Forest Strata and Abiotic Factors Primarily Regulate Understory Species Richness Rather than Forest Type in a Temperate Forest of South Korea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Data Collection
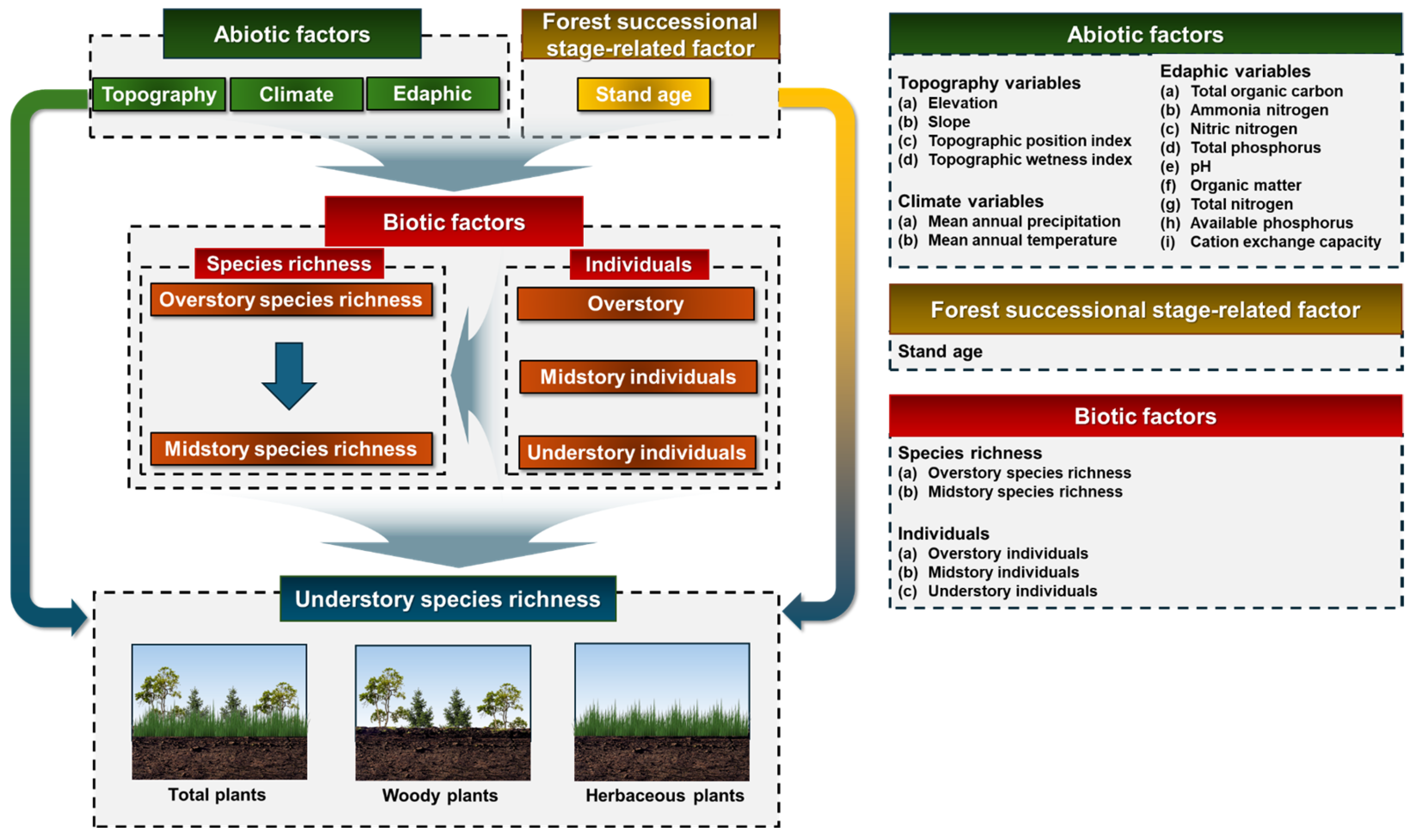
2.2. Quantification of Abiotic, Biotic, and Forest Development Stage Factors
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of tree species on understory vegetation diversity and mechanisms involved–a critical review for temperate and boreal forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Suchar, V.A.; Crookston, N.L. Understory cover and biomass indices predictions for forest ecosystems of the Northwestern United States. Ecol. Indic. 2010, 10, 602–609. [Google Scholar] [CrossRef]
- Gilliam, F.S. The ecological significance of the herbaceous layer in temperate forest ecosystems. BioScience 2007, 57, 845–858. [Google Scholar] [CrossRef]
- McCann, K.S. The diversity stability debate. Nature 2000, 405, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Hector, A.; Bagchi, R. Biodiversity and ecosystem multifunctionality. Nature 2007, 448, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.N. Herbaceous layer in deciduous forest ecosystems. In The Herbaceous Layer in Forests of Eastern North America; Gilliam, F.S., Roberts, M.R., Eds.; Oxford University Press: New York, NY, USA, 2003; pp. 15–37. [Google Scholar]
- Muller, R.N.; Bormann, F.H. Role of Erythronium Americanum Ker. In energy flow and nutrient dynamics of a northern hardwood forest ecosystem. Science 1976, 193, 1126–1128. [Google Scholar] [CrossRef] [PubMed]
- Whigham, D.F. Ecology of woodland herbs in temperate deciduous forests. Annu. Rev. Ecol. Evol. Syst. 2004, 35, 583–621. [Google Scholar] [CrossRef]
- Eriksson, O.; Ehrlen, J. Seedling recruitment and population ecology. In Seedling Ecology and Evolution; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Leck, M.A.; Parker, V.T.; Simpson, R. (Eds.) Seedling Ecology and Evolution; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Huston, M.A. A General Hypothesis of Species Diversity. Am. Nat. 1979, 113, 81–101. [Google Scholar] [CrossRef]
- Stein, A.; Gerstner, K.; Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 2014, 17, 866–880. [Google Scholar] [CrossRef]
- Zhang, L.; Mi, X.; Harrison, R.D.; Yang, B.; Man, X.; Ren, H.; Ma, K. Resource Heterogeneity, Not Resource Quantity, Plays an Important Role in Determining Tree Species Diversity in Two Species-Rich Forests. Front. Ecol. Evol. 2020, 8, 224. [Google Scholar] [CrossRef]
- Bartels, S.F.; Chen, H.Y. Is understory plant species diversity driven by resource quantity or resource heterogeneity? Ecology 2010, 91, 1931–1938. [Google Scholar] [CrossRef]
- Stevens, M.H.H.; Carson, W.P. Resource quantity, not resource heterogeneity, maintains plant diversity. Ecol. Lett. 2002, 5, 420–426. [Google Scholar] [CrossRef]
- Woo, J.H.; Lee, M.K.; Lee, H.I.; Lee, C.B. Joint Influence of Forest Strata Attributes and Abiotic Factors on Herbaceous Plant Abundance in South Korean Forest Restoration Sites: Native Versus Alien Species. Forests 2024, 15, 1924. [Google Scholar] [CrossRef]
- Richard, B.; Dupouey, J.L.; Corcket, E.; Alard, D.; Archaux, F.; Aubert, M.; Boulanger, V.; Gillet, F.; Langlois, E.; Macé, S.; et al. The climatic debt is growing in the understorey of temperate forests: Stand characteristics matter. Glob. Ecol. Biogeogr. 2021, 30, 1474–1487. [Google Scholar] [CrossRef]
- Zema, D.A.; Plaza-Alvarez, P.A.; Xu, X.; Carra, B.G.; Lucas-Borja, M.E. Influence of forest stand age on soil water repellency and hydraulic conductivity in the Mediterranean environment. Sci. Total Environ. 2021, 753, e142006. [Google Scholar] [CrossRef] [PubMed]
- Bátori, Z.; Tölgyesi, C.; Li, G.; Erdős, L.; Gajdács, M.; Kelemen, A. Forest age and topographic position jointly shape the species richness and composition of vascular plants in karstic habitats. Ann. For. Sci. 2023, 80, 16. [Google Scholar] [CrossRef]
- Kumar, P.; Chen, H.Y.; Thomas, S.C.; Shahi, C. Linking resource availability and heterogeneity to understorey species diversity through succession in boreal forest of Canada. J. Ecol. 2018, 106, 1266–1276. [Google Scholar] [CrossRef]
- Han, J.; Yin, H.; Xue, J.; Zhang, Z.; Xing, Z.; Wang, S.; Yu, B. Vertical distribution differences of the understory herbs and their driving factors on shady and sunny slopes in high altitude mountainous areas. Front. For. Glob. Change 2023, 6, 1138317. [Google Scholar] [CrossRef]
- Tian, K.; Chai, P.; Wang, Y.; Chen, L.; Qian, H.; Chen, S.; Mi, X.; Ren, H.; Ma, K.; Chen, J. Species diversity pattern and its drivers of the understory herbaceous plants in a Chinese subtropical forest. Front. Ecol. Evol. 2023, 10, 1113742. [Google Scholar] [CrossRef]
- Leuschner, C.; Lendzion, J. Air humidity, soil moisture and soil chemistry as determinants of the herb layer composition in European beech forests. J. Veg. Sci. 2009, 20, 288–298. [Google Scholar] [CrossRef]
- Baskett, C.A.; Schemske, D.W. Latitudinal patterns of herbivore pressure in a temperate herb support the biotic interactions hypothesis. Ecol. Lett. 2018, 21, 578–587. [Google Scholar] [CrossRef]
- Kim, G.T.; Um, T.W. A study on the distribution of wild edible herb species in Mt. Kariwang. J. Korean Soc. For. Sci. 1997, 86, 422–429. [Google Scholar]
- Baek, W.G.; Park, W.G.; Lee, U.C. Flora and vegetation of resources plants in the Mt. Kariwang (Kangwon-do). Korean J. Plant Res. 1998, 11, 217. [Google Scholar]
- Lee, C.B.; Kim, H.H. Elevational patterns of plant species richness and relative importance of climatic and topographic factors on the mt. seorak, South Korea. J. Agric. Life Sci. 2018, 52, 1–11. [Google Scholar] [CrossRef]
- Weiss, A. Topographic position and landforms analysis. In Proceedings of the Poster presentation, ESRI User Conference, San Diego, CA, USA, 9–13 July 2001. [Google Scholar]
- Drake, M.; Vengris, J.; Colby, W.G. Cation-exchange capacity of plant roots. Soil Sci. 1951, 72, 139–148. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Nitrogen Analysis of Soil and Plant Tissues. J. Assoc. Off. Anal. Chem. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- McCauley, A.; Jones, C.; Jacobsen, J. Soil pH and organic matter. Nutr. Manag. Modul. 2009, 8, 1–12. [Google Scholar]
- Korea Forest Service. F-GiS: Forest Geospatial Information Service. Available online: https://map.forest.go.kr/forest/ (accessed on 15 August 2023).
- Kim, J.C.; Jeong, J.H.; Kim, S.H.; Kim, W.B.; Ryu, J.H.; Kim, J.S.; Seo, S.A.; Yoo, B.O. The 4th National Forest Inventory; Section of National Forest: Seoul, Republic of Korea, 2009. [Google Scholar]
- Lee, H.I.; Seo, Y.O.; Kim, H.; Ali, A.; Lee, C.B.; Chung, Y. Species evenness declines but specific functional strategy enhances aboveground biomass across strata in subtropical Warm-temperate forests of South Korea. For. Ecol. Manag. 2022, 512, 120179. [Google Scholar] [CrossRef]
- Chun, J.H.; Ali, A.; Lee, C.B. Topography and forest diversity facets regulate overstory and understory aboveground biomass in a temperate forest of South Korea. Sci. Total Environ. 2020, 744, 140783. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, H.I.; Lee, C.B.; Lee, K.H.; Kim, R.H.; Ali, A. Abiotic and stand age-induced changes in tree diversity and size inequality regulate aboveground biomass and soil organic carbon stock in temperate forests of South Korea. Catena 2024, 237, 107827. [Google Scholar] [CrossRef]
- LaFrankie, J.V.; Ashton, P.S.; Chuyong, G.B.; Co, L.; Condit, R.; Davies, S.J.; Foster, R.; Hubbell, S.P.; Kenfack, D.; Lagunzard, D.; et al. Contrasting structure and composition of the understory in species—Rich tropical rain forests. Ecology 2006, 87, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Practical Use of the Information-Theoretic Approach; Springer: New York, NY, USA, 1998; pp. 75–117. [Google Scholar] [CrossRef]
- Lefcheck, J.S. piecewiseSEM: Piecewise structural equation modelling in r for ecology, evolution, and systematics. Methods Ecol. Evol. 2016, 7, 573–579. [Google Scholar] [CrossRef]
- Blanck, Y.L.; Gowda, J.; Martensson, L.M.; Sandberg, J.; Fransson, A.M. Plant species richness in a natural Argentinian matorral shrub-land correlates negatively with levels of plant phosphorus. Plant Soil 2011, 345, 11–21. [Google Scholar] [CrossRef]
- Ligot, G.; Ameztegui, A.; Courbaud, B.; Coll, L.; Kneeshaw, D. Tree light capture and spatial variability of understory light increase with species mixing and tree size heterogeneity. Can. J. For. Res. 2016, 46, 968–977. [Google Scholar] [CrossRef]
- Gong, C.; Tan, Q.; Liu, G.; Xu, M. Impacts of tree mixtures on understory plant diversity in China. For. Ecol. Manag. 2021, 498, 119545. [Google Scholar] [CrossRef]
- Neufeld, H.S.; Donald, R.Y. Ecophysiology of the Herbaceous Layer in Temperate Deciduous Forests. In The Herbaceous Layer in Forests of Eastern North America, 2nd ed.; Gilliam, F., Ed.; Oxford Academic: New York, NY, USA, 2003. [Google Scholar]
- Tilman, D. Resource Competition and Community Structure; Princeton University Press: Princeton, NJ, USA, 1982. [Google Scholar]
- Graves, J.H.; Peet, R.K.; White, P.S. The influence of carbon—Nutrient balance on herb and woody plant abundance in temperate forest understories. J. Veg. Sci. 2006, 17, 217–226. [Google Scholar] [CrossRef]
- Murphy, S.J.; Salpeter, K.; Comita, L.S. Higher β-diversity observed for herbs over woody plants is driven by stronger habitat filtering in a tropical understory. Ecology 2016, 97, 2074–2084. [Google Scholar] [CrossRef]
- Spicer, M.E.; Radhamoni, H.V.N.; Duguid, M.C.; Queenborough, S.A.; Comita, L.S. Herbaceous plant diversity in forest ecosystems: Patterns, mechanisms, and threats. Plant Ecol. 2022, 223, 117–129. [Google Scholar] [CrossRef]
- Massante, J.C.; Gotzenberger, L.; Takkis, K.; Hallikma, T.; Kaasik, A.; Laanisto, L.; Gerhold, P. Contrasting latitudinal patterns in phylogenetic diversity between woody and herbaceous communities. Sci. Rep. 2019, 9, 6443. [Google Scholar] [CrossRef]
- Rahbek, C.; Borregaard, M.K.; Colwell, R.K.; Dalsgaard, B.O.; Holt, B.G.; Morueta-Holme, N.; Fjeldså, J. Humboldt’s enigma: What causes global patterns of mountain biodiversity? Science 2019, 365, 1108–1113. [Google Scholar] [CrossRef]
- Lütz, C. (Ed.) Plants in Alpine Regions: Cell Physiology of Adaptation and Survival Strategies; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar] [CrossRef]
- Billings, W.D.; Mooney, H.A. The ecology of arctic and alpine plants. Biol. Rev. 1968, 43, 481–529. [Google Scholar] [CrossRef]
- Billings, W.D. Adaptations and origins of alpine plants. Arct. Alp. Res. 1974, 6, 129–142. [Google Scholar] [CrossRef]
- Gusta, L.V.; Wisniewski, M. Understanding plant cold hardiness: An opinion. Physiol. Plant. 2013, 147, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Billah, M.; Khan, M.; Bano, A.; Hassan, T.U.; Munir, A.; Gurmani, A.R. Phosphorus and phosphate solubilizing bacteria: Keys for sustainable agriculture. Geomicrobiol. J. 2019, 36, 904–916. [Google Scholar] [CrossRef]
- Sakachep, Z.K.; Rai, P.K. Effects of invasive alien plants on floristic diversity and soil physico-chemical characteristics in Hailakandi district, Assam, an Indo Burma hotspot region. Trop. Ecol. 2025, 66, 303–320. [Google Scholar] [CrossRef]
- Huang, W.; Pohjonen, V.; Johansson, S.; Nashanda, M.; Katigula, M.I.L.; Luukkanen, O. Species diversity, forest structure and species composition in Tanzanian tropical forests. For. Ecol. Manag. 2003, 173, 11–24. [Google Scholar] [CrossRef]




| Forest Types | Strata | Frequency of Occurrence | |
|---|---|---|---|
| Most Frequent Species | Second Most Frequent Species | ||
| Larix kaempferi forests | Overstory | Larix kaempferi (Lamb.) Carrière | Fraxinus rhynchophylla Hance |
| Midstory | Quercus mongolica Fisch. ex Ledeb. | Aralia elata (Miq.) Seem. | |
| Understory | Tripterygium wilfordii Hook.f. | Rubus pungens Cambess. | |
| Pinus densiflora forests | Overstory | Pinus densiflora Siebold & Zucc. | Quercus mongolica Fisch. ex Ledeb. |
| Midstory | Quercus mongolica Fisch. ex Ledeb. | Fraxinus rhynchophylla Hance | |
| Understory | Lindera obtusiloba Blume | Parthenocissus tricuspidata (Siebold & Zucc.) Planch. | |
| Pinus koraiensis forests | Overstory | Pinus koraiensis Siebold & Zucc. | Fraxinus rhynchophylla Hance |
| Midstory | Aralia elata (Miq.) Seem. | Quercus mongolica Fisch. ex Ledeb. | |
| Understory | Fraxinus rhynchophylla Hance | Rubus pungens Cambess. | |
| Quercus mongolica forests | Overstory | Quercus mongolica Fisch. ex Ledeb. | Acer pictum Thunb. |
| Midstory | Acer pseudosieboldianum (Pax) Kom. | Symplocos sawafutagi Nagam. | |
| Understory | Pseudostellaria palibiniana (Takeda) Ohwi | Ainsliaea acerifolia Sch.Bip. | |
| Other Quercus forests | Overstory | Quercus mongolica Fisch. ex Ledeb. | Acer pictum Thunb. |
| Midstory | Lindera obtusiloba Blume | Acer pseudosieboldianum (Pax) Kom. | |
| Understory | Tripterygium wilfordii Hook.f. | Fraxinus rhynchophylla Hance | |
| Other broadleaved forests | Overstory | Acer pictum Thunb. | Fraxinus rhynchophylla Hance |
| Midstory | Acer pictum Thunb. | Morus indica L. | |
| Understory | Fraxinus rhynchophylla Hance | Dryopteris crassirhizoma Nakai | |
| Subalpine forests | Overstory | Abies nephrolepis (Trautv. ex. Maxim.) | Taxus cuspidata Siebold & Zucc. |
| Midstory | Acer tschonoskii Maxim. | Euonymus macropterus Rupr. | |
| Understory | Carex siderosticta Hance | Pseudostellaria palibiniana (Takeda) Ohwi | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, J.-H.; Lee, M.-K.; Chun, J.-H.; Lee, C.-B. Forest Strata and Abiotic Factors Primarily Regulate Understory Species Richness Rather than Forest Type in a Temperate Forest of South Korea. Biology 2025, 14, 1565. https://doi.org/10.3390/biology14111565
Woo J-H, Lee M-K, Chun J-H, Lee C-B. Forest Strata and Abiotic Factors Primarily Regulate Understory Species Richness Rather than Forest Type in a Temperate Forest of South Korea. Biology. 2025; 14(11):1565. https://doi.org/10.3390/biology14111565
Chicago/Turabian StyleWoo, Jun-Hyuk, Min-Ki Lee, Jung-Hwa Chun, and Chang-Bae Lee. 2025. "Forest Strata and Abiotic Factors Primarily Regulate Understory Species Richness Rather than Forest Type in a Temperate Forest of South Korea" Biology 14, no. 11: 1565. https://doi.org/10.3390/biology14111565
APA StyleWoo, J.-H., Lee, M.-K., Chun, J.-H., & Lee, C.-B. (2025). Forest Strata and Abiotic Factors Primarily Regulate Understory Species Richness Rather than Forest Type in a Temperate Forest of South Korea. Biology, 14(11), 1565. https://doi.org/10.3390/biology14111565





