Temporal Change Rate in Sound Velocity Caused by Ultrasonic Heating for Evaluation of Steatotic Liver
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
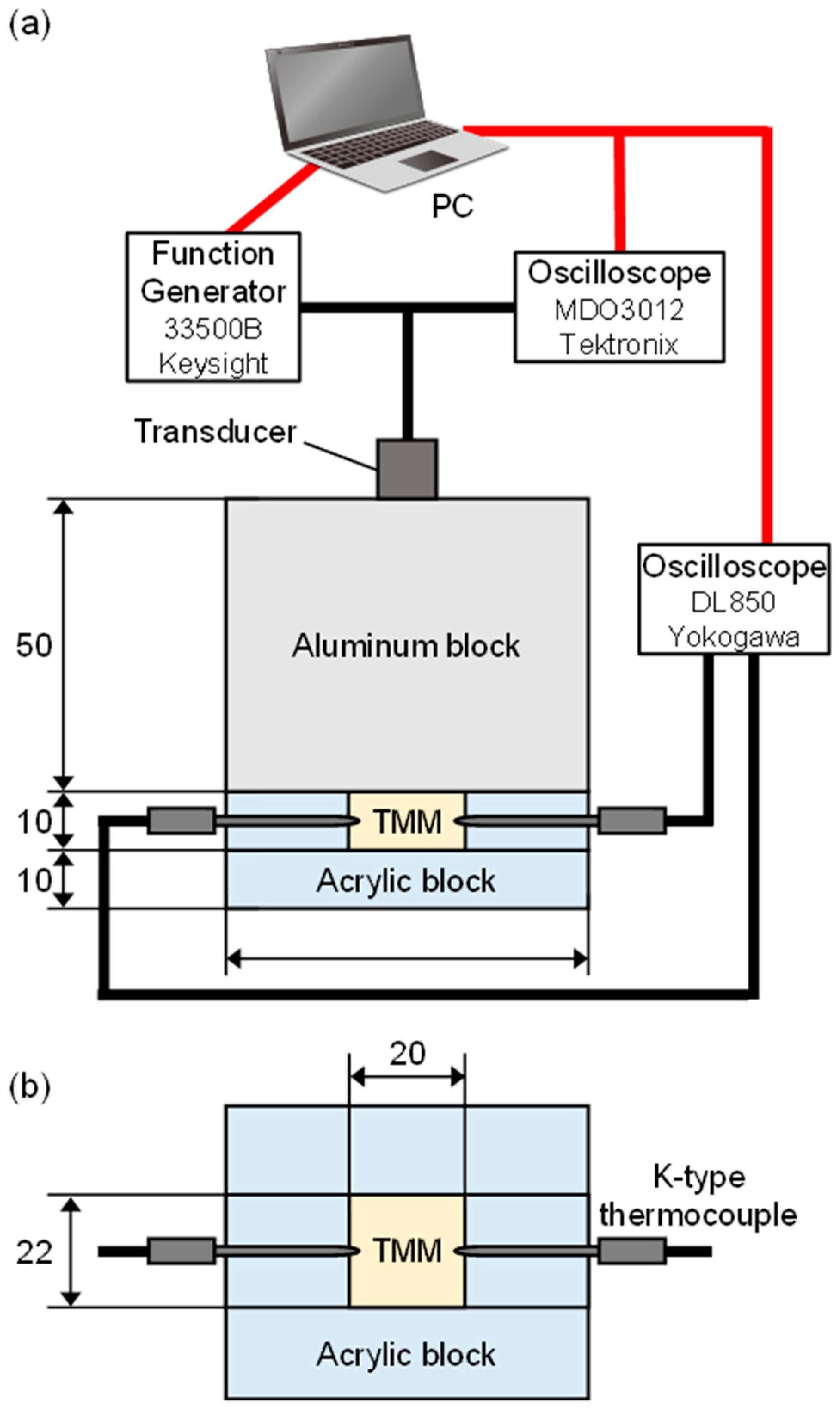
2.1. Measurement of Temperature Coefficient of Sound Velocity in Tissue-Mimicking Material (TMM) Phantoms
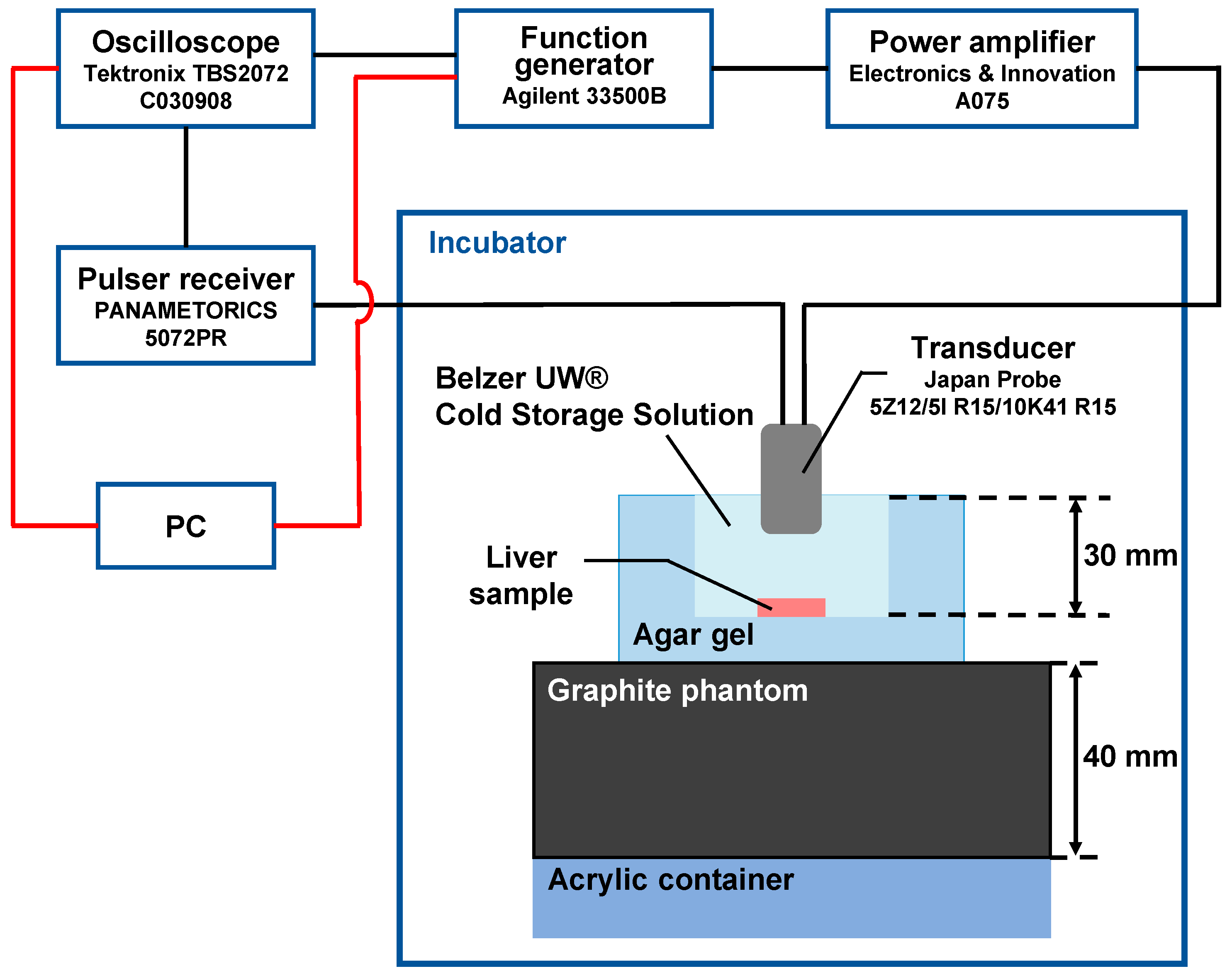
2.2. In Vitro Measurement of Change Rate in Sound Velocity
2.3. In Vivo Measurement of Change Rate in Sound Velocity
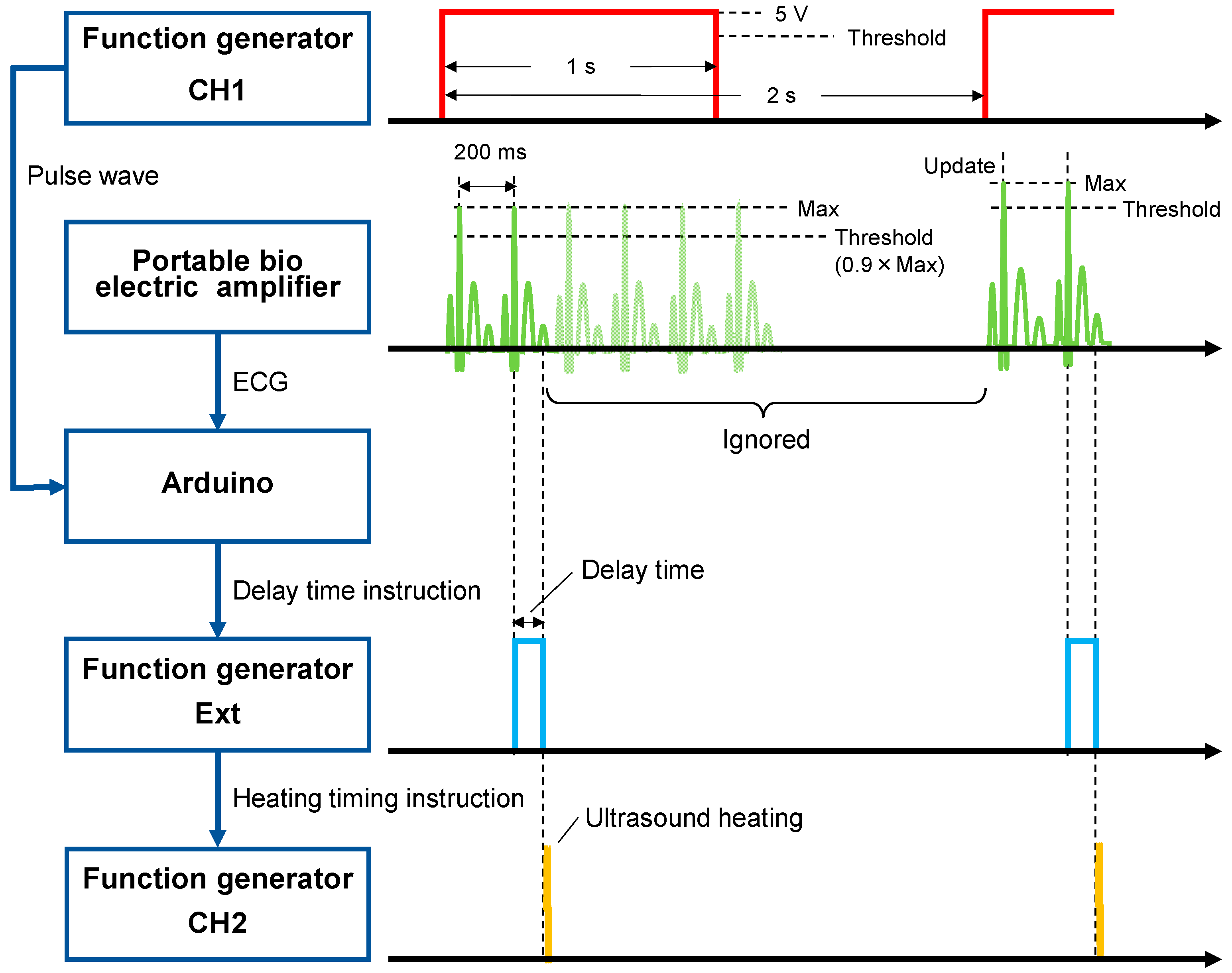
2.3.1. Electrocardiogram (ECG)-Synchronized System
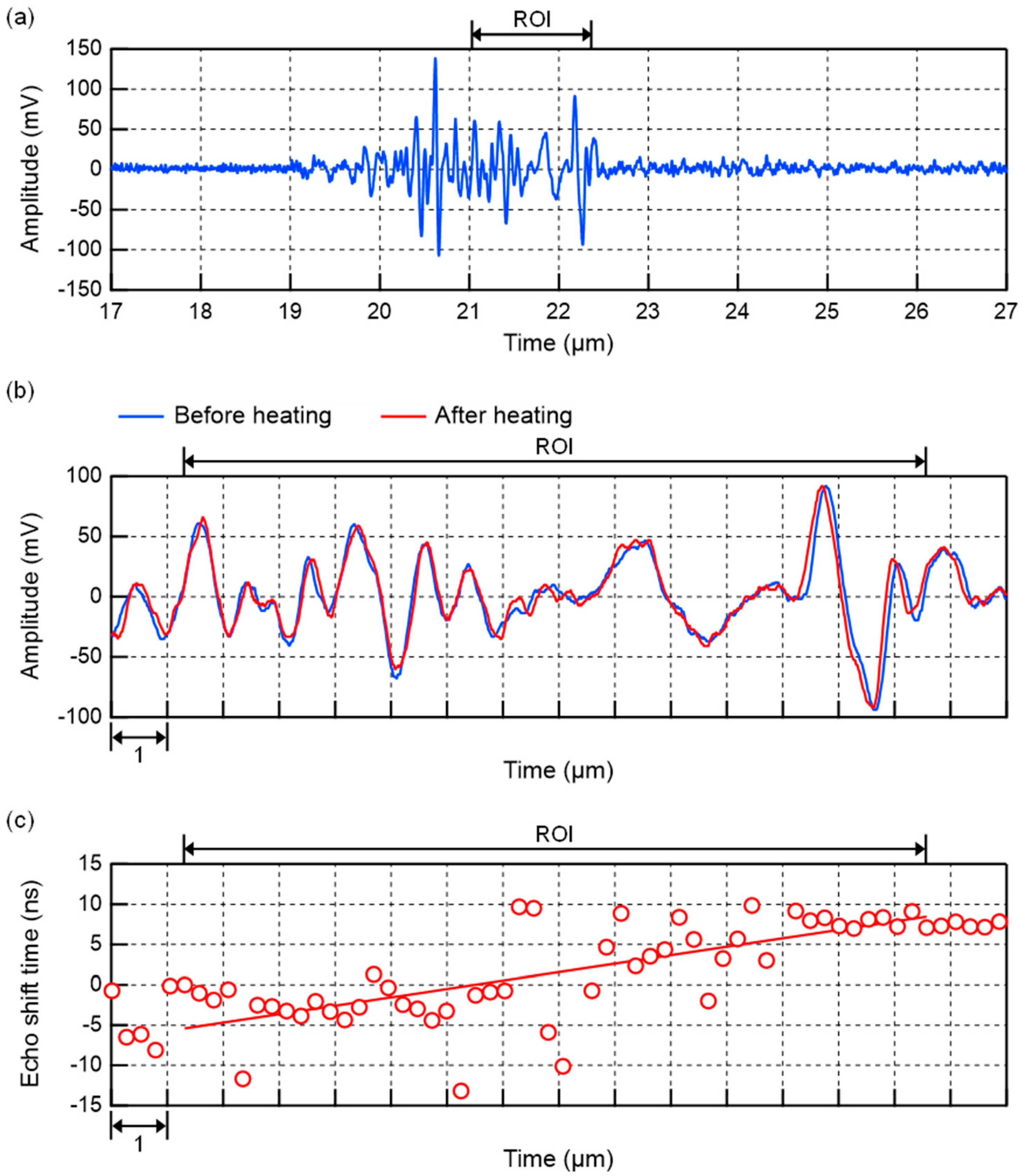
2.3.2. Method of In Vivo Measurement
3. Results
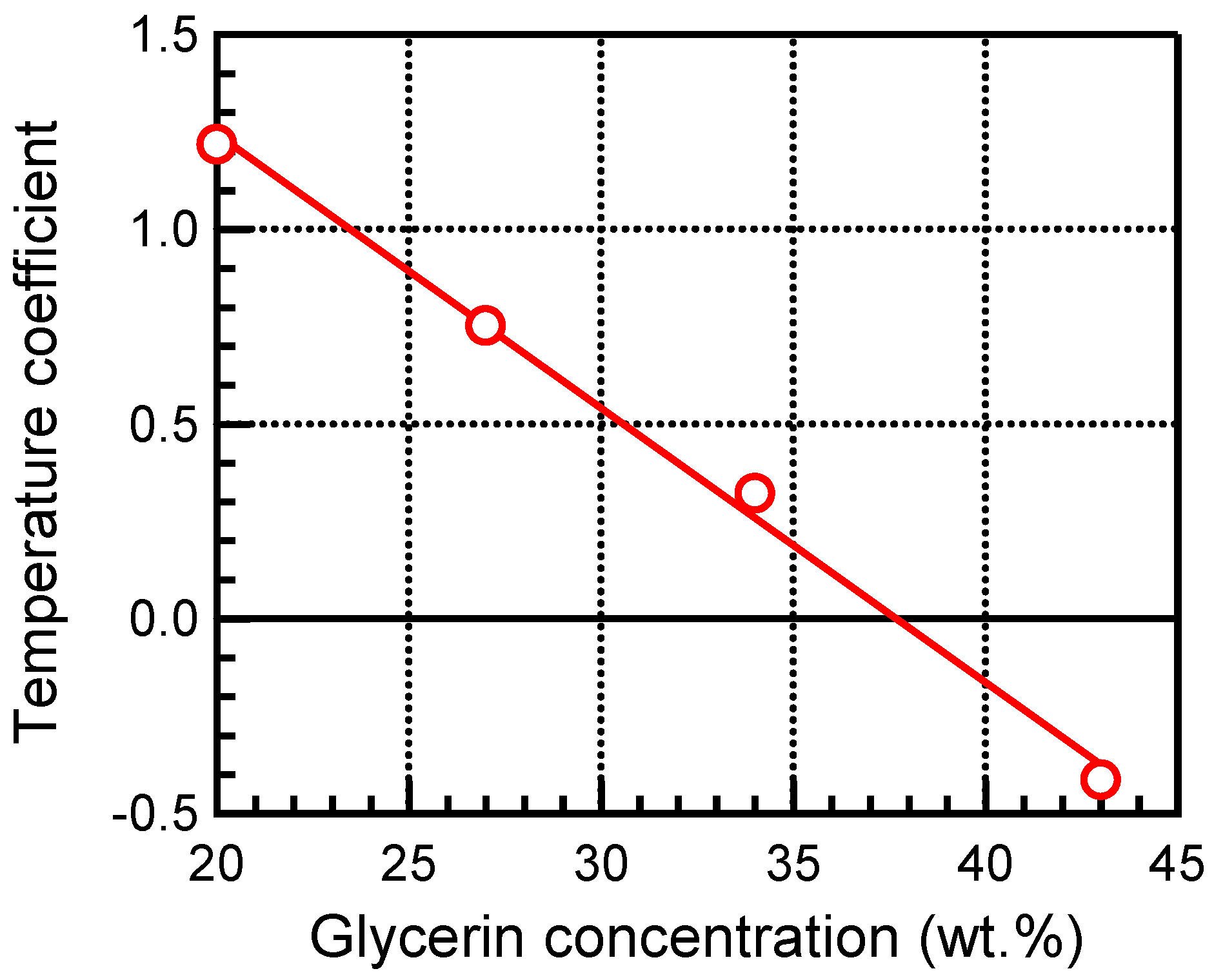
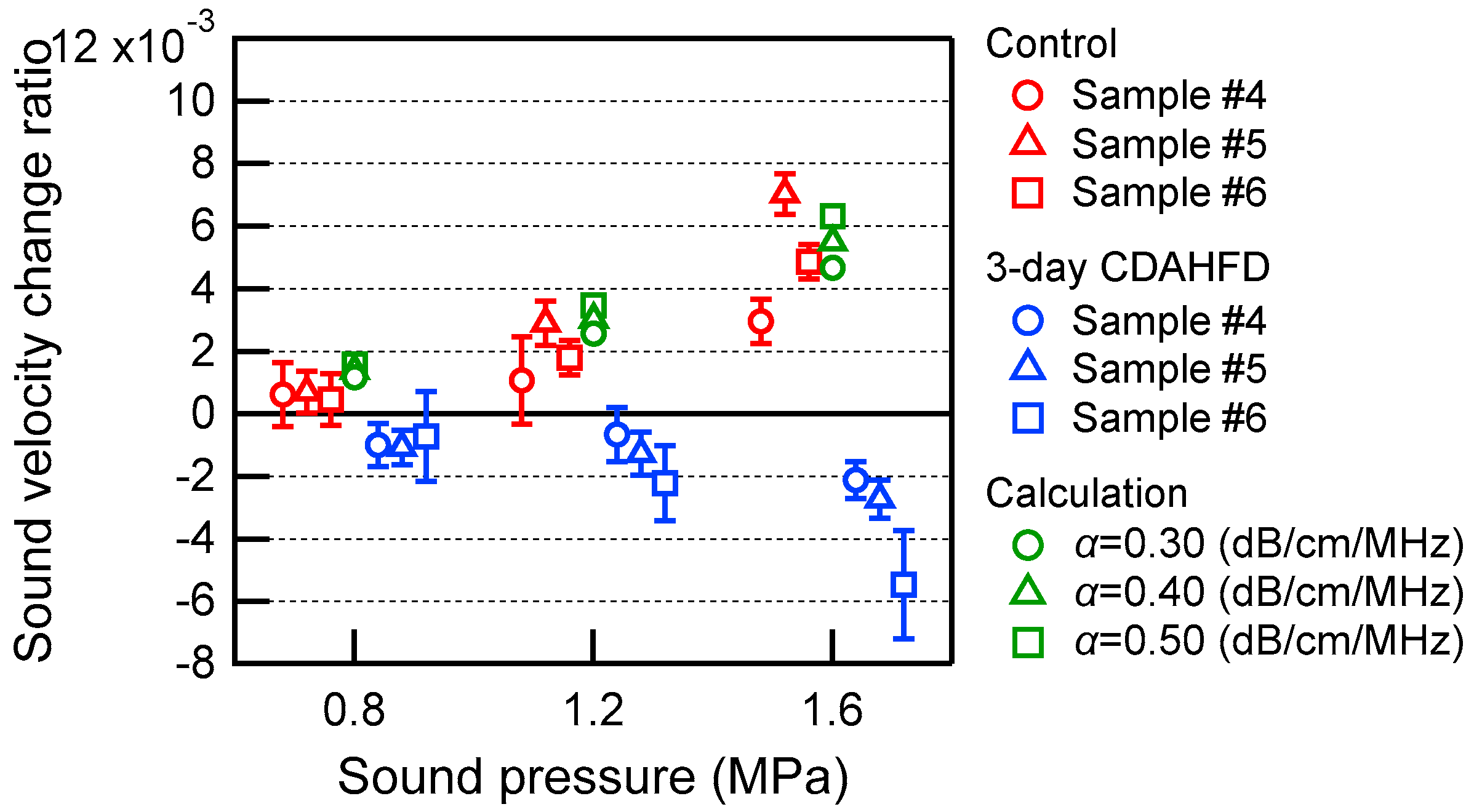
3.1. TMM Experiments
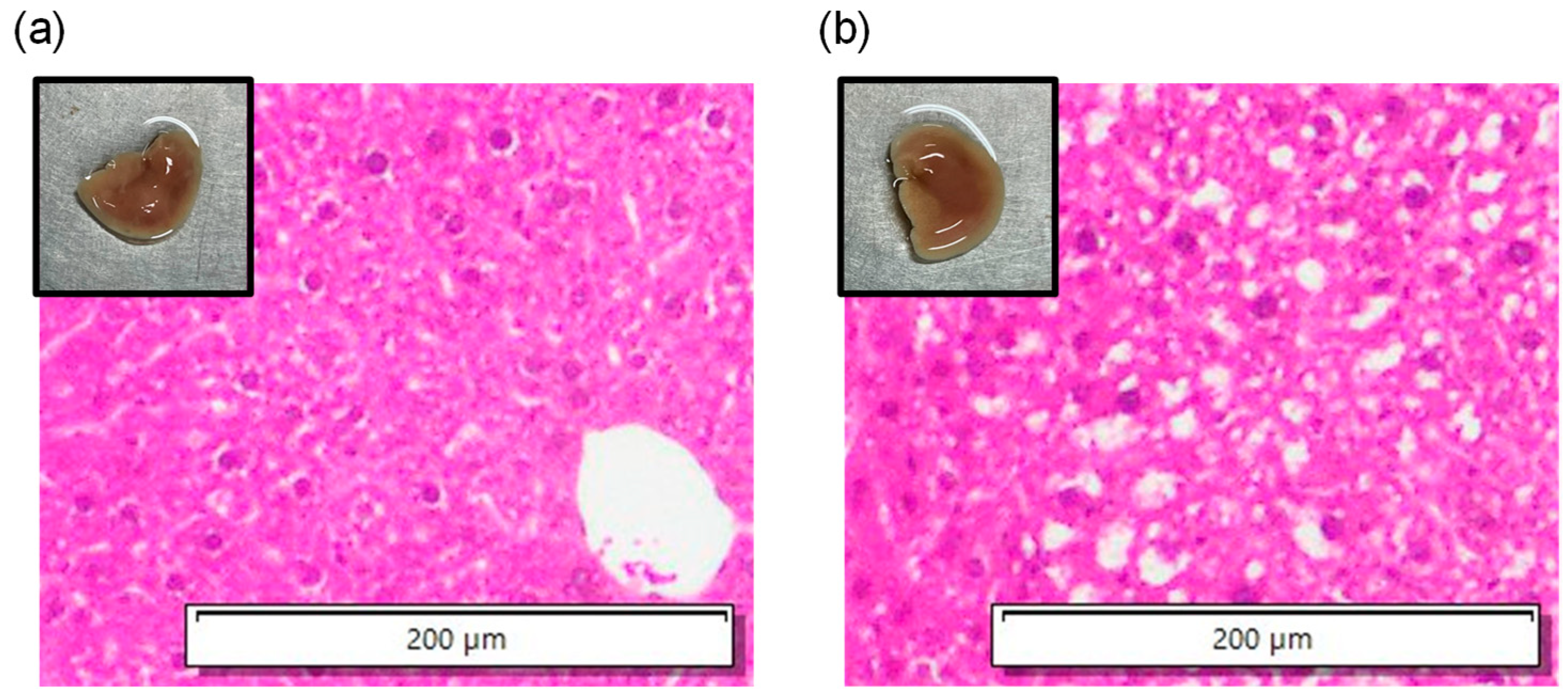
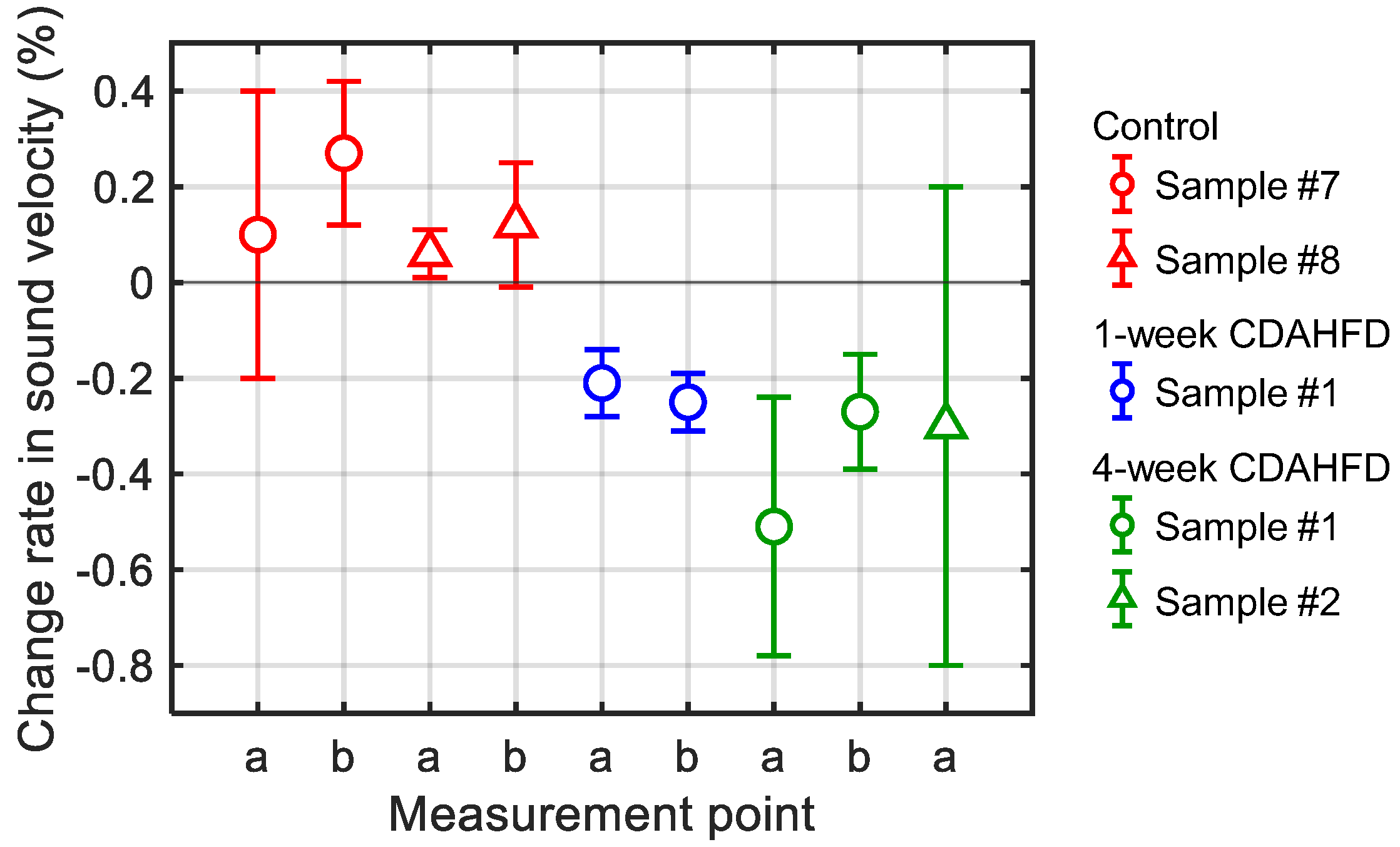
3.2. In Vitro Experiments of Mouse Livers
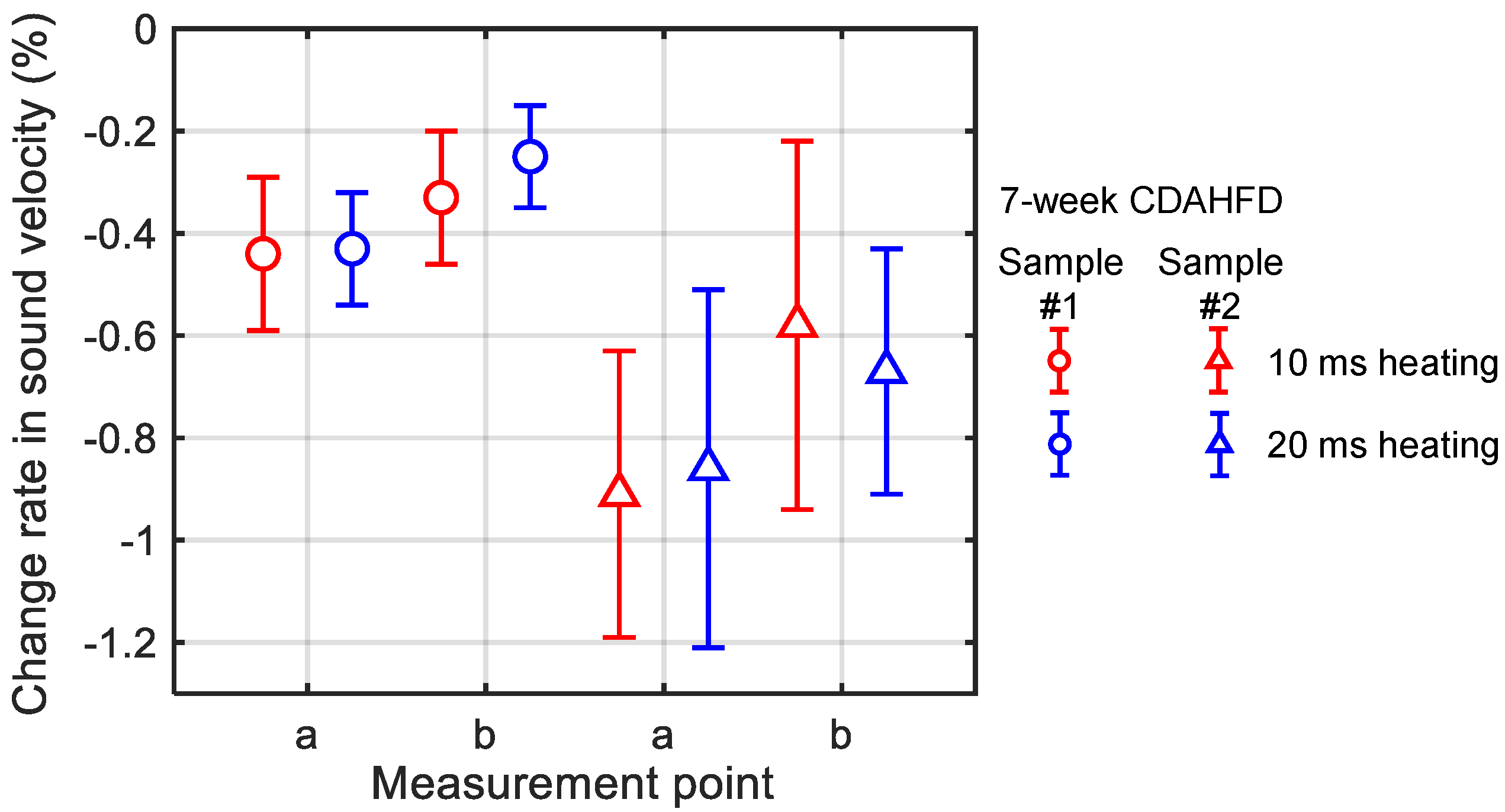
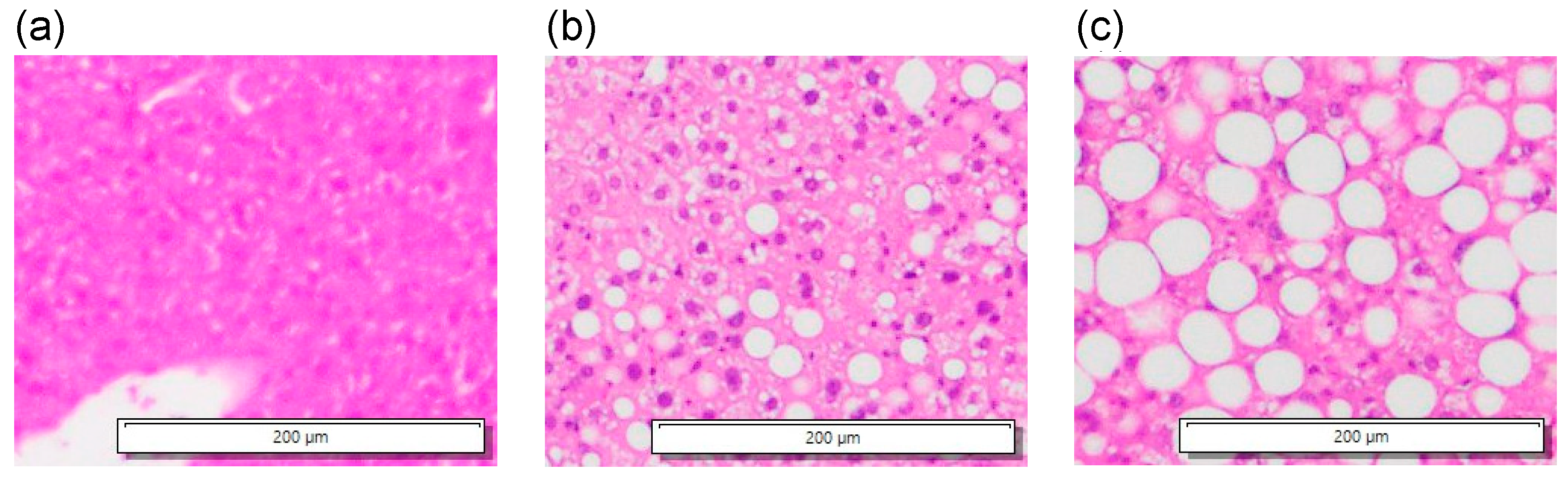
3.3. In Vivo Experiments of Mouse Livers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| TMM | Tissue-mimicking material |
| CDAHFD | Choline-deficient, L-amino acid-defined, and high-fat diet |
| ECG | Electrocardiogram |
| LCF | Low cut-off frequency |
| HCF | How cut-off frequency |
| HE | Hematoxylin-eosin |
| ROI | Region of interest |
References
- Scott, L.F.; Brent, A.N.; Mary, R.; Arun, J.S. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Zobair, M.Y. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar]
- Amélio, F.G.; Wellington, S.S.; Cynthia, M.V. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020, 12, 60–80. [Google Scholar] [CrossRef]
- Elizabeth, E.P.; Vincent, W.W.; Mary, R. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef] [PubMed]
- Vincent, W.W.; Mattias, E.; Grace, L.W.; Hannes, H. Changing epidemiology, global trends and implications for ourcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar]
- Elisabetta, B.; Nicola, L.; Ester, V.; Giulio, M.; Franco, B.; Patrizia, C.; Alessandro, M.; Paolo, D.P.; Lorenzo, C.; Mauro, S.; et al. Expanding the natural history of nonalcoholic steatohepatitis: From cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology 2002, 123, 134–140. [Google Scholar] [CrossRef]
- Brad, Q.S.; Christopher, J.C.; Stephen, A.H. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology 2010, 51, 1820–1832. [Google Scholar] [CrossRef]
- Rohit, L.; Scott, L.F.; Gerald, I.S. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021, 184, 2537–2564. [Google Scholar] [CrossRef]
- George, N.I. Epidemiology and risk-strafication of NAFLD-associated HCC. J. Hepatol. 2021, 75, 1476–1484. [Google Scholar]
- Rod, S.T.; Rebecca, J.T.; Sue, B.; Hannes, H.; Patrik, N.; Jorn, M.S.; Ishigami, M.; Toyoda, H.; Vincent, W.W.; Noam, P.; et al. Association Between Fibrosis Stage and Analysis. Gastroenterology 2020, 158, 1611–1625. [Google Scholar]
- Lizzi, F.L.; Greenebaum, M.; Feleppa, E.J.; Elbaum, M.; Coleman, D.J. Theoretical framework for spectrum analysis in ultrasonic tissue characterization. J. Acoust. Soc. Am. 1983, 73, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Fellingham, L.L.; Sommer, F.G. Ultrasonic Characterization of Tissue Structure in the In Vivo Human Liver and Spleen. IEEE Trans. Sonics Ultrason. 1984, 31, 418–428. [Google Scholar] [CrossRef]
- Robert, C.W. A Review of Tissue Characterization from Ultrasonic Scattering. IEEE Trans. Biomed. Eng. 1984, 31, 884–893. [Google Scholar]
- Nicholas, D.; Nassiri, D.K.; Garbutt, P.; Hill, C.R. Tissue characterization from ultrasound B-scan data. Ultrasound Med. Biol. 1986, 12, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Michael, L.O.; Jonathan, M. Review of Quantitative Ultrasound: Envelope Statistics and Backscatter Coefficient Imaging and Contributions to Diagnostic Ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2016, 63, 336–351. [Google Scholar] [CrossRef]
- Bamber, J.C.; Hill, C.R. Acoustic properties of normal and cancerous human liver—I. Dependence on pathological condition. Ultrasound Med. Biol. 1981, 7, 121–133. [Google Scholar] [CrossRef]
- Laurent, S.; Bertrand, F.; Jean-Michel, H.; Sylvain, Y.; Céline, F.; Frédéric, M.; Christos, C.; Marianne, Z.; Bruno, P.; Farad, K.; et al. Transient elastography: A new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med. Biol. 2003, 29, 1705–1713. [Google Scholar] [CrossRef]
- Andres, L.C.; Roberto, L. Regularized Spectral Log Difference Technique for Ultrasonic Attenuation Imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control. 2018, 65, 378–389. [Google Scholar]
- Haowei, T.; Mawia, K.; Kenneth, H. Adaptive attenuation correction during H-scan ultrasound imaging using K-means clustering. Ultrasonics 2020, 102, 105987. [Google Scholar]
- Tsujimoto, Y.; Morimoto, M.; Nitta, N.; Akiyama, I. Ultrasonic measurement of sound velocity fluctuations in biological tissue due to ultrasonic heating and estimation of thermo-physical properties. J. Med. Ultrason. 2019, 46, 35–43. [Google Scholar] [CrossRef]
- Bamber, J.C.; Hill, C.R. Ultrasonic attenuation and propagation speed in mammalian tissues as a function of temperature. Ultrasound Med. Biol. 1979, 5, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Techavipoo, U.; Varghese, T.; Chen, Q.; Stiles, T.A.; Zagzebski, J.A.; Frank, G.R. Temperature dependence of ultrasonic propagation speed and attenuation in excised canine liver tissue measured using transmitted and reflected pulses. J. Acoust. Soc. Am. 2004, 115, 2859–2865. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, Y.; Matsuda, D.; Minamiguchi, K.; Tanaka, T.; Hirai, T.; Akiyama, I. Measurement of temperature dependence of sound velocity in biological tissues. Jpn. J. Appl. Phys. 2019, 58, SGGE01. [Google Scholar] [CrossRef]
- IEC 60601–2-37:2007; Medical Electrical Equipment—Part 2–37: Particular Requirements for the Basic Safety and Essential Performance of Ultrasonic Medical Diagnostic and Monitoring Equipment. IEC (International Electrotechnical Commission): Geneva, Switzerland, 2010.
- Saito, N.; Tanaka, T.; Minamiguchi, K.; Taiji, R.; Nishiofuku, H.; Matsumoto, T.; Hirai, T.; Kichikawa, K.; Kawahara, N.; Matsuda, D.; et al. Ultrasonic heating detects lipiodol deposition within liver tumors after transarterial embolization: An in vivo approach: An In Vivo Approach. Biology 2021, 10, 901. [Google Scholar] [CrossRef]
- Zahiri-Azar, R.; Salcudean, S.E. Motion estimation in ultrasound images using time domain cross correlation with prior estimates. IEEE Trans. Biomed. Eng. 2006, 53, 1990–2000. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Itsubo, M.; Kobayashi, Y.; Yamamoto, M.; Takayanagi, S.; Akiyama, I. Temporal Change Rate in Sound Velocity Caused by Ultrasonic Heating for Evaluation of Steatotic Liver. Biology 2025, 14, 1585. https://doi.org/10.3390/biology14111585
Itsubo M, Kobayashi Y, Yamamoto M, Takayanagi S, Akiyama I. Temporal Change Rate in Sound Velocity Caused by Ultrasonic Heating for Evaluation of Steatotic Liver. Biology. 2025; 14(11):1585. https://doi.org/10.3390/biology14111585
Chicago/Turabian StyleItsubo, Machi, Yume Kobayashi, Masaki Yamamoto, Shinji Takayanagi, and Iwaki Akiyama. 2025. "Temporal Change Rate in Sound Velocity Caused by Ultrasonic Heating for Evaluation of Steatotic Liver" Biology 14, no. 11: 1585. https://doi.org/10.3390/biology14111585
APA StyleItsubo, M., Kobayashi, Y., Yamamoto, M., Takayanagi, S., & Akiyama, I. (2025). Temporal Change Rate in Sound Velocity Caused by Ultrasonic Heating for Evaluation of Steatotic Liver. Biology, 14(11), 1585. https://doi.org/10.3390/biology14111585




