LIMK1 Deficiency Disrupts Hippocampal–Cortical Memory Consolidation and Attenuates Trauma-Induced PTSD-like Behavior
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cannula Implantation and Drug Micro-Infusion
2.3. Object Location Recognition Task
2.4. EEG and EMG Electrode Implantation
2.5. Sleep Scoring and Quantification
2.6. In Vivo Electrophysiology
2.7. Electrophysiological Analysis
2.8. Water-Associated Zero Maze (WAZM)
2.9. Underwater Trauma and Re-Exposure
2.10. Western Blot
2.11. Statistical Analysis
3. Results
3.1. LIMK1 Deficiency Significantly Prevents Memory Consolidation
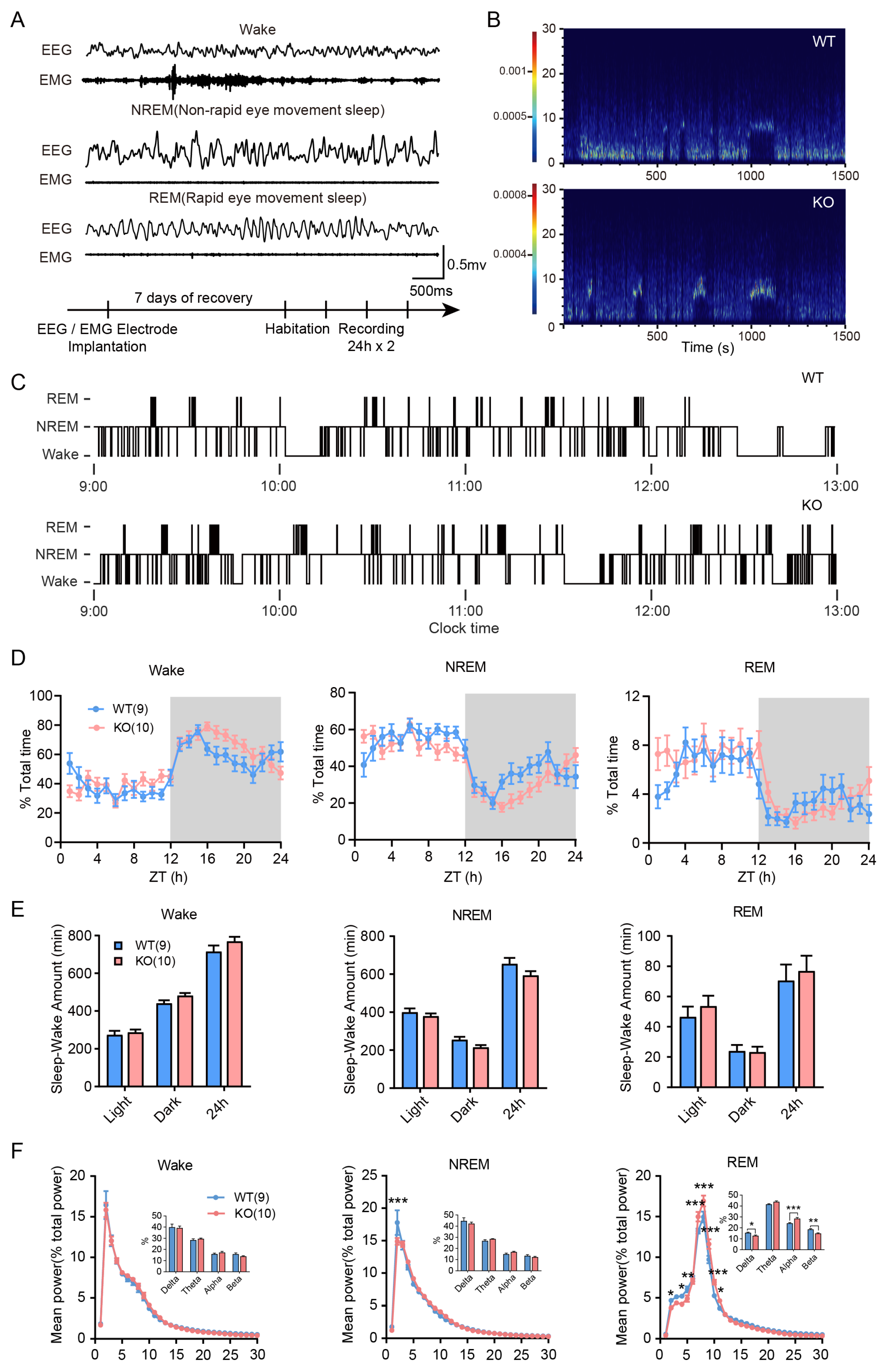
3.2. Sleep/Wake Time and Architecture of Limk1 KO Mice
3.3. Mice Deficient in LIMK1 Show Impaired Coupling Between the mPFC and Hippocampus
3.4. Hippocampus and mPFC LIMK1/Cofilin Are Activated After Underwater Trauma to Develop PTSD-like Behavior
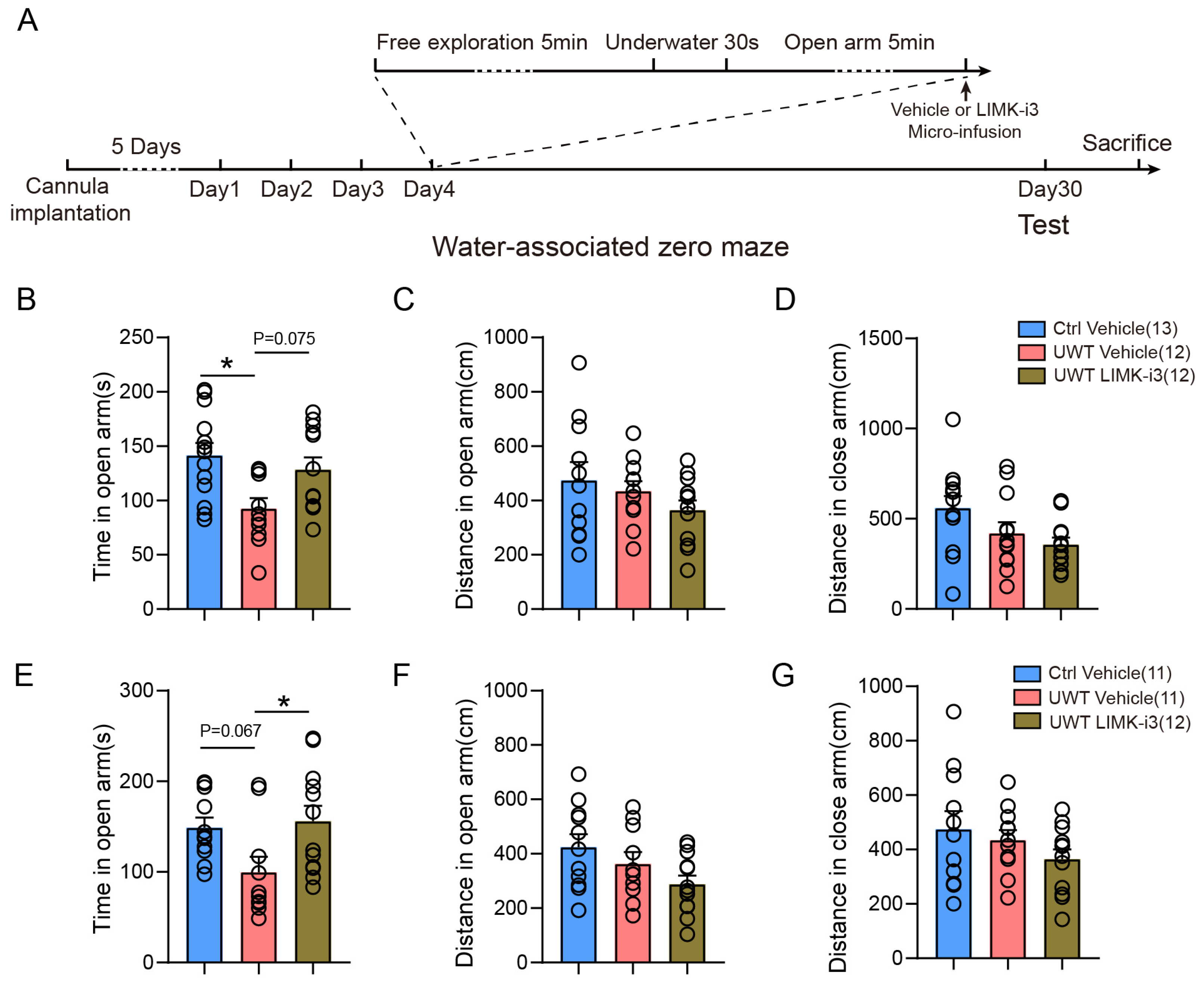
3.5. Hippocampus and mPFC LIMK1 Inhibition During Memory Consolidation in Reducing Trauma-Induced PTSD-like Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Squire, L.R.; Genzel, L.; Wixted, J.T.; Morris, R.G. Memory consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021766. [Google Scholar] [CrossRef]
- Staresina, B.P. Coupled sleep rhythms for memory consolidation. Trends Cogn. Sci. 2024, 28, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, T.; Gal-Ben-Ari, S.; Dieterich, D.C.; Kreutz, M.R.; Ziv, N.E.; Gundelfinger, E.D.; Rosenblum, K. The roles of protein expression in synaptic plasticity and memory consolidation. Front. Mol. Neurosci. 2014, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, R. Actin Cytoskeleton Role in the Maintenance of Neuronal Morphology and Long-Term Memory. Cells 2021, 10, 1795. [Google Scholar] [CrossRef]
- Klinzing, J.G.; Niethard, N.; Born, J. Mechanisms of systems memory consolidation during sleep. Nat. Neurosci. 2019, 22, 1598–1610. [Google Scholar] [CrossRef]
- Skelin, I.; Kilianski, S.; McNaughton, B.L. Hippocampal coupling with cortical and subcortical structures in the context of memory consolidation. Neurobiol. Learn. Mem. 2019, 160, 21–31. [Google Scholar] [CrossRef]
- Khodagholy, D.; Gelinas, J.N.; Buzsáki, G. Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 2017, 358, 369–372. [Google Scholar] [CrossRef]
- Maingret, N.; Girardeau, G.; Todorova, R.; Goutierre, M.; Zugaro, M. Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 2016, 19, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, G.; Eban, E.; Frank, L.M. A cortical-hippocampal-cortical loop of information processing during memory consolidation. Nat. Neurosci. 2017, 20, 251–259. [Google Scholar] [CrossRef]
- Wang, H.; Hu, Y.; Tsien, J.Z. Molecular and systems mechanisms of memory consolidation and storage. Prog. Neurobiol. 2006, 79, 123–135. [Google Scholar] [CrossRef]
- Brodt, S.; Inostroza, M.; Niethard, N.; Born, J. Sleep-A brain-state serving systems memory consolidation. Neuron 2023, 111, 1050–1075. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.; Zou, Y.; Zhang, S.; Xia, M.; Fu, L.; Vollger, M.R.; Chen, N.C.; Taylor, D.J.; Harvey, W.T.; et al. Characterization of large-scale genomic differences in the first complete human genome. Genome Biol. 2023, 24, 157. [Google Scholar] [CrossRef]
- Kozel, B.A.; Barak, B.; Kim, C.A.; Mervis, C.B.; Osborne, L.R.; Porter, M.; Pober, B.R. Williams syndrome. Nat. Rev. Dis. Primers 2021, 7, 42. [Google Scholar] [CrossRef]
- Scott, R.W.; Olson, M.F. LIM kinases: Function, regulation and association with human disease. J. Mol. Med. 2007, 85, 555–568. [Google Scholar] [CrossRef]
- Ben Zablah, Y.; Zhang, H.; Gugustea, R.; Jia, Z. LIM-Kinases in Synaptic Plasticity, Memory, and Brain Diseases. Cells 2021, 10, 2079. [Google Scholar] [CrossRef]
- Todorovski, Z.; Asrar, S.; Liu, J.; Saw, N.M.; Joshi, K.; Cortez, M.A.; Snead, O.C., 3rd; Xie, W.; Jia, Z. LIMK1 regulates long-term memory and synaptic plasticity via the transcriptional factor CREB. Mol. Cell Biol. 2015, 35, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhang, Y.; Tregoubov, V.; Janus, C.; Cruz, L.; Jackson, M.; Lu, W.Y.; MacDonald, J.F.; Wang, J.Y.; Falls, D.L.; et al. Abnormal spine morphology and enhanced LTP in LIMK-1 knockout mice. Neuron 2002, 35, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; He, G.; Zhang, X.; Chen, L.; Kong, Y.; Xie, W.; Jia, Z.; Liu, W.T.; Zhou, Z. Transient inhibition of LIMKs significantly attenuated central sensitization and delayed the development of chronic pain. Neuropharmacology 2017, 125, 284–294. [Google Scholar] [CrossRef]
- Meng, Y.; Takahashi, H.; Meng, J.; Zhang, Y.; Lu, G.; Asrar, S.; Nakamura, T.; Jia, Z. Regulation of ADF/cofilin phosphorylation and synaptic function by LIM-kinase. Neuropharmacology 2004, 47, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.T.; Wang, Y.; Liu, L.; Wang, J.L.; Wang, Y.J.; Guan, W.; Chen, T.T.; Zhao, J.; Jiang, B. LIMK1/2 in the mPFC Plays a Role in Chronic Stress-Induced Depressive-Like Effects in Mice. Int. J. Neuropsychopharmacol. 2020, 23, 821–836. [Google Scholar] [CrossRef]
- Lunardi, P.; Sachser, R.M.; Sierra, R.O.; Pedraza, L.K.; Medina, C.; de la Fuente, V.; Romano, A.; Quillfeldt, J.A.; de Oliveira Alvares, L. Effects of Hippocampal LIMK Inhibition on Memory Acquisition, Consolidation, Retrieval, Reconsolidation, and Extinction. Mol. Neurobiol. 2018, 55, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Ressler, K.J.; Berretta, S.; Bolshakov, V.Y.; Rosso, I.M.; Meloni, E.G.; Rauch, S.L.; Carlezon, W.A., Jr. Post-traumatic stress disorder: Clinical and translational neuroscience from cells to circuits. Nat. Rev. Neurol. 2022, 18, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Bryant, R.A. Post-traumatic stress disorder: A state-of-the-art review of evidence and challenges. World Psychiatry 2019, 18, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.J.; Lien, J.C.; Wu, C.R. Puerarin Attenuates Cycloheximide-Induced Oxidative Damage and Memory-Consolidation Impairment in Rats. J. Integr. Neurosci. 2024, 23, 17. [Google Scholar] [CrossRef]
- Natraj, N.; Neylan, T.C.; Yack, L.M.; Metzler, T.J.; Woodward, S.H.; Hubachek, S.Q.; Dukes, C.; Udupa, N.S.; Mathalon, D.H.; Richards, A. Sleep Spindles Favor Emotion Regulation Over Memory Consolidation of Stressors in Posttraumatic Stress Disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2023, 8, 899–908. [Google Scholar] [CrossRef]
- van der Heijden, A.C.; Hofman, W.F.; de Boer, M.; Nijdam, M.J.; van Marle, H.J.F.; Jongedijk, R.A.; Olff, M.; Talamini, L.M. Sleep spindle dynamics suggest over-consolidation in post-traumatic stress disorder. Sleep 2022, 45, zsac139. [Google Scholar] [CrossRef]
- Mariottini, C.; Munari, L.; Gunzel, E.; Seco, J.M.; Tzavaras, N.; Hansen, J.; Stern, S.A.; Gao, V.; Aleyasin, H.; Sharma, A.; et al. Wilm’s tumor 1 promotes memory flexibility. Nat. Commun. 2019, 10, 3756. [Google Scholar] [CrossRef]
- Barchiesi, R.; Chanthongdee, K.; Petrella, M.; Xu, L.; Söderholm, S.; Domi, E.; Augier, G.; Coppola, A.; Wiskerke, J.; Szczot, I.; et al. An epigenetic mechanism for over-consolidation of fear memories. Mol. Psychiatry 2022, 27, 4893–4904. [Google Scholar] [CrossRef] [PubMed]
- Vogel-Ciernia, A.; Wood, M.A. Examining object location and object recognition memory in mice. Curr. Protoc. Neurosci. 2014, 69, 8.31.1–17. [Google Scholar] [CrossRef]
- Yuan, J.; Chang, S.-Y.; Yin, S.-G.; Liu, Z.-Y.; Cheng, X.; Liu, X.-J.; Jiang, Q.; Gao, G.; Lin, D.-Y.; Kang, X.-L. Two conserved epigenetic regulators prevent healthy ageing. Nature 2020, 579, 118–122. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, X.; Zhao, Q.; Li, C.; Zhang, T.; Yang, X. Acute stress promotes brain oscillations and hippocampal-cortical dialog in emotional processing. Biochem. Biophys. Res. Commun. 2022, 598, 55–61. [Google Scholar] [CrossRef]
- Ritov, G.; Richter-Levin, G. Water associated zero maze: A novel rat test for long term traumatic re-experiencing. Front. Behav. Neurosci. 2014, 8, 1. [Google Scholar] [CrossRef]
- Yang, N.; Higuchi, O.; Ohashi, K.; Nagata, K.; Wada, A.; Kangawa, K.; Nishida, E.; Mizuno, K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 1998, 393, 809–812. [Google Scholar] [CrossRef]
- Arber, S.; Barbayannis, F.A.; Hanser, H.; Schneider, C.; Stanyon, C.A.; Bernard, O.; Caroni, P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998, 393, 805–809. [Google Scholar] [CrossRef]
- Edwards, D.C.; Sanders, L.C.; Bokoch, G.M.; Gill, G.N. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat. Cell Biol. 1999, 1, 253–259. [Google Scholar] [CrossRef]
- Bailey, C.H.; Kandel, E.R.; Harris, K.M. Structural Components of Synaptic Plasticity and Memory Consolidation. Cold Spring Harb. Perspect. Biol. 2015, 7, a021758. [Google Scholar] [CrossRef]
- Bhasin, B.J.; Raymond, J.L.; Goldman, M.S. Synaptic weight dynamics underlying memory consolidation: Implications for learning rules, circuit organization, and circuit function. Proc. Natl. Acad. Sci. USA 2024, 121, e2406010121. [Google Scholar] [CrossRef] [PubMed]
- Takehara-Nishiuchi, K. Neurobiology of systems memory consolidation. Eur. J. Neurosci. 2021, 54, 6850–6863. [Google Scholar] [CrossRef] [PubMed]
- Cuberos, H.; Vallée, B.; Vourc’h, P.; Tastet, J.; Andres, C.R.; Bénédetti, H. Roles of LIM kinases in central nervous system function and dysfunction. FEBS Lett. 2015, 589, 3795–3806. [Google Scholar] [CrossRef]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, Y.; Koymans, K.J.; Poe, G.R.; Kessels, H.W.; Van Someren, E.J.W.; Wassing, R. Overnight neuronal plasticity and adaptation to emotional distress. Nat. Rev. Neurosci. 2024, 25, 253–271. [Google Scholar] [CrossRef]
- Girardeau, G.; Lopes-Dos-Santos, V. Brain neural patterns and the memory function of sleep. Science 2021, 374, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Kjaerby, C.; Andersen, M.; Hauglund, N.; Untiet, V.; Dall, C.; Sigurdsson, B.; Ding, F.; Feng, J.; Li, Y.; Weikop, P.; et al. Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nat. Neurosci. 2022, 25, 1059–1070. [Google Scholar] [CrossRef]
- Siegel, J.M. The REM sleep-memory consolidation hypothesis. Science 2001, 294, 1058–1063. [Google Scholar] [CrossRef]
- Geva-Sagiv, M.; Mankin, E.A.; Eliashiv, D.; Epstein, S.; Cherry, N.; Kalender, G.; Tchemodanov, N.; Nir, Y.; Fried, I. Augmenting hippocampal-prefrontal neuronal synchrony during sleep enhances memory consolidation in humans. Nat. Neurosci. 2023, 26, 1100–1110. [Google Scholar] [CrossRef]
- Steadman, P.E.; Xia, F.; Ahmed, M.; Mocle, A.J.; Penning, A.R.A.; Geraghty, A.C.; Steenland, H.W.; Monje, M.; Josselyn, S.A.; Frankland, P.W. Disruption of Oligodendrogenesis Impairs Memory Consolidation in Adult Mice. Neuron 2020, 105, 150–164.e6. [Google Scholar] [CrossRef] [PubMed]
- Dahal, P.; Rauhala, O.J.; Khodagholy, D.; Gelinas, J.N. Hippocampal-cortical coupling differentiates long-term memory processes. Proc. Natl. Acad. Sci. USA 2023, 120, e2207909120. [Google Scholar] [CrossRef] [PubMed]
- Zhurakovskaya, E.; Ishchenko, I.; Gureviciene, I.; Aliev, R.; Gröhn, O.; Tanila, H. Impaired hippocampal-cortical coupling but preserved local synchrony during sleep in APP/PS1 mice modeling Alzheimer’s disease. Sci. Rep. 2019, 9, 5380. [Google Scholar] [CrossRef]
- Havekes, R.; Park, A.J.; Tudor, J.C.; Luczak, V.G.; Hansen, R.T.; Ferri, S.L.; Bruinenberg, V.M.; Poplawski, S.G.; Day, J.P.; Aton, S.J.; et al. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. eLife 2016, 5, e13424. [Google Scholar] [CrossRef]
- Medina, C.; de la Fuente, V.; Tom Dieck, S.; Nassim-Assir, B.; Dalmay, T.; Bartnik, I.; Lunardi, P.; de Oliveira Alvares, L.; Schuman, E.M.; Letzkus, J.J.; et al. LIMK, Cofilin 1 and actin dynamics involvement in fear memory processing. Neurobiol. Learn. Mem. 2020, 173, 107275. [Google Scholar] [CrossRef]
- Holland, P.; Lewis, P.A. Emotional memory: Selective enhancement by sleep. Curr. Biol. 2007, 17, R179–R181. [Google Scholar] [CrossRef]
- Murkar, A.L.A.; De Koninck, J. Consolidative mechanisms of emotional processing in REM sleep and PTSD. Sleep. Med. Rev. 2018, 41, 173–184. [Google Scholar] [CrossRef]
- Ritov, G.; Ardi, Z.; Richter-Levin, G. Differential activation of amygdala, dorsal and ventral hippocampus following an exposure to a reminder of underwater trauma. Front. Behav. Neurosci. 2014, 8, 18. [Google Scholar] [CrossRef]
- Buzsáki, G. The hippocampo-neocortical dialogue. Cereb. Cortex 1996, 6, 81–92. [Google Scholar] [CrossRef]
- Ji, D.; Wilson, M.A. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat. Neurosci. 2007, 10, 100–107. [Google Scholar] [CrossRef]
- Peyrache, A.; Khamassi, M.; Benchenane, K.; Wiener, S.I.; Battaglia, F.P. Replay of rule-learning related neural patterns in the prefrontal cortex during sleep. Nat. Neurosci. 2009, 12, 919–926. [Google Scholar] [CrossRef]
- Siapas, A.G.; Wilson, M.A. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron 1998, 21, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Rust, M.B.; Gurniak, C.B.; Renner, M.; Vara, H.; Morando, L.; Görlich, A.; Sassoè-Pognetto, M.; Banchaabouchi, M.A.; Giustetto, M.; Triller, A.; et al. Learning, AMPA receptor mobility and synaptic plasticity depend on n-cofilin-mediated actin dynamics. Embo J. 2010, 29, 1889–1902. [Google Scholar] [CrossRef]
- Niethard, N.; Ngo, H.V.; Ehrlich, I.; Born, J. Cortical circuit activity underlying sleep slow oscillations and spindles. Proc. Natl. Acad. Sci. USA 2018, 115, E9220–E9229. [Google Scholar] [CrossRef] [PubMed]
- Berabez, R.; Routier, S.; Bénédetti, H.; Plé, K.; Vallée, B. LIM Kinases, Promising but Reluctant Therapeutic Targets: Chemistry and Preclinical Validation In Vivo. Cells 2022, 11, 2090. [Google Scholar] [CrossRef] [PubMed]
- Park, J.B.; Agnihotri, S.; Golbourn, B.; Bertrand, K.C.; Luck, A.; Sabha, N.; Smith, C.A.; Byron, S.; Zadeh, G.; Croul, S.; et al. Transcriptional profiling of GBM invasion genes identifies effective inhibitors of the LIM kinase-Cofilin pathway. Oncotarget 2014, 5, 9382–9395. [Google Scholar] [CrossRef]
- Astill Wright, L.; Horstmann, L.; Holmes, E.A.; Bisson, J.I. Consolidation/reconsolidation therapies for the prevention and treatment of PTSD and re-experiencing: A systematic review and meta-analysis. Transl. Psychiatry 2021, 11, 453. [Google Scholar] [CrossRef]
- Cowan, E.T.; Schapiro, A.C.; Dunsmoor, J.E.; Murty, V.P. Memory consolidation as an adaptive process. Psychon. Bull. Rev. 2021, 28, 1796–1810. [Google Scholar] [CrossRef]
- Brewin, C.R. Memory and forgetting. Curr. Psychiatry Rep. 2018, 20, 87. [Google Scholar] [CrossRef]
- Jiang, Y.; Shi, J.; Tai, J.; Yan, L. Circadian Regulation in Diurnal Mammals: Neural Mechanisms and Implications in Translational Research. Biology 2024, 13, 958. [Google Scholar] [CrossRef] [PubMed]
- Maguire, E.A. Memory consolidation in humans: New evidence and opportunities. Exp. Physiol. 2014, 99, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Vorster, A.P.; Born, J. Sleep and memory in mammals, birds and invertebrates. Neurosci. Biobehav. Rev. 2015, 50, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Leake, J.; Cardona, L.S.; Mencevski, F.; Westbrook, R.F.; Holmes, N.M. Context and Time Regulate Fear Memory Consolidation and Reconsolidation in the Basolateral Amygdala Complex. J. Neurosci. 2024, 44, e1698232023. [Google Scholar] [CrossRef]
- Roesler, R.; Parent, M.B.; LaLumiere, R.T.; McIntyre, C.K. Amygdala-hippocampal interactions in synaptic plasticity and memory formation. Neurobiol. Learn. Mem. 2021, 184, 107490. [Google Scholar] [CrossRef]
- Bruzsik, B.; Biro, L.; Zelena, D.; Sipos, E.; Szebik, H.; Sarosdi, K.R.; Horvath, O.; Farkas, I.; Csillag, V.; Finszter, C.K.; et al. Somatostatin Neurons of the Bed Nucleus of Stria Terminalis Enhance Associative Fear Memory Consolidation in Mice. J. Neurosci. 2021, 41, 1982–1995. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Wu, Z.; Wang, Z.; Wang, L.; Xia, S.; Li, W.; He, G. LIMK1 Deficiency Disrupts Hippocampal–Cortical Memory Consolidation and Attenuates Trauma-Induced PTSD-like Behavior. Biology 2025, 14, 1560. https://doi.org/10.3390/biology14111560
Yang X, Wu Z, Wang Z, Wang L, Xia S, Li W, He G. LIMK1 Deficiency Disrupts Hippocampal–Cortical Memory Consolidation and Attenuates Trauma-Induced PTSD-like Behavior. Biology. 2025; 14(11):1560. https://doi.org/10.3390/biology14111560
Chicago/Turabian StyleYang, Xiangyu, Zhengping Wu, Ziying Wang, Lihui Wang, Shuting Xia, Weidong Li, and Guiqin He. 2025. "LIMK1 Deficiency Disrupts Hippocampal–Cortical Memory Consolidation and Attenuates Trauma-Induced PTSD-like Behavior" Biology 14, no. 11: 1560. https://doi.org/10.3390/biology14111560
APA StyleYang, X., Wu, Z., Wang, Z., Wang, L., Xia, S., Li, W., & He, G. (2025). LIMK1 Deficiency Disrupts Hippocampal–Cortical Memory Consolidation and Attenuates Trauma-Induced PTSD-like Behavior. Biology, 14(11), 1560. https://doi.org/10.3390/biology14111560





