of Stimulating Action
4.5. Dependence of the Efficiency of γ-Stimulation on the Target
4.6. Dependence of the Effectiveness of γ-Stimulation on Pathology
5. Mechanisms of γ-Stimulation Action
5.1. Visual Stimulation
5.2. Modulation of GABAergic Interneuron Activity
5.3. Acetylcholine Mechanism of γ-Oscillation Activation
5.4. Modulation of Neural Network Activity
5.4.1. Basic Modes of Brain Function and γ-Stimulation
5.4.2. The Default Mode Network
Functions of the DMN
Effects of γ-Stimulation on the Functioning of the DMN
5.4.3. Central Executive Network
Central Executive Network Functions
- Concentration and attention: maintaining focus on the task.
- Working memory involves retaining and manipulating information in the mind.
- Planning and control involves developing a strategy of actions and controlling their implementation.
- Inhibiting impulses and preventing rash actions.
Effects of γ-Stimulation on Central Executive Network
5.4.4. Salience Network Executive Network
Salience Network Functions
Effects of γ-Stimulation on the Salience Network
5.5. Activation of Neurogenesis
5.6. Adenosine Hypothesis
5.7. Effect of γ-Stimulation on Microglia and Inflammation
6. Optimization of γ-Stimulation
6.1. Visual γ-Stimulation in Young Adults
6.2. Visual γ-Stimulation in Older Adults
6.3. The Problem of Stroboscopic Flicker and Its Solution
6.4. Increasing the Exposure Time by Stimulation During Sleep
6.5. Cognitive Task During Stimulation
6.6. Motor (Behavioral) Activity During Stimulation
6.7. Sound Wave Characteristics During Auditory Stimulation
6.8. The Influence of Glucose Metabolism on the γ-Rhythm
7. Clinical Trials
8. Limitations and Prospects
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ACh | Acetylcholine |
| AD | Alzheimer’s disease |
| ANOVA | Analysis of variance |
| CNS | Central nervous system |
| CSF1 | Colony-stimulating factor 1 |
| DMN | default mode network |
| EEG | Electroencephalogram |
| GABA | γ-aminobutyric acid |
| GENUS | Gamma ENtrainment Using Sensory stimuli |
| IFN-γ | Interferon gamma |
| IL | Interleukin |
| ING | Purely inhibitory populations of neurons |
| iNOS | inducible nitric oxide synthase |
| IR | Infrared |
| ISF | Invisible spectral flicker |
| JNK | c-Jun N-terminal kinase |
| LED | Light-emitting diode |
| mAChRs | Muscarinic ACh receptors |
| MAPK | Mitogen-activated protein kinase |
| M-CSF | Macrophage colony-stimulating factor |
| MIP-1β | Macrophage inflammatory protein-1β |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B |
| NMDA | N-Methyl-D-aspartic acid |
| PING | Excitatory–inhibitory networks of neurons |
| PKR | Protein kinase R |
| PLX3397 | Inhibitor of the colony-stimulating factor 1 |
| PV | Parvalbumin |
| SST | Somatostatin |
| SVEP | Steady-state visual evoked potentials |
| TLR | Toll-like receptors |
| TNF-α | Tumor necrosis factor-alpha |
| UV | Ultraviolet |
| VIP | vasoactive intestinal peptide |
| Ym1 | Heparin-binding lectin |
Appendix A
| # | Organism | Age, Years | Pathology | Type of Impact | Type of Exhibition | Duration, s | Additional Conditions | Additional Conditions Characteristics | Analyzed Parameter | Effect Module % | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Human | 35 | Healthy | Audio | Binaural | 120 | Eyes | Open | Central–frontal region eeg 25–50 hz oscillations | 25 | [304] |
| Human | 35 | Healthy | Audio | Binaural | 120 | Eyes | Closed | Central–frontal region eeg 25–50 hz oscillations | 40 | ||
| Human | 35 | Healthy | Audio | Binaural | 120 | Eyes | Open | Central–frontal region eeg 25–50 hz oscillations | 26 | ||
| Human | 35 | Healthy | Audio | Binaural | 120 | Eyes | Closed | Central–frontal region eeg 25–50 hz oscillations | 25 | ||
| 2 | Human | 20 | Healthy | Audio | Binaural | 120 | Eyes | Open | Alpha rhythm | 53 | [303] |
| Human | 20 | Healthy | Audio | Binaural | 120 | Eyes | Closed | Alpha rhythm | 29 | ||
| Human | 20 | Healthy | Audio | Binaural | 1920 | Eyes | Open | Alpha rhythm | 24 | ||
| Human | 20 | Healthy | Audio | Binaural | 1920 | Eyes | Closed | Alpha rhythm | 53 | ||
| Human | 20 | Healthy | Audio | Binaural | 120 | Eyes | Open | Deplta rhythm | 23 | ||
| Human | 20 | Healthy | Audio | Binaural | 120 | Eyes | Closed | Deplta rhythm | 5 | ||
| Human | 20 | Healthy | Audio | Binaural | 1920 | Eyes | Open | Deplta rhythm | 32 | ||
| Human | 20 | Healthy | Audio | Binaural | 1920 | Eyes | Closed | Deplta rhythm | 14 | ||
| Human | 20 | Healthy | Audio | Binaural | 120 | Eyes | Open | Theta rhythm | 22 | ||
| Human | 20 | Healthy | Audio | Binaural | 120 | Eyes | Closed | Theta rhythm | 11 | ||
| Human | 20 | Healthy | Audio | Binaural | 1920 | Eyes | Open | Theta rhythm | 33 | ||
| Human | 20 | Healthy | Audio | Binaural | 1920 | Eyes | Closed | Theta rhythm | 22 | ||
| 3 | Human | 9 | Insomnia | Light | Visually | 1800 | Wavelength, nm | 750 | Sol duration | 81 | [299] |
| Human | 9 | Insomnia | Light | Visually | 1800 | Wavelength, nm | 750 | Tst duration | 12 | ||
| Human | 9 | Insomnia | Light | Visually | 1800 | Wavelength, nm | 750 | Se duration | 15 | ||
| Human | 9 | Insomnia | Light | Visually | 1800 | Wavelength, nm | 750 | Rem amount | 6 | ||
| 4 | Human | 60 | BA | Light | Visually | “-” | Wavelength, nm | 750 | N-back test accuracy | 11 | [293] |
| Human | 60 | BA | Light | Visually | “-” | Wavelength, nm | 750 | Reaction time in a 1-back test | 8 | ||
| 5 | Human | 22 | Healthy | Light | Visually | 350 | Wavelength, nm | 810 | Score of the computerized visual memory test | 29 | [273] |
| Human | 22 | Healthy | Light | Visually | 350 | Wavelength, nm | 810 | Longest correct span of the computerized visual memory test | 16 | ||
| Human | 22 | Healthy | Light | Visually | 350 | Wavelength, nm | 810 | Oxi-hb changedduring early phase of test | 83 | ||
| Human | 22 | Healthy | Light | Visually | 350 | Wavelength, nm | 810 | Oxi-hb changedduring late phase of test | 38 | ||
| 6 | Human | 70 | BA | Light | Visually | 126,000 | Wavelength, nm | 810 | Mini-mental state exam results | 15 | [312] |
| Human | 70 | BA | Light | Visually | 126,000 | Wavelength, nm | 810 | Alzheimer’s disease assessment scale-cognitive subscale scores | 19 | ||
| Human | 70 | BA | Light | Visually | 63,000 | Wavelength, nm | 810 | Mini-mental state exam results | 14 | ||
| Human | 70 | BA | Light | Visually | 63,000 | Wavelength, nm | 810 | Alzheimer’s disease assessment scale-cognitive subscale scores | 19 | ||
| 7 | Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Alzheimer’s disease assessment scale-cognitive scores | 5 | [313] |
| Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Alzheimer’s disease assessment scale-cognitive scores | 14 | ||
| Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Neuropsychiatric inventory scopes | 35 | ||
| Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Neuropsychiatric inventory scopes | 61 | ||
| Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Total perfusion | 37 | ||
| Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Superior frontal perfusion | 3 | ||
| Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Superior parietal perfusion | 88 | ||
| Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Supramarginal perfusion | 28 | ||
| Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Connectivy between posterior cingulate cortex and left lateral parietal | 233 | ||
| Human | 80 | BA | Light | Visually | 21,600 | Wavelength, nm | 810 | Connectivy between posterior cingulate cortex and right lateral parietal | 181 | ||
| 8 | Human | 65 | Parkinson’s disease | Light | Visually | 8568 | Wavelength, nm | 1080 | Trail making a | 63 | [313] |
| Human | 65 | Parkinson’s disease | Light | Visually | 8568 | Wavelength, nm | 1080 | Adas- cog ideational praxis | 22 | ||
| Human | 65 | Parkinson’s disease | Light | Visually | 8568 | Wavelength, nm | 1080 | Adas- cog boston naming | 11 | ||
| Human | 65 | Parkinson’s disease | Light | Visually | 8568 | Wavelength, nm | 1080 | Cortical perfusion | 5 | ||
| 9 | Human | 76 | Dementia | Light | Visually | 72,000 | Wavelength, nm | 700 | Emotional state | 71 | [284] |
| Human | 76 | Dementia | Light | Visually | 72,000 | Wavelength, nm | 700 | Psychiatric symptom | 43 | ||
| Human | 76 | Dementia | Light | Visually | 144,000 | Wavelength, nm | 700 | Psychiatric symptom | 79 | ||
| Human | 76 | Dementia | Light | Visually | 72,000 | Wavelength, nm | 700 | Sleep disturbances | 73 | ||
| Human | 76 | Dementia | Light | Visually | 144,000 | Wavelength, nm | 700 | Sleep disturbances | 82 | ||
| Human | 76 | Dementia | Light | Visually | 72,000 | Wavelength, nm | 700 | Orientation | 54 | ||
| Human | 76 | Dementia | Light | Visually | 144,000 | Wavelength, nm | 700 | Orientation | 62 | ||
| 10 | Human | 25 | Healthy | Light | Visually | 1800 | Maximum wavelength, nm | 630 | Gamma-rhythm power | 1200 | [282] |
| Human | 25 | Healthy | Light | Visually | 1800 | Maximum wavelength, nm | 450 | Gamma-rhythm power | 350 | ||
| 11 | Human | 28 | Healthy | Light | Visually | 300 | Wavelength, nm | 500 | Gamma-rhythm power | 300 | [281] |
| Human | 28 | Healthy | Light | Visually | 300 | Wavelength, nm | 450 | Gamma-rhythm power | 600 | ||
| Human | 28 | Healthy | Light | Visually | 300 | Wavelength, nm | 550 | Gamma-rhythm power | 700 | ||
| 12 | Human | 24.8 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Alpha rhythm power | 200 | [272] |
| Human | 24.8 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Alpha rhythm power | 150 | ||
| 13 | Human | 71.5 | BA | Light | Visually | 72,000 | Wavelength, nm | 555 | Amyloid-β (a β) content | 1 | [270] |
| 14 | Human | 25 | Healthy | Light | Visually | 300 | Wavelength, nm | 555 | Gamma-rhythm power without cognitive task | 900 | [123] |
| Human | 25 | Healthy | Light | Visually | 300 | Wavelength, nm | 555 | Gamma-rhythm power with cognitive task | 700 | ||
| Human | 25 | Healthy | Light | Visually | 300 | Wavelength, nm | 555 | Different in p300 amplitude | 300 | ||
| Human | 25 | Healthy | Light | Visually | 300 | Wavelength, nm | 555 | Different in p300 latency | 4500 | ||
| 15 | Human | 71 | BA | Lighting and audio | Combination | 16,470 | Wavelength, nm | 745 | Gamma-rhythm power | 1400 | [192] |
| Human | 71 | BA | Lighting and audio | Combination | 16,470 | Wavelength, nm | 745 | Ventricular volume change | 60 | ||
| Human | 71 | BA | Lighting and audio | Combination | 16,470 | Wavelength, nm | 745 | Hippocampal atrophy | 55 | ||
| Human | 71 | BA | Lighting and audio | Combination | 16,470 | Wavelength, nm | 745 | Pcc connectivity | 125 | ||
| Human | 71 | BA | Lighting and audio | Combination | 16,470 | Wavelength, nm | 745 | Mediam visual network connectivity | 161 | ||
| Human | 71 | BA | Lighting and audio | Combination | 16,470 | Wavelength, nm | 745 | Interdaily sleep stability | 200 | ||
| Human | 71 | BA | Lighting and audio | Combination | 16,470 | Wavelength, nm | 745 | Wake after sleep onset | 1300 | ||
| Human | 71 | BA | Lighting and audio | Combination | 16,470 | Wavelength, nm | 745 | Accuracy | 580 | ||
| 16 | Human | 72 | BA | Lighting and audio | Combination | 201,600 | Wavelength, nm | 555 | Pcc-pcu functional connectivity | 30 | [191] |
| Human | 72 | BA | Lighting and audio | Combination | 201,600 | Wavelength, nm | 555 | Tweak immune factor expression | 10 | ||
| 17 | Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power | 1300 | [138] |
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power | 1000 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power | 600 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power change in k29 | 250 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power change in k29 | 220 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power change in k29 | 120 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power change in k18 | 330 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power change in k18 | 290 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power change in k18 | 90 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power change in k19 | 360 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power change in k19 | 320 | ||
| Human | 11.5 | Healthy | Image rotation | Visually | 2100 | Wavelength, nm | 555 | Gamma-rhythm power change in k19 | 205 | ||
| 18 | Human | 27.5 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Gamma-rhythm power by fmri | 14 | [314] |
| Human | 27.5 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Gamma-rhythm power by fmri | 38 | ||
| Human | 27.5 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Gamma-rhythm power by fmri | 43 | ||
| Human | 27.5 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Gamma-rhythm power by fmri | 46 | ||
| Human | 27.5 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Gamma-rhythm power by meg | 29 | ||
| Human | 27.5 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Gamma-rhythm power by meg | 6 | ||
| Human | 27.5 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Gamma-rhythm power by meg | 40 | ||
| Human | 27.5 | Healthy | Light | Visually | 600 | Wavelength, nm | 555 | Gamma-rhythm power by meg | 43 | ||
| 19 | Human | 33 | Healthy | Light | Visually | 10 | Wavelength, nm | 555 | Gamma-rhythm power by meg in v1 | 100 | [117] |
| Human | 33 | Healthy | Light | Visually | 10 | Wavelength, nm | 555 | Gamma-rhythm power by meg in v1 lfp | 560 | ||
| Human | 33 | Healthy | Light | Visually | 10 | Wavelength, nm | 555 | Meg in v1 lfp signle spike density | 567 | ||
| Human | 33 | Healthy | Light | Visually | 10 | Michelson contrast, % | 20 | Gamma-rhythm power by meg | 20 | ||
| Human | 33 | Healthy | Light | Visually | 10 | Michelson contrast, % | 36 | Gamma-rhythm power by meg | 200 | ||
| Human | 33 | Healthy | Light | Visually | 10 | Michelson contrast, % | 48 | Gamma-rhythm power by meg | 300 | ||
| Human | 33 | Healthy | Light | Visually | 10 | Michelson contrast, % | 66 | Gamma-rhythm power by meg | 340 | ||
| Human | 33 | Healthy | Light | Visually | 10 | Michelson contrast, % | 96 | Gamma-rhythm power by meg | 490 | ||
| 20 | Human | 37.8 | Healthy | Light | Visually | 420 | Wavelength, nm | 370 | Theta rhythm power | 205 | [104] |
| Human | 37.8 | Healthy | Light | Visually | 420 | Wavelength, nm | 555 | Theta rhythm power | 215 | ||
| Human | 37.8 | Healthy | Light | Visually | 420 | Wavelength, nm | 370 | Visually evoked potential | 215 | ||
| Human | 37.8 | Healthy | Light | Visually | 420 | Wavelength, nm | 370 | Alpha-gamma coupling f5 | 1400 | ||
| Human | 37.8 | Healthy | Light | Visually | 420 | Wavelength, nm | 370 | Alpha-gamma coupling c4 | 200 | ||
| 21 | Human | 23.9 | Healthy | Light | Visually | 5 | Wavelength, nm | 390 | Mean ssvep power | 15 | [103] |
| Human | 23.9 | Healthy | Light | Visually | 5 | Wavelength, nm | 430 | Mean ssvep power | 19 | ||
| Human | 23.9 | Healthy | Light | Visually | 5 | Wavelength, nm | 390 | Mean msi power | 33 | ||
| Human | 23.9 | Healthy | Light | Visually | 5 | Wavelength, nm | 430 | Mean msi power | 42 | ||
| Human | 23.9 | Healthy | Light | Visually | 5 | Wavelength, nm | 430 | Mean of cca coeddicient | 25 | ||
| 22 | Human | 28.5 | Healthy | Light | Visually | 15 | Wavelength, nm | 440 | Maximum gamma rhythm peaks values | 63 | [99] |
| Human | 28.5 | Healthy | Light | Visually | 15 | Wavelength, nm | 600 | Maximum gamma rhythm peaks values | 53 | ||
| Human | 28.5 | Healthy | Light | Visually | 15 | Wavelength, nm | 600 | Maximum gamma rhythm peaks values | 133 | ||
| Human | 28.5 | Healthy | Light | Visually | 15 | Wavelength, nm | 600 | Maximum gamma rhythm peaks values | 150 | ||
| Human | 28.5 | Healthy | Light | Visually | 15 | Wavelength, nm | 600 | Maximum gamma rhythm peaks values | 113 | ||
| Human | 28.5 | Healthy | Light | Visually | 15 | Wavelength, nm | 560 | Maximum gamma rhythm peaks values | 67 | ||
| 23 | Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in pz region | 800 | [72] |
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in pz region | 850 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in pz region | 830 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in pz region | 820 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in pz region | 790 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in pz region | 780 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in fz region | 430 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in fz region | 420 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in fz region | 400 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in fz region | 380 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Event-related synchronization in fz region | 370 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Strength of connectivity | 280 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Strength of connectivity | 320 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Strength of connectivity | 400 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Strength of connectivity | 370 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Strength of connectivity | 330 | ||
| Human | 69.9 | Healthy | Light | Visually | 695 | Wavelength, nm | 560 | Strength of connectivity | 240 | ||
| 24 | Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 700 | Event-related synchronization in pz region | 230 | [96] |
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 530 | Event-related synchronization in pz region | 200 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 440 | Event-related synchronization in pz region | 125 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 95 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 700 | Event-related synchronization in fz region | 50 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 530 | Event-related synchronization in fz region | 40 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 440 | Event-related synchronization in fz region | 20 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 16 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 400 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 570 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 590 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 140 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 210 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 230 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 400 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 450 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 500 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 530 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 450 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 380 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 360 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in pz region | 360 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 200 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 190 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 205 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 185 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 145 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 120 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 110 | ||
| Human | 21.4 | Healthy | Light | Visually | 695 | Wavelength, nm | 610 | Event-related synchronization in fz region | 111 | ||
| 25 | Human | 20 | Healthy | Light | Visually | 30 | Eyes | Open | Gamma-rhythm power | 150 | [95] |
| Human | 20 | Healthy | Light | Visually | 30 | Eyes | Closed | Gamma-rhythm power | 150 | ||
| Human | 20 | Healthy | Light | Visually | 30 | Eyes | Closed | Alhpa-rhythm power | 300 | ||
| Human | 20 | Healthy | Light | Visually | 30 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 1300 | ||
| Human | 20 | Healthy | Light | Visually | 30 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 1700 | ||
| Human | 20 | Healthy | Light | Visually | 30 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 500 | ||
| Human | 20 | Healthy | Light | Visually | 30 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 1500 | ||
| Human | 20 | Healthy | Light | Visually | 30 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 300 | ||
| Human | 20 | Healthy | Light | Visually | 30 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 200 | ||
| Human | 20 | Healthy | Light | Visually | 20 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 2100 | ||
| Human | 20 | Healthy | Light | Visually | 10 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 1900 | ||
| Human | 20 | Healthy | Light | Visually | 5 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 1200 | ||
| Human | 20 | Healthy | Light | Visually | 2 | Eyes | Open | Number of electrodes regisctrared gamma-rhythm enhacne | 1000 | ||
| 26 | Human | 25.3 | Healthy | Lighting and audio | Visually | 2 | Tactile stimuli | Eat | Test response time | 8 | [94] |
| Human | 25.3 | Healthy | Lighting and audio | Visually | 2 | Tactile stimuli | Eat | Imaginary gamma-rhythm cogerence | 100 | ||
| 27 | Human | 67.4 | Parkinson’s disease | Magnetic | Cranial | 1200 | “-” | “-” | Fingers velocity | 5 | [315] |
| Human | 67.4 | Parkinson’s disease | Magnetic | Cranial | 1200 | “-” | “-” | Fingers velocity | 4 | ||
| Human | 67.4 | Parkinson’s disease | Magnetic | Cranial | 1200 | “-” | “-” | Fingers move amplitude | 7 | ||
| Human | 67.4 | Parkinson’s disease | Magnetic | Cranial | 1200 | “-” | “-” | Fingers move amplitude | 5 | ||
| Human | 67.4 | Parkinson’s disease | Magnetic | Cranial | 1200 | “-” | “-” | Short-interval intracortical inhibition | 33 | ||
| Human | 67.4 | Parkinson’s disease | Magnetic | Cranial | 1200 | “-” | “-” | Short-interval intracortical inhibition | 17 | ||
| Human | 67.4 | Parkinson’s disease | Magnetic | Cranial | 1200 | “-” | “-” | Short-latency afferent inhibition | 22 | ||
| Human | 67.4 | Parkinson’s disease | Magnetic | Cranial | 1200 | “-” | “-” | Short-latency afferent inhibition | 22 | ||
| 28 | Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | First harmonic oscillation amplitude | 1700 | [54] |
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | Second harmonic oscillation amplitude | 200 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | First harmonic oscillation amplitude | 600 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | Second harmonic oscillation amplitude | 200 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | First harmonic oscillation amplitude | 500 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | Second harmonic oscillation amplitude | 100 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | First harmonic oscillation amplitude | 240 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | First harmonic oscillation amplitude | 440 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | First harmonic oscillation amplitude | 220 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | First harmonic oscillation amplitude | 1100 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | Other harmonics oscillation amplitude | 300 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | First harmonic oscillation amplitude | 3100 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | Other harmonics oscillation amplitude | 5900 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | Other harmonics oscillation amplitude | 3900 | ||
| Human | 24.5 | Healthy | Light | Visually | 30 | Wavelength, nm | 555 | Other harmonics oscillation amplitude | 2900 | ||
| 29 | Human | 20.6 | Healthy | Audio | Binourally | 300 | “-” | “-” | Gamma rhythm power in f4 | 20 | [64] |
| Human | 20.6 | Healthy | Audio | Binourally | 600 | “-” | “-” | Gamma rhythm power in f4 | 12 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 900 | “-” | “-” | Gamma rhythm power in f4 | 36 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1200 | “-” | “-” | Gamma rhythm power in f4 | 18 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1500 | “-” | “-” | Gamma rhythm power in f4 | 2 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Gamma rhythm power in f4 | 4 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 300 | “-” | “-” | Gamma rhythm power in fp2 | 32 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 600 | “-” | “-” | Gamma rhythm power in fp2 | 11 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 900 | “-” | “-” | Gamma rhythm power in fp2 | 23 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1200 | “-” | “-” | Gamma rhythm power in fp2 | 18 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1500 | “-” | “-” | Gamma rhythm power in fp2 | 5 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Gamma rhythm power in fp2 | 7 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 300 | “-” | “-” | Beta rhythm power in f4 | 15 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 600 | “-” | “-” | Beta rhythm power in f4 | 35 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 900 | “-” | “-” | Beta rhythm power in f4 | 23 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1200 | “-” | “-” | Beta rhythm power in f4 | 12 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1500 | “-” | “-” | Beta rhythm power in f4 | 15 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Beta rhythm power in f4 | 8 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 300 | “-” | “-” | Beta rhythm power in fp2 | 27 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 600 | “-” | “-” | Beta rhythm power in fp2 | 34 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 900 | “-” | “-” | Beta rhythm power in fp2 | 41 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1200 | “-” | “-” | Beta rhythm power in fp2 | 32 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1500 | “-” | “-” | Beta rhythm power in fp2 | 18 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Beta rhythm power in fp2 | 15 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 300 | “-” | “-” | Alpha rhythm power in f4 | 42 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 600 | “-” | “-” | Alpha rhythm power in f4 | 39 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 900 | “-” | “-” | Alpha rhythm power in f4 | 39 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1200 | “-” | “-” | Alpha rhythm power in f4 | 18 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1500 | “-” | “-” | Alpha rhythm power in f4 | 11 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Alpha rhythm power in f4 | 11 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 300 | “-” | “-” | Alpha rhythm power in fp2 | 30 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 600 | “-” | “-” | Alpha rhythm power in fp2 | 7 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 900 | “-” | “-” | Alpha rhythm power in fp2 | 11 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1200 | “-” | “-” | Alpha rhythm power in fp2 | 17 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1500 | “-” | “-” | Alpha rhythm power in fp2 | 3 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Alpha rhythm power in fp2 | 3 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Word recall test result | 100 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Brunel mood scale worried | 51 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Brunel mood scale nervous | 44 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Brunel mood scale annoyed | 104 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Brunel mood scale miserable | 56 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Brunel mood scale sleepy | 25 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Brunel mood energy scale | 39 | ||
| Human | 20.6 | Healthy | Audio | Binourally | 1800 | “-” | “-” | Brunel mood scale muddled | 32 | ||
| 30 | Human | 66.5 | BA | Lighting and audio | Visually | 648,000 | “-” | “-” | Active periods during nighttime | 10 | [63] |
| Human | 66.5 | BA | Lighting and audio | Visually | 648,000 | “-” | “-” | Normalized active periods during nighttime | 4 | ||
| Human | 66.5 | BA | Lighting and audio | Visually | 648,000 | “-” | “-” | Activities of daily living | 133 | ||
| 31 | Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 2 | Escape latency | 7 | [316] |
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 3 | Escape latency | 38 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 4 | Escape latency | 42 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Escape latency | 64 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Step-through latency time | 67 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Time in probe quadrant | 75 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | P-act expression level | 50 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | P-gsk3beta expression level | 40 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | P-tay expression level | 30 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | App expression level | 47 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Number of abeta-positive cells | 57 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Number of gfap-positive astrocytes in ca | 19 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Number of gfap-positive astrocytes in dentate gyrus | 25 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Number of iba-1-positive astrocytes in ca | 25 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Number of iba-1-positive astrocytes in dentate gyrus | 33 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Tnfapha secretion | 28 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Il-6 secretion | 27 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Mitochondrial Ca2+ retention capacity | 70 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Mitochondrial ptp inhibition | 67 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Mitochondrial H2O2 generation | 12 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Bax protein expression level | 33 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Cyt c protein expression level | 27 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Caspase-3 protein expression level | 31 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Bcl-2 protein expression level | 25 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Tunel-positive cells number in dentate gyrus | 21 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Dcx-positive cells number in dentate gyrus | 38 | ||
| Mouse | 0.5 | BA | Light | Cranial | 216,000 | Training day | 5 | Neun/brdu-positive cells number in dentate gyrus | 90 | ||
| 32 | Mouse | 0.5 | Healthy | Light | Cranial | 1800 | Wavelength, nm | 545 | Intracellular adenosine concentration in v1 | 400 | [299] |
| Mouse | 0.5 | Healthy | Light | Cranial | 1800 | Wavelength, nm | 545 | Intracellular adenosine concentration in v2 | 900 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 1800 | Wavelength, nm | 545 | Intracellular adenosine concentration in v3 | 1100 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 1800 | Wavelength, nm | 545 | Caicium infux | 100 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 1800 | Wavelength, nm | 545 | Caicium infux | 180 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 1800 | Wavelength, nm | 545 | Local field potential oscillations | 20 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 1800 | Wavelength, nm | 545 | Local field potential oscillations | 30 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 14,400 | Wavelength, nm | 545 | Ado concentration in neurons | 50 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 14,400 | Wavelength, nm | 545 | Adp concentration in neurons | 25 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 14,400 | Wavelength, nm | 545 | Amp concentration in neurons | 88 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 14,400 | Wavelength, nm | 545 | Imp concentration in neurons | 57 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 14,400 | Wavelength, nm | 545 | Hcy concentration in neurons | 50 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 14,400 | Wavelength, nm | 545 | Met concentration in neurons | 92 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 14,400 | Wavelength, nm | 545 | P-amk-alpha2 concentration | 20 | ||
| Mouse | 0.5 | Healthy | Light | Cranial | 14,400 | Wavelength, nm | 545 | Slow-wave sleep amount | 133 | ||
| 33 | Mouse | 0.25 | Healthy | Light | Cranial | 72,000 | Wavelength, nm | 630 | Crossing numbers | 40 | [278] |
| Mouse | 0.25 | SAMP8 aging model | Light | Cranial | 72,000 | Wavelength, nm | 630 | Crossing numbers | 36 | ||
| Mouse | 0.25 | Healthy | Light | Cranial | 72,000 | Wavelength, nm | 630 | Escape latency | 16 | ||
| Mouse | 0.25 | Healthy | Light | Cranial | 72,000 | Wavelength, nm | 630 | Brain fdh protein expression | 20 | ||
| Mouse | 0.25 | SAMP8 aging model | Light | Cranial | 72,000 | Wavelength, nm | 630 | Brain fdh protein expression | 117 | ||
| Mouse | 0.25 | Healthy | Light | Cranial | 72,000 | Wavelength, nm | 630 | Brain catalase protein expression | 80 | ||
| Mouse | 0.25 | SAMP8 aging model | Light | Cranial | 72,000 | Wavelength, nm | 630 | Brain catalase protein expression | 255 | ||
| 34 | Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | M-cgf cytokine expression | 100 | [263] |
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Il-6 cytokine expression | 500 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Mig cytokine expression | 52 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Il-4 cytokine expression | 77 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Ifn-gamma cytokine expression | 2400 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Il-12 p70 cytokine expression | 50 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Gm-csf cytokine expression | 79 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Il-7 cytokine expression | 100 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Il-1beta cytokine expression | 22 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Gm-csf secretion | 100 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Il-2 secretion | 30 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Il-12 p70 secretion | 60 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Eotaxin secretion | 40 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Tnfapha secretion | 100 | ||
| Mouse | 0.2 | Healthy | Light | Visually | 3600 | Wavelength, nm | 555 | Mcp1 secretion | 180 | ||
| 35 | Mouse | 0.5 | Healthy | Light | Visually | 108,000 | “-” | “-” | Number of shocks to learning | 75 | [206] |
| Mouse | 0.5 | Healthy | Light | Visually | 108,000 | “-” | “-” | Brdu + cells number in dg region | 89 | ||
| Mouse | 0.5 | Healthy | Light | Visually | 108,000 | “-” | “-” | Dcx + cells number in dg region | 86 | ||
| Mouse | 0.5 | Healthy | Light | Visually | 108,000 | “-” | “-” | Brdu + dcx + cells number in dg region | 70 | ||
| 36 | Mouse | 0.1 7 | BA | Lighting and audio | Visually | 10 | “-” | “-” | Abeta1-42 amyloid concentration solible | 55 | [37] |
| Mouse | 0.1 7 | BA | Lighting and audio | Visually | 10 | “-” | “-” | Abeta1-42 amyloid concentration insolible | 35 | ||
| Mouse | 0.1 7 | BA | Lighting and audio | Visually | 10 | “-” | “-” | Plague numbers in ac | 41 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Plague numbers in ca1 | 50 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Plague core area in ac | 55 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Plague core area in ca1 | 45 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Abeta 12f4 amyloid concentration in ac | 45 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Abeta 12f4 amyloid concentration in ca1 | 40 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Sb100b + astrocytes count in ac | 26 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Sb100b + astrocytes count in ca1 | 12 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Gfab + astrocyte count in ac | 38 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Gfab + astrocyte count in ca1 | 17 | ||
| Mouse | 0.1 7 | BA | Audio | “-” | 10 | “-” | “-” | Lrp1-abeta colocalization | 157 | ||
| Mouse | 0.1 7 | BA | Lighting and audio | Visually | 10 | “-” | “-” | Microglia cell body diameter | 110 | ||
| Mouse | 0.1 7 | BA | Lighting and audio | Visually | 10 | “-” | “-” | Microglia avarage processes leunght | 25 | ||
| Mouse | 0.1 7 | BA | Lighting and audio | Visually | 10 | “-” | “-” | Microglia count | 95 | ||
| Mouse | 0.1 7 | BA | Lighting and audio | Visually | 10 | “-” | “-” | Migroglia per plague rate | 33 | ||
| 37 | Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 725 | Microglia cell body diameter | 75 | [39] |
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 725 | Process length | 40 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 725 | Alpha-beta + microglia | 42 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 725 | Plague count | 69 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 725 | Plague core area | 65 | ||
| 38 | Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Discrimination index | 22 | [40] |
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Latency in behavior test | 26 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Latency in behavior test | 10 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Number of plagues in hpc | 25 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Number of plagues in hpc | 20 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Number of plagues in ca1 | 43 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Aplhabeta load in hpc | 53 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Aplhabeta load in ca1 | 33 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Vessel-associated microglia | 7 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Vessel-associated microglia | 11 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Perivascilar microglia | 9 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Perivascilar microglia | 9 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Pverg concentration | 40 | ||
| Mouse | 0.8 | BA | Light | Cranial | 5,184,000 | Wavelength, nm | 1070 | Perk concentration | 45 | ||
| 39 | Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Total eeg power in m1 | 150 | [41] |
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Total eeg power in pta | 39 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Theta rhythm power in m1 | 120 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Theta rhythm power in pta | 300 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Beta rhythm power in m1 | 150 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Beta rhythm power in pta | 700 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Gamma rhythm power in m1 | 150 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Gamma rhythm power in pta | 233 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Blood flow | 33 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Brain swelling | 125 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Contralesional foot faults | 60 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Contralesional foot faults | 80 | ||
| Mouse | 0.25 | Stroke | Light | Cranial | 2880 | Wavelength, nm | 473 | Neurodeficite scope | 50 | ||
| 40 | Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Alpha beta1-40 amyloid content | 55 | [58] |
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Alpha beta1-42 amyloid content | 45 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | App ntf content | 25 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | App ctfs content | 20 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Aplha beta maen intensity value | 40 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Eea1 mean intensity value | 35 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Csf1 gene expression | 340 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Csf11r gene expression | 230 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Csf2ra gene expression | 50 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Icam1 gene expression | 40 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | B2m | 250 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Spp1 gene expression | 120 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Lyz2 gene expression | 290 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Cd68 gene expression | 210 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Irf7 gene expression | 220 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Bst2 gene expression | 130 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Microglia count | 114 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Microglia aβ+ | 187 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Microglia cell body diameter | 130 | ||
| Mouse | 0.25 | BA | Light | Cranial | 3600 | Wavelength, nm | 4793 | Process length | 60 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 555 | Alpha beta1-40 amyloid content | 65 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 555 | Alpha beta1-42 amyloid content | 70 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 555 | Microglia cell body diameter | 70 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 555 | Process length | 40 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 555 | Microglia aβ+ | 57 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 555 | Plaque number | 64 | ||
| Mouse | 0.25 | BA | Light | Visually | 3600 | Wavelength, nm | 555 | Plaque core area | 65 | ||
| 41 | Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 2 | Gamma-rhythm power by meg | 5 | [117] |
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 3.5 | Gamma-rhythm power by meg | 5 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 6 | Gamma-rhythm power by meg | 10 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 9.7 | Gamma-rhythm power by meg | 20 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 16.3 | Gamma-rhythm power by meg | 160 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 35.9 | Gamma-rhythm power by meg | 390 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 50.3 | Gamma-rhythm power by meg | 500 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 72 | Gamma-rhythm power by meg | 630 | ||
| 42 | Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 50.3 | Gamma-rhythm power by meg in v1 lfp | 600 | [116] |
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 35.9 | Gamma-rhythm power by meg in v1 lfp | 1000 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 16.3 | Gamma-rhythm power by meg in v1 lfp | 550 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 9.7 | Gamma-rhythm power by meg in v1 lfp | 200 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 6.1 | Gamma-rhythm power by meg in v1 lfp | 150 | ||
| Rhesus macaque | 3 | Healthy | Light | Visually | 10 | Michelson contrast, % | 3.7 | Gamma-rhythm power by meg in v1 lfp | 20 |
References
- Gudkov, S.V.; Sarimov, R.M.; Astashev, M.E.; Pishchalnikov, R.Y.; Yanykin, D.V.; Simakin, A.V.; Shkirin, A.V.; Serov, D.A.; Konchekov, E.M.; Gusein-zade Namik Guseynaga, O.; et al. Modern physical methods and technologies in agriculture. Physics-Uspekhi 2023, 67, 194–210. [Google Scholar] [CrossRef]
- Semenova, N.A.; Burmistrov, D.E.; Shumeyko, S.A.; Gudkov, S.V. Fertilizers Based on Nanoparticles as Sources of Macro- and Microelements for Plant Crop Growth: A Review. Agronomy 2024, 14, 1646. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Shumeyko, S.A.; Semenova, N.A.; Dorokhov, A.S.; Gudkov, S.V. Selenium Nanoparticles (Se NPs) as Agents for Agriculture Crops with Multiple Activity: A Review. Agronomy 2025, 15, 1591. [Google Scholar] [CrossRef]
- Burmistrov, D.E.; Gudkov, S.V.; Franceschi, C.; Vedunova, M.V. Sex as a Determinant of Age-Related Changes in the Brain. Int. J. Mol. Sci. 2024, 25, 7122. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Kondakova, E.V.; Sarimov, R.M.; Yarkov, R.S.; Franceschi, C.; Vedunova, M.V. An emerging role of astrocytes in aging/neuroinflammation and gut-brain axis with consequences on sleep and sleep disorders. Ageing Res. Rev. 2023, 83, 101775. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef]
- Cummings, J.; Lee, G.; Zhong, K.; Fonseca, J.; Taghva, K. Alzheimer’s disease drug development pipeline: 2021. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, 12179. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, Y.; Qiu, C.; Cheng, F. Global and regional economic costs of dementia: A systematic review. Lancet 2017, 390, S47. [Google Scholar] [CrossRef]
- Cole, S.R.; Voytek, B. Brain Oscillations and the Importance of Waveform Shape. Trends Cogn. Sci. 2017, 21, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Mathalon, D.H.; Sohal, V.S. Neural Oscillations and Synchrony in Brain Dysfunction and Neuropsychiatric Disorders. JAMA Psychiatry 2015, 72, 840–844. [Google Scholar] [CrossRef]
- Zhang, H.; Watrous, A.J.; Patel, A.; Jacobs, J. Theta and Alpha Oscillations Are Traveling Waves in the Human Neocortex. Neuron 2018, 98, 1269–1281.e4. [Google Scholar] [CrossRef]
- Guan, A.; Wang, S.; Huang, A.; Qiu, C.; Li, Y.; Li, X.; Wang, J.; Wang, Q.; Deng, B. The role of gamma oscillations in central nervous system diseases: Mechanism and treatment. Front. Cell. Neurosci. 2022, 16, 962957. [Google Scholar] [CrossRef]
- Başar, E.; Başar-Eroğlu, C.; Karakaş, S.; Schürmann, M. Brain oscillations in perception and memory. Int. J. Psychophysiol. 2000, 35, 95–124. [Google Scholar] [CrossRef]
- Roux, F.; Wibral, M.; Mohr, H.M.; Singer, W.; Uhlhaas, P.J. Gamma-Band Activity in Human Prefrontal Cortex Codes for the Number of Relevant Items Maintained in Working Memory. J. Neurosci. 2012, 32, 12411–12420. [Google Scholar] [CrossRef]
- Swanson, O.K.; Maffei, A. From Hiring to Firing: Activation of Inhibitory Neurons and Their Recruitment in Behavior. Front. Mol. Neurosci. 2019, 12, 168. [Google Scholar] [CrossRef]
- Eckhorn, R.; Bauer, R.; Jordan, W.; Brosch, M.; Kruse, W.; Munk, M.; Reitboeck, H.J. Coherent oscillations: A mechanism of feature linking in the visual cortex? Biol. Cybern. 1988, 60, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.J.; Cash, S.S. Finding synchrony in the desynchronized EEG: The history and interpretation of gamma rhythms. Front. Integr. Neurosci. 2013, 7, 49503. [Google Scholar] [CrossRef]
- Wu, L.; Wei, Y.; He, K.; Gao, Q. The Effects and Safety of Gamma Rhythm Stimulation on Cognitive Function in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Neurorehabil. Neural Repair 2025. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, C.; Parker, E.; Zhu, J.; Liu, T.C.-Y.; Duan, R.; Yang, L. Mystery of gamma wave stimulation in brain disorders. Mol. Neurodegener. 2024, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Tiesinga, P.H.; Fellous, J.-M.; Salinas, E.; José, J.V.; Sejnowski, T.J. Inhibitory synchrony as a mechanism for attentional gain modulation. J. Physiol.-Paris 2004, 98, 296–314. [Google Scholar] [CrossRef] [PubMed]
- Antonoudiou, P.; Tan, Y.L.; Kontou, G.; Upton, A.L.; Mann, E.O. Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations. J. Neurosci. 2020, 40, 7668–7687. [Google Scholar] [CrossRef] [PubMed]
- Buzsáki, G.; Wang, X.-J. Mechanisms of Gamma Oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef]
- Takada, N.; Pi, H.J.; Sousa, V.H.; Waters, J.; Fishell, G.; Kepecs, A.; Osten, P. A developmental cell-type switch in cortical interneurons leads to a selective defect in cortical oscillations. Nat. Commun. 2014, 5, 5333. [Google Scholar] [CrossRef]
- Adaikkan, C.; Tsai, L.-H. Gamma Entrainment: Impact on Neurocircuits, Glia, and Therapeutic Opportunities. Trends Neurosci. 2020, 43, 24–41. [Google Scholar] [CrossRef]
- Beyna, T.; Gerges, C. Clinical Management of Bile Duct Diseases: Role of Endoscopic Ultrasound in a Personalized Approach. J. Pers. Med. 2020, 11, 1. [Google Scholar] [CrossRef]
- Lasztóczi, B.; Klausberger, T. Layer-Specific GABAergic Control of Distinct Gamma Oscillations in the CA1 Hippocampus. Neuron 2014, 81, 1126–1139. [Google Scholar] [CrossRef]
- Muller, M.; Rouleau, E.A.M.Y.; van der Gaag, S.; Zutt, R.; Hoffmann, C.F.; van der Gaag, N.A.; van Essen, T.A.; Schouten, A.C.; Contarino, M.F. Decoding finely-tuned gamma oscillations in chronic deep brain stimulation for Parkinson’s disease. medRxiv 2025. [Google Scholar] [CrossRef]
- Olaru, M.; Hahn, A.; Shcherbakova, M.; Little, S.; Neumann, W.-J.; Abbasi-Asl, R.; Starr, P.A. Deep brain stimulation-entrained gamma oscillations in chronic home recordings in Parkinson’s disease. Brain Stimul. 2025, 18, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Tsai, L.-H. Innovations in noninvasive sensory stimulation treatments to combat Alzheimer’s disease. PLoS Biol. 2025, 23, e3003046. [Google Scholar] [CrossRef] [PubMed]
- Traikapi, A.; Konstantinou, N. Gamma Oscillations in Alzheimer’s Disease and Their Potential Therapeutic Role. Front. Syst. Neurosci. 2021, 15, 782399. [Google Scholar] [CrossRef]
- Rojas, D.C.; Wilson, L.B. γ-Band Abnormalities as Markers of Autism Spectrum Disorders. Biomark. Med. 2014, 8, 353–368. [Google Scholar] [CrossRef]
- Metzner, C.; Mäki-Marttunen, T.; Karni, G.; McMahon-Cole, H.; Steuber, V. The effect of alterations of schizophrenia-associated genes on gamma band oscillations. Schizophrenia 2022, 8, 46. [Google Scholar] [CrossRef]
- McNally, J.M.; McCarley, R.W. Gamma band oscillations. Curr. Opin. Psychiatry 2016, 29, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Mucke, L. Network abnormalities and interneuron dysfunction in Alzheimer disease. Nat. Rev. Neurosci. 2016, 17, 777–792. [Google Scholar] [CrossRef] [PubMed]
- Strüber, D.; Herrmann, C.S. Modulation of gamma oscillations as a possible therapeutic tool for neuropsychiatric diseases: A review and perspective. Int. J. Psychophysiol. 2020, 152, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Manippa, V.; Palmisano, A.; Filardi, M.; Vilella, D.; Nitsche, M.A.; Rivolta, D.; Logroscino, G. An update on the use of gamma (multi)sensory stimulation for Alzheimer’s disease treatment. Front. Aging Neurosci. 2022, 14, 1095081. [Google Scholar] [CrossRef]
- Martorell, A.J.; Paulson, A.L.; Suk, H.-J.; Abdurrob, F.; Drummond, G.T.; Guan, W.; Young, J.Z.; Kim, D.N.-W.; Kritskiy, O.; Barker, S.J.; et al. Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition. Cell 2019, 177, 256–271.e22. [Google Scholar] [CrossRef]
- Soula, M.; Martín-Ávila, A.; Zhang, Y.; Dhingra, A.; Nitzan, N.; Sadowski, M.J.; Gan, W.-B.; Buzsáki, G. Forty-hertz light stimulation does not entrain native gamma oscillations in Alzheimer’s disease model mice. Nat. Neurosci. 2023, 26, 570–578. [Google Scholar] [CrossRef]
- Singer, A.C.; Martorell, A.J.; Douglas, J.M.; Abdurrob, F.; Attokaren, M.K.; Tipton, J.; Mathys, H.; Adaikkan, C.; Tsai, L.-H. Noninvasive 40-Hz light flicker to recruit microglia and reduce amyloid beta load. Nat. Protoc. 2018, 13, 1850–1868. [Google Scholar] [CrossRef]
- Tao, L.; Liu, Q.; Zhang, F.; Fu, Y.; Zhu, X.; Weng, X.; Han, H.; Huang, Y.; Suo, Y.; Chen, L.; et al. Microglia modulation with 1070-nm light attenuates Aβ burden and cognitive impairment in Alzheimer’s disease mouse model. Light Sci. Appl. 2021, 10, 179. [Google Scholar] [CrossRef]
- Balbi, M.; Xiao, D.; Jativa Vega, M.; Hu, H.; Vanni, M.P.; Bernier, L.-P.; LeDue, J.; MacVicar, B.; Murphy, T.H. Gamma frequency activation of inhibitory neurons in the acute phase after stroke attenuates vascular and behavioral dysfunction. Cell Rep. 2021, 34, 108696. [Google Scholar] [CrossRef]
- Obleser, J.; Kayser, C. Neural Entrainment and Attentional Selection in the Listening Brain. Trends Cogn. Sci. 2019, 23, 913–926. [Google Scholar] [CrossRef]
- Sheer, D.E. Focused arousal and the cognitive 40-Hz event-related potentials: Differential diagnosis of Alzheimer’s disease. Prog. Clin. Biol. Res. 1989, 317, 79–94. [Google Scholar]
- Bressler, S.L.; Freeman, W.J. Frequency analysis of olfactory system EEG in cat, rabbit, and rat. Electroencephalogr. Clin. Neurophysiol. 1980, 50, 19–24. [Google Scholar] [CrossRef]
- Galambos, R.; Makeig, S.; Talmachoff, P.J. A 40-Hz auditory potential recorded from the human scalp. Proc. Natl. Acad. Sci. USA 1981, 78, 2643–2647. [Google Scholar] [CrossRef] [PubMed]
- Ferster, D. Orientation selectivity of synaptic potentials in neurons of cat primary visual cortex. J. Neurosci. 1986, 6, 1284–1301. [Google Scholar] [CrossRef] [PubMed]
- Bouyer, J.J.; Montaron, M.F.; Vahnée, J.M.; Albert, M.P.; Rougeul, A. Anatomical localization of cortical beta rhythms in cat. Neuroscience 1987, 22, 863–869. [Google Scholar] [CrossRef]
- Gray, C.M.; Singer, W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc. Natl. Acad. Sci. USA 1989, 86, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.M.; König, P.; Engel, A.K.; Singer, W. Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 1989, 338, 334–337. [Google Scholar] [CrossRef]
- Engel, A.K.; König, P.; Kreiter, A.K.; Schillen, T.B.; Singer, W. Temporal coding in the visual cortex: New vistas on integration in the nervous system. Trends Neurosci. 1992, 15, 218–226. [Google Scholar] [CrossRef]
- Traub, R.D.; Whittington, M.A.; Stanford, I.M.; Jefferys, J.G.R. A mechanism for generation of long-range synchronous fast oscillations in the cortex. Nature 1996, 383, 621–624. [Google Scholar] [CrossRef]
- Traub, R.D.; Jefferys, J.G.R.; Whittington, M.A. Simulation of Gamma Rhythms in Networks of Interneurons and Pyramidal Cells. J. Comput. Neurosci. 1997, 4, 141–150. [Google Scholar] [CrossRef]
- Elliott, M.A.; Müller, H.J. Synchronous Information Presented in 40-HZ Flicker Enhances Visual Feature Binding. Psychol. Sci. 1998, 9, 277–283. [Google Scholar] [CrossRef]
- Herrmann, C.S. Human EEG responses to 1–100 Hz flicker: Resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp. Brain Res. 2001, 137, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Hadler, M.D.; Tzilivaki, A.; Schmitz, D.; Alle, H.; Geiger, J.R.P. Gamma oscillation plasticity is mediated via parvalbumin interneurons. Sci. Adv. 2024, 10, eadj7427. [Google Scholar] [CrossRef]
- Onorato, I.; Tzanou, A.; Schneider, M.; Uran, C.; Broggini, A.C.; Vinck, M. Distinct roles of PV and Sst interneurons in visually induced gamma oscillations. Cell Rep. 2025, 44, 115385. [Google Scholar] [CrossRef]
- Zhang, N.; Hu, B.-W.; Li, X.-M.; Huang, H. Rethinking parvalbumin: From passive marker to active modulator of hippocampal circuits. IBRO Neurosci. Rep. 2025, 19, 760–773. [Google Scholar] [CrossRef]
- Iaccarino, H.F.; Singer, A.C.; Martorell, A.J.; Rudenko, A.; Gao, F.; Gillingham, T.Z.; Mathys, H.; Seo, J.; Kritskiy, O.; Abdurrob, F.; et al. Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 2016, 540, 230–235. [Google Scholar] [CrossRef]
- Rajji, T.K.; Chan, D.; Suk, H.-J.; Jackson, B.L.; Milman, N.P.; Stark, D.; Klerman, E.B.; Kitchener, E.; Fernandez Avalos, V.S.; de Weck, G.; et al. Gamma frequency sensory stimulation in mild probable Alzheimer’s dementia patients: Results of feasibility and pilot studies. PLoS ONE 2022, 17, e0278412. [Google Scholar] [CrossRef]
- Tian, T.; Qin, X.; Wang, Y.; Shi, Y.; Yang, X. 40 Hz Light Flicker Promotes Learning and Memory via Long Term Depression in Wild-Type Mice. J. Alzheimer’s Dis. 2021, 84, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Duque, C.; Chan, D.; Kahn, M.C.; Murdock, M.H.; Tsai, L.H. Audiovisual gamma stimulation for the treatment of neurodegeneration. J. Intern. Med. 2023, 295, 146–170. [Google Scholar] [CrossRef]
- Adaikkan, C.; Middleton, S.J.; Marco, A.; Pao, P.-C.; Mathys, H.; Kim, D.N.-W.; Gao, F.; Young, J.Z.; Suk, H.-J.; Boyden, E.S.; et al. Gamma Entrainment Binds Higher-Order Brain Regions and Offers Neuroprotection. Neuron 2019, 102, 929–943.e8. [Google Scholar] [CrossRef]
- Cimenser, A.; Hempel, E.; Travers, T.; Strozewski, N.; Martin, K.; Malchano, Z.; Hajós, M. Sensory-Evoked 40-Hz Gamma Oscillation Improves Sleep and Daily Living Activities in Alzheimer’s Disease Patients. Front. Syst. Neurosci. 2021, 15, 746859. [Google Scholar] [CrossRef] [PubMed]
- Jirakittayakorn, N.; Wongsawat, Y. Brain responses to 40-Hz binaural beat and effects on emotion and memory. Int. J. Psychophysiol. 2017, 120, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Li, K.T.; Liang, J.; Zhou, C.; Su, J. Gamma Oscillations Facilitate Effective Learning in Excitatory-Inhibitory Balanced Neural Circuits. Neural Plast. 2021, 2021, 6668175. [Google Scholar] [CrossRef]
- Park, S.-S.; Park, H.-S.; Kim, C.-J.; Kang, H.-S.; Kim, D.-H.; Baek, S.-S.; Kim, T.-W. Physical exercise during exposure to 40-Hz light flicker improves cognitive functions in the 3xTg mouse model of Alzheimer’s disease. Alzheimer’s Res. Ther. 2020, 12, 62. [Google Scholar] [CrossRef]
- Prichard, A.; Garza, K.M.; Shridhar, A.; He, C.; Bitarafan, S.; Pybus, A.; Wang, Y.; Snyder, E.; Goodson, M.C.; Franklin, T.C.; et al. Brain rhythms control microglial response and cytokine expression via NF-κB signaling. Sci. Adv. 2023, 9, eadf5672. [Google Scholar] [CrossRef] [PubMed]
- Ross, B.; Fujioka, T. 40-Hz oscillations underlying perceptual binding in young and older adults. Psychophysiology 2016, 53, 974–990. [Google Scholar] [CrossRef]
- Zheng, L.; Yu, M.; Lin, R.; Wang, Y.; Zhuo, Z.; Cheng, N.; Wang, M.; Tang, Y.; Wang, L.; Hou, S.-T. Rhythmic light flicker rescues hippocampal low gamma and protects ischemic neurons by enhancing presynaptic plasticity. Nat. Commun. 2020, 11, 3012. [Google Scholar] [CrossRef]
- Murdock, M.H.; Yang, C.-Y.; Sun, N.; Pao, P.-C.; Blanco-Duque, C.; Kahn, M.C.; Kim, T.; Lavoie, N.S.; Victor, M.B.; Islam, M.R.; et al. Multisensory gamma stimulation promotes glymphatic clearance of amyloid. Nature 2024, 627, 149–156. [Google Scholar] [CrossRef]
- Sarimov, R.M.; Serov, D.A.; Gudkov, S.V. Biological Effects of Magnetic Storms and ELF Magnetic Fields. Biology 2023, 12, 1506. [Google Scholar] [CrossRef]
- Park, Y.; Lee, K.; Park, J.; Bae, J.B.; Kim, S.-S.; Kim, D.-W.; Woo, S.J.; Yoo, S.; Kim, K.W. Optimal flickering light stimulation for entraining gamma rhythms in older adults. Sci. Rep. 2022, 12, 15550. [Google Scholar] [CrossRef]
- Hashim, A.A.; Majid, M.A.; Mustafa, B.A. Legibility of Web Page on Full High Definition Display. In Proceedings of the 2013 International Conference on Advanced Computer Science Applications and Technologies, Kuching, Malaysia, 23–24 December 2013; pp. 521–524. [Google Scholar]
- Sánchez-Lacambra, M.; Orduna-Hospital, E.; Arcas-Carbonell, M.; Sánchez-Cano, A. Effects of Light on Visual Function, Alertness, and Cognitive Performance: A Computerized Test Assessment. Appl. Sci. 2024, 14, 6424. [Google Scholar] [CrossRef]
- Vetter, C.; Pattison, P.M.; Houser, K.; Herf, M.; Phillips, A.J.K.; Wright, K.P.; Skene, D.J.; Brainard, G.C.; Boivin, D.B.; Glickman, G. A Review of Human Physiological Responses to Light: Implications for the Development of Integrative Lighting Solutions. Leukos 2021, 18, 387–414. [Google Scholar] [CrossRef]
- Dittmar, M. Changing Colour Preferences with Ageing: A Comparative Study on Younger and Older Native Germans Aged 19–90 Years. Gerontology 2001, 47, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Spitschan, M.; Yokoyama, S.; Tanaka, Y.; Kojima, T.; Horai, R.; Kato, Y.; Nakamura, H.; Sato, H.; Mitamura, M.; Tanaka, K.; et al. Age-related changes of color visual acuity in normal eyes. PLoS ONE 2021, 16, e0260525. [Google Scholar] [CrossRef]
- Klimesch, W. An algorithm for the EEG frequency architecture of consciousness and brain body coupling. Front. Hum. Neurosci. 2013, 7, 66103. [Google Scholar] [CrossRef] [PubMed]
- Fries, P. Rhythms for Cognition: Communication through Coherence. Neuron 2015, 88, 220–235. [Google Scholar] [CrossRef] [PubMed]
- Murty, D.V.P.S.; Shirhatti, V.; Ravishankar, P.; Ray, S. Large Visual Stimuli Induce Two Distinct Gamma Oscillations in Primate Visual Cortex. J. Neurosci. 2018, 38, 2730–2744. [Google Scholar] [CrossRef]
- Arutiunian, V.; Arcara, G.; Buyanova, I.; Gomozova, M.; Dragoy, O. The age-related changes in 40 Hz Auditory Steady-State Response and sustained Event-Related Fields to the same amplitude-modulated tones in typically developing children: A magnetoencephalography study. Hum. Brain Mapp. 2022, 43, 5370–5383. [Google Scholar] [CrossRef]
- Grachev, I.D.; Apkarian, A.V. Aging alters regional multichemical profile of the human brain: An in vivo1H-MRS study of young versus middle-aged subjects. J. Neurochem. 2008, 76, 582–593. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Singh, K.D. Spatiotemporal frequency tuning of BOLD and gamma band MEG responses compared in primary visual cortex. NeuroImage 2008, 40, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Bennett, P.J.; Sekuler, R.; Sekuler, A.B. The effects of aging on motion detection and direction identification. Vis. Res. 2007, 47, 799–809. [Google Scholar] [CrossRef]
- Betts, L.R.; Taylor, C.P.; Sekuler, A.B.; Bennett, P.J. Aging Reduces Center-Surround Antagonism in Visual Motion Processing. Neuron 2005, 45, 361–366. [Google Scholar] [CrossRef]
- Leventhal, A.G.; Wang, Y.; Pu, M.; Zhou, Y.; Ma, Y. GABA and Its Agonists Improved Visual Cortical Function in Senescent Monkeys. Science 2003, 300, 812–815. [Google Scholar] [CrossRef]
- Schmolesky, M.T.; Wang, Y.; Pu, M.; Leventhal, A.G. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat. Neurosci. 2000, 3, 384–390. [Google Scholar] [CrossRef]
- Murty, D.V.P.S.; Manikandan, K.; Kumar, W.S.; Ramesh, R.G.; Purokayastha, S.; Javali, M.; Rao, N.P.; Ray, S. Gamma oscillations weaken with age in healthy elderly in human EEG. NeuroImage 2020, 215, 116826. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Pustovoy, V.I.; Sarimov, R.M.; Serov, D.A.; Simakin, A.V.; Shcherbakov, I.A. Diversity of Effects of Mechanical Influences on Living Systems and Aqueous Solutions. Int. J. Mol. Sci. 2025, 26, 5556. [Google Scholar] [CrossRef]
- Davey, Z.; Gupta, P.B.; Li, D.R.; Nayak, R.U.; Govindarajan, P. Rapid Response EEG: Current State and Future Directions. Curr. Neurol. Neurosci. Rep. 2022, 22, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Rohenkohl, G.; Bosman, C.A.; Fries, P. Gamma Synchronization between V1 and V4 Improves Behavioral Performance. Neuron 2018, 100, 953–963.e953. [Google Scholar] [CrossRef] [PubMed]
- Astashev, M.; Serov, D.; Gudkov, S. Application of Spectral Methods of Analysis for Description of Ultradian Biorhythms at the Levels of Physiological Systems, Cells and Molecules (Review). Mathematics 2023, 11, 3307. [Google Scholar] [CrossRef]
- Pack, C.; Han, C.; Wang, T.; Yang, Y.; Wu, Y.; Li, Y.; Dai, W.; Zhang, Y.; Wang, B.; Yang, G.; et al. Multiple gamma rhythms carry distinct spatial frequency information in primary visual cortex. PLoS Biol. 2021, 19, e3001466. [Google Scholar] [CrossRef]
- Misselhorn, J.; Schwab, B.C.; Schneider, T.R.; Engel, A.K. Synchronization of Sensory Gamma Oscillations Promotes Multisensory Communication. eNeuro 2019, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; McDermott, B.; Oliveira, B.L.; O’Brien, A.; Coogan, D.; Lang, M.; Moriarty, N.; Dowd, E.; Quinlan, L.; Mc Ginley, B.; et al. Gamma Band Light Stimulation in Human Case Studies: Groundwork for Potential Alzheimer’s Disease Treatment. J. Alzheimer’s Dis. 2019, 70, 171–185. [Google Scholar] [CrossRef]
- Lee, K.; Park, Y.; Suh, S.W.; Kim, S.-S.; Kim, D.-W.; Lee, J.; Park, J.; Yoo, S.; Kim, K.W. Optimal flickering light stimulation for entraining gamma waves in the human brain. Sci. Rep. 2021, 11, 16206. [Google Scholar] [CrossRef] [PubMed]
- Min, B.-K.; Dähne, S.; Ahn, M.-H.; Noh, Y.-K.; Müller, K.-R. Decoding of top-down cognitive processing for SSVEP-controlled BMI. Sci. Rep. 2016, 6, 36267. [Google Scholar] [CrossRef]
- Regan, D. An Effect of Stimulus Colour on Average Steady-state Potentials evoked in Man. Nature 1966, 210, 1056–1057. [Google Scholar] [CrossRef] [PubMed]
- Tello, R.J.M.G.; Müller, S.M.T.; Ferreira, A.; Bastos, T.F. Comparison of the influence of stimuli color on Steady-State Visual Evoked Potentials. Res. Biomed. Eng. 2015, 31, 218–231. [Google Scholar] [CrossRef]
- Roorda, A.; Williams, D.R. The arrangement of the three cone classes in the living human eye. Nature 1999, 397, 520–522. [Google Scholar] [CrossRef]
- Roorda, A.; Metha, A.B.; Lennie, P.; Williams, D.R. Packing arrangement of the three cone classes in primate retina. Vis. Res. 2001, 41, 1291–1306. [Google Scholar] [CrossRef]
- Stauch, B.J.; Peter, A.; Ehrlich, I.; Nolte, Z.; Fries, P. Human visual gamma for color stimuli. eLife 2022, 11, 75897. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Fernández-Vargas, J.; Kita, K.; Yu, W. Influence of Stimulus Color on Steady State Visual Evoked Potentials. In Intelligent Autonomous Systems 14; Advances in Intelligent Systems and Computing; Springer: New York, NY, USA, 2017; pp. 499–509. [Google Scholar]
- Noda, Y.; Takano, M.; Hayano, M.; Li, X.; Wada, M.; Nakajima, S.; Mimura, M.; Kondo, S.; Tsubota, K. Photobiological Neuromodulation of Resting-State EEG and Steady-State Visual-Evoked Potentials by 40 Hz Violet Light Optical Stimulation in Healthy Individuals. J. Pers. Med. 2021, 11, 557. [Google Scholar] [CrossRef]
- Osorio, D.; Wuerger, S. Colour Constancy Across the Life Span: Evidence for Compensatory Mechanisms. PLoS ONE 2013, 8, e63921. [Google Scholar] [CrossRef]
- Wijk, H.; Berg, S.; Sivik, L.; Steen, B. Color discrimination, color naming and color preferences in 80-year olds. Aging Clin. Exp. Res. 1999, 11, 176–185. [Google Scholar] [CrossRef]
- Raven, F.; Aton, S.J. The Engram’s Dark Horse: How Interneurons Regulate State-Dependent Memory Processing and Plasticity. Front. Neural Circuits 2021, 15, 750541. [Google Scholar] [CrossRef]
- Kloc, M.; Maffei, A. Target-Specific Properties of Thalamocortical Synapses onto Layer 4 of Mouse Primary Visual Cortex. J. Neurosci. 2014, 34, 15455–15465. [Google Scholar] [CrossRef]
- Abubaker, M.; Al Qasem, W.; Pilátová, K.; Ježdík, P.; Kvašňák, E. Theta-gamma-coupling as predictor of working memory performance in young and elderly healthy people. Mol. Brain 2024, 17, 74. [Google Scholar] [CrossRef]
- Cardin, J.A.; Carlén, M.; Meletis, K.; Knoblich, U.; Zhang, F.; Deisseroth, K.; Tsai, L.-H.; Moore, C.I. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009, 459, 663–667. [Google Scholar] [CrossRef]
- Carlén, M.; Meletis, K.; Siegle, J.H.; Cardin, J.A.; Futai, K.; Vierling-Claassen, D.; Rühlmann, C.; Jones, S.R.; Deisseroth, K.; Sheng, M.; et al. A critical role for NMDA receptors in parvalbumin interneurons for gamma rhythm induction and behavior. Mol. Psychiatry 2011, 17, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, A.I.; Szabó, G.G.; Ulbert, I.; Holderith, N.; Monyer, H.; Erdélyi, F.; Szabó, G.; Freund, T.F.; Hájos, N. Parvalbumin-Containing Fast-Spiking Basket Cells Generate the Field Potential Oscillations Induced by Cholinergic Receptor Activation in the Hippocampus. J. Neurosci. 2010, 30, 15134–15145. [Google Scholar] [CrossRef] [PubMed]
- Whittington, M.A.; Traub, R.D.; Kopell, N.; Ermentrout, B.; Buhl, E.H. Inhibition-based rhythms: Experimental and mathematical observations on network dynamics. Int. J. Psychophysiol. 2000, 38, 315–336. [Google Scholar] [CrossRef]
- Segneri, M.; Bi, H.; Olmi, S.; Torcini, A. Theta-Nested Gamma Oscillations in Next Generation Neural Mass Models. Front. Comput. Neurosci. 2020, 14, 47. [Google Scholar] [CrossRef] [PubMed]
- Friedman-Hill, S. Dynamics of Striate Cortical Activity in the Alert Macaque: I. Incidence and Stimulus-dependence of Gamma-band Neuronal Oscillations. Cereb. Cortex 2000, 10, 1105–1116. [Google Scholar] [CrossRef]
- Ermentrout, B.; Lowet, E.; Roberts, M.; Hadjipapas, A.; Peter, A.; van der Eerden, J.; De Weerd, P. Input-Dependent Frequency Modulation of Cortical Gamma Oscillations Shapes Spatial Synchronization and Enables Phase Coding. PLoS Comput. Biol. 2015, 11, e1004072. [Google Scholar] [CrossRef] [PubMed]
- Hadjipapas, A.; Lowet, E.; Roberts, M.J.; Peter, A.; De Weerd, P. Parametric variation of gamma frequency and power with luminance contrast: A comparative study of human MEG and monkey LFP and spike responses. NeuroImage 2015, 112, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.J.; Lowet, E.; Brunet, N.M.; Ter Wal, M.; Tiesinga, P.; Fries, P.; De Weerd, P. Robust Gamma Coherence between Macaque V1 and V2 by Dynamic Frequency Matching. Neuron 2013, 78, 523–536. [Google Scholar] [CrossRef]
- Ferando, I.; Mody, I. In vitro gamma oscillations following partial and complete ablation of δ subunit-containing GABAA receptors from parvalbumin interneurons. Neuropharmacology 2015, 88, 91–98. [Google Scholar] [CrossRef]
- Mann, E.O.; Mody, I. Control of hippocampal gamma oscillation frequency by tonic inhibition and excitation of interneurons. Nat. Neurosci. 2009, 13, 205–212. [Google Scholar] [CrossRef]
- Robson, S.E.; Muthukumarawswamy, S.D.; John Evans, C.; Shaw, A.; Brealy, J.; Davis, B.; McNamara, G.; Perry, G.; Singh, K.D. Structural and neurochemical correlates of individual differences in gamma frequency oscillations in human visual cortex. J. Anat. 2015, 227, 409–417. [Google Scholar] [CrossRef]
- Gaetz, W.; Edgar, J.C.; Wang, D.J.; Roberts, T.P.L. Relating MEG measured motor cortical oscillations to resting γ-Aminobutyric acid (GABA) concentration. NeuroImage 2011, 55, 616–621. [Google Scholar] [CrossRef]
- Khachatryan, E.; Wittevrongel, B.; Reinartz, M.; Dauwe, I.; Carrette, E.; Meurs, A.; Van Roost, D.; Boon, P.; Van Hulle, M.M. Cognitive tasks propagate the neural entrainment in response to a visual 40 Hz stimulation in humans. Front. Aging Neurosci. 2022, 14, 1010765. [Google Scholar] [CrossRef]
- Jeffries, K.J.; Fritz, J.B.; Braun, A.R. Words in melody: An H215O PET study of brain activation during singing and speaking. NeuroReport 2003, 14, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, J.; Hertrich, I.; Ackermann, H.; Mathiak, K.; Lutzenberger, W. Hearing Lips: Gamma-band Activity During Audiovisual Speech Perception. Cereb. Cortex 2005, 15, 646–653. [Google Scholar] [CrossRef]
- Kaiser, J.; Lutzenberger, W. Induced Gamma-Band Activity and Human Brain Function. Neurosci. 2003, 9, 475–484. [Google Scholar] [CrossRef]
- Kaiser, J.; Ripper, B.; Birbaumer, N.; Lutzenberger, W. Dynamics of gamma-band activity in human magnetoencephalogram during auditory pattern working memory. NeuroImage 2003, 20, 816–827. [Google Scholar] [CrossRef]
- Lu, Y.; Sarter, M.; Zochowski, M.; Booth, V. Phasic cholinergic signaling promotes emergence of local gamma rhythms in excitatory–inhibitory networks. Eur. J. Neurosci. 2020, 52, 3545–3560. [Google Scholar] [CrossRef]
- Howe, W.M.; Gritton, H.J.; Lusk, N.A.; Roberts, E.A.; Hetrick, V.L.; Berke, J.D.; Sarter, M. Acetylcholine Release in Prefrontal Cortex Promotes Gamma Oscillations and Theta–Gamma Coupling during Cue Detection. J. Neurosci. 2017, 37, 3215–3230. [Google Scholar] [CrossRef]
- Bianciardi, B.; Uhlhaas, P.J. Do NMDA-R antagonists re-create patterns of spontaneous gamma-band activity in schizophrenia? A systematic review and perspective. Neurosci. Biobehav. Rev. 2021, 124, 308–323. [Google Scholar] [CrossRef]
- Cornford, J.H.; Mercier, M.S.; Leite, M.; Magloire, V.; Häusser, M.; Kullmann, D.M. Dendritic NMDA receptors in parvalbumin neurons enable strong and stable neuronal assemblies. eLife 2019, 8, 49872. [Google Scholar] [CrossRef] [PubMed]
- Jadi, M.P.; Behrens, M.M.; Sejnowski, T.J. Abnormal Gamma Oscillations in N-Methyl-D-Aspartate Receptor Hypofunction Models of Schizophrenia. Biol. Psychiatry 2016, 79, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Luo, B.; Gao, N.; Li, H.; Wang, H.; Li, L.; Cui, W.; Zhang, L.; Sun, D.; Liu, F.; et al. Neddylation stabilizes Nav1.1 to maintain interneuron excitability and prevent seizures in murine epilepsy models. J. Clin. Investig. 2021, 131, e136956. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Losa, M.; Tracy, T.E.; Ma, K.; Verret, L.; Clemente-Perez, A.; Khan, A.S.; Cobos, I.; Ho, K.; Gan, L.; Mucke, L.; et al. Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a Mouse Model of Alzheimer’s Disease. Neuron 2018, 98, 75–89.e75. [Google Scholar] [CrossRef]
- Bressler, S.L.; Menon, V. Large-scale brain networks in cognition: Emerging methods and principles. Trends Cogn. Sci. 2010, 14, 277–290. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Kuptsov, P.V.; Stankevich, N.V. Reconstruction of neuromorphic dynamics from a single scalar time series using variational autoencoder and neural network map. Chaos Solitons Fractals 2025, 191, 115818. [Google Scholar] [CrossRef]
- Orekhova, E.V.; Butorina, A.V.; Sysoeva, O.V.; Prokofyev, A.O.; Nikolaeva, A.Y.; Stroganova, T.A. Frequency of gamma oscillations in humans is modulated by velocity of visual motion. J. Neurophysiol. 2015, 114, 244–255. [Google Scholar] [CrossRef]
- Bressler, S. Neurocognitive networks. Scholarpedia 2008, 3, 1567. [Google Scholar] [CrossRef]
- Pechenkova, E.; Rachinskaya, M.; Vasilenko, V.; Blazhenkova, O.; Mershina, E. Brain Functional Connectivity During First- and Third-Person Visual Imagery. Vision 2025, 9, 30. [Google Scholar] [CrossRef]
- Kiviniemi, V.; Tervonen, O.; Timonen, M.; Nissilä, J.; Nikkinen, J.; Starck, T.; Remes, J.; Littow, H.; Abou Elseoud, A. Group-ICA Model Order Highlights Patterns of Functional Brain Connectivity. Front. Syst. Neurosci. 2011, 5, 37. [Google Scholar] [CrossRef]
- Sinitsyn, D.O.; Bakulin, I.S.; Poydasheva, A.G.; Legostaeva, L.A.; Kremneva, E.I.; Lagoda, D.Y.; Chernyavskiy, A.Y.; Medyntsev, A.A.; Suponeva, N.A.; Piradov, M.A. Brain Activations and Functional Connectivity Patterns Associated with Insight-Based and Analytical Anagram Solving. Behav. Sci. 2020, 10, 170. [Google Scholar] [CrossRef]
- Thomas Yeo, B.T.; Krienen, F.M.; Sepulcre, J.; Sabuncu, M.R.; Lashkari, D.; Hollinshead, M.; Roffman, J.L.; Smoller, J.W.; Zöllei, L.; Polimeni, J.R.; et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011, 106, 1125–1165. [Google Scholar] [CrossRef]
- Griffiths, K.R.; Braund, T.A.; Kohn, M.R.; Clarke, S.; Williams, L.M.; Korgaonkar, M.S. Structural brain network topology underpinning ADHD and response to methylphenidate treatment. Transl. Psychiatry 2021, 11, 150. [Google Scholar] [CrossRef]
- Puchkov, N.A.; Tarumov, D.A.; Markin, K.V.; Prochik, Y.E.; Tyomniy, A.V.; Maslov, V.E. Pathology of microstructural brain connectivity in paranoid schizophrenia (according to diffusion-tensor tractography data). Russ. Mil. Med. Acad. Rep. 2021, 40, 35–43. [Google Scholar] [CrossRef]
- Rubinov, M.; Bullmore, E. Schizophrenia and abnormal brain network hubs. Dialogues Clin. Neurosci. 2022, 15, 339–349. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Zheng, L.; Zhu, X.; Shao, B.; Fan, G.; Liu, T.; Wang, J. Abnormal Brain Network Connectivity in a Triple-Network Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 69, 237–252. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Sperling, R.A. Large-scale functional brain network abnormalities in Alzheimer’s disease: Insights from functional neuroimaging. Behav. Neurol. 2009, 21, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Binnewijzend, M.A.A.; Schoonheim, M.M.; Sanz-Arigita, E.; Wink, A.M.; van der Flier, W.M.; Tolboom, N.; Adriaanse, S.M.; Damoiseaux, J.S.; Scheltens, P.; van Berckel, B.N.M.; et al. Resting-state fMRI changes in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2012, 33, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Greicius, M.D.; Srivastava, G.; Reiss, A.L.; Menon, V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proc. Natl. Acad. Sci. USA 2004, 101, 4637–4642. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Qin, W.; Liu, Y.; Zhang, X.; Duan, Y.; Song, J.; Li, K.; Jiang, T.; Yu, C. Abnormal salience network in normal aging and in amnestic mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp. 2014, 35, 3446–3464. [Google Scholar] [CrossRef]
- Teipel, S.J. S5-02-06: DTI and resting state fMRI as biomarker of Alzheimer’s disease: Present state and perspectives. Alzheimer’s Dement. 2010, 6, S169. [Google Scholar] [CrossRef]
- Wang, K.; Liang, M.; Wang, L.; Tian, L.; Zhang, X.; Li, K.; Jiang, T. Altered functional connectivity in early Alzheimer’s disease: A resting-state fMRI study. Hum. Brain Mapp. 2006, 28, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhou, B.; Yao, H.; Zhan, Y.; Zhang, Z.; Cui, Y.; Xu, K.; Ma, J.; Wang, L.; An, N.; et al. Aberrant intra- and inter-network connectivity architectures in Alzheimer’s disease and mild cognitive impairment. Sci. Rep. 2015, 5, 14824. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Huang, L.; Cai, S.; Zhang, Y.; von Deneen, K.M.; Ren, A.; Ren, J. Altered effective connectivity patterns of the default mode network in Alzheimer’s disease: An fMRI study. Neurosci. Lett. 2014, 578, 171–175. [Google Scholar] [CrossRef]
- Gavron, A.A.; Deza-Araujo, Y.I.; Sharova, E.V.; Smirnov, A.S.; Knyazev, G.G.; Chelyapina, M.V.; Fadeeva, L.M.; Abdulaev, A.A.; Kulikov, M.A.; Zhavoronkova, L.A.; et al. Group and Individual fMRI Analysis of the Main Resting State Networks in Healthy Subjects. Neurosci. Behav. Physiol. 2020, 50, 288–297. [Google Scholar] [CrossRef]
- Lim, H.K.; Nebes, R.; Snitz, B.; Cohen, A.; Mathis, C.; Price, J.; Weissfeld, L.; Klunk, W.; Aizenstein, H.J. Regional amyloid burden and intrinsic connectivity networks in cognitively normal elderly subjects. Brain 2014, 137, 3327–3338. [Google Scholar] [CrossRef]
- Zhou, J.; Greicius, M.D.; Gennatas, E.D.; Growdon, M.E.; Jang, J.Y.; Rabinovici, G.D.; Kramer, J.H.; Weiner, M.; Miller, B.L.; Seeley, W.W. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 2010, 133, 1352–1367. [Google Scholar] [CrossRef]
- Onoda, K.; Ishihara, M.; Yamaguchi, S. Decreased Functional Connectivity by Aging Is Associated with Cognitive Decline. J. Cogn. Neurosci. 2012, 24, 2186–2198. [Google Scholar] [CrossRef]
- Li, R.; Wu, X.; Chen, K.; Fleisher, A.S.; Reiman, E.M.; Yao, L. Alterations of Directional Connectivity among Resting-State Networks in Alzheimer Disease. Am. J. Neuroradiol. 2013, 34, 340–345. [Google Scholar] [CrossRef]
- Liu, X.; Chen, X.; Zheng, W.; Xia, M.; Han, Y.; Song, H.; Li, K.; He, Y.; Wang, Z. Altered Functional Connectivity of Insular Subregions in Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 107. [Google Scholar] [CrossRef]
- Tanashyan, M.M.; Lagoda, O.V.; Medvedev, R.B.; Krotenkova, M.V.; Konovalov, R.N.; Ponomareva, N.V.; Fokin, V.F. Resting-state neural networks in cognitive decline in patients with vascular encephalopathy. Ann. Clin. Exp. Neurol. 2020, 14, 39–45. [Google Scholar] [CrossRef]
- Galanin, I.V.; Naryshkin, A.G.; Liaskina, I.Y.; Skoromets, T.A. Morphometric and Functional Changes of the Brain in Mental Disorders and Their Dynamics during Drug Treatment. Hum. Physiol. 2022, 48, 306–312. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Nieto-Castanon, A. Conn: A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks. Brain Connect. 2012, 2, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Northoff, G.; Wu, Y.T. Resting network is composed of more than one neural pattern: An fMRI study. Neuroscience 2014, 274, 198–208. [Google Scholar] [CrossRef]
- Mulders, P.C.; van Eijndhoven, P.F.; Schene, A.H.; Beckmann, C.F.; Tendolkar, I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015, 56, 330–344. [Google Scholar] [CrossRef]
- Stoyanov, D.; Khorev, V.; Paunova, R.; Kandilarova, S.; Simeonova, D.; Badarin, A.; Hramov, A.; Kurkin, S. Resting-State Functional Connectivity Impairment in Patients with Major Depressive Episode. Int. J. Environ. Res. Public Health 2022, 19, 14045. [Google Scholar] [CrossRef]
- Raichle, M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.W.; Xue, S.W. Functional connectivity maps based on hippocampal and thalamic dynamics may account for the default-mode network. Eur. J. Neurosci. 2018, 47, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Gusnard, D.A.; Akbudak, E.; Shulman, G.L.; Raichle, M.E. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 4259–4264. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, S.; Konishi, M.; McLaren, D.G.; Engen, H.; Smallwood, J. Shaped by the Past: The Default Mode Network Supports Cognition that Is Independent of Immediate Perceptual Input. PLoS ONE 2015, 10, e0132209. [Google Scholar] [CrossRef]
- Østby, Y.; Walhovd, K.B.; Tamnes, C.K.; Grydeland, H.; Westlye, L.T.; Fjell, A.M. Mental time travel and default-mode network functional connectivity in the developing brain. Proc. Natl. Acad. Sci. USA 2012, 109, 16800–16804. [Google Scholar] [CrossRef]
- Sali, A.W.; Courtney, S.M.; Yantis, S. Spontaneous Fluctuations in the Flexible Control of Covert Attention. J. Neurosci. 2016, 36, 445–454. [Google Scholar] [CrossRef]
- Vatansever, D.; Manktelow, A.E.; Sahakian, B.J.; Menon, D.K.; Stamatakis, E.A. Cognitive Flexibility: A Default Network and Basal Ganglia Connectivity Perspective. Brain Connect. 2016, 6, 201–207. [Google Scholar] [CrossRef]
- Davey, C.G.; Pujol, J.; Harrison, B.J. Mapping the self in the brain’s default mode network. NeuroImage 2016, 132, 390–397. [Google Scholar] [CrossRef]
- Qin, P.; Grimm, S.; Duncan, N.W.; Fan, Y.; Huang, Z.; Lane, T.; Weng, X.; Bajbouj, M.; Northoff, G. Spontaneous activity in default-mode network predicts ascription of self-relatedness to stimuli. Soc. Cogn. Affect. Neurosci. 2016, 11, 693–702. [Google Scholar] [CrossRef]
- Li, W.; Mai, X.; Liu, C. The default mode network and social understanding of others: What do brain connectivity studies tell us. Front. Hum. Neurosci. 2014, 8, 74. [Google Scholar] [CrossRef]
- Mars, R.B.; Neubert, F.-X.; Noonan, M.P.; Sallet, J.; Toni, I.; Rushworth, M.F.S. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 2012, 6, 189. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, N. A journey into chaos: Creativity and the unconsciousFNx08. Mens Sana Monogr. 2011, 9, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Beaty, R.E.; Benedek, M.; Wilkins, R.W.; Jauk, E.; Fink, A.; Silvia, P.J.; Hodges, D.A.; Koschutnig, K.; Neubauer, A.C. Creativity and the default network: A functional connectivity analysis of the creative brain at rest. Neuropsychologia 2014, 64, 92–98. [Google Scholar] [CrossRef]
- Knyazev, G.G.; Ushakov, V.L.; Orlov, V.A.; Malakhov, D.G.; Kartashov, S.I.; Savostyanov, A.N.; Bocharov, A.V.; Velichkovsky, B.M. EEG and fMRI Correlates of Insight: A Pilot Study. Symmetry 2021, 13, 330. [Google Scholar] [CrossRef]
- Ino, T.; Nakai, R.; Azuma, T.; Kimura, T.; Fukuyama, H. Brain Activation During Autobiographical Memory Retrieval with Special Reference to Default Mode Network. Open Neuroimaging J. 2011, 5, 14–23. [Google Scholar] [CrossRef]
- Yang, J.; Weng, X.; Zang, Y.; Xu, M.; Xu, X. Sustained activity within the default mode network during an implicit memory task. Cortex 2010, 46, 354–366. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ford, J.M. Default Mode Network Activity and Connectivity in Psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef]
- Northoff, G.; Bermpohl, F. Cortical midline structures and the self. Trends Cogn. Sci. 2004, 8, 102–107. [Google Scholar] [CrossRef]
- Hou, X.; Liu, R.; Zhou, Y.; Guan, L.; Zhou, J.; Liu, J.; Liu, M.; Yuan, X.; Feng, Y.; Chen, X.; et al. Shared and unique alterations of large-scale network connectivity in drug-free adolescent-onset and adult-onset major depressive disorder. Transl. Psychiatry 2024, 14, 255. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R.H.; Andrews-Hanna, J.R.; Wager, T.D.; Pizzagalli, D.A. Large-Scale Network Dysfunction in Major Depressive Disorder. JAMA Psychiatry 2015, 72, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Cui, H.; Sun, Q.; Li, Y.; Bai, T.; Wang, K.; Zhang, J.; Tian, Y.; Wang, J. Individual large-scale functional network mapping for major depressive disorder with electroconvulsive therapy. J. Affect. Disord. 2024, 360, 116–125. [Google Scholar] [CrossRef]
- Dong, D.; Wang, Y.; Chang, X.; Luo, C.; Yao, D. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophr. Bull. 2018, 44, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Kraguljac, N.V.; White, D.M.; Hadley, J.A.; Visscher, K.; Knight, D.; ver Hoef, L.; Falola, B.; Lahti, A.C. Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. NeuroImage Clin. 2016, 10, 146–158. [Google Scholar] [CrossRef]
- He, Q.; Colon-Motas, K.M.; Pybus, A.F.; Piendel, L.; Seppa, J.K.; Walker, M.L.; Manzanares, C.M.; Qiu, D.; Miocinovic, S.; Wood, L.B.; et al. A feasibility trial of gamma sensory flicker for patients with prodromal Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2021, 7, e12178. [Google Scholar] [CrossRef]
- Chan, D.; Suk, H.-J.; Jackson, B.; Milman, N.P.; Stark, D.; Klerman, E.B.; Kitchener, E.; Avalos, V.S.F.; Banerjee, A.; Beach, S.D.; et al. 40Hz sensory stimulation induces gamma entrainment and affects brain structure, sleep and cognition in patients with Alzheimer’s dementia. medRxiv 2021. [Google Scholar] [CrossRef]
- Gusnard, D.A.; Raichle, M.E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001, 2, 685–694. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Scolari, M.; Seidl-Rathkopf, K.N.; Kastner, S. Functions of the human frontoparietal attention network: Evidence from neuroimaging. Curr. Opin. Behav. Sci. 2015, 1, 32–39. [Google Scholar] [CrossRef]
- Uddin, L.Q.; Yeo, B.T.T.; Spreng, R.N. Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. Brain Topogr. 2019, 32, 926–942. [Google Scholar] [CrossRef]
- Kornblith, S.; Buschman, T.J.; Miller, E.K. Stimulus Load and Oscillatory Activity in Higher Cortex. Cereb. Cortex 2016, 26, 3772–3784. [Google Scholar] [CrossRef]
- Palva, S.; Kulashekhar, S.; Hämäläinen, M.; Palva, J.M. Localization of Cortical Phase and Amplitude Dynamics during Visual Working Memory Encoding and Retention. J. Neurosci. 2011, 31, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.; Chang, Y.-T.; Chang, C.-F.; Liang, W.-K.; Juan, C.-H. The critical role of phase difference in gamma oscillation within the temporoparietal network for binding visual working memory. Sci. Rep. 2016, 6, 32138. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Brain Mapping; Wiley: Hoboken, NJ, USA, 2015; pp. 643–651. [Google Scholar]
- Steimke, R.; Nomi, J.S.; Calhoun, V.D.; Stelzel, C.; Paschke, L.M.; Gaschler, R.; Goschke, T.; Walter, H.; Uddin, L.Q. Salience network dynamics underlying successful resistance of temptation. Soc. Cogn. Affect. Neurosci. 2017, 12, 1928–1939. [Google Scholar] [CrossRef] [PubMed]
- Bertolero, M.; Bassett, D.S.; Studios, M.R. How Matter Becomes Mind. Sci. Am. 2024, 321, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L. The serendipitous discovery of the brain’s default network. NeuroImage 2012, 62, 1137–1145. [Google Scholar] [CrossRef]
- Riedl, V.; Utz, L.; Castrillón, G.; Grimmer, T.; Rauschecker, J.P.; Ploner, M.; Friston, K.J.; Drzezga, A.; Sorg, C. Metabolic connectivity mapping reveals effective connectivity in the resting human brain. Proc. Natl. Acad. Sci. USA 2015, 113, 428–433. [Google Scholar] [CrossRef]
- Bailey, S.K.; Aboud, K.S.; Nguyen, T.Q.; Cutting, L.E. Applying a network framework to the neurobiology of reading and dyslexia. J. Neurodev. Disord. 2018, 10, 37. [Google Scholar] [CrossRef]
- Yan, H.; Deng, X.; Wang, Y.; Wu, S.; Du, J.; Yu, M.; Liu, B.; Wang, H.; Pan, Y.; Zhang, Z.; et al. Enhancement of spatial learning by 40 Hz visual stimulation requires parvalbumin interneuron-dependent hippocampal neurogenesis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Brown, J.; Cooper-Kuhn, C.M.; Kempermann, G.; Van Praag, H.; Winkler, J.; Gage, F.H.; Kuhn, H.G. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur. J. Neurosci. 2003, 17, 2042–2046. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, M.; Perfilieva, E.; Johansson, U.; Orwar, O.; Eriksson, P.S. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J. Neurobiol. 1999, 39, 569–578. [Google Scholar] [CrossRef]
- van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. USA 1999, 96, 13427–13431. [Google Scholar] [CrossRef]
- Kempermann, G.; Kuhn, H.G.; Gage, F.H. Experience-Induced Neurogenesis in the Senescent Dentate Gyrus. J. Neurosci. 1998, 18, 3206–3212. [Google Scholar] [CrossRef]
- Song, J.; Olsen, R.H.J.; Sun, J.; Ming, G.-l.; Song, H. Neuronal Circuitry Mechanisms Regulating Adult Mammalian Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018937. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Gan, J.; Jonas, P. Fast-spiking, parvalbumin+ GABAergic interneurons: From cellular design to microcircuit function. Science 2014, 345, 1255263. [Google Scholar] [CrossRef] [PubMed]
- Lodge, M.; Bischofberger, J. Synaptic properties of newly generated granule cells support sparse coding in the adult hippocampus. Behav. Brain Res. 2019, 372, 112036. [Google Scholar] [CrossRef]
- Song, J.; Sun, J.; Moss, J.; Wen, Z.; Sun, G.J.; Hsu, D.; Zhong, C.; Davoudi, H.; Christian, K.M.; Toni, N.; et al. Parvalbumin interneurons mediate neuronal circuitry–neurogenesis coupling in the adult hippocampus. Nat. Neurosci. 2013, 16, 1728–1730. [Google Scholar] [CrossRef]
- Catavero, C.; Bao, H.; Song, J. Neural mechanisms underlying GABAergic regulation of adult hippocampal neurogenesis. Cell Tissue Res. 2017, 371, 33–46. [Google Scholar] [CrossRef]
- Markwardt, S.J.; Wadiche, J.I.; Overstreet-Wadiche, L.S. Input-Specific GABAergic Signaling to Newborn Neurons in Adult Dentate Gyrus. J. Neurosci. 2009, 29, 15063–15072. [Google Scholar] [CrossRef]
- Sibbe, M.; Kulik, A. GABAergic Regulation of Adult Hippocampal Neurogenesis. Mol. Neurobiol. 2016, 54, 5497–5510. [Google Scholar] [CrossRef]
- Tozuka, Y.; Fukuda, S.; Namba, T.; Seki, T.; Hisatsune, T. GABAergic Excitation Promotes Neuronal Differentiation in Adult Hippocampal Progenitor Cells. Neuron 2005, 47, 803–815. [Google Scholar] [CrossRef]
- Pallotto, M.; Deprez, F. Regulation of adult neurogenesis by GABAergic transmission: Signaling beyond GABAA-receptors. Front. Cell. Neurosci. 2014, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- Pontes, A.; Zhang, Y.; Hu, W. Novel functions of GABA signaling in adult neurogenesis. Front. Biol. 2013, 8, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Lord, L.-D.; Expert, P.; Huckins, J.F.; Turkheimer, F.E. Cerebral Energy Metabolism and the Brain’s Functional Network Architecture: An Integrative Review. J. Cereb. Blood Flow Metab. 2013, 33, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.D.; Li, B. Neural–metabolic coupling in the central visual pathway. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150357. [Google Scholar] [CrossRef]
- Niessing, J.r.; Ebisch, B.; Schmidt, K.E.; Niessing, M.; Singer, W.; Galuske, R.A.W. Hemodynamic Signals Correlate Tightly with Synchronized Gamma Oscillations. Science 2005, 309, 948–951. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Chu, S.; Xiong, W.; Zhang, D.; Soylu, H.; Sun, C.; Albensi, B.C.; Parkinson, F.E. Regulation of adenosine levels during cerebral ischemia. Acta Pharmacol. Sin. 2012, 34, 60–66. [Google Scholar] [CrossRef]
- Garcia-Gil, M.; Camici, M.; Allegrini, S.; Pesi, R.; Tozzi, M.G. Metabolic Aspects of Adenosine Functions in the Brain. Front. Pharmacol. 2021, 12, 672182. [Google Scholar] [CrossRef]
- Chen, J.-F.; Eltzschig, H.K.; Fredholm, B.B. Adenosine receptors as drug targets—What are the challenges? Nat. Rev. Drug Discov. 2013, 12, 265–286. [Google Scholar] [CrossRef]
- Cunha, R.A. How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 2016, 139, 1019–1055. [Google Scholar] [CrossRef]
- Shlyakhto, E.V.; Barantsevich, E.P.; Shcherbak, N.S.; Galagudza, M.M. Molecular Mechanisms of Development of Cerebral Tolerance to Ischemia. Part 1. Ann. Russ. Acad. Med. Sci. 2012, 67, 42–50. [Google Scholar] [CrossRef]
- Pomytkin, I.A.; Karkischenko, N.N. Metabolic Control of High-Frequency Gamma Oscillations in the Brain. Biomeditsina 2019, 15, 43–53. [Google Scholar] [CrossRef]
- Fielder, E.; Tweedy, C.; Wilson, C.; Oakley, F.; LeBeau, F.E.N.; Passos, J.F.; Mann, D.A.; von Zglinicki, T.; Jurk, D. Anti-inflammatory treatment rescues memory deficits during aging in nfkb1−/− mice. Aging Cell 2020, 19, e13188. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Chausse, B.; Dikmen, H.O.; Almouhanna, F.; Hollnagel, J.O.; Lewen, A.; Kann, O. TLR2- and TLR3-activated microglia induce different levels of neuronal network dysfunction in a context-dependent manner. Brain Behav. Immun. 2021, 96, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.-T.; Dikmen, H.O.; Schilling, S.; Chausse, B.; Lewen, A.; Hollnagel, J.-O.; Kann, O. Priming of microglia with IFN-γ slows neuronal gamma oscillations in situ. Proc. Natl. Acad. Sci. USA 2019, 116, 4637–4642. [Google Scholar] [CrossRef]
- Assogna, M.; Casula, E.P.; Borghi, I.; Bonnì, S.; Samà, D.; Motta, C.; Di Lorenzo, F.; D’Acunto, A.; Porrazzini, F.; Minei, M.; et al. Effects of Palmitoylethanolamide Combined with Luteoline on Frontal Lobe Functions, High Frequency Oscillations, and GABAergic Transmission in Patients with Frontotemporal Dementia. J. Alzheimer’s Dis. 2020, 76, 1297–1308. [Google Scholar] [CrossRef]
- Chausse, B.; Lewen, A.; Poschet, G.; Kann, O. Selective inhibition of mitochondrial respiratory complexes controls the transition of microglia into a neurotoxic phenotype in situ. Brain Behav. Immun. 2020, 88, 802–814. [Google Scholar] [CrossRef]
- Salter, M.W.; Stevens, B. Microglia emerge as central players in brain disease. Nat. Med. 2017, 23, 1018–1027. [Google Scholar] [CrossRef]
- Ferro, A.; Auguste, Y.S.S.; Cheadle, L. Microglia, Cytokines, and Neural Activity: Unexpected Interactions in Brain Development and Function. Front. Immunol. 2021, 12, 703527. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.-È.; Stevens, B.; Sierra, A.; Wake, H.; Bessis, A.; Nimmerjahn, A. The Role of Microglia in the Healthy Brain: Figure 1. J. Neurosci. 2011, 31, 16064–16069. [Google Scholar] [CrossRef]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflamm. 2016, 13, 297. [Google Scholar] [CrossRef]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative Activation of Macrophages: An Immunologic Functional Perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Miller-Fleming, L.; Pais, T.F. Microglia and inflammation: Conspiracy, controversy or control? Cell. Mol. Life Sci. 2014, 71, 3969–3985. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Chiba, K. Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol. Ther. 2015, 154, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Le, W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2015, 53, 1181–1194. [Google Scholar] [CrossRef]
- Lyamtsev, A.S.; Sentyabreva, A.V.; Kosyreva, A.M. The Role of Prenatal Microglial Activation and Its Sex Differences in the Development of Neuropsychiatric Disorders and Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 9250. [Google Scholar] [CrossRef] [PubMed]
- Miron, V.E.; Boyd, A.; Zhao, J.-W.; Yuen, T.J.; Ruckh, J.M.; Shadrach, J.L.; van Wijngaarden, P.; Wagers, A.J.; Williams, A.; Franklin, R.J.M.; et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat. Neurosci. 2013, 16, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Hines, D.J.; Hines, R.M.; Mulligan, S.J.; Macvicar, B.A. Microglia processes block the spread of damage in the brain and require functional chloride channels. Glia 2009, 57, 1610–1618. [Google Scholar] [CrossRef]
- Spittau, B. Aging Microglia—Phenotypes, Functions and Implications for Age-Related Neurodegenerative Diseases. Front. Aging Neurosci. 2017, 9, 194. [Google Scholar] [CrossRef]
- Hanisch, U.K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Lyons, Y.A.; Pradeep, S.; Wu, S.Y.; Haemmerle, M.; Hansen, J.M.; Wagner, M.J.; Villar-Prados, A.; Nagaraja, A.S.; Dood, R.L.; Previs, R.A.; et al. Macrophage depletion through colony stimulating factor 1 receptor pathway blockade overcomes adaptive resistance to anti-VEGF therapy. Oncotarget 2017, 8, 96496–96505. [Google Scholar] [CrossRef]
- Anderson, W.D.; Greenhalgh, A.D.; Takwale, A.; David, S.; Vadigepalli, R. Novel Influences of IL-10 on CNS Inflammation Revealed by Integrated Analyses of Cytokine Networks and Microglial Morphology. Front. Cell. Neurosci. 2017, 11, 233. [Google Scholar] [CrossRef]
- Balasingam, V.; Yong, V.W. Attenuation of Astroglial Reactivity by Interleukin-10. J. Neurosci. 1996, 16, 2945–2955. [Google Scholar] [CrossRef]
- Ledeboer, A.; Brevé, J.J.P.; Wierinckx, A.; Van Der Jagt, S.; Bristow, A.F.; Leysen, J.E.; Tilders, F.J.H.; Van Dam, A.M. Expression and regulation of interleukin-10 and interleukin-10 receptor in rat astroglial and microglial cells. Eur. J. Neurosci. 2002, 16, 1175–1185. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, X.; Insolera, R.; Fink, D.J.; Mata, M. IL-10 promotes neuronal survival following spinal cord injury. Exp. Neurol. 2009, 220, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Font-Nieves, M.; Van den Haute, C.; Baekelandt, V.; Planas, A.M.; Pozas, E. IL-10 regulates adult neurogenesis by modulating ERK and STAT3 activity. Front. Cell. Neurosci. 2015, 9, 57. [Google Scholar] [CrossRef]
- Perez-Asensio, F.J.; Perpiñá, U.; Planas, A.M.; Pozas, E. Interleukin-10 regulates progenitor differentiation and modulates neurogenesis on adult brain. J. Cell Sci. 2013, 126, 4208–4219. [Google Scholar] [CrossRef]
- Zhou, Z.; Peng, X.; Insolera, R.; Fink, D.J.; Mata, M. Interleukin-10 provides direct trophic support to neurons. J. Neurochem. 2009, 110, 1617–1627. [Google Scholar] [CrossRef]
- Synchikova, A.P.; Korneva, E.A. Morphological and functional characteristics of LPS-stimulated microglial cells under the action of orexin A. Russ. J. Immunol. 2022, 25, 333–338. [Google Scholar] [CrossRef]
- Kiyota, T.; Ingraham, K.L.; Swan, R.J.; Jacobsen, M.T.; Andrews, S.J.; Ikezu, T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2011, 19, 724–733. [Google Scholar] [CrossRef]
- Van Boxel-Dezaire, A.H.H.; Hoff, S.C.J.; Van Oosten, B.W.; Verweij, C.L.; Dräger, A.M.; Adèr, H.J.; Van Houwelingen, J.C.; Barkhof, F.; Polman, C.H.; Nagelkerken, L. Decreased interleukin-10 and increased interleukin-12p40 mRNA are associated with disease activity and characterize different disease stages in multiple sclerosis. Ann. Neurol. 1999, 45, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Hesse, D.; Krakauer, M.; Lund, H.; Søndergaard, H.B.; Limborg, S.J.W.; Soelberg Sørensen, P.; Sellebjerg, F. Disease protection and interleukin-10 induction by endogenous interferon-β in multiple sclerosis? Eur. J. Neurol. 2011, 18, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, T.; Choi, D.-Y.; Lu, X.; Liu, M.; Nguyen, X.V.; Zheng, N.; Stewart, C.A.; Kim, H.-C.; Bing, G. Interleukin-10 protects against inflammation-mediated degeneration of dopaminergic neurons in substantia nigra. Neurobiol. Aging 2007, 28, 894–906. [Google Scholar] [CrossRef] [PubMed]
- George, A.; Kleinschnitz, C.; Zelenka, M.; Brinkhoff, J.; Stoll, G.; Sommer, C. Wallerian degeneration after crush or chronic constriction injury of rodent sciatic nerve is associated with a depletion of endoneurial interleukin-10 protein. Exp. Neurol. 2004, 188, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Garza, K.M.; Zhang, L.; Borron, B.; Wood, L.B.; Singer, A.C. Gamma Visual Stimulation Induces a Neuroimmune Signaling Profile Distinct from Acute Neuroinflammation. J. Neurosci. 2020, 40, 1211–1225. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Astakhova, A.A.; Azbukina, N.V.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G. Cellular Model of Endotoxin Tolerance in Astrocytes: Role of Interleukin 10 and Oxylipins. Cells 2019, 8, 1553. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Azbukina, N.V.; Astakhova, A.A.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G. Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes. Int. J. Mol. Sci. 2018, 19, 2793. [Google Scholar] [CrossRef]
- Park, C.; Lee, S.; Cho, I.H.; Lee, H.K.; Kim, D.; Choi, S.Y.; Oh, S.B.; Park, K.; Kim, J.S.; Lee, S.J. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: Differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia 2005, 53, 248–256. [Google Scholar] [CrossRef]
- Chakrabarty, P.; Li, A.; Ceballos-Diaz, C.; Eddy, J.A.; Funk, C.C.; Moore, B.; DiNunno, N.; Rosario, A.M.; Cruz, P.E.; Verbeeck, C.; et al. IL-10 Alters Immunoproteostasis in APP Mice, Increasing Plaque Burden and Worsening Cognitive Behavior. Neuron 2015, 85, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Guillot-Sestier, M.-V.; Doty, K.R.; Gate, D.; Rodriguez, J.; Leung, B.P.; Rezai-Zadeh, K.; Town, T. Il10 Deficiency Rebalances Innate Immunity to Mitigate Alzheimer-Like Pathology. Neuron 2015, 85, 534–548. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef]
- Ismail, R.; Hansen, A.K.; Parbo, P.; Brændgaard, H.; Gottrup, H.; Brooks, D.J.; Borghammer, P. The Effect of 40-Hz Light Therapy on Amyloid Load in Patients with Prodromal and Clinical Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2018, 2018, 6852303. [Google Scholar] [CrossRef]
- Notbohm, A.; Herrmann, C.S. Flicker Regularity Is Crucial for Entrainment of Alpha Oscillations. Front. Hum. Neurosci. 2016, 10, 503. [Google Scholar] [CrossRef]
- Notbohm, A.; Kurths, J.; Herrmann, C.S. Modification of Brain Oscillations via Rhythmic Light Stimulation Provides Evidence for Entrainment but Not for Superposition of Event-Related Responses. Front. Hum. Neurosci. 2016, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.S.; Lee, T.-l.; Hamblin, M.R.; Cheung, M.-c. Photobiomodulation Enhances Memory Processing in Older Adults with Mild Cognitive Impairment: A Functional Near-Infrared Spectroscopy Study. J. Alzheimer’s Dis. 2021, 83, 1471–1480. [Google Scholar] [CrossRef]
- Yao, D.; Horwitz, A.; Dyhr Thomsen, M.; Wiegand, I.; Horwitz, H.; Klemp, M.; Nikolic, M.; Rask, L.; Lauritzen, M.; Benedek, K. Visual steady state in relation to age and cognitive function. PLoS ONE 2017, 12, e0171859. [Google Scholar] [CrossRef]
- Schneck, M.E.; Haegerstrom-Portnoy, G.; Lott, L.A.; Brabyn, J.A. Comparison of Panel D-15 Tests in a Large Older Population. Optom. Vis. Sci. 2014, 91, 284–290. [Google Scholar] [CrossRef]
- Nguyen-Tri, D.; Overbury, O.; Faubert, J. The Role of Lenticular Senescence in Age-Related Color Vision Changes. Investig. Opthalmol. Vis. Sci. 2003, 44, 3698–3704. [Google Scholar] [CrossRef] [PubMed]
- Tsoneva, T.; Garcia-Molina, G.; Desain, P. Neural dynamics during repetitive visual stimulation. J. Neural Eng. 2015, 12, 066017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yue, X.; Luo, H.; Jiang, W.; Mei, Y.; Ai, L.; Gao, G.; Wu, Y.; Yang, H.; An, J.; et al. Illumination with 630 nm Red Light Reduces Oxidative Stress and Restores Memory by Photo-Activating Catalase and Formaldehyde Dehydrogenase in SAMP8 Mice. Antioxid. Redox Signal. 2019, 30, 1432–1449. [Google Scholar] [CrossRef]
- Megerian, J.; Hajos, M.; Hempel, E.; Cimenser, A.; Boasso, A.; Cotter, C.; Williams, M.; Kwan, K.; Nicodemus-Johnson, J.; Hendrix, S.; et al. Feasibility, Safety, and Efficacy of Gamma Sensory Stimulation as a Novel Therapeutic Intervention for Alzheimer’s Disease (N1.001). Neurology 2022, 98, 1936. [Google Scholar] [CrossRef]
- Shady, S.; MacLeod, D.I.A.; Fisher, H.S. Adaptation from invisible flicker. Proc. Natl. Acad. Sci. USA 2004, 101, 5170–5173. [Google Scholar] [CrossRef] [PubMed]
- Henney, M.A.; Carstensen, M.; Thorning-Schmidt, M.; Kubińska, M.; Grønberg, M.G.; Nguyen, M.; Madsen, K.H.; Clemmensen, L.K.H.; Petersen, P.M. Brain stimulation with 40 Hz heterochromatic flicker extended beyond red, green, and blue. Sci. Rep. 2024, 14, 2147. [Google Scholar] [CrossRef]
- Agger, M.P.; Carstensen, M.S.; Henney, M.A.; Hansen, L.S.; Baandrup, A.O.; Nguyen, M.; Petersen, P.M.; Madsen, K.H.; Kjær, T.W. Novel Invisible Spectral Flicker Induces 40 Hz Neural Entrainment with Similar Spatial Distribution as 40 Hz Stroboscopic Light. J. Alzheimer’s Dis. 2022, 88, 335–344. [Google Scholar] [CrossRef]
- Terman, M.; Terman, J.S. Light Therapy for Seasonal and Nonseasonal Depression: Efficacy, Protocol, Safety, and Side Effects. CNS Spectr. 2014, 10, 647–663. [Google Scholar] [CrossRef]
- Liu, C.-R.; Liou, Y.M.; Jou, J.-H. Pilot Study of the Effects of Bright Ambient Therapy on Dementia Symptoms and Cognitive Function. Front. Psychol. 2021, 12, 782160. [Google Scholar] [CrossRef]
- Hainke, L.; Dowsett, J.; Spitschan, M.; Priller, J. 40 Hz visual stimulation during sleep evokes neuronal gamma activity in NREM and REM stages. Sleep 2025, 48, zsae299. [Google Scholar] [CrossRef]
- Cordone, S.; Annarumma, L.; Rossini, P.M.; De Gennaro, L. Sleep and β-Amyloid Deposition in Alzheimer Disease: Insights on Mechanisms and Possible Innovative Treatments. Front. Pharmacol. 2019, 10, 695. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y.; Thiyagarajan, M.; O’Donnell, J.; Christensen, D.J.; Nicholson, C.; Iliff, J.J.; et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Fedosov, I.; Penzel, T.; Li, D.; Yu, T.; Telnova, V.; Kaybeleva, E.; Saranceva, E.; Terskov, A.; Khorovodov, A.; et al. Brain Waste Removal System and Sleep: Photobiomodulation as an Innovative Strategy for Night Therapy of Brain Diseases. Int. J. Mol. Sci. 2023, 24, 3221. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.; Yamakawa, G.R.; Shultz, S.R.; Mychasiuk, R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? Examining the implications and uncertainties. Prog. Neurobiol. 2021, 198, 101917. [Google Scholar] [CrossRef] [PubMed]
- Terskov, A.; Evsukova, A.; Blokhina, I.; Tzoy, M.; Zlatogorskaya, D.; Adushkina, V. Photo-sleep therapy of Alzheimer’s disease. Eur. Phys. J. Spec. Top. 2024, 233, 685–690. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Penzel, T.; Poluektov, M.; Fedosov, I.; Tzoy, M.; Terskov, A.; Blokhina, I.; Sidorov, V.; Kurths, J. Phototherapy of Alzheimer’s Disease: Photostimulation of Brain Lymphatics during Sleep: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 10946. [Google Scholar] [CrossRef] [PubMed]
- Lim, L. Modifying Alzheimer’s disease pathophysiology with photobiomodulation: Model, evidence, and future with EEG-guided intervention. Front. Neurol. 2024, 15, 1407785. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Du, W.; Jiang, J.; Han, Y.; Zhu, L.-Q. Brain Photobiomodulation Improves Sleep Quality in Subjective Cognitive Decline: A Randomized, Sham-Controlled Study. J. Alzheimer’s Dis. 2022, 87, 1581–1589. [Google Scholar] [CrossRef]
- Mitrofanis, J.; Valverde, A.; Hamilton, C.; Moro, C.; Billeres, M.; Magistretti, P. Lights at night: Does photobiomodulation improve sleep? Neural Regen. Res. 2023, 18, 474–477. [Google Scholar] [CrossRef] [PubMed]
- van Hattem, T.; Verkaar, L.; Krugliakova, E.; Adelhöfer, N.; Zeising, M.; Drinkenburg, W.H.I.M.; Claassen, J.A.H.R.; Bódizs, R.; Dresler, M.; Rosenblum, Y. Targeting Sleep Physiology to Modulate Glymphatic Brain Clearance. Physiology 2025, 40, 271–290. [Google Scholar] [CrossRef]
- Murphy, M.; Öngür, D. Decreased peak alpha frequency and impaired visual evoked potentials in first episode psychosis. NeuroImage Clin. 2019, 22, 101693. [Google Scholar] [CrossRef] [PubMed]
- Khuwaja, G.A.; Haghighi, S.J.; Hatzinakos, D. 40-Hz ASSR fusion classification system for observing sleep patterns. EURASIP J. Bioinform. Syst. Biol. 2015, 2015, 2. [Google Scholar] [CrossRef] [PubMed]
- Lustenberger, C.; Patel, Y.A.; Alagapan, S.; Page, J.M.; Price, B.; Boyle, M.R.; Fröhlich, F. High-density EEG characterization of brain responses to auditory rhythmic stimuli during wakefulness and NREM sleep. NeuroImage 2018, 169, 57–68. [Google Scholar] [CrossRef]
- Zhou, X.; He, Y.; Xu, T.; Wu, Z.; Guo, W.; Xu, X.; Liu, Y.; Zhang, Y.; Shang, H.; Huang, L.; et al. 40 Hz light flickering promotes sleep through cortical adenosine signaling. Cell Res. 2024, 34, 214–231. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Liu, S.; Liang, T.; Wu, Y.; Wang, X.; Li, Y.; Xu, Y. Study on Gamma sensory flicker for Insomnia. Int. J. Neurosci. 2024, 135, 1023–1033. [Google Scholar] [CrossRef]
- Gross, D.W.; Gotman, J. Correlation of high-frequency oscillations with the sleep–wake cycle and cognitive activity in humans. Neuroscience 1999, 94, 1005–1018. [Google Scholar] [CrossRef]
- Park, S.-S.; Park, H.-S.; Kim, C.-J.; Baek, S.-S.; Park, S.-Y.; Anderson, C.; Kim, M.-K.; Park, I.-R.; Kim, T.-W. Combined effects of aerobic exercise and 40-Hz light flicker exposure on early cognitive impairments in Alzheimer’s disease of 3×Tg mice. J. Appl. Physiol. 2022, 132, 1054–1068. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef]
- Han, C.; Zhao, X.; Li, M.; Haihambo, N.; Teng, J.; Li, S.; Qiu, J.; Feng, X.; Gao, M. Enhancement of the neural response during 40 Hz auditory entrainment in closed-eye state in human prefrontal region. Cogn. Neurodyn. 2022, 17, 399–410. [Google Scholar] [CrossRef]
- Perry, G.; Cash, A.D.; Smith, M.A. Alzheimer Disease and Oxidative Stress. BioMed Res. Int. 2002, 2, 120–123. [Google Scholar] [CrossRef]
- Schubert, D. Glucose metabolism and Alzheimer’s disease. Ageing Res. Rev. 2005, 4, 240–257. [Google Scholar] [CrossRef] [PubMed]
- Hajós, M.; Boasso, A.; Hempel, E.; Shpokayte, M.; Konisky, A.; Seshagiri, C.V.; Fomenko, V.; Kwan, K.; Nicodemus-Johnson, J.; Hendrix, S.; et al. Safety, tolerability, and efficacy estimate of evoked gamma oscillation in mild to moderate Alzheimer’s disease. Front. Neurol. 2024, 15, 1343588. [Google Scholar] [CrossRef] [PubMed]
- Deuschl, G.; Schade-Brittinger, C.; Krack, P.; Volkmann, J.; Schäfer, H.; Bötzel, K.; Daniels, C.; Deutschländer, A.; Dillmann, U.; Eisner, W.; et al. A Randomized Trial of Deep-Brain Stimulation for Parkinson’s Disease. N. Engl. J. Med. 2006, 355, 896–908. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, M.; Bange, M.; Koirala, N.; Ciolac, D.; Pintea, B.; Glaser, M.; Tinkhauser, G.; Brown, P.; Deuschl, G.; Groppa, S. Cross-frequency coupling between gamma oscillations and deep brain stimulation frequency in Parkinson’s disease. Brain 2020, 143, 3393–3407. [Google Scholar] [CrossRef]
- Okun, M.S.; Fernandez, H.H.; Wu, S.S.; Kirsch-Darrow, L.; Bowers, D.; Bova, F.; Suelter, M.; Jacobson, C.E.; Wang, X.; Gordon, C.W.; et al. Cognition and mood in Parkinson’s disease in subthalamic nucleus versus globus pallidus interna deep brain stimulation: The COMPARE Trial. Ann. Neurol. 2009, 65, 586–595. [Google Scholar] [CrossRef]
- Baptista, M.A.T.; Kimura, N.; Lacerda, I.B.; Silva, F.d.O.; Dourado, M.C.N.; Caramelli, P. Domains of Awareness in Young and Late Onset Dementia. J. Alzheimer’s Dis. 2021, 81, 169–178. [Google Scholar] [CrossRef]
- Saltmarche, A.E.; Naeser, M.A.; Ho, K.F.; Hamblin, M.R.; Lim, L. Significant Improvement in Cognition in Mild to Moderately Severe Dementia Cases Treated with Transcranial Plus Intranasal Photobiomodulation: Case Series Report. Photomed. Laser Surg. 2017, 35, 432–441. [Google Scholar] [CrossRef]
- Chao, L.L. Effects of Home Photobiomodulation Treatments on Cognitive and Behavioral Function, Cerebral Perfusion, and Resting-State Functional Connectivity in Patients with Dementia: A Pilot Trial. Photobiomodul. Photomed. Laser Surg. 2019, 37, 133–141. [Google Scholar] [CrossRef]
- Ewbank, M.P.; Andrews, T.J. Differential sensitivity for viewpoint between familiar and unfamiliar faces in human visual cortex. NeuroImage 2008, 40, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.; Colella, D.; Giangrosso, M.; Cannavacciuolo, A.; Paparella, G.; Fabbrini, G.; Suppa, A.; Berardelli, A.; Bologna, M. Driving motor cortex oscillations modulates bradykinesia in Parkinson’s disease. Brain 2022, 145, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Senefeld, J.W.; Hunter, S.K.; Coleman, D.; Joyner, M.J. Case Studies in Physiology: Male to female transgender swimmer in college athletics. J. Appl. Physiol. 2023, 134, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
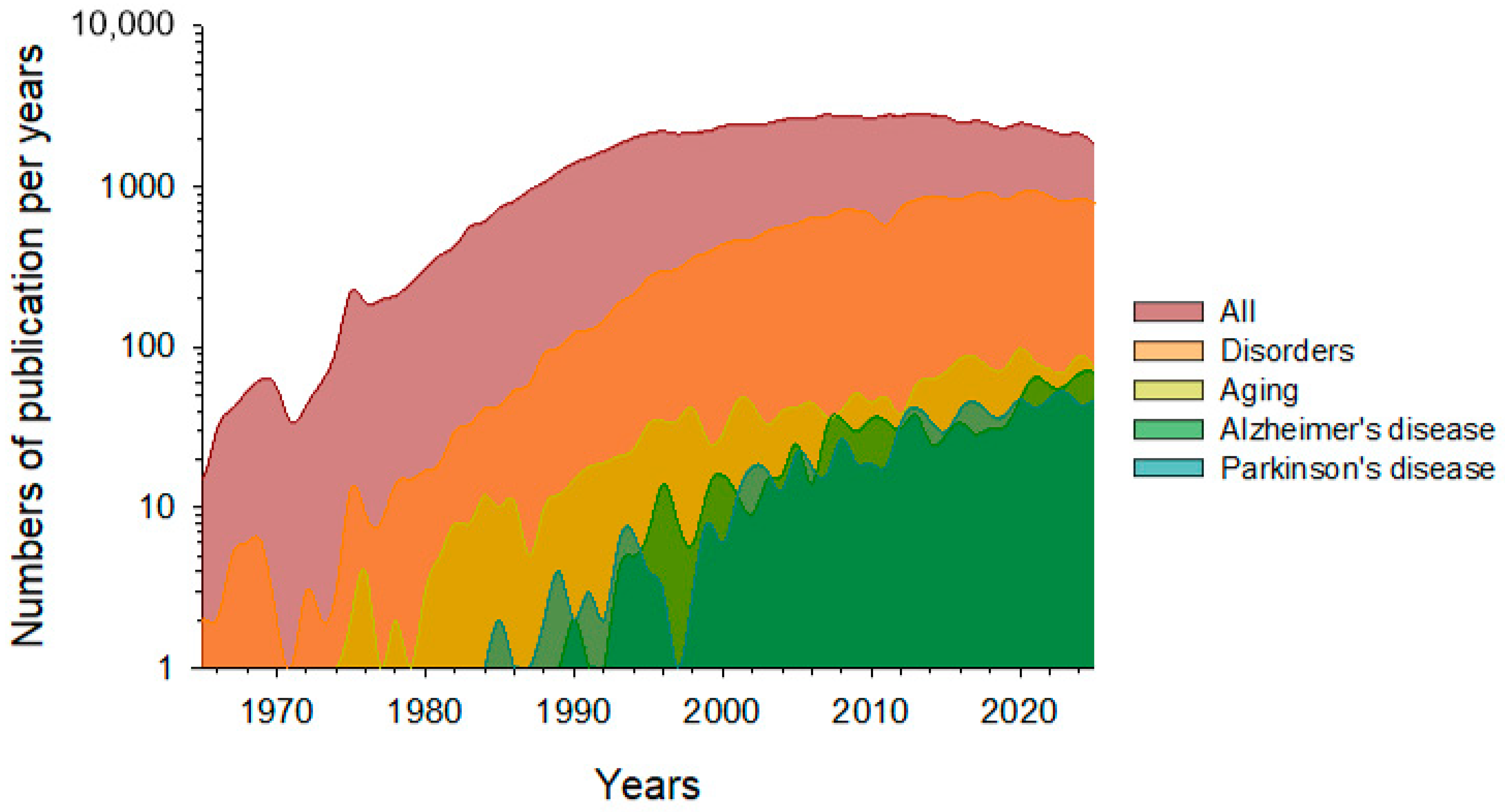
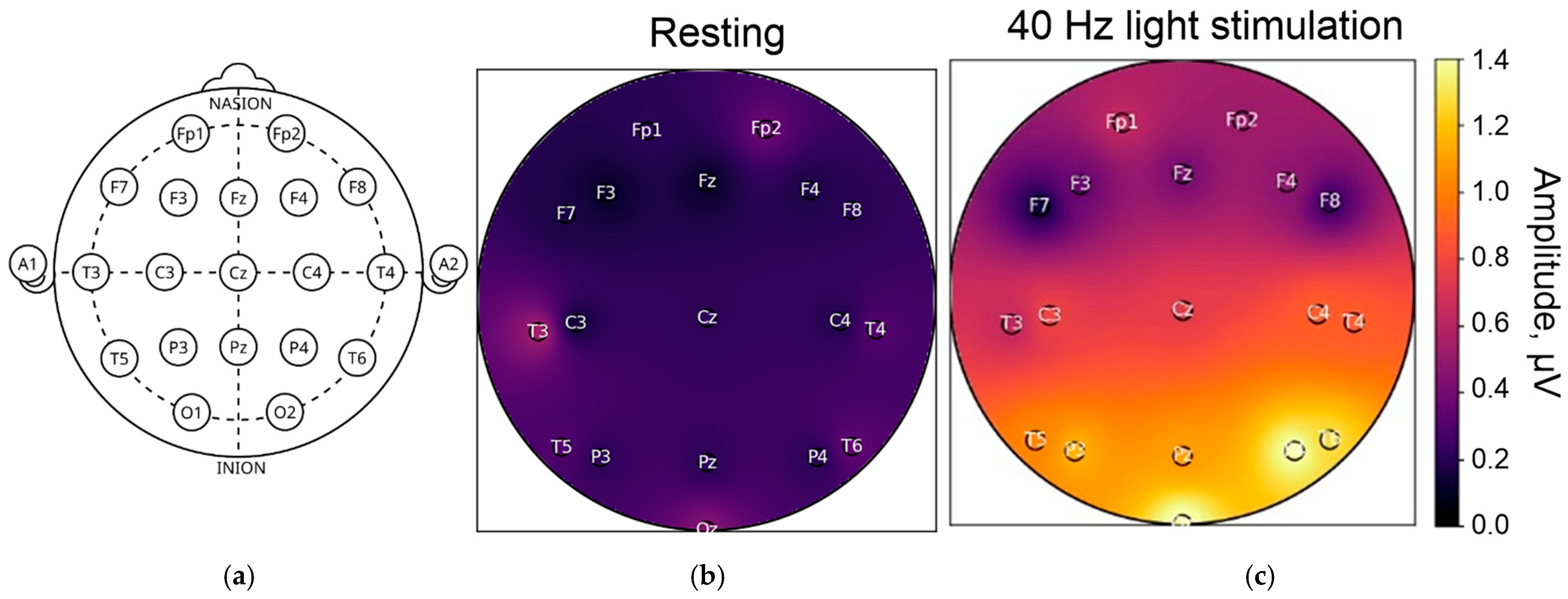
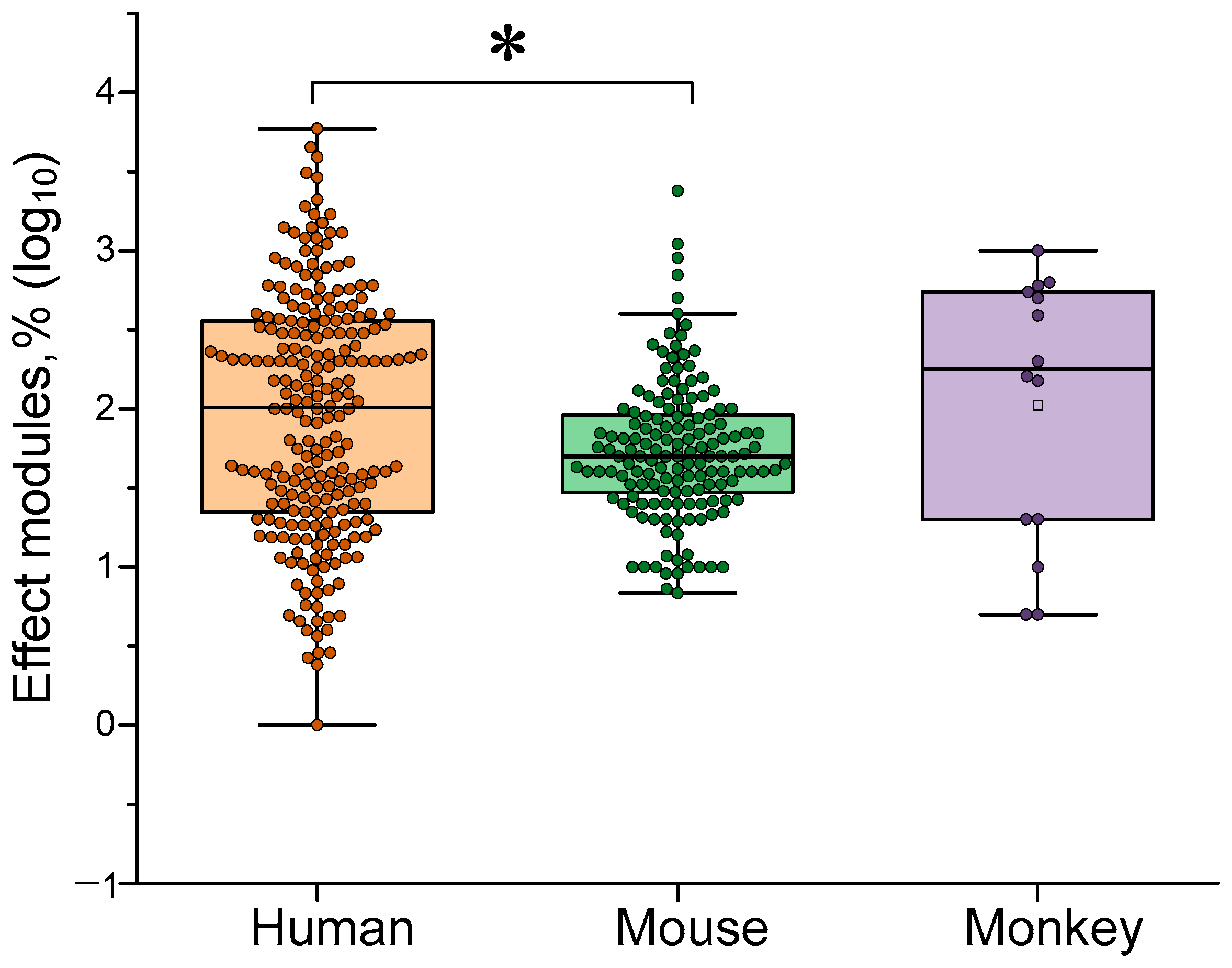
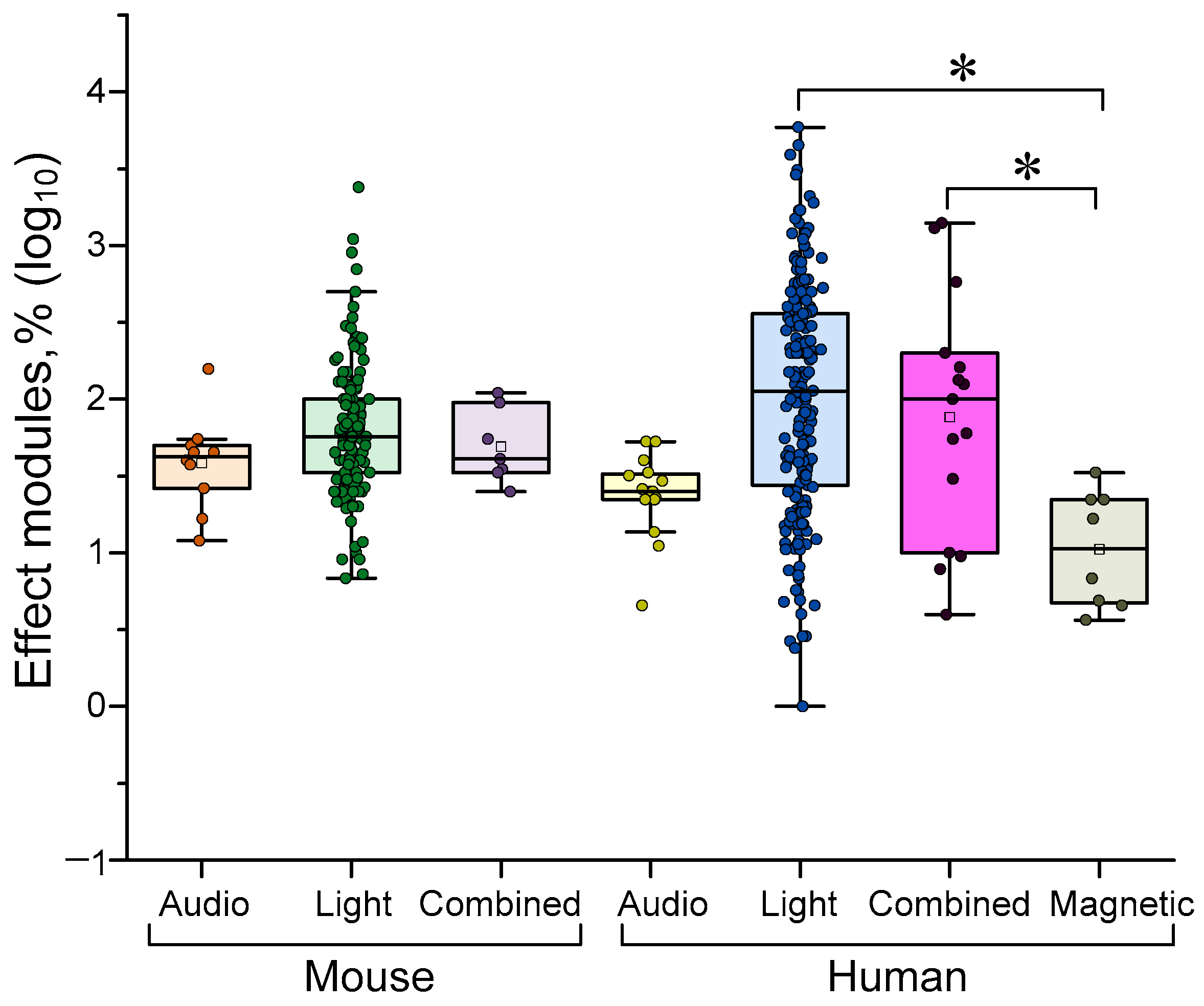
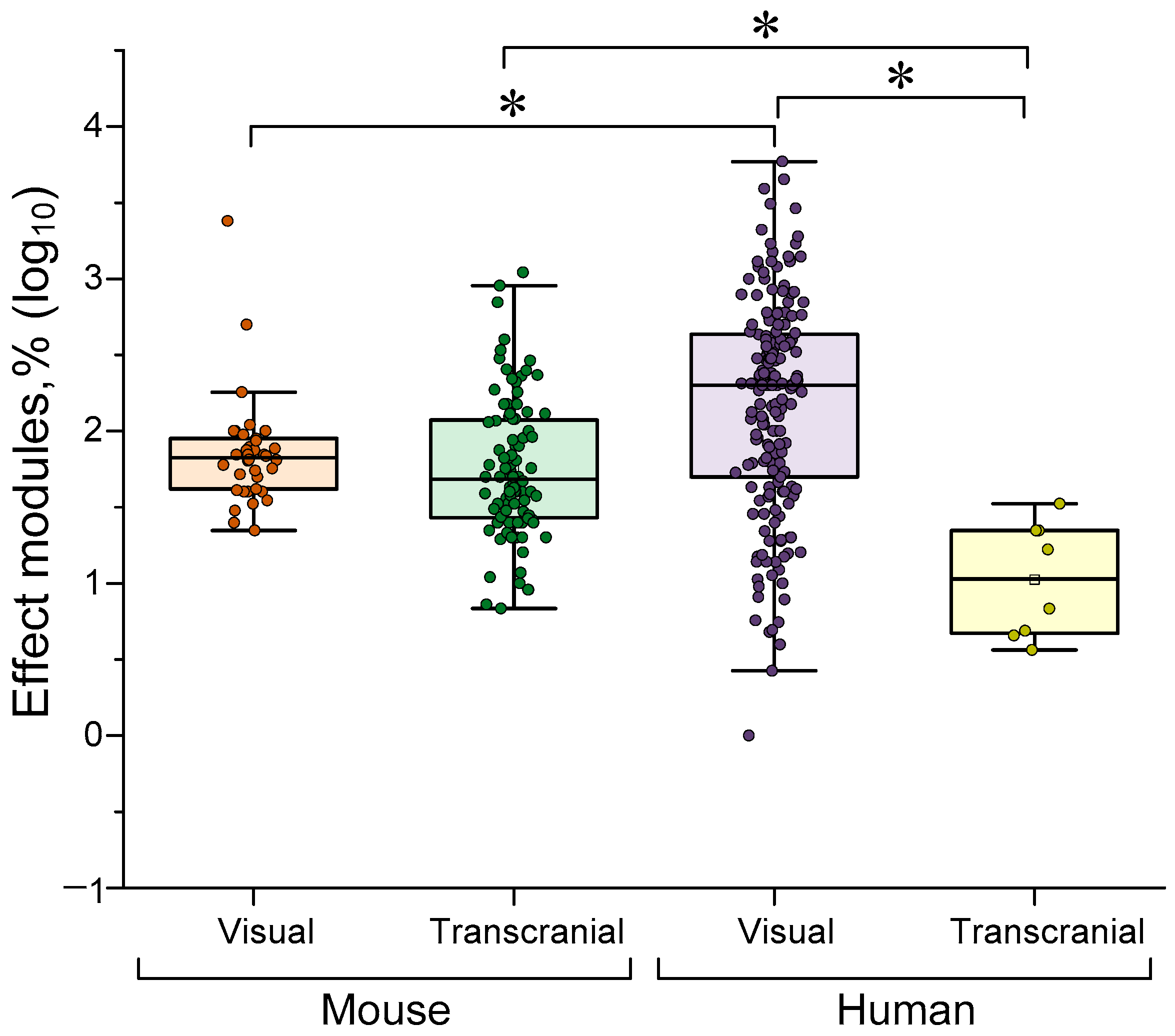
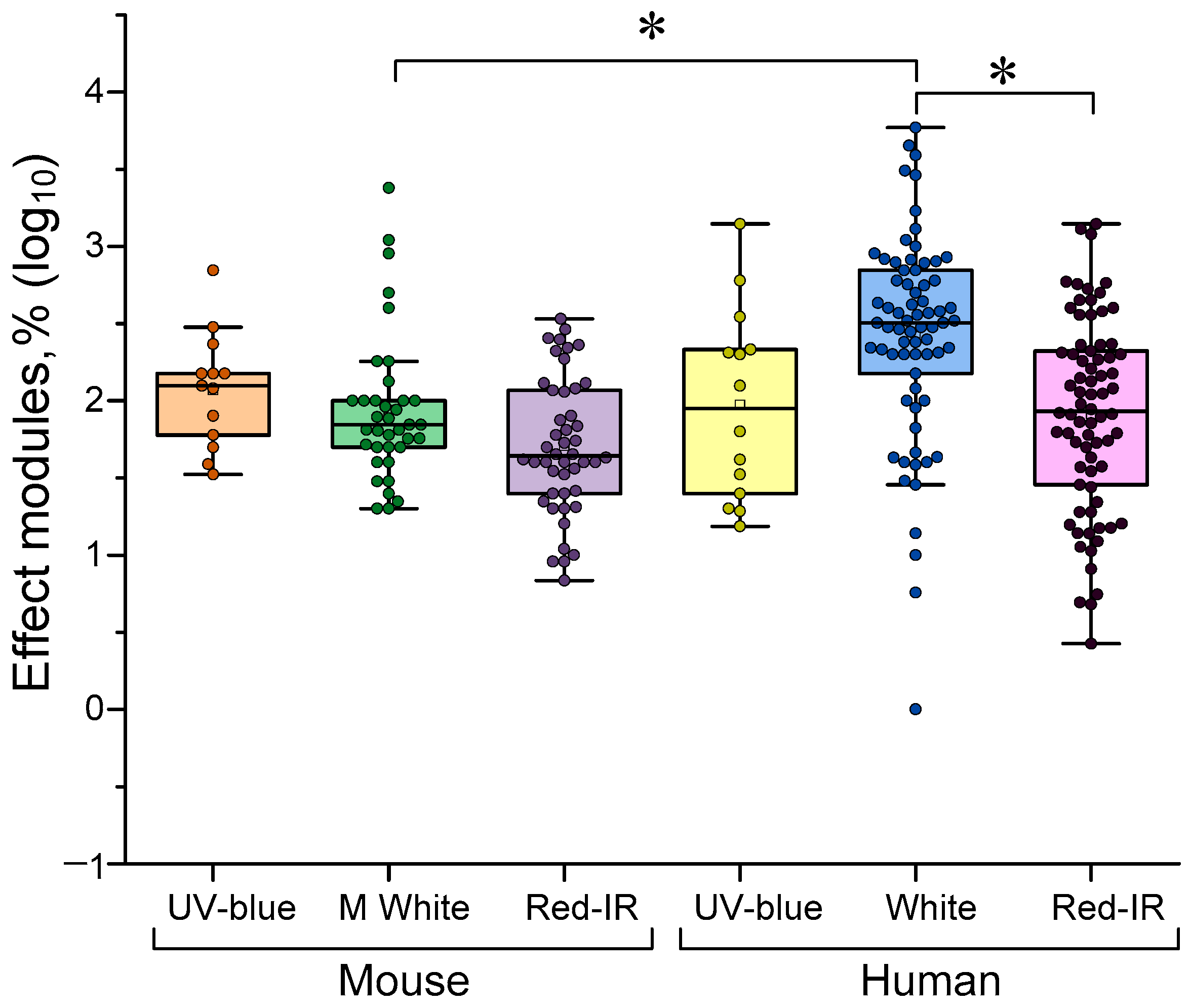
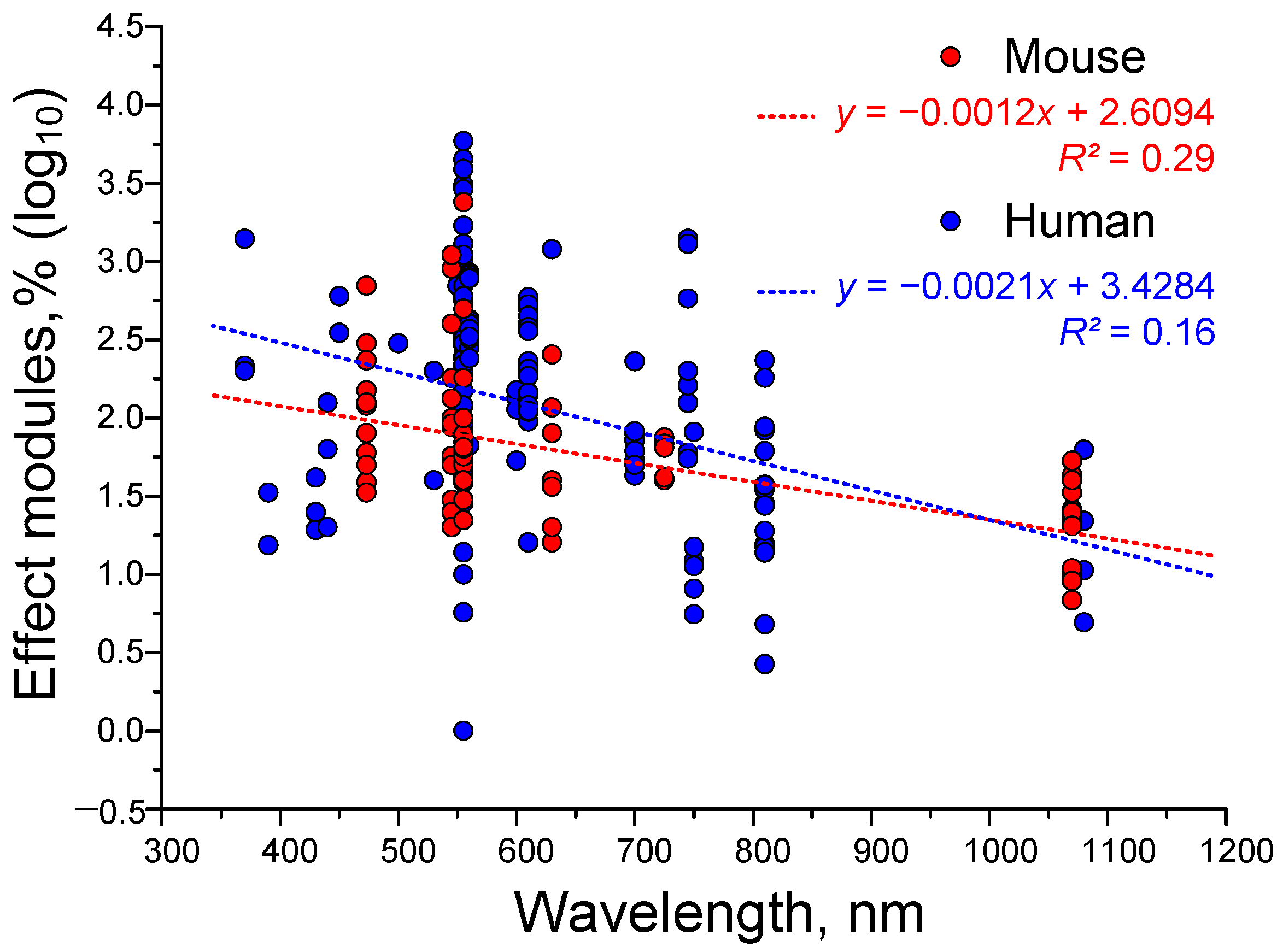
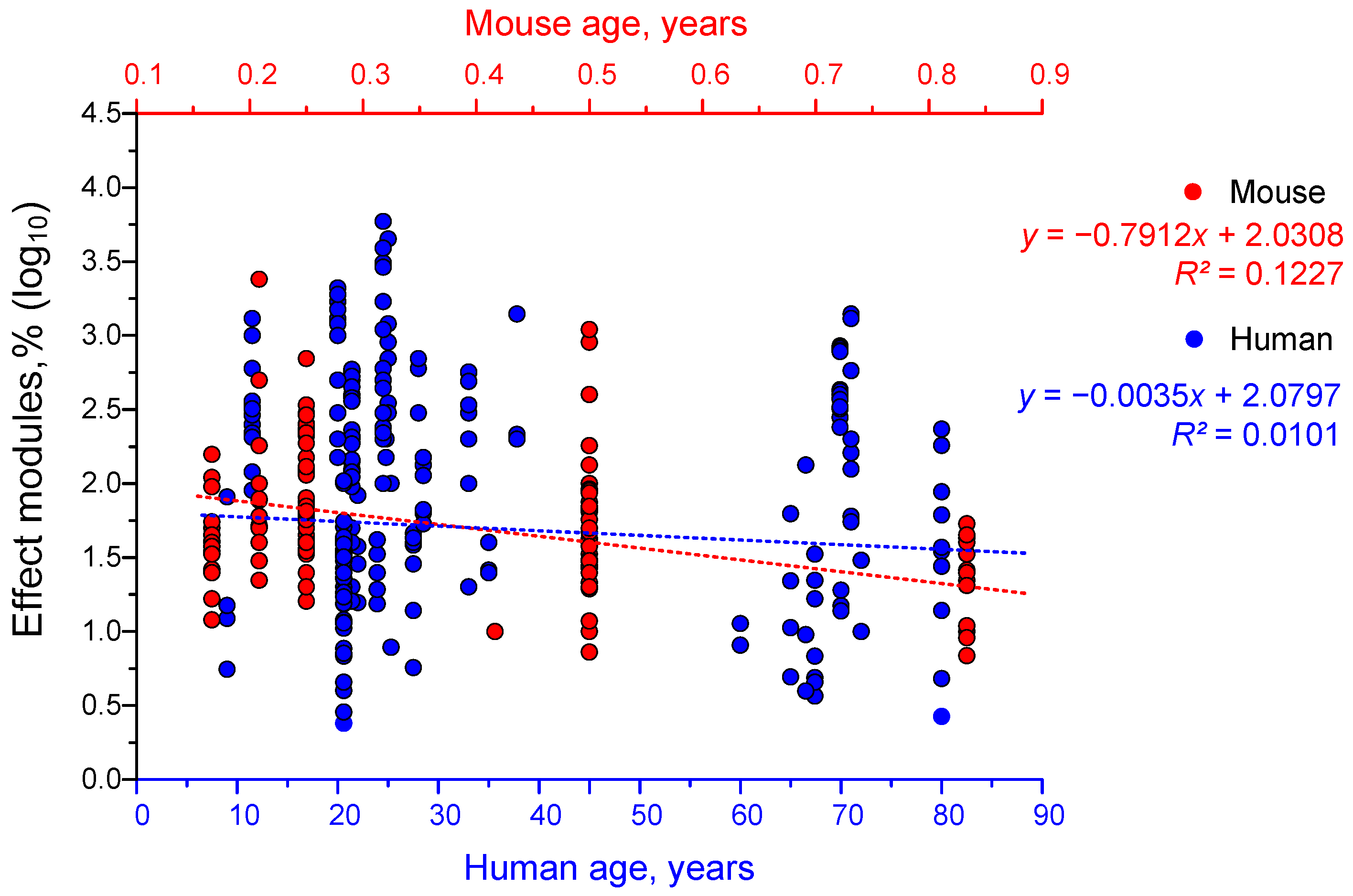
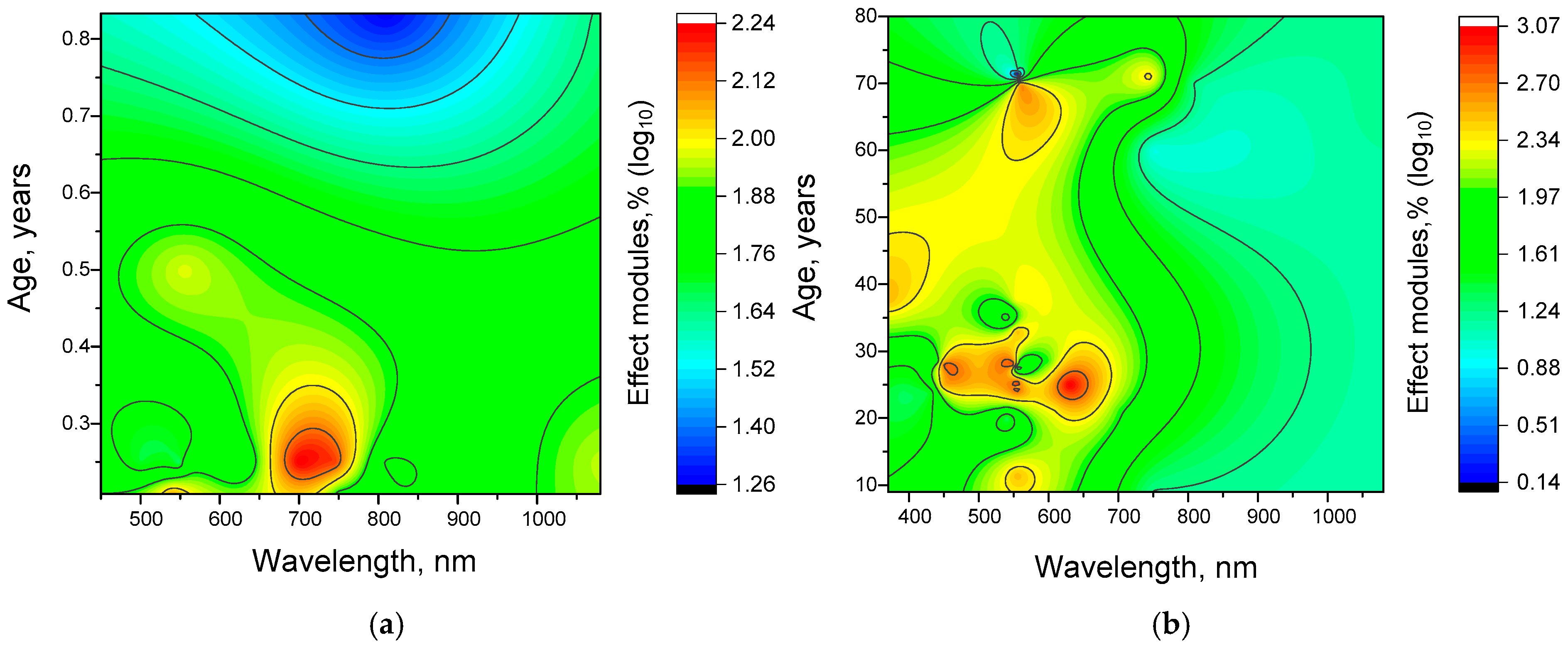
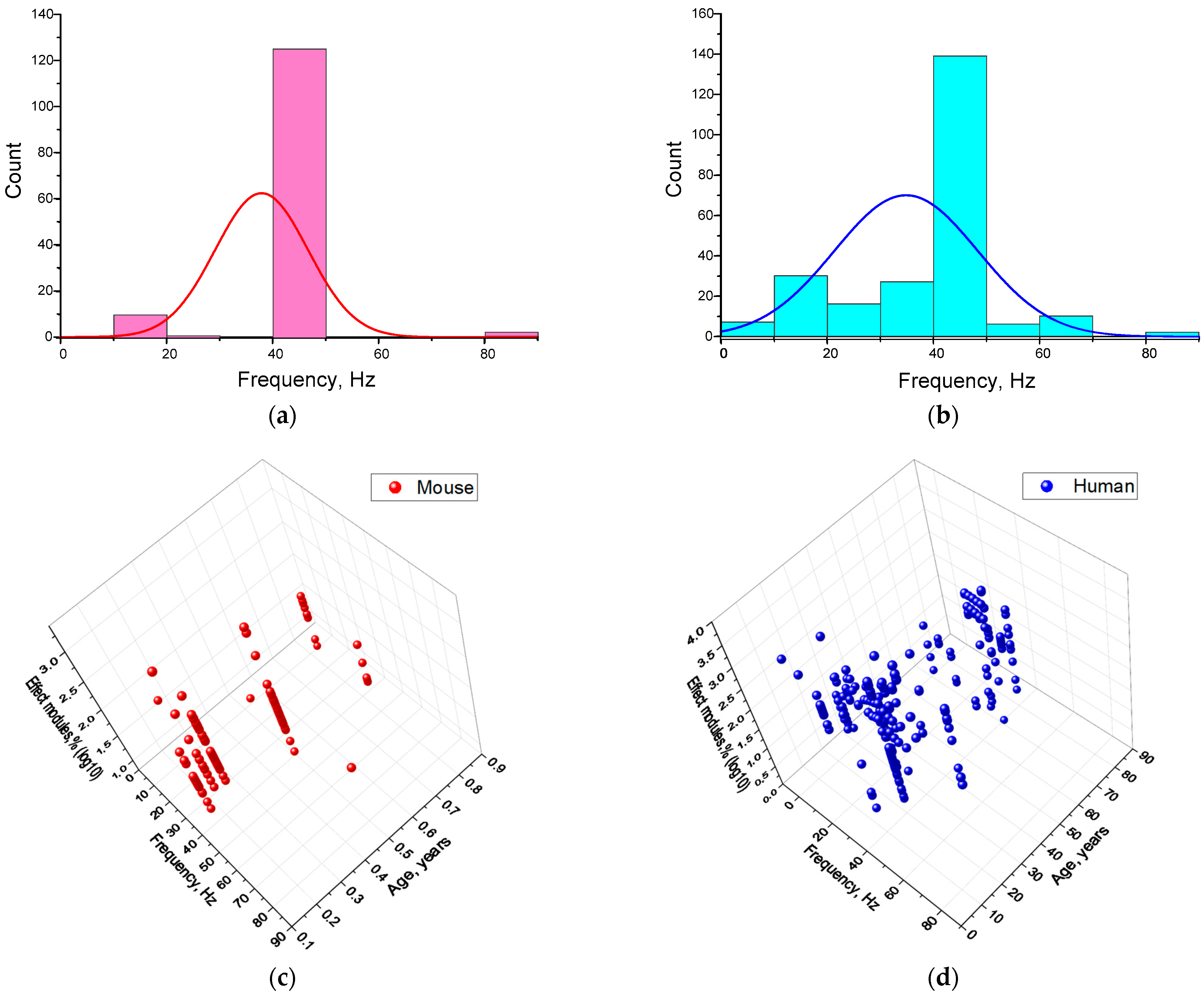
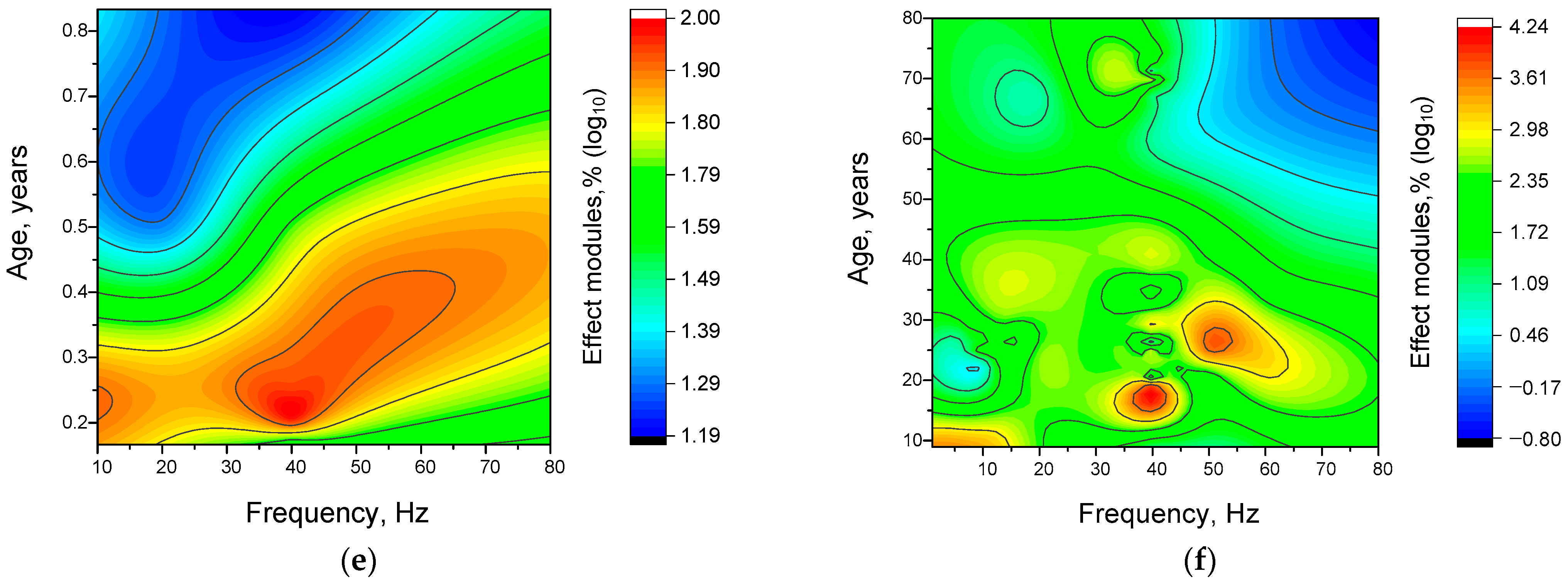
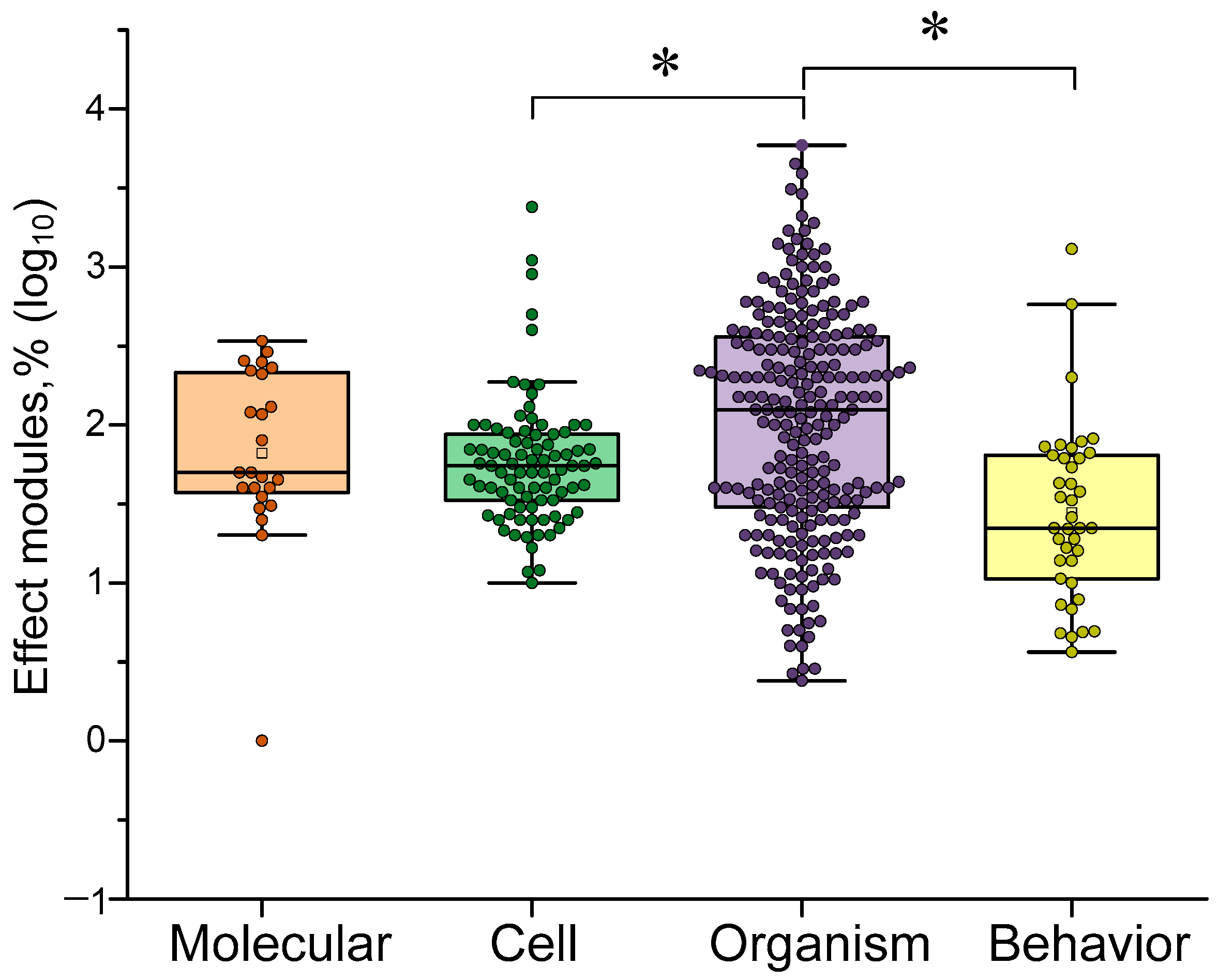
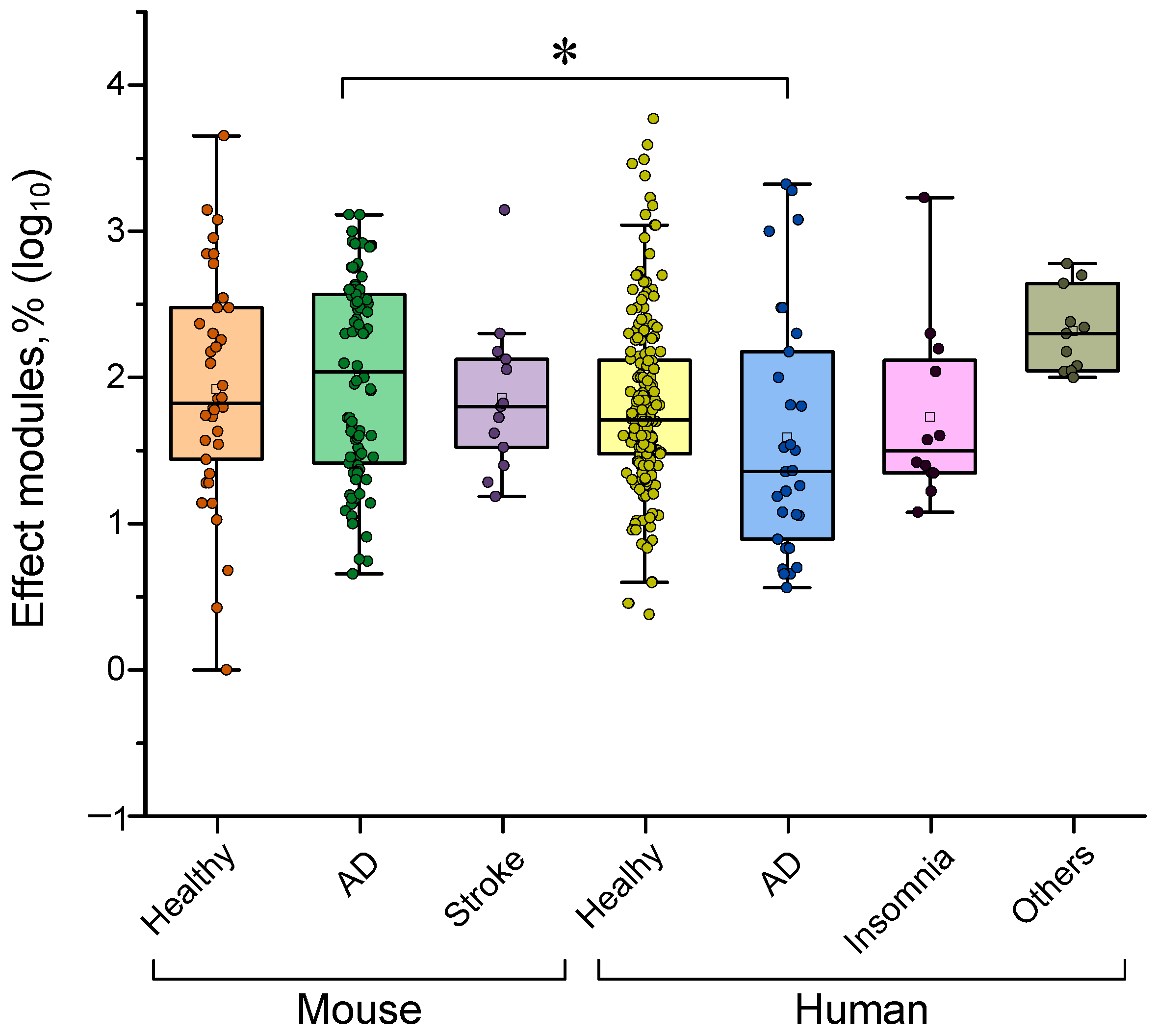
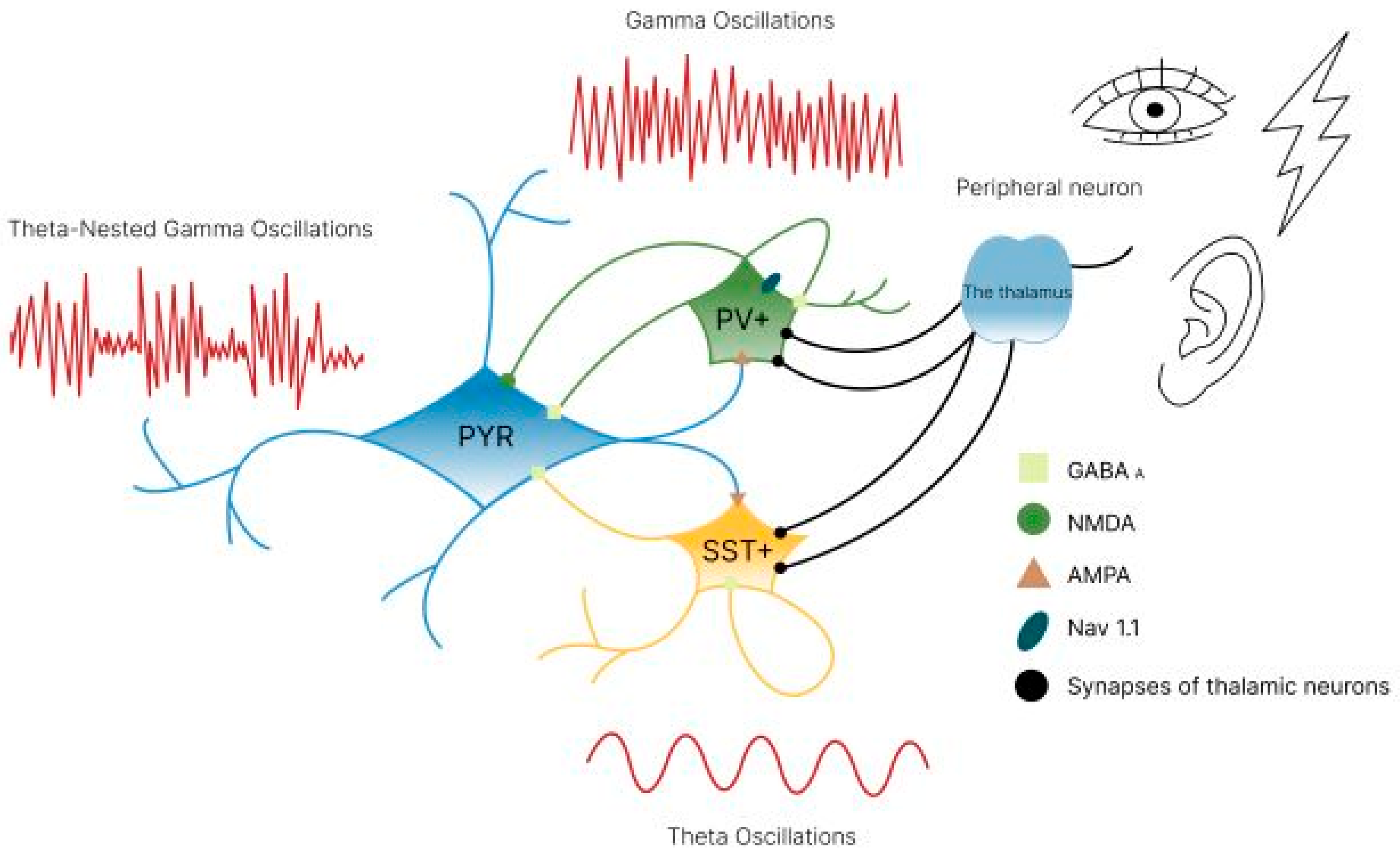
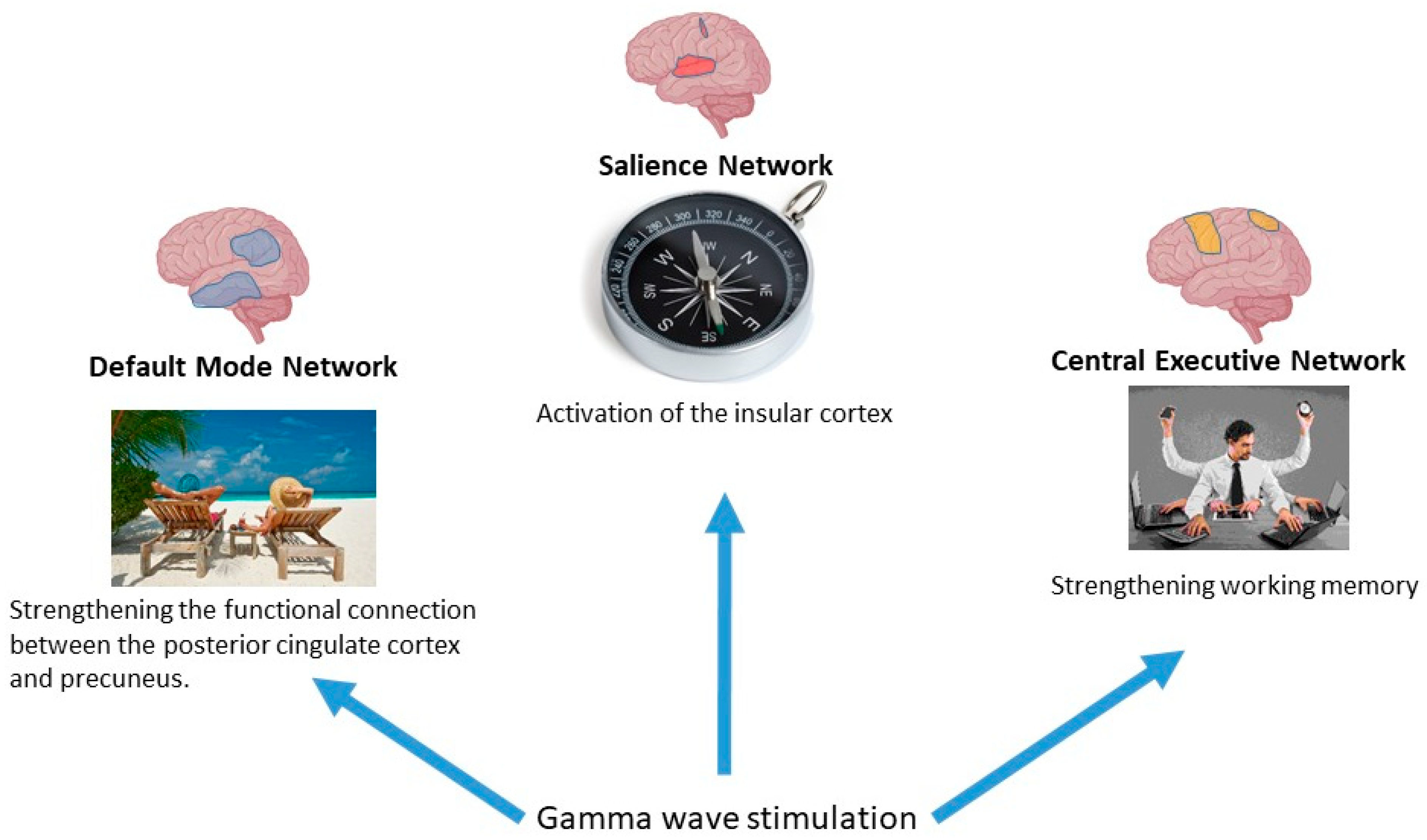
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lushnikov, K.V.; Serov, D.A.; Astashev, M.E.; Kozlov, V.A.; Melerzanov, A.; Vedunova, M.V. Brain Gamma-Stimulation: Mechanisms and Optimization of Impact. Biology 2025, 14, 1722. https://doi.org/10.3390/biology14121722
Lushnikov KV, Serov DA, Astashev ME, Kozlov VA, Melerzanov A, Vedunova MV. Brain Gamma-Stimulation: Mechanisms and Optimization of Impact. Biology. 2025; 14(12):1722. https://doi.org/10.3390/biology14121722
Chicago/Turabian StyleLushnikov, Konstantin V., Dmitriy A. Serov, Maxim E. Astashev, Valeriy A. Kozlov, Alexander Melerzanov, and Maria V. Vedunova. 2025. "Brain Gamma-Stimulation: Mechanisms and Optimization of Impact" Biology 14, no. 12: 1722. https://doi.org/10.3390/biology14121722
APA StyleLushnikov, K. V., Serov, D. A., Astashev, M. E., Kozlov, V. A., Melerzanov, A., & Vedunova, M. V. (2025). Brain Gamma-Stimulation: Mechanisms and Optimization of Impact. Biology, 14(12), 1722. https://doi.org/10.3390/biology14121722







