1. Introduction
Antarctic krill (
Euphausia superba, hereafter krill), a keystone species in the Southern Ocean, plays an important role in the food-web [
1,
2], mainly because it is a major component of the diet of marine mammals, birds, and fishes [
3,
4], and it also contributes to biogeochemical cycles [
5,
6] and carbon flux in the Southern Ocean [
7,
8,
9]. Krill is one of the largest and longest-lived epipelagic zooplankton in the world, with an average length of more than 60 mm and a lifespan of more than six years [
9,
10]. They are widely distributed in the waters around Antarctica, but mostly abundant in the Atlantic sector of the Southern Ocean, where the biomass of krill resources account for approximately 30% of the total [
11,
12].
Krill are one of the most abundant metazoan species in the world [
13], whose biomass has been estimated at between 300 and 500 million tonnes in Antarctic waters [
11], and it is also the main target species in the largest fishery in the Southern Ocean [
14]. The krill commercial fishery started in the early 1970s [
15], which has been going on for more than 50 years, with great fluctuation in the spatio-temporal distribution of fishing grounds and catches (
Figure 1). Since the late 1990s, krill fishing activities have been almost entirely concentrated in the Antarctic Peninsula and South Shetland Islands (SSI), as well as South Georgia and the South Orkney Islands [
16,
17,
18]. The Bransfield Strait (BS), located between the SSI and the West Antarctic Peninsula (WAP), is both an important spawning ground [
19] and a major fishing ground [
20,
21].
The WAP is one of the areas under faster warming rates worldwide [
22,
23], with an average atmospheric temperature increase of 6 °C since 1950 [
24], resulting in a continuous deterioration of sea ice coverage [
25,
26,
27]. Climate warming and the reduction in the amount of sea ice may lead to a dramatic decrease in habitat quality [
28,
29,
30] and the relative gross growth potential (RGGP) [
31] of krill, thereby causing krill habitats and fishing grounds to shift to higher latitudes in the WAP [
18,
21,
31]. Against the background of climate warming, most of the fishery management policies and studies focus on the changes in the biomass, abundance and distribution of krill resources [
32,
33,
34], ignoring the composition and changes in the structure of krill populations, especially the relationship between the population structure and krill density. However, the size and population structure of krill should be given sufficient attention by managers.
Body length is very important for fishery management and resource assessment, because they can not only show the growth status of fish, but also be the key indicator to assess the status of fish population in some resource assessment models. However, it is worth noting that the size of krill has not been regarded as a management index in krill fishery management, which leads to our limited understanding of the growth of the krill population, especially its impact on the ecosystem. Studies have shown that krill is the main food source of penguins, which account for 100% of the diet of
Pygoscelis penguins in the WAP [
35]. Among them, gentoo and chinstrap penguins prefer larger krill, even though small krill individuals are dominant in their habitat waters [
36]. Furthermore, the krill body size exerts a dominant control on the particulate organic carbon (POC) flux, which oscillates synchronously with krill body length [
9]. Therefore, it is necessary to pay attention to the changes in the length of krill for the protection of ecology and the guidance of fishery production. Due to the lack of attention to krill body size in the past, it is a challenge for Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) to balance the relationships among the dynamics of krill biomass, fishing ground, and the population structure under the premise of climate warming.
The CCAMLR is the sole multilateral body responsible for managing marine living resources under the framework of The Antarctic Treaty. It undertakes key tasks such as formulating fishery resource conservation measures, reviewing fishing applications, and releasing statistical data. The Ecosystem-Based Quota Management System is currently the primary management approach for the Antarctic marine ecosystem [
37,
38,
39]; for krill, this means setting differentiated quotas based on variations in krill biomass, natural resource fluctuations, and predator distribution (such as seabirds, seals, whales, and penguins) across different statistical subareas [
40]. The sustainable development of the krill fishery is safeguarded through the implementation of fishery restrictions. These restrictions ensure that a sufficient quantity of krill remains after fishing operations, thereby maintaining an adequate spawning stock and providing sufficient prey for predators [
41]. The sustainability of the krill fishery is contingent upon the size of the catch relative to the krill population [
39]. However, the current krill management overlooks the impacts of changes in krill population structure on the entire ecosystem, which constitutes a highly research-worthy issue.
Based on midwater trawling data of 6 years, including more than 160,000 krill length data, this study analyzed the distribution of catches, density, length, and age of krill in the BS and SSI. Furthermore, we conducted additional analyses to elucidate the relationship between krill length and environmental variables using a Generalized Additive Model (GAM), specifically examining how morphological variations in krill populations correlate with gradients in key abiotic factors across sampled habitats. This study found that there was a correlation between the krill body size and the depth and temperature of the water layer of krill cluster, krill density, year, month, latitude, and longitude. Although there is no direct evidence at present that the size of krill has a negative impact on the ecosystem, whether it should be added into fishery management needs to be considered carefully by CCAMLR.
4. Discussion
The BS and the SSI constitute the core distribution area of Antarctic krill and a hotspot for commercial fishing [
20]. However, dramatic environmental changes in the region in recent years have raised significant ecological concerns: sea surface temperature (SST) has continued to rise at a rate of 0.31 °C per decade since the beginning of the 21st century [
53], and the extent of winter sea ice cover has shown a significant contraction since 2016 [
54]. In the Southwest Atlantic sector, Antarctic krill distribution has exhibited a persistent southward contraction over the past nine decades. Along its northern boundary, population density has undergone a sharp decline, with the stock becoming increasingly concentrated on the Antarctic continental shelf [
18,
21]. Under this climatic regime, commercial fishing grounds have demonstrated progressive poleward latitudinal shifts [
18,
21,
55], mirroring krill population redistribution toward higher latitudes where remnant sea ice persists. The southward shift of the Antarctic krill fishing ground’s center of gravity is the most intuitive response to climate warming, which has led to the southward movement of krill’s suitable habitats [
55] and will also exert a significant impact on the Antarctic marine ecosystem. The southward shift of the krill fishing ground’s center of gravity is bound to increase fishing effort, which in turn will raise the pressure on the marine ecosystem in this sea area to maintain biodiversity. Therefore, incorporating climate-driven changes in krill habitats into the sustainable fishery management framework is crucial.
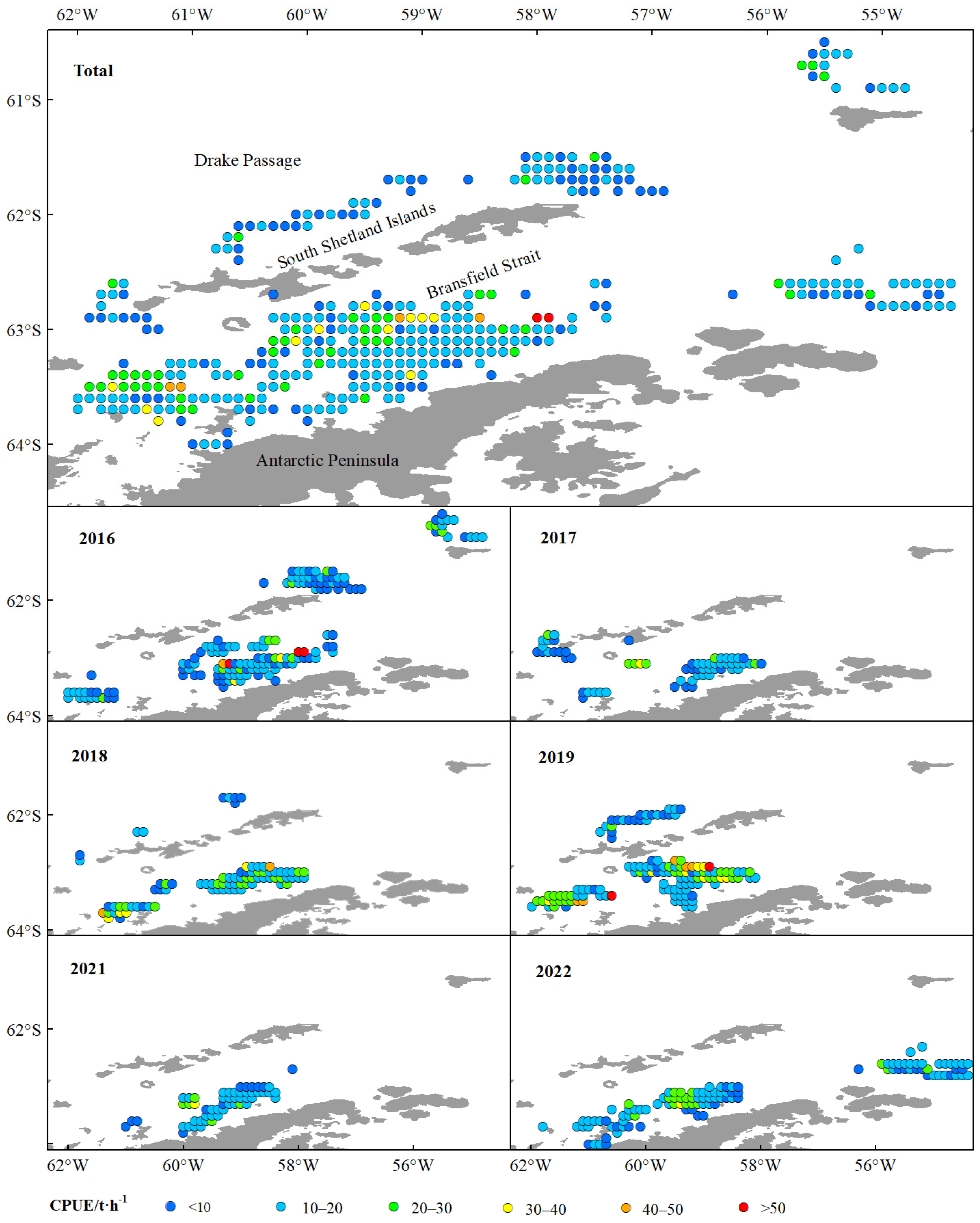
This study showed that krill green weight and fishing effort were significantly higher in the BS than in the Drake Passage, and that the area of high green weight values was concentrated in the southern end of the BS adjacent to the continental shelf of the Antarctic Peninsula (
Figure 2 and
Figure 5). This spatial pattern is closely linked to the habitat preferences of Antarctic krill, as continental shelf margins and upwelling zones typically exhibit higher primary productivity, providing abundant food resources for krill [
56,
57]. Further analysis indicated that the high-value areas of CPUE and krill density were primarily distributed in the BS region rather than the Drake Passage (
Figure 4 and
Figure 5). This difference may originate from the more stable hydrological conditions (e.g., circulation systems) and higher resource aggregation in the BS, which significantly enhance fishing efficiency [
14]. Quantitatively, the correlation between krill swarm distribution and catch volume (r = 0.767) was significantly stronger than that between CPUE and catch (r = 0.505) (
Figure 6,
Table 5), validating density as more reliable indicators of fishable aggregations than effort-adjusted catch rates. This inconsistency may reflect the sensitivity of CPUE data to fishing strategies (e.g., dynamic adjustments of fishing vessel operations), whereas density estimates more directly reflect the actual distribution of biomass [
58,
59].
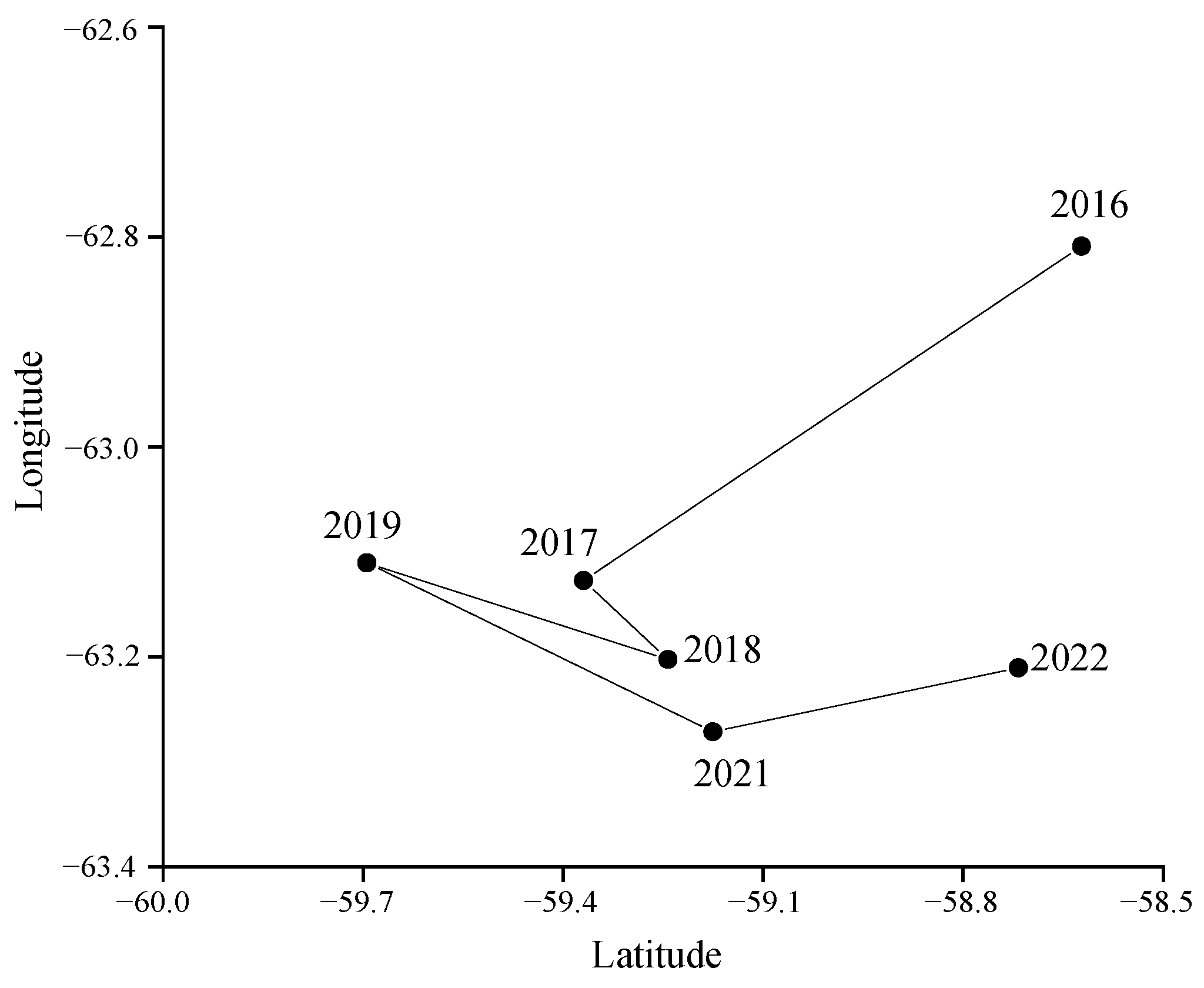
Although there were no statistically significant differences in mean annual fluctuations in catch, CPUE, and density between 2016 and 2022 (
Figure 6), trends in the southward shift of the fishery (
Figure 9) suggest potential climate-driven mechanisms. For example, rapid warming (0.31 °C/decade) [
53] in the waters off the western side of the Antarctic Peninsula, coupled with plummeting winter sea ice cover (~20% reduction after 2016) [
54], may indirectly affect krill distribution boundaries by altering plankton community structure or ice algal productivity. It is crucial to note that food competition and SST anomalies may drive reductions in Antarctic krill abundance, coupled with their southward shifts toward the Antarctic Peninsula or migration to deeper, colder habitats [
60]. Furthermore, environmental perturbations—such as SST anomalies or shifts in water masses across depth gradients—could act as indirect drivers of ecosystem destabilization, altering the composition and biomass of zooplankton communities in regions like the Bransfield Strait [
61]. However, the time span of the current data (6 years) may not be sufficient to capture long-term trends, and longer time series of environmental and harvest data need to be combined to verify causal associations.
In this study, the spatial and temporal heterogeneity of krill body length distributions and its driving mechanisms were systematically resolved for the first time by a GAM based on 6 years of mesocosm trawl data (covering 160,000 individual body length measurements) from BS and SSI. The results of model residual analysis and spatial cross-validation showed that the GAM was able to effectively capture nonlinear environmental responses and provided a reliable tool for resolving complex ecological relationships. The results showed that the spatial distribution of krill body length showed significant nonlinear characteristics, with an extreme point at 62.5° S on the latitudinal gradient (
p < 0.01), while the longitudinal extremes were concentrated around 60° W. This distribution pattern may be closely linked to regional ocean dynamical processes: the 57° W sea area is located at the entrance to BS on the eastern side of the SSI, which is subject to the interaction of the Antarctic Circumpolar Current branch with land-slope upwelling to form a highly productive frontal system [
62,
63,
64], which provides a steady supply of bait for krill clusters. At the same time, the peak body length on the latitudinal gradient (62° S) was highly coincident with the edge of summer sea ice retreat, supporting an adaptive strategy of small individuals to the ecological niche of the ice-marginal zone. It is noteworthy that the mean body length of krill individuals caught in the high-latitude region (>63° S) was lower than in the northern fishing ground, which may be related to the dependence of larvae on sea ice microhabitats (e.g., ice algal resources) [
65]. Positive correlations of krill body length with depth (r = 0.36,
p < 0.01) and temperature (r = 0.26,
p < 0.01) reveal the ecological advantages of the deep-water layer for large individuals, where lower predation pressure and a stable thermal environment in deeper water may promote individual survival and growth [
66]. Aggregation of large individuals in deeper water may be related to a gradient in predation pressure, and reduced light availability in deeper waters reduces the predation efficiency of seabirds and cetaceans. The negative correlation between krill body length and density (r = −0.22,
p < 0.01), which was similar to the results of [
9], and its “parabola-shaped” density-dependent curve, reflected the dynamic balance between intra-population competition and resource availability. In low-density areas (<0.2 kg/m
2), the abundance of resources may promote individual growth. However, when population density exceeds this threshold, adult individuals likely achieve ecological niche partitioning through vertical migration to deeper water layers.
While this study elucidates multiscale drivers of Antarctic krill size distribution, the following limitations warrant consideration: 1. Temporal Resolution Constraints. The six-year dataset remains insufficient to resolve long-term climate oscillation effects. Decadal-scale mechanistic analysis requires the integration of historical specimen archives spanning the climate phases of more years. 2. Vertical Migration Mechanism Gaps. Trawl sampling biases toward krill-intensive areas and may have underestimated the biomass of large individual krill in deeper waters. Future efforts should incorporate underwater glider hydroacoustic surveys to quantify diel vertical migration-driven size stratification. 3. Multifactorial Interaction Oversights. The synergistic effects between sea ice coverage and climate change remain unquantified. Mechanistic disentanglement necessitates coupled biogeochemical models with ice-ocean atmosphere-forcing modules.
5. Conclusions
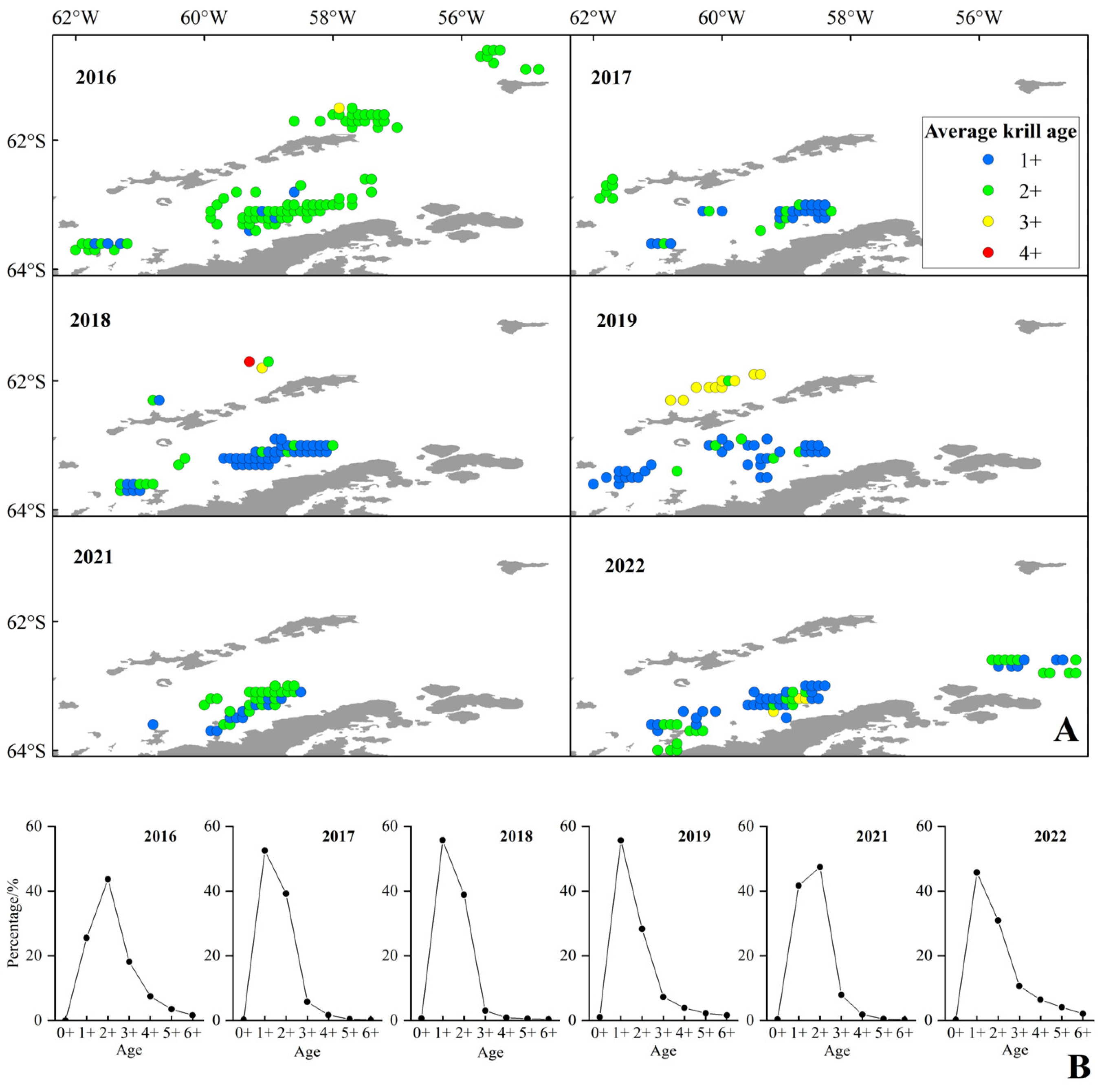
The key findings of this research are as follows: First, krill fishing grounds showed a significant southward-shifting trend, with smaller krill preferring ice-rich areas at higher southern latitudes, while commercial fishing targeted high-density krill areas rather than selecting larger individuals. Notably, the continuous increase in fishing effort in recent years did not lead to a reduction in krill body size. Second, the mean body length of krill had a significant positive correlation with the depth and temperature of krill clusters, and a significant negative correlation with density and latitude; body size also exhibited nonlinear relationships with latitude, longitude, and temperature (increasing first then stabilizing with temperature). Additionally, the krill population in the study area was dominated by juvenile individuals, with 1+ and 2+ age classes accounting for 82.2% of the total samples. The southward shift of Antarctic krill fishing grounds caused by climate warming should be given sufficient attention, as it exerts a significant impact on the Antarctic marine ecosystem. CCAMLR should incorporate this situation into its ecological management framework to achieve the goals of protecting the Antarctic marine ecology and promoting the sustainable development of the krill fishery.
This study provides critical insights into the population dynamics of Antarctic krill in the BS and SSI waters, and highlights that environmental factors, geographical location, and krill density jointly shape krill body size and population structure. Given the ecological importance of krill body size and the ongoing climate warming in the Antarctic Peninsula, it is recommended that the CCAMLR consider incorporating krill body size into fishery management indicators to balance the sustainability of krill fisheries and the stability of the Southern Ocean ecosystem. We must point out that changes in the krill population structure require longer-term data accumulation. Additionally, it is also necessary to incorporate other indicators, such as sea ice coverage rate and ocean currents, to conduct more in-depth research.