Soy Isoflavones Mitigate High-Fat Diet-Induced Oxidative Stress and Inflammation in the Gut of Monopterus albus via Gut Microbiota Remodeling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Feeding Management
2.3. Ethic Statement
2.4. Sampling
2.5. Determination of MDA Content and Enzyme Activity
2.6. Intestine Microscope Observations and Image Fitting
2.7. Gene Expression Detection
2.8. Amplicon Sequencing and Microbial Community Profiling
2.9. Functional Prediction of Microbial Metabolism
2.10. General Statistical Analyses
3. Results
3.1. Intestinal Histomorphology Observation and Tight Juction-Related Gene Expression Patterns
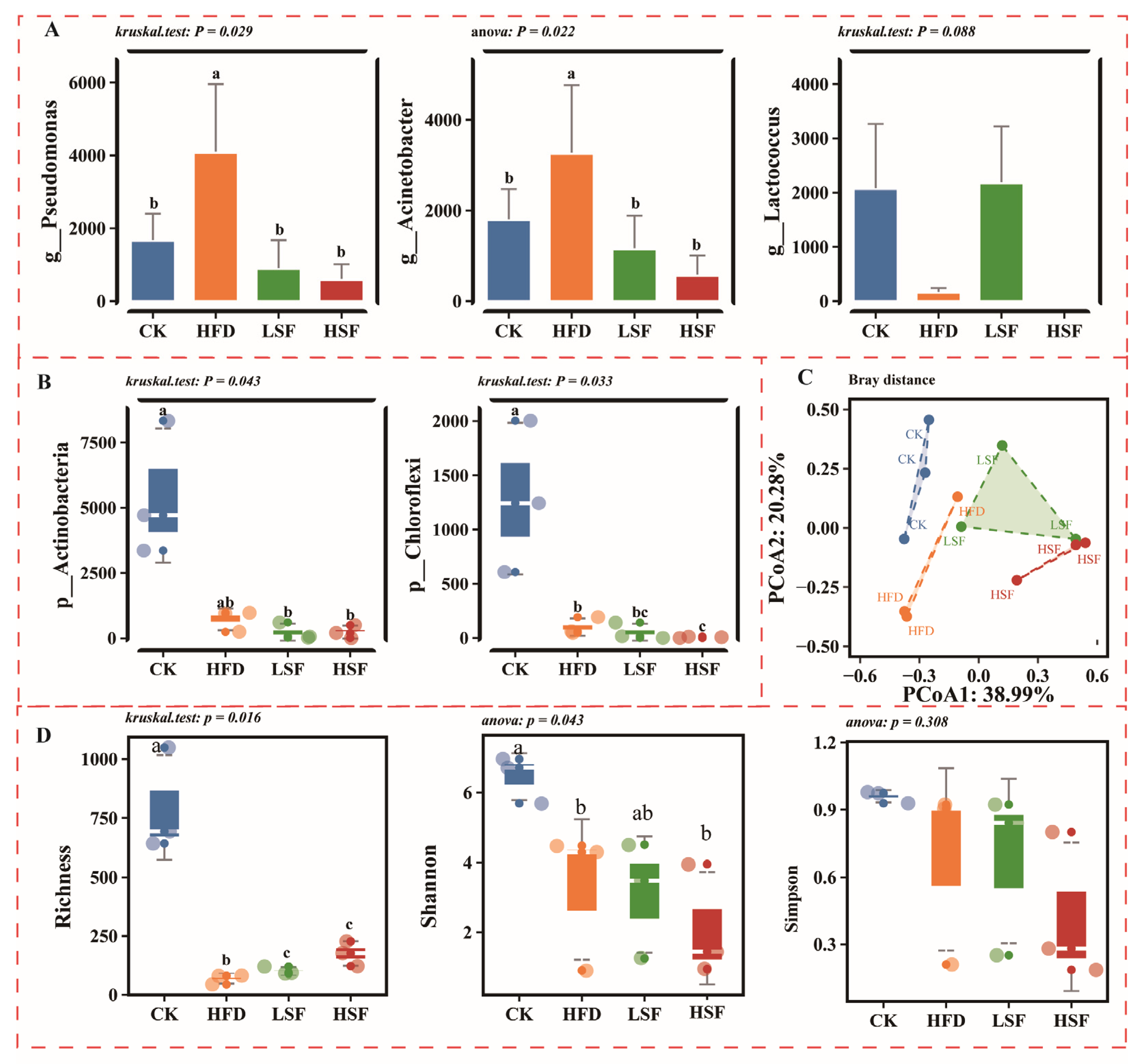
3.2. Intestinal Antioxidant-Related Indicators and Gene Expression Patterns
3.3. Intestinal Inflammation-Related Indicators and Gene Expression Patterns
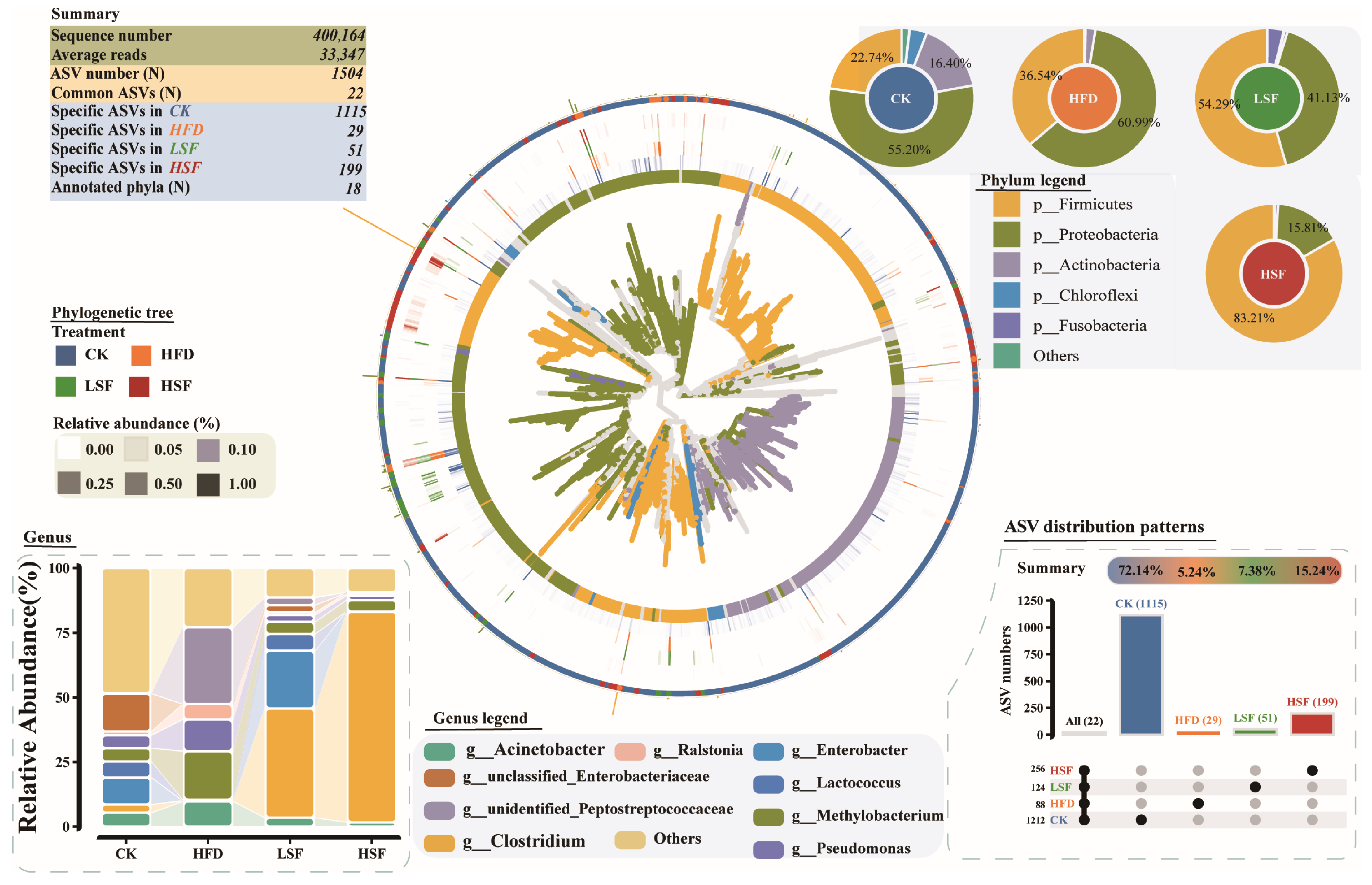
3.4. Gut Microbiota Alterations
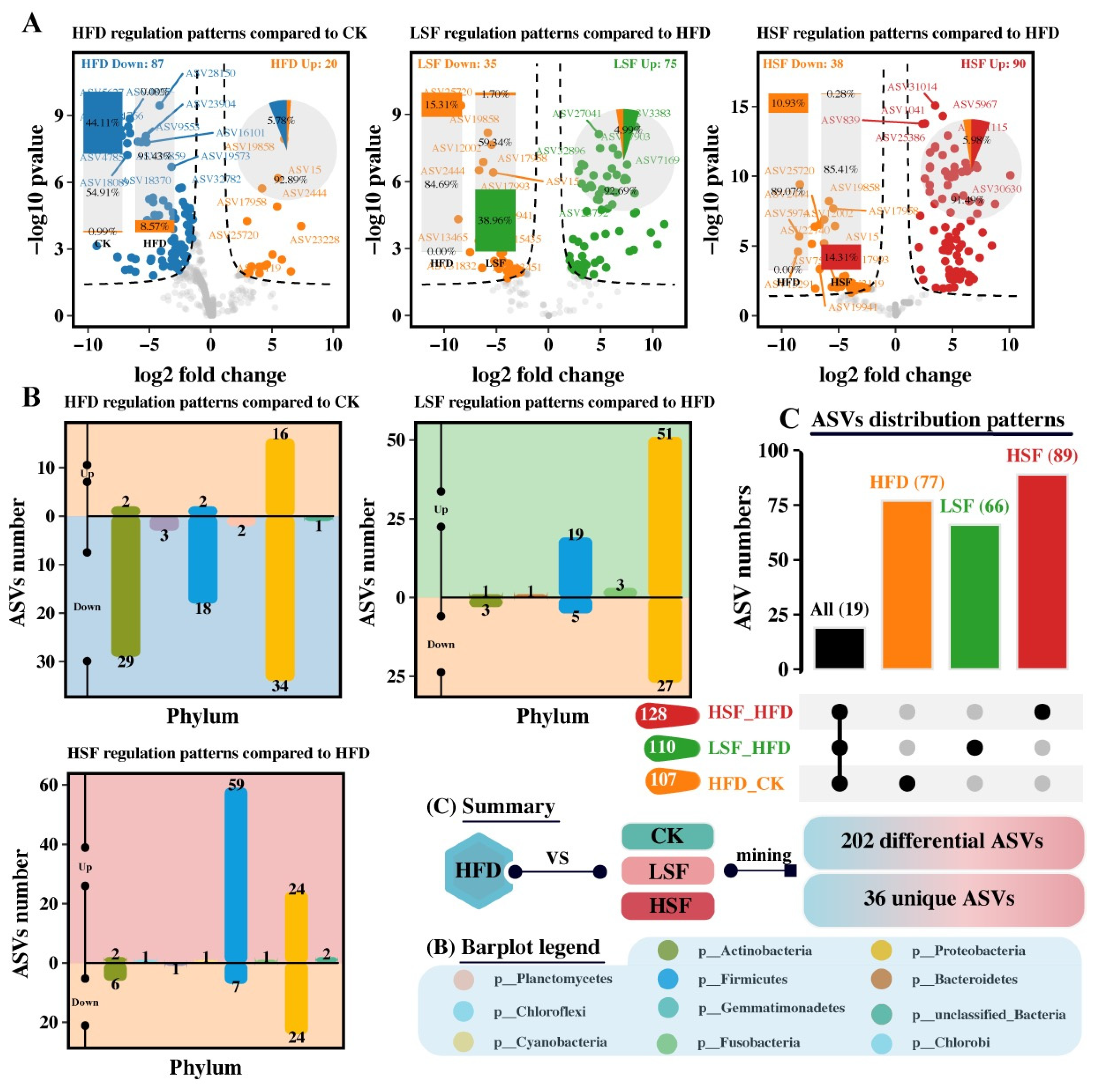
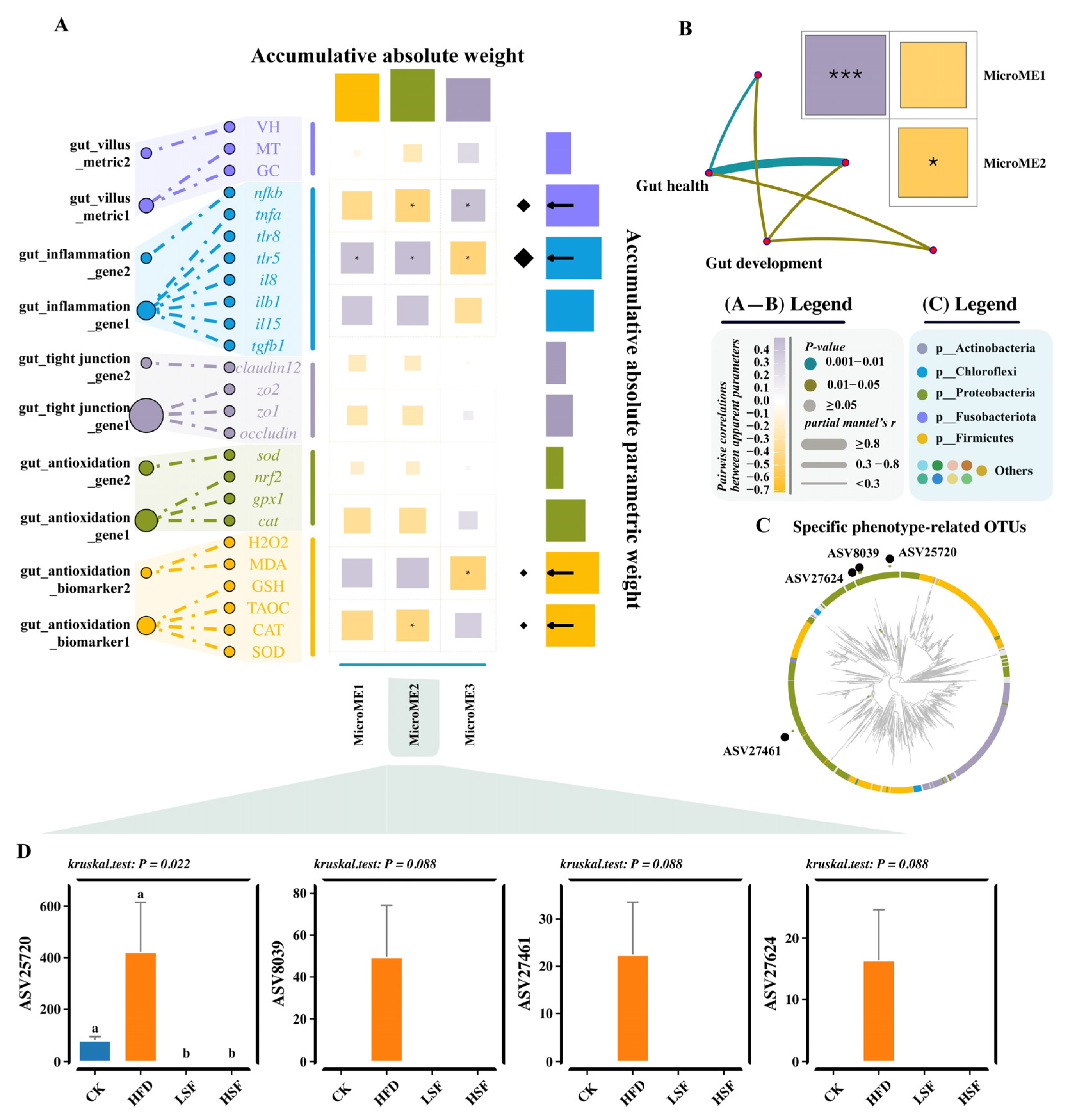
3.5. Differential Analysis and Specific-Phenotypes Biomarkers Mining
3.6. Molecular Ecological Network Analyses
3.7. Functional Annotation Prediction
4. Discussion
5. Limitations and Future Perspectives
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Begho, T.; Irabor, A.E. Fish feed formulation: Does Nigerian farmers’ risk and time preference play a part in choosing feed protein sources for intensively farmed fish? Aquaculture 2024, 585, 740723. [Google Scholar] [CrossRef]
- Kikuchi, K.; Furuta, T.; Iwata, N.; Onuki, K.; Noguchi, T. Effect of dietary lipid levels on the growth, feed utilization, body composition and blood characteristics of tiger puffer Takifugu rubripes. Aquaculture 2009, 298, 111–117. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, M.; Liu, M.; Zhang, W.; Zhi, S.; Qu, L.; Xiong, J.; Wang, L.; Qin, C.; Nie, G. Effects of Genistein on Lipid Metabolism, Antioxidant Activity, and Immunity of Common Carp (Cyprinus carpio L.) Fed with High-Carbohydrate and High-Fat Diets. Aquac. Nutr. 2023, 2023, 9555855. [Google Scholar] [CrossRef]
- Han, S.-L.; Wang, J.; Li, L.-Y.; Lu, D.-L.; Chen, L.-Q.; Zhang, M.-L.; Du, Z.-Y. The regulation of rapamycin on nutrient metabolism in Nile tilapia fed with high-energy diet. Aquaculture 2020, 520, 734975. [Google Scholar] [CrossRef]
- Lin, Z.; Bu, X.; Wang, N.; Lei, Y.; Liu, S.; Wang, X.; Gu, Z.; Du, Z.; Qin, J.; Chen, L. Dietary phospholipid alleviates the adverse effects of high-lipid diet in Chinese mitten crab (Eriocheir sinensis). Aquaculture 2021, 531, 735899. [Google Scholar] [CrossRef]
- Wang, X.; Gong, Y.; Li, W.; Liu, N.; Fang, Z.; Zhang, N.; Chen, N.; Li, S. The beneficial effects of metformin inclusion on growth performance, glucose utilization, antioxidant capacity and apoptosis of largemouth bass (Micropterus salmoides) fed with high dietary carbohydrates. Aquaculture 2024, 588, 740957. [Google Scholar] [CrossRef]
- Welengane, E.; Sado, R.Y.; Bicudo, Á.J.d.A. Protein-sparing effect by dietary lipid increase in juveniles of the hybrid fish tambatinga (♀Colossoma macropomum × ♂Piaractus brachypomus). Aquac. Nutr. 2019, 25, 1272–1280. [Google Scholar] [CrossRef]
- Skalli, A.; Hidalgo, M.C.; Abellán, E.; Arizcun, M.; Cardenete, G. Effects of the dietary protein/lipid ratio on growth and nutrient utilization in common dentex (Dentex dentex L.) at different growth stages. Aquaculture 2004, 235, 1–11. [Google Scholar] [CrossRef]
- Peng, M.; Xue, J.; Hu, Y.; Wen, C.; Hu, B.; Jian, S.; Liang, L.; Yang, G. Disturbance in the homeostasis of intestinal microbiota by a high-fat diet in the rice field eel (Monopterus albus). Aquaculture 2019, 502, 347–355. [Google Scholar] [CrossRef]
- Das, R.; Sahu, N.P.; Sardar, P.; Jana, P.; Varghese, T.; Deo, A.D.; Bedekar, M.K.; Nanda, C. Glycerol monolaurate improves growth, lipid utilization and antioxidative status of white-leg shrimp, Penaeus vannamei fed with varying protein-lipid diets reared in inland saline water. Anim. Feed Sci. Technol. 2023, 306, 115794. [Google Scholar] [CrossRef]
- Hamidoghli, A.; Won, S.; Aya, F.A.; Yun, H.; Bae, J.; Jang, I.-K.; Bai, S.C. Dietary lipid requirement of whiteleg shrimp Litopenaeus vannamei juveniles cultured in biofloc system. Aquac. Nutr. 2020, 26, 603–612. [Google Scholar] [CrossRef]
- Zhao, W.; Yao, R.; Wei, H.-L.; Guo, Y.-C.; Chen, A.-Q.; Chen, B.-Y.; Jin, N. Astaxanthin, bile acid and chlorogenic acid attenuated the negative effects of high-fat diet on the growth, lipid deposition, and liver health of Oncorhynchus mykiss. Aquaculture 2023, 567, 739255. [Google Scholar] [CrossRef]
- Catacutan, M.R. Growth and body composition of juvenile mud crab, Scylla serrata, fed different dietary protein and lipid levels and protein to energy ratios. Aquaculture 2002, 208, 113–123. [Google Scholar] [CrossRef]
- Wu, W.; Ji, H.; Yu, H.; Sun, J.; Zhou, J. Effect of refeeding dietary containing different protein and lipid levels on growth performance, body composition, digestive enzyme activities and metabolic related gene expression of grass carp (Ctenopharyngodon idellus) after overwinter starvation. Aquaculture 2020, 523, 735196. [Google Scholar] [CrossRef]
- Theodoro, J.M.V.; Martinez, O.D.M.; Grancieri, M.; Toledo, R.C.L.; Dias Martins, A.M.; Dias, D.M.; Carvalho, C.W.P.; Martino, H.S.D. Germinated millet flour (Pennisetum glaucum (L.) R. Br.) reduces inflammation, oxidative stress, and liver steatosis in rats fed with high-fat high-fructose diet. J. Cereal Sci. 2021, 99, 103207. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, W.; Zhang, Y.; Gao, X.; Huang, Y.; Ren, H.; Chang, K.; Sun, P.; Gao, S. Dietary guar gum supplementation reduces the adverse effects of high-fat diets on the growth performance, antioxidant capacity, inflammation, and apoptosis of juvenile largemouth bass (Micropterus salmoides). Anim. Feed Sci. Technol. 2024, 308, 115881. [Google Scholar] [CrossRef]
- Huang, Y.; Wen, X.; Li, S.; Li, W.; Zhu, D. Effects of dietary lipid levels on growth, feed utilization, body composition, fatty acid profiles and antioxidant parameters of juvenile chu’s croaker Nibea coibor. Aquac. Int. 2016, 24, 1229–1245. [Google Scholar] [CrossRef]
- Raghuvaran, N.; Sardar, P.; Sahu, N.P.; Shamna, N.; Jana, P.; Paul, M.; Bhusare, S.; Bhavatharaniya, U. Effect of L-carnitine supplemented diets with varying protein and lipid levels on growth, body composition, antioxidant status and physio-metabolic changes of white shrimp, Penaeus vannamei juveniles reared in inland saline water. Anim. Feed Sci. Technol. 2023, 296, 115548. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, J.; Xue, J.; Chu, W.; Hu, Y. Effects of dietary soy isoflavone and soy saponin on growth performance, intestinal structure, intestinal immunity and gut microbiota community on rice field eel (Monopterus albus). Aquaculture 2021, 537, 736506. [Google Scholar] [CrossRef]
- Shi, M.; He, Y.; Zheng, J.; Xu, Y.; Tan, Y.; Jia, L.; Chen, L.; Ye, J.; Qi, C. Effects of Soybean Isoflavones on the Growth Performance and Lipid Metabolism of the Juvenile Chinese Mitten Crab Eriocheir sinensis. Fishes 2024, 9, 335. [Google Scholar] [CrossRef]
- He, L.; Wang, H.; Li, E.; Huang, Q.; Wang, X.; Qiao, F.; Qin, C.; Qin, J.; Chen, L. Effects of soy isoflavones on growth performance, antioxidant capacity, non-specific immunity and lipid metabolism of juvenile Chinese mitten crab, Eriocheir sinensis. Aquaculture 2024, 581, 740470. [Google Scholar] [CrossRef]
- De Santis, C.; Taylor, J.F.; Martinez-Rubio, L.; Boltana, S.; Tocher, D.R. Influence of Development and Dietary Phospholipid Content and Composition on Intestinal Transcriptome of Atlantic Salmon (Salmo salar). PLoS ONE 2015, 10, e0140964. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ma, X.-y.; Jiang, Z.-y.; Hu, Y.-j.; Zheng, C.-t.; Yang, X.-f.; Wang, L.; Gao, K.-g. Effects of soybean isoflavone on intestinal antioxidant capacity and cytokines in young piglets fed oxidized fish oil. J. Zhejiang Univ.-Sci. 2016, 17, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Lin, H.; Ge, X.; Niu, J.; Wang, J.; Wang, Y.; Chen, L.; Huang, Z.; Yu, W.; Tan, X. The Effects of dietary soybean isoflavones on growth, innate immune responses, hepatic antioxidant abilities and disease resistance of juvenile golden pompano Trachinotus ovatus. Fish Shellfish Immunol. 2015, 43, 158–166. [Google Scholar] [CrossRef]
- Cao, S.; Xiong, D.; Luo, W.; Tang, J.; Qu, F.; Zhou, Y.; He, Z.; Xie, S.; Liu, Z. Effects of dietary soy isoflavones on growth, antioxidant status, immune response and resistance of juvenile grass carp (Ctenopharyngodon idella) to Aeromonas hydrophila challenge. Aquac. Res. 2020, 51, 2472–2482. [Google Scholar] [CrossRef]
- Semova, I.; Carten, J.D.; Stombaugh, J.; Mackey, L.C.; Knight, R.; Farber, S.A.; Rawls, J.F. Microbiota Regulate Intestinal Absorption and Metabolism of Fatty Acids in the Zebrafish. Cell Host Microbe 2012, 12, 277–288. [Google Scholar] [CrossRef]
- Fu, J.; Bonder, M.J.; Cenit, M.C.; Tigchelaar, E.F.; Maatman, A.; Dekens, J.A.; Brandsma, E.; Marczynska, J.; Imhann, F.; Weersma, R.K.; et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ. Res. 2015, 117, 817–824. [Google Scholar] [CrossRef]
- Gao, S.; Chen, W.; Zhang, Y.; Zhao, X.; Chang, K.; Huang, Y. Guar gum improves growth performance, intestinal microbiota homeostasis, and hepatic lipid metabolism in juvenile largemouth bass (Micropterus salmoides) fed high-fat diets. Int. J. Biol. Macromol. 2023, 235, 123807. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Tang, Z.; Shi, Y.; Deng, Z.T.; Cai, M.L.; Dai, J.H.; Shao, C.; Zhang, J.Z.; Hu, Y.; Li, D. Effects of Corbicula fluminea meal in high-fat diet on growth, lipid metabolism and intestinal microbiota of juvenile rice field eel Monopterus albus. Aquaculture 2024, 590, 741064. [Google Scholar] [CrossRef]
- Xue, M.; Xu, P.; Wen, H.; Chen, J.; Wang, Q.; He, J.; He, C.; Kong, C.; Li, X.; Li, H.; et al. A High-Fat-Diet-Induced Microbiota Imbalance Correlates with Oxidative Stress and the Inflammatory Response in the Gut of Freshwater Drum (Aplodinotus grunniens). Antioxidants 2024, 13, 363. [Google Scholar] [CrossRef]
- Tremaroli, V.; Bäckhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef]
- Petriz, B.A.; Castro, A.P.; Almeida, J.A.; Gomes, C.P.C.; Fernandes, G.R.; Kruger, R.H.; Pereira, R.W.; Franco, O.L. Exercise induction of gut microbiota modifications in obese, non-obese and hypertensive rats. BMC Genom. 2014, 15, 511. [Google Scholar] [CrossRef]
- Luche, E.; Cousin, B.; Garidou, L.; Serino, M.; Waget, A.; Barreau, C.; André, M.; Valet, P.; Courtney, M.; Casteilla, L.; et al. Metabolic endotoxemia directly increases the proliferation of adipocyte precursors at the onset of metabolic diseases through a CD14-dependent mechanism. Mol. Metab. 2013, 2, 281–291. [Google Scholar] [CrossRef]
- Hu, Y.; Cai, M.; Zhang, J.; Dai, J.; Zhong, H.; Chu, W.; Xu, W.; Hu, Y.; Chen, K. Methionine supplemented in a high fat-diet: Effects of growth performance and lipid metabolism in rice field eel (Monopterus albus). Aquac. Rep. 2023, 32, 101701. [Google Scholar] [CrossRef]
- GB/T 6433-2025; Determination of Crude Fat in Feeds. National Standard of the People’s Republic of China: Beijing, China, 2025.
- GB/T 6432-2018; Determination of Crude Protein in Feeds—Kjeldahl Method. National Standard of the People’s Republic of China: Beijing, China, 2018.
- Cai, M.; Dai, W.; Qiu, X.; He, Z.; Wang, A.; Chen, K.; Hu, Y. A study on the effects of replacing fishmeal with soybean meal in the feed of Procambarus clarkii: Assessing growth performance, immunity, and gut microbiota. Aquac. Rep. 2024, 36, 102184. [Google Scholar] [CrossRef]
- Liu, F.; Shao, G.-Y.; Tian, Q.-Q.; Cheng, B.-X.; Shen, C.; Wang, A.-M.; Zhang, J.-H.; Tian, H.-Y.; Yang, W.-P.; Yu, Y.-B. Enhanced growth performance, immune responses, immune-related gene expression and disease resistance of red swamp crayfish (Procambarus clarkii) fed dietary glycyrrhizic acid. Aquaculture 2021, 533, 736202. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Holmes, S.P. Exact sequence variants should replace operational taxonomic units in marker-gene data analysis. ISME J. 2017, 11, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The silva ribosomal rna gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Muscle5: High-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat. Commun. 2022, 13, 6968. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. Trimal: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; Von Haeseler, A.; Lanfear, R. Iq-tree 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 20 August 2025).
- Jobling, M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2012, 20, 601–602. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Y.; Xie, K.; Zhang, J.; Wang, Y.; Hu, Y.; Zhong, L. Sanguinarine Improves Intestinal Health in Grass Carp Fed High-Fat Diets: Involvement of Antioxidant, Physical and Immune Barrier, and Intestinal Microbiota. Antioxidants 2023, 12, 1366. [Google Scholar] [CrossRef]
- Chen, W.; Gao, S.; Sun, P.; Han, L.; Zhang, Z. Sodium butyrate ameliorates high-fat diet-induced growth retardation and gut injury in largemouth bass (Micropterus salmoides) by modulating gut mucosal barrier and microbiota. Anim. Feed Sci. Technol. 2025, 323, 116283. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Yu, H.; Li, Y.; Zhang, C.; Zhang, X.; Li, H.; Li, T.; Ji, H. Glycerol monolaurate reduces lipid deposition and promotes the health of liver and intestine in grass carp fed with high-fat diet. Anim. Feed Sci. Technol. 2025, 325, 116341. [Google Scholar] [CrossRef]
- Lombardo, G.E.; Navarra, M.; Cremonini, E. A flavonoid-rich extract of bergamot juice improves high-fat diet-induced intestinal permeability and associated hepatic damage in mice. Food Funct. 2024, 15, 9941–9953. [Google Scholar] [CrossRef]
- Du, J.; Cao, L.-p.; Jia, R.; Gu, Z.; He, Q.; Xu, P.; Yin, G.; Ma, Y. Alleviative effects of total flavones of Glycyrrhiza uralensis Fisch on oxidative stress and lipid metabolism disorder induced by high-fat diet in intestines of Tilapia (Oreochromis niloticus). 3 Biotech 2021, 11, 348. [Google Scholar] [CrossRef]
- Jalili, M.; Vahedi, H.; Poustchi, H.; Hekmatdoost, A. Soy isoflavones and cholecalciferol reduce inflammation, and gut permeability, without any effect on antioxidant capacity in irritable bowel syndrome: A randomized clinical trial. Clin. Nutr. ESPEN 2019, 34, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Jiang, X.R.; Wei, Z.X.; Cai, L.; Yin, J.D.; Li, X.L. Effects of soybean isoflavones on the growth performance, intestinal morphology and antioxidative properties in pigs. Animal 2020, 14, 2262–2270. [Google Scholar] [CrossRef]
- Al-Nakkash, L.; Kubinski, A. Soy Isoflavones and Gastrointestinal Health. Curr. Nutr. Rep. 2020, 9, 193–201. [Google Scholar] [CrossRef]
- Huang, W.; Liu, H.; Yang, S.; Song, H.; Cai, W.; Tan, B.; Zhang, S.; Yang, Y.; Zhang, H.; Dong, X. Impacts of dietary phosphorus level on growth, antioxidant capacity and immunity in juvenile hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) fed with high-lipid diets. Aquac. Rep. 2024, 35, 101958. [Google Scholar] [CrossRef]
- Arshadi, A.; Azhdari, A.; Oujifard, A. Dietary supplementation with hesperidin increased growth, antioxidant capacity, and transcription of immune-related genes in Litopenaeus vannamei (Boone 1931). Aquac. Int. 2025, 33, 90. [Google Scholar] [CrossRef]
- Hasanthi, M.; Jo, S.; Kim, H.-s.; Yun, K.-S.; Lee, Y.; Lee, K.-J. Dietary supplementation of micelle silymarin enhances the antioxidant status, innate immunity, growth performance, resistance against Vibrio parahaemolyticus infection, and gut morphology in Pacific white shrimp (Litopenaeus vannamei). Anim. Feed Sci. Technol. 2024, 311, 115953. [Google Scholar] [CrossRef]
- Smith, B.N.; Dilger, R.N. Immunomodulatory potential of dietary soybean-derived isoflavones and saponins in pigs1. J. Anim. Sci. 2018, 96, 1288–1304. [Google Scholar] [CrossRef]
- Kamada, N.; Seo, S.-U.; Chen, G.Y.; Núñez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 2013, 13, 321–335. [Google Scholar] [CrossRef]
- Szabo, G.; Bala, S.; Petrasek, J.; Gattu, A. Gut-Liver Axis and Sensing Microbes. Dig. Dis. 2011, 28, 737–744. [Google Scholar] [CrossRef]
- Compare, D.; Coccoli, P.; Rocco, A.; Nardone, O.M.; De Maria, S.; Cartenì, M.; Nardone, G. Gut-liver axis: The impact of gut microbiota on non alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.H.; Scott, K.P.; Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc. Nutr. Soc. 2015, 74, 13–22. [Google Scholar] [CrossRef]
- Jang, K.B.; Purvis, J.M.; Kim, S.W. Supplemental effects of dietary lysophospholipids in lactation diets on sow performance, milk composition, gut health, and gut-associated microbiome of offspring. J. Anim. Sci. 2020, 98, skaa227. [Google Scholar] [CrossRef] [PubMed]
- Taghavizadeh, M.; Shekarabi, S.P.H.; Mehrgan, M.S.; Islami, H.R. Efficacy of dietary lysophospholipids (Lipidol™) on growth performance, serum immuno-biochemical parameters, and the expression of immune and antioxidant-related genes in rainbow trout (Oncorhynchus mykiss). Aquaculture 2020, 525, 735315. [Google Scholar] [CrossRef]
- Guo, L.; Jin, X.; Yang, D.; Wei, L.; Chen, J.; Lin, Z.; Ma, L. Identification and characterization of Serratia nematophila and Acinetobacter guillouiae from putrid-skin disease lesions in farmed Chinese spiny frog (Quasipaa spinosa). Microbiol. Spectr. 2025, 13, e02096-24. [Google Scholar] [CrossRef]
- Abd-Elrasoul, O.; Soliman, H.; Marzouk Fouad, A. Protective efficacy and immune response to adjuvanted Pseudomonas putida ghost vaccine in Nile tilapia. Fish Shellfish. Immunol. 2025, 166, 110648. [Google Scholar] [CrossRef]
- Okasha, L.A.; Tawfeek, W.S.; Elbaz, S.; Sherif, A.H. Lactobacillus plantarum ameliorates stress caused by Pseudomonas aeruginosa infection in Nile tilapia. Res. Vet. Sci. 2025, 192, 105721. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Zhang, J.; Zou, W.; Yan, Q. Isolation, Characterization, and Assessment of Probiotic Lactococcus lactis from the Intestinal Tract of Largemouth Bass (Micropterus salmoides). Fishes 2025, 10, 291. [Google Scholar] [CrossRef]
- Purnamasari, L.; dela Cruz, J.F.; Cho, D.-Y.; Lee, K.-H.; Cho, S.-M.; Chung, S.-S.; Choi, Y.-J.; Yi, J.-K.; Hwang, S.-G. Effect of Lactococcus lactis JNU 534 Supplementation on the Performance, Blood Parameters and Meat Characteristics of Salmonella enteritidis Inoculated Broilers. Microorganisms 2025, 13, 525. [Google Scholar] [CrossRef]
- Thepot, V.; Campbell, A.H.; Rimmer, M.A.; Jelocnik, M.; Johnston, C.; Evans, B.; Paul, N.A. Dietary inclusion of the red seaweed Asparagopsis taxiformis boosts production, stimulates immune response and modulates gut microbiota in Atlantic salmon, Salmo salar. Aquaculture 2022, 546, 737286. [Google Scholar] [CrossRef]
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and Gut Microbiota: Interaction and Implication for Human Health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef]
- Berezow, A.B.; Ernst, R.K.; Coats, S.R.; Braham, P.H.; Karimi-Naser, L.M.; Darveau, R.P. The structurally similar, penta-acylated lipopolysaccharides of Porphyromonas gingivalis and Bacteroides elicit strikingly different innate immune responses. Microb. Pathog. 2009, 47, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Morris, D.L.; Copelin, J.E.; Hettick, J.M.; Kwon, I.H. Effects of lysophospholipids on short-term production, nitrogen utilization, and rumen fermentation and bacterial population in lactating dairy cows. J. Dairy Sci. 2019, 102, 3110–3120. [Google Scholar] [CrossRef]
- Vanhoof, R.; Sonck, P.; Hannecart-Pokorni, E. The role of lipopolysaccharide anionic binding sites in aminoglycoside uptake in Stenotrophomonas (Xanthomonas) maltophilia. J. Antimicrob. Chemother. 1995, 35, 167–171. [Google Scholar] [CrossRef]
- Chockalingam, A.; McKinney, C.E.; Rinaldi, M.; Zarlenga, D.S.; Bannerman, D.D. A peptide derived from human bactericidal/permeability-increasing protein (BPI) exerts bactericidal activity against Gram-negative bacterial isolates obtained from clinical cases of bovine mastitis. Vet. Microbiol. 2007, 125, 80–90. [Google Scholar] [CrossRef]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
- Chen, W.; Ren, K.; Isabwe, A.; Chen, H.; Liu, M.; Yang, J. Stochastic processes shape microeukaryotic community assembly in a subtropical river across wet and dry seasons. Microbiome 2019, 7, 138. [Google Scholar] [CrossRef]
- Coyte, K.Z.; Schluter, J.; Foster, K.R. The ecology of the microbiome: Networks, competition, and stability. Science 2015, 350, 663–666. [Google Scholar] [CrossRef]
- McWilliams, C.; Lurgi, M.; Montoya, J.M.; Sauve, A.; Montoya, D. The stability of multitrophic communities under habitat loss. Nat. Commun. 2019, 10, 2322. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.M.; Pimm, S.L.; Solé, R.V. Ecological networks and their fragility. Nature 2006, 442, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef] [PubMed]
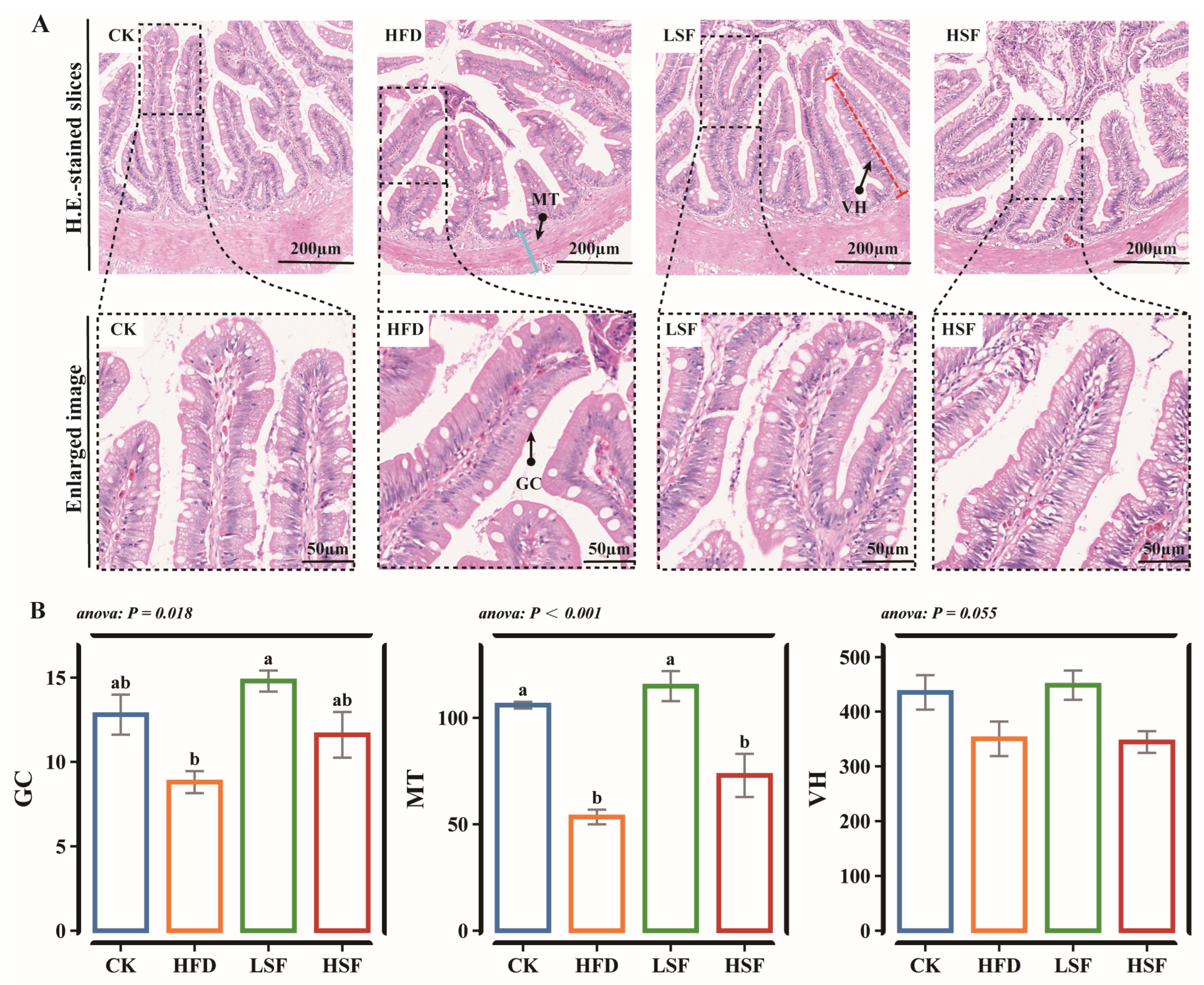
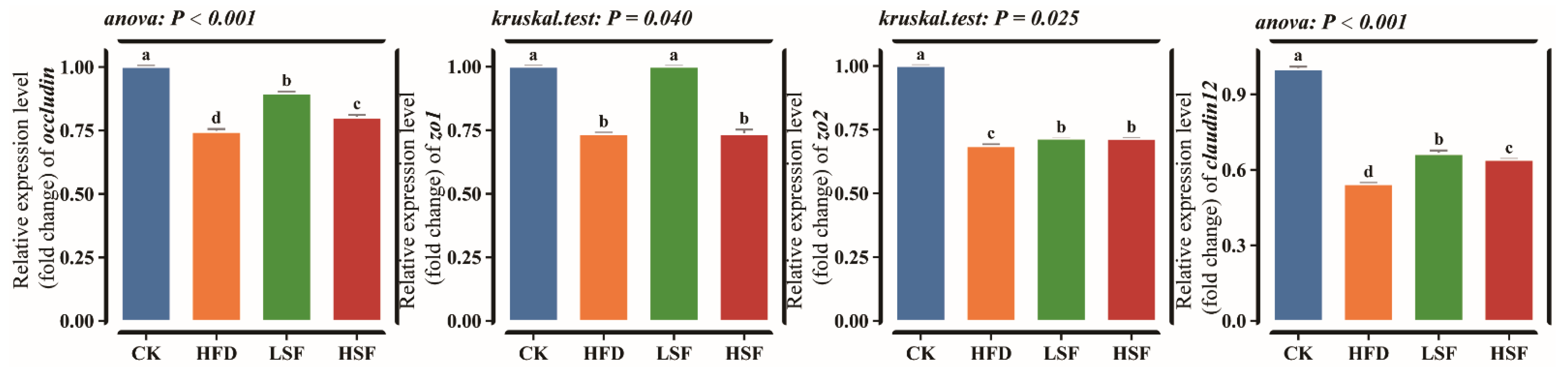
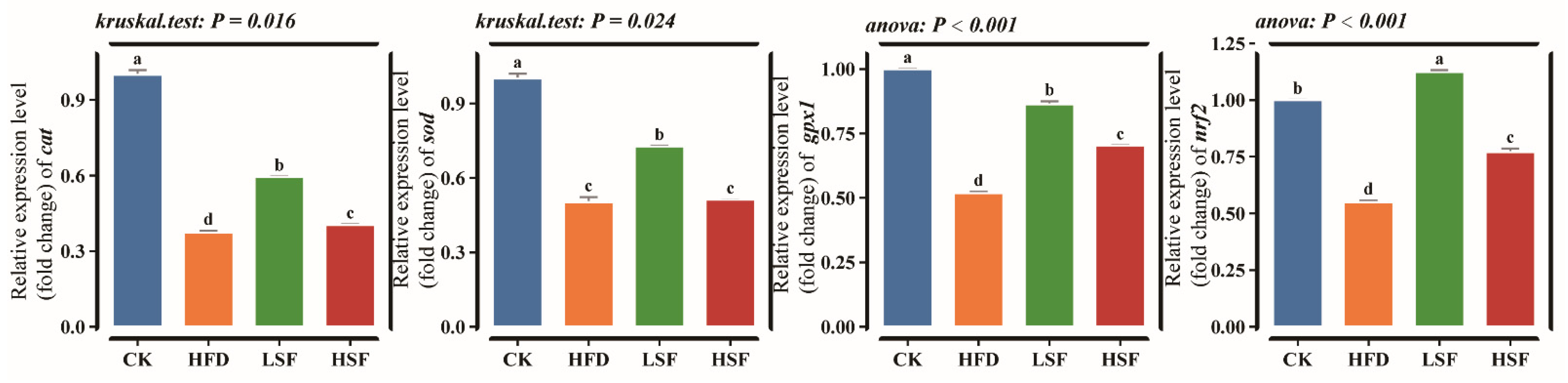
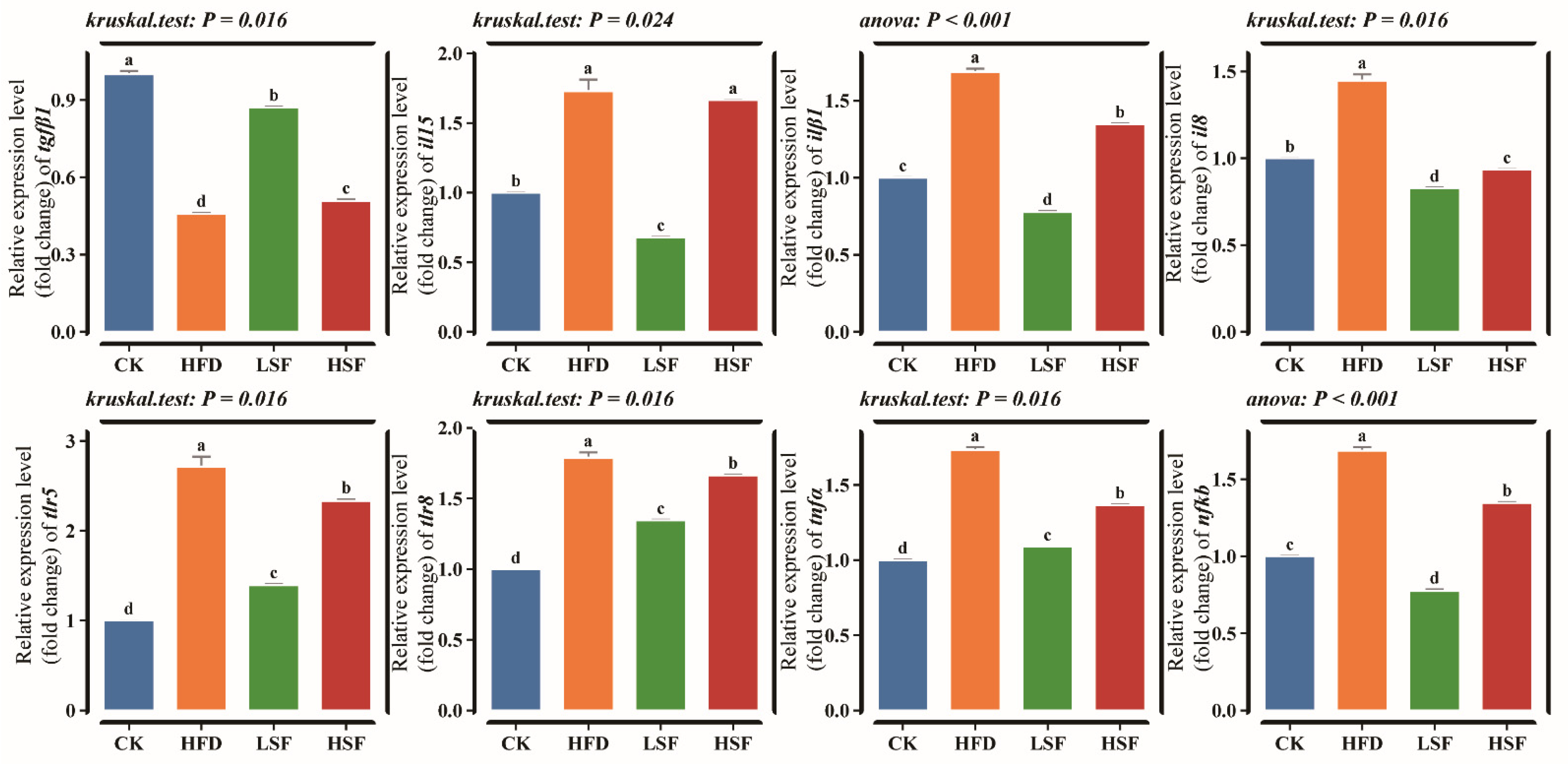
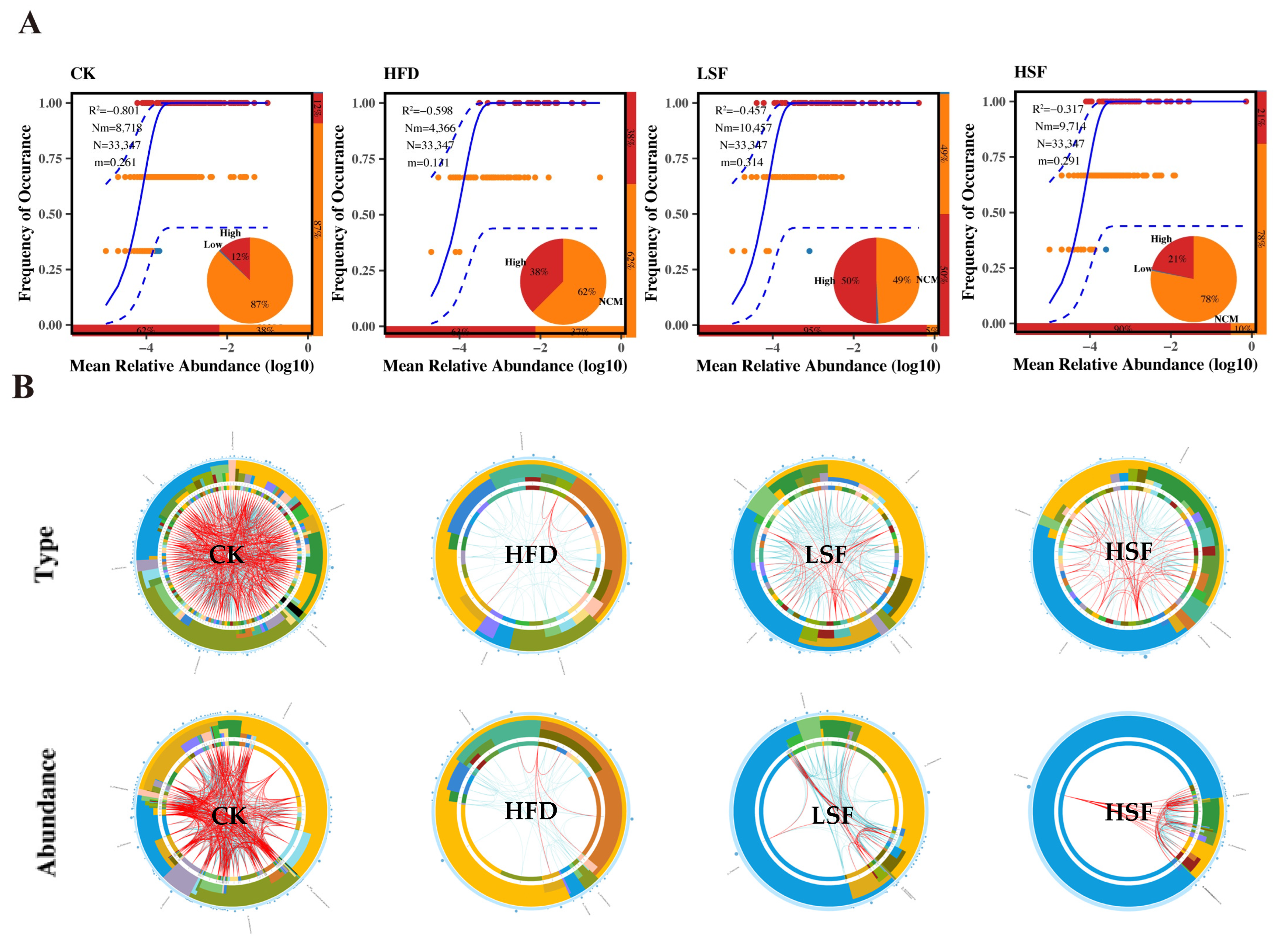
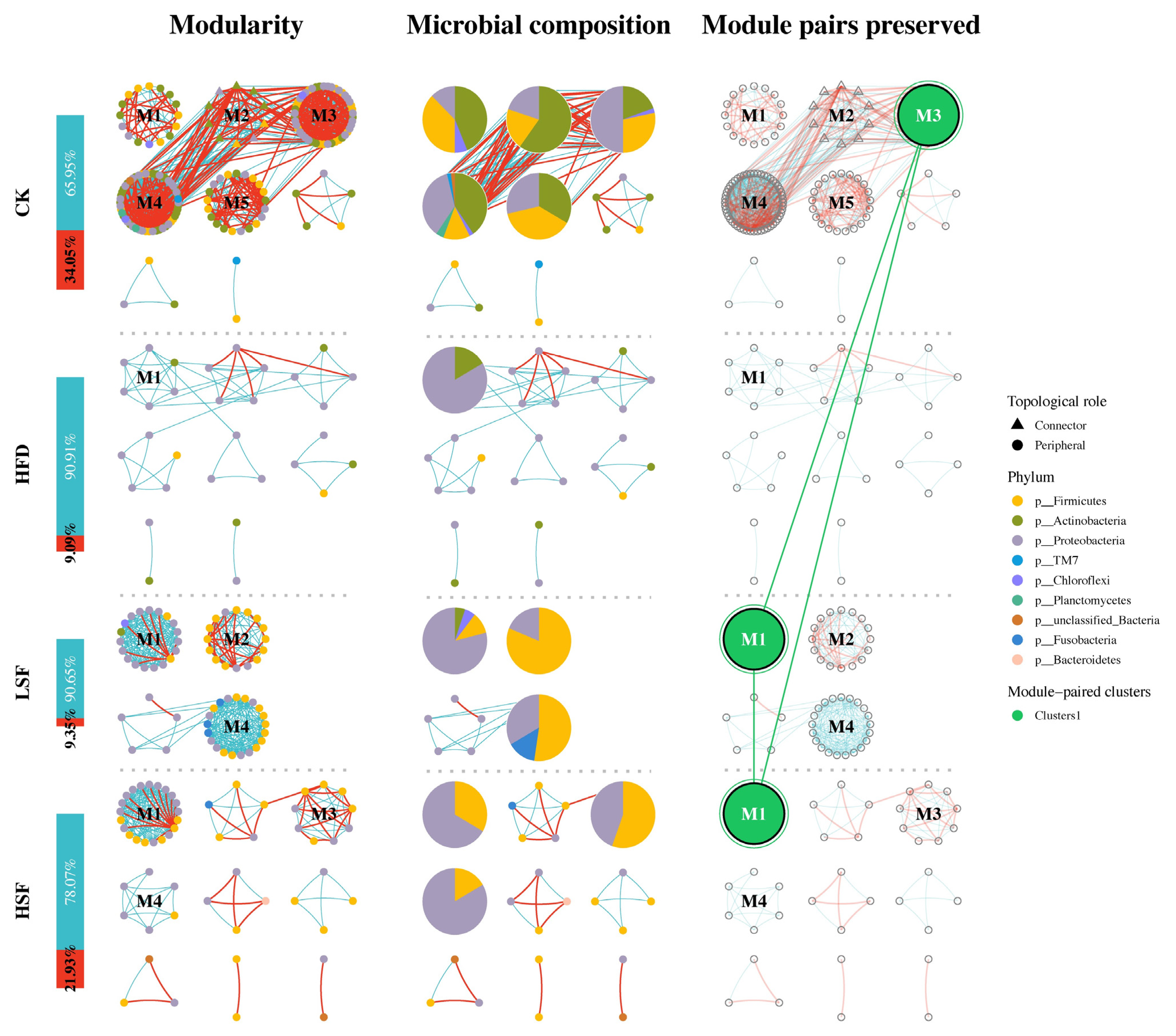
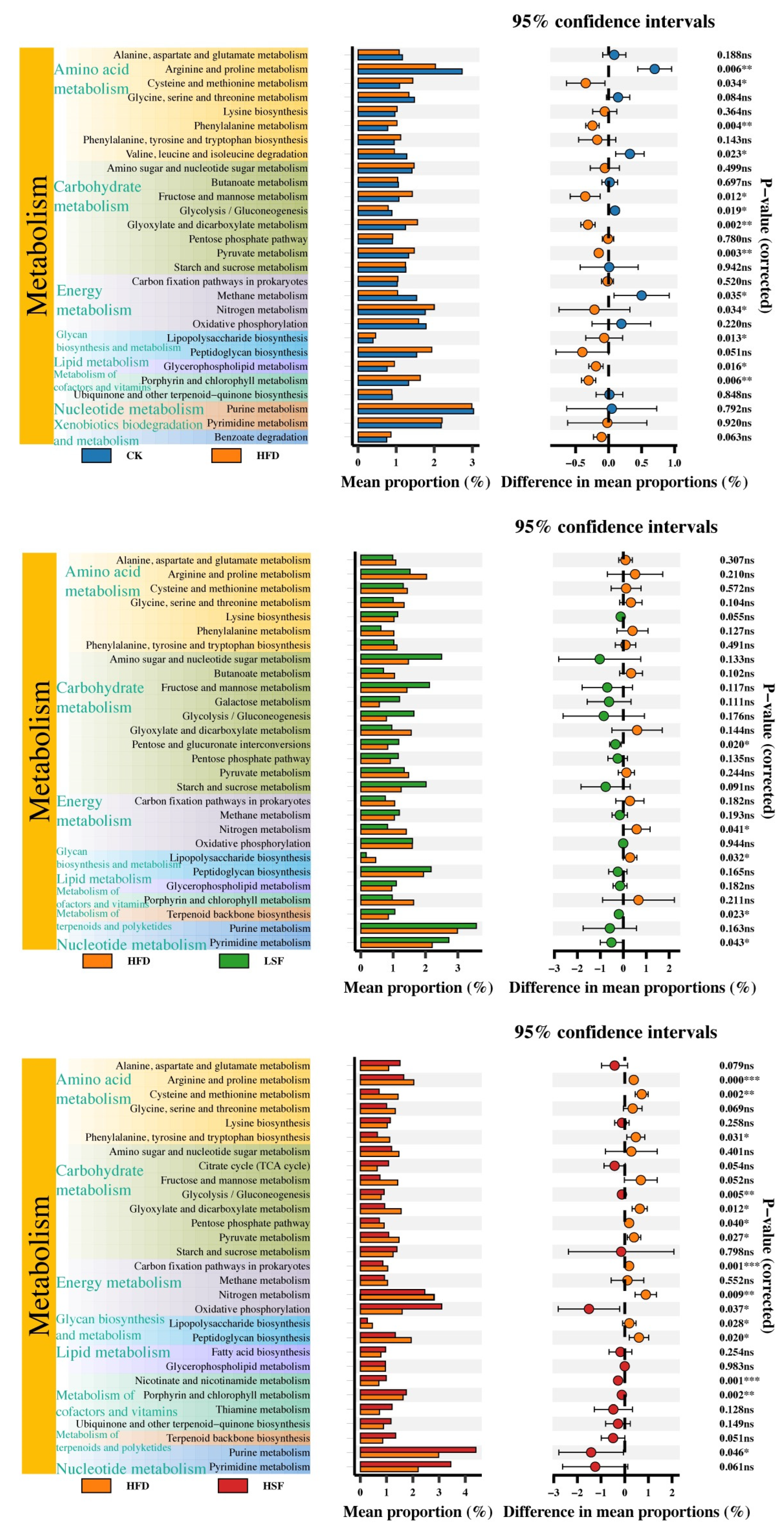
| Ingredients (%) | CK | HFD |
|---|---|---|
| Fish meal | 40.00 | 40.00 |
| Soy protein concentrate | 16.00 | 16.00 |
| Poultry by-product meal | 3.00 | 3.00 |
| Brewer yeast powder | 5.00 | 5.00 |
| Fish oil | 2.20 | 8.20 |
| Microcrystalline cellulose | 10.26 | 4.26 |
| Corn starch | 20.00 | 20.00 |
| Choline | 0.50 | 0.50 |
| Premix a | 1.00 | 1.00 |
| Ca(H2PO4)2 | 2.00 | 2.00 |
| Antioxidant | 0.01 | 0.01 |
| Anti-mildew agent | 0.03 | 0.03 |
| Total | 100.00 | 100.00 |
| Proximate nutritional analysis (%) | ||
| Crude protein | 41.36 | 41.26 |
| Crude lipid | 5.96 | 11.96 |
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Amplification Efficiency (%) | Source |
|---|---|---|---|---|
| rpl17 | GTTGTAGCGACGGAAAGGGAC | GACTAAATCATGCAAGTCGAGGG | 96.34 | XM_020587712.1 |
| cat | GTCCAAGTCTAAGGCATCTCC | CTCCTCTTCGTTCAGCACC | 102.11 | XM_020624985.1 |
| gpx1 | TGTGAATGGGAAGGATGCC | CCTGCTGTAACGCTTGAACG | 103.51 | XM_020607739.1 |
| sod | AGCTGGCTAAGTTCTCATTCAC | GCAGTAACATTGCCCAAGTCT | 99.67 | XM_020598413.1 |
| nrf2 | CTTCAGACAGCGGTGACAGG | GCCTCATTCAGTTGGTGCTT | 91.14 | XM_020596409.1 |
| zo1 | GGCATCATCCCCAACAAA | GCGAAGACCACGGAACCT | 108.42 | XM_020621576.1 |
| zo2 | AGCCGAGGTCGCACTTTA | GCTTTGCTTCTGTGGTTGAT | 106.88 | XM_020615114.1 |
| occludin | TGTCGGGGAGTGGGTAAA | TCCAGGCAAATAAAGAGGCT | 105.03 | XM_020599328.1 |
| claudin12 | TCACCTTCAATCGCAACG | ATGTCTGGCTCAGGCTTATCT | 92.55 | XM_020607277.1 |
| nfkb | ACCCTACCGTGACACTAACCT | TGCCGTCTATCTTGTGGAAT | 96.32 | XM_020616319.1 |
| tgfβ1 | AACCCACTACCTCACTACCCG | GCCGAAGTTGGAAACCCT | 104.17 | XM_020623392.1 |
| tlr5 | TGTAGCCAACTGTGCCTTCCG | CACATTCATGCCGAGCACCAG | 98.76 | XM_020617011.1 |
| tlr8 | GTGATGAGAAATCTGCGAGTG | GAGGTTATCTACCAGCGGGAC | 107.29 | XM_020596483.1 |
| tnfa | TTTCAAGGAGGGCTGGTTCT | CTTGACCAGCGCATCACTGT | 93.88 | XM_020622780.1 |
| il15 | AGAAATGCCCCATCTCCA | CCCTGTCTCCGTCTTGTTG | 101.36 | XM_020622724.1 |
| il8 | TACTGGTTCTGCTTACTGTCGC | CAAATCTTTTGCCCATCCCT | 95.47 | XM_020597077.1 |
| il1β | GAGATGTGGAGCCCAAACTT | CTGCCTCTGACCTTCTGGACTT | 104.05 | KM113037.1 |
| Items | Group | |||
|---|---|---|---|---|
| CK | HFD | LSF | HSF | |
| Empirical network | ||||
| Similarity threshold (St) | 0.995 | 0.995 | 0.995 | 0.995 |
| Node number (N) | 155 | 31 | 61 | 56 |
| Edge number (N) | 1028 | 55 | 310 | 187 |
| Positive edges (%) | 65.95 | 90.91 | 90.65 | 78.07 |
| Negative edges (%) | 34.05 | 9.09 | 9.35 | 21.93 |
| Average degree (avgK) | 13.265 | 3.548 | 10.164 | 6.679 |
| Module number (N) | 8 | 8 | 4 | 9 |
| Average clustering coefficient (avgCC) | 0.763 | 0.576 | 0.763 | 0.789 |
| Modularity (M) | 0.559 | 0.613 | 0.638 | 0.55 |
| Average path distance (GD) | 6.509 | 4.027 | 1.896 | 1.726 |
| Random network | ||||
| Average clustering coefficient (avgCC) | 0.136 ± 0.006 | 0.131 ± 0.006 | 0.225 ± 0.006 | 0.226 ± 0.006 |
| Modularity (M) | 0.151 ± 0.004 | 0.359 ± 0.005 | 0.158 ± 0.005 | 0.190 ± 0.005 |
| Average path distance (GD) | 2.309 ± 0.042 | 2.766 ± 0.016 | 2.077 ± 0.003 | 2.344 ± 0.007 |
| Items | Group | ANOVA | |||
|---|---|---|---|---|---|
| CK | HFD | LSF | HSF | p | |
| MDA (nmol g−1 protein) | 2.12 ± 0.02 c | 5.21 ± 0.01 a | 1.06 ± 0.00 d | 3.94 ± 0.04 b | p < 0.001 |
| H2O2 (mmol g−1 protein) | 9.92 ± 0.04 c | 19.02 ± 0.17 a | 6.06 ± 0.01 d | 14.64 ± 0.05 b | p < 0.001 |
| GSH (µmol g−1 protein) | 7.50 ± 0.09 a | 2.87 ± 0.10 c | 6.11 ± 0.15 b | 3.23 ± 0.19 c | p < 0.001 |
| SOD (U mg−1 protein) | 1.02 ± 0.01 a | 0.34 ± 0.02 d | 0.78 ± 0.00 b | 0.75 ± 0.00 c | 0.016 |
| CAT (U mg−1 protein) | 1.36 ± 0.02 b | 0.61 ± 0.03 d | 1.54 ± 0.00 a | 1.02 ± 0.00 c | p < 0.001 |
| T-AOC (mmol g−1 protein) | 0.05 ± 0.00 b | 0.01 ± 0.00 d | 0.06 ± 0.00 a | 0.04 ± 0.00 c | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Cai, M.; Li, Q.; Wei, H.; Hu, Y.; Zhang, J. Soy Isoflavones Mitigate High-Fat Diet-Induced Oxidative Stress and Inflammation in the Gut of Monopterus albus via Gut Microbiota Remodeling. Biology 2025, 14, 1586. https://doi.org/10.3390/biology14111586
Wang S, Cai M, Li Q, Wei H, Hu Y, Zhang J. Soy Isoflavones Mitigate High-Fat Diet-Induced Oxidative Stress and Inflammation in the Gut of Monopterus albus via Gut Microbiota Remodeling. Biology. 2025; 14(11):1586. https://doi.org/10.3390/biology14111586
Chicago/Turabian StyleWang, Shao, Minglang Cai, Quan Li, Huahong Wei, Yi Hu, and Junzhi Zhang. 2025. "Soy Isoflavones Mitigate High-Fat Diet-Induced Oxidative Stress and Inflammation in the Gut of Monopterus albus via Gut Microbiota Remodeling" Biology 14, no. 11: 1586. https://doi.org/10.3390/biology14111586
APA StyleWang, S., Cai, M., Li, Q., Wei, H., Hu, Y., & Zhang, J. (2025). Soy Isoflavones Mitigate High-Fat Diet-Induced Oxidative Stress and Inflammation in the Gut of Monopterus albus via Gut Microbiota Remodeling. Biology, 14(11), 1586. https://doi.org/10.3390/biology14111586






