Exercise as Osteoarthritis Treatment in Wistar Rats Promotes Frequency-Dependent Benefits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Osteoarthritis Model
2.3. Exercise Protocols
2.4. Euthanasia and Tissue Preparation
2.5. Analysis
2.5.1. Metabolic Activity
Citrate Synthase
Succinate Dehydrogenase
Complex I
Complex II
2.5.2. Weight Gain Assessment
2.5.3. Oxidants
2.5.4. Markers of Oxidative Damage
2.5.5. Antioxidant Enzyme Activities
2.5.6. Protein Content Determination
2.5.7. Pro-Inflammatory and Anti-Inflammatory Mediators
2.5.8. Gene Expression
2.5.9. Tissue Morphology
2.5.10. Histology
2.5.11. Statistical Analysis
3. Results
3.1. Metabolic Activity
3.2. Weight Gain
3.3. Oxidative Stress
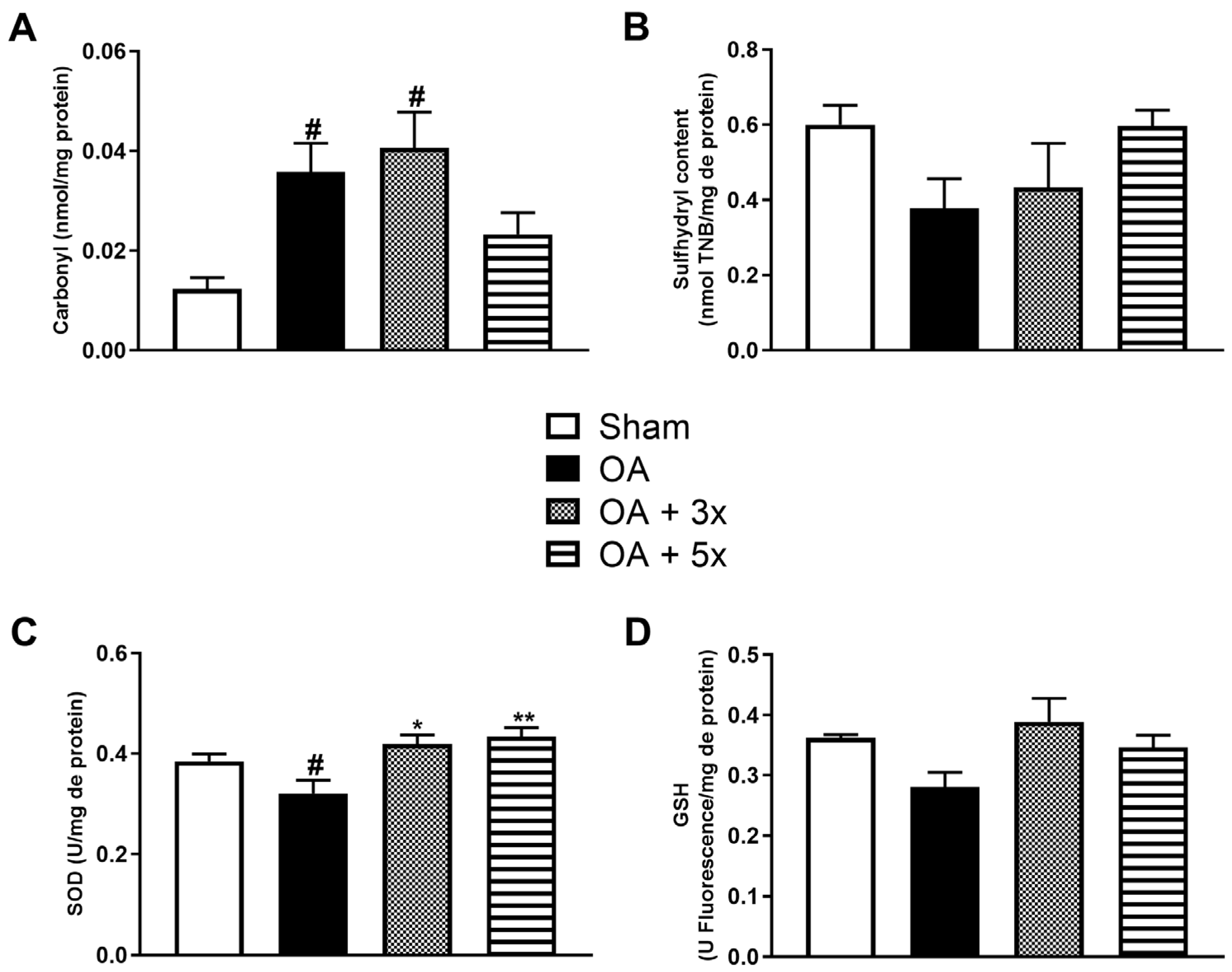
3.3.1. Oxidants
3.3.2. Oxidative Damage and Antioxidants
3.4. Inflammatory Mediators
3.4.1. Pro-Inflammatory Cytokines
3.4.2. Anti-Inflammatory Cytokines
3.5. Gene Expression
3.6. Tissue Morphology
3.7. Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ding, D.-F.; Xue, Y.; Wu, X.-C.; Zhu, Z.-H.; Ding, J.-Y.; Song, Y.-J.; Xu, X.-L.; Xu, J.-G. Recent advances in reactive oxygen species (ros)-responsive polyfunctional nanosystems 3.0 for the treatment of osteoarthritis. J. Inflamm. Res. 2022, 15, 5009–5026. [Google Scholar] [CrossRef]
- Hunter, D.J.; Felson, D.T. Osteoarthritis. BMJ 2006, 332, 639–642. [Google Scholar] [CrossRef]
- Lammert, E.; Zeeb, M. Metabolism of Human Diseases: Organ Physiology and Pathophysiology; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Iijima, H.; Aoyama, T.; Ito, A.; Yamaguchi, S.; Nagai, M.; Tajino, J.; Zhang, X.; Kuroki, H. Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1563–1574. [Google Scholar] [CrossRef]
- Rausch Osthoff, A.K.; Niedermann, K.; Braun, J.; Adams, J.; Brodin, N.; Dagfinrud, H.; Duruoz, T.; Esbensen, B.A.; Günther, K.P.; Hurkmans, E.; et al. 2018 EULAR recommendations for physical activity in people with inflammatory arthritis and osteoarthritis. Ann. Rheum. Dis. 2018, 77, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.A.; Thirupathi, A.; Colares, M.C.; Haupenthal, D.; Venturini, L.M.; Corrêa, M.; Silveira, G.B.; Haupenthal, A.; do Bomfim, F.R.C.; de Andrade, T.A.M.; et al. The effectiveness of treadmill and swimming exercise in an animal model of osteoarthritis. Front. Physiol. 2023, 14, 1101159. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 american college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res. 2020, 72, 149–162. [Google Scholar] [CrossRef]
- Wittenauer, R.; Smith, L.; Aden, K. Background Paper 6.12 Osteoarthritis; World Health Organisation: Geneva, Switzerland, 2013. [Google Scholar]
- Iijima, H.; Aoyama, T.; Tajino, J.; Ito, A.; Nagai, M.; Yamaguchi, S.; Zhang, X.; Kiyan, W.; Kuroki, H. Subchondral plate porosity colocalizes with the point of mechanical load during ambulation in a rat knee model of post-traumatic osteoarthritis. Osteoarthr. Cartil. 2016, 24, 354–363. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Di Rosa, M.; Ravalli, S.; Castorina, A.; Guglielmino, C.; Imbesi, R.; Vecchio, M.; Drago, F.; Szychlinska, M.A.; Musumeci, G. Moderate physical activity as a prevention method for knee osteoarthritis and the role of synoviocytes as biological key. Int. J. Mol. Sci. 2019, 20, 511. [Google Scholar] [CrossRef]
- Iijima, H.; Ito, A.; Nagai, M.; Tajino, J.; Yamaguchi, S.; Kiyan, W.; Nakahata, A.; Zhang, J.; Wang, T.; Aoyama, T.; et al. Physiological exercise loading suppresses post-traumatic osteoarthritis progression via an increase in bone morphogenetic proteins expression in an experimental rat knee model. Osteoarthr. Cartil. 2017, 25, 964–975. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Aoyama, T.; Ito, A.; Nagai, M.; Iijima, H.; Zhang, X.; Tajino, J.; Kuroki, H. Effects of exercise level on biomarkers in a rat knee model of osteoarthritis. J. Orthop. Res. 2013, 31, 1026–1031. [Google Scholar] [CrossRef]
- Allen, J.; Imbert, I.; Havelin, J.; Henderson, T.; Stevenson, G.; Liaw, L.; King, T. Effects of treadmill exercise on advanced osteoarthritis pain in rats. Arthritis Rheumatol. 2017, 69, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory effects of high and moderate intensity exercise—A systematic review. Front. Physiol. 2019, 10, 1550. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Sun, Y.; Woods, J.A. Exercise and the regulation of inflammatory responses. Prog. Mol. Biol. Transl. Sci. 2015, 135, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Gomes, E.C.; Silva, A.N.; de Oliveira, M.R. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid. Med. Cell. Longev. 2012, 2012, 756132. [Google Scholar] [CrossRef]
- Cruz, R.; Ramírez, C.; Rojas, O.I.; Casas-Mejía, O.; Kouri, J.B.; Vega-López, M.A. Menisectomized miniature vietnamese pigs develop articular cartilage pathology resembling osteoarthritis. Pathol. Res. Pract. 2015, 211, 829–838. [Google Scholar] [CrossRef]
- Fang, L.; Lin, L.; Lv, Y.; Huang, Z.; Lin, X.; Wang, X.; Chen, B. The mechanism of aerobic exercise combined with glucosamine therapy and circUNK in improving knee osteoarthritis in rabbits. Life Sci. 2021, 275, 119375. [Google Scholar] [CrossRef]
- Griffin, T.M.; Batushansky, A.; Hudson, J.; Lopes, E.B.P. Correlation network analysis shows divergent effects of a long-term, high-fat diet and exercise on early stage osteoarthritis phenotypes in mice. J. Sport. Health Sci. 2020, 9, 119–131. [Google Scholar] [CrossRef]
- Gronau, T.; Krüger, K.; Prein, C.; Aszodi, A.; Gronau, I.; Iozzo, R.V.; Mooren, F.C.; Clausen-Schaumann, H.; Bertrand, J.; Pap, T.; et al. Forced exercise-induced osteoarthritis is attenuated in mice lacking the small leucine-rich proteoglycan decorin. Ann. Rheum. Dis. 2017, 76, 442–449. [Google Scholar] [CrossRef]
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Yamaguchi, S.; Nagai, M.; Kiyan, W.; Zhang, X.; Kuroki, H. Exercise intervention increases expression of bone morphogenetic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthr. Cartil. 2016, 24, 1092–1102. [Google Scholar] [CrossRef]
- Kim, B.J.; Kim, D.W.; Kim, S.H.; Cho, J.H.; Lee, H.J.; Park, D.Y.; Park, S.R.; Choi, B.H.; Min, B.H. Establishment of a reliable and reproducible murine osteoarthritis model. Osteoarthr. Cartil. 2013, 21, 2013–2020. [Google Scholar] [CrossRef] [PubMed]
- Almonte-Becerril, M.; Gimeno, L.I.; Villarroya, O.; Benito-Jardón, M.; Kouri, J.B.; Costell, M. Genetic abrogation of the fibronectin-α5β1 integrin interaction in articular cartilage aggravates osteoarthritis in mice. PLoS ONE 2018, 13, e0198559. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.; Almeida, T.; Milares, L.P.; dos Passos, N.; Araújo, B.; Bublitz, C.; Veronez, S.; Renno, A.C. Musculoskeletal atrophy in an experimental model of knee osteoarthritis: The effects of exercise training and low-level laser therapy. Am. J. Phys. Med. Rehabil. 2015, 94, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.; Milares, L.P.; Almeida, T.; Tim, C.; Magri, A.; Fernandes, K.R.; Medalha, C.; Renno, A.C. Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthr. Cartil. 2016, 24, 169–177. [Google Scholar] [CrossRef]
- Beckett, J.; Jin, W.; Schultz, M.; Chen, A.; Tolbert, D.; Moed, B.R.; Zhang, Z. Excessive running induces cartilage degeneration in knee joints and alters gait of rats. J. Orthop. Res. 2012, 30, 1604–1610. [Google Scholar] [CrossRef]
- Liu, S.S.; Zhou, P.; Zhang, Y. Abnormal expression of key genes and proteins in the canonical wnt/β-catenin pathway of articular cartilage in a rat model of exercise-induced osteoarthritis. Mol. Med. Rep. 2016, 13, 1999–2006. [Google Scholar] [CrossRef]
- Ma, N.; Wang, T.; Bie, L.; Zhao, Y.; Zhao, L.; Zhang, S.; Gao, L.; Xiao, J. Comparison of the effects of exercise with chondroitin sulfate on knee osteoarthritis in rabbits. J. Orthop. Surg. Res. 2018, 13, 16. [Google Scholar] [CrossRef]
- Milares, L.P.; Assis, L.; Siqueira, A.; Claudino, V.; Domingos, H.; Almeida, T.; Tim, C.; Renno, A.C. Effectiveness of an aquatic exercise program and low-level laser therapy on articular cartilage in an experimental model of osteoarthritis in rats. Connect. Tissue Res. 2016, 57, 398–407. [Google Scholar] [CrossRef]
- Miyatake, K.; Muneta, T.; Ojima, M.; Yamada, J.; Matsukura, Y.; Abula, K.; Sekiya, I.; Tsuji, K. Coordinate and synergistic effects of extensive treadmill exercise and ovariectomy on articular cartilage degeneration. BMC Musculoskelet. Disord. 2016, 17, 238. [Google Scholar] [CrossRef]
- Moriyama, H.; Kanemura, N.; Brouns, I.; Pintelon, I.; Adriaensen, D.; Timmermans, J.P.; Ozawa, J.; Kito, N.; Gomi, T.; Deie, M. Effects of aging and exercise training on the histological and mechanical properties of articular structures in knee joints of male rat. Biogerontology 2012, 13, 369–381. [Google Scholar] [CrossRef]
- Peng, K.T.; Liu, J.F.; Chiang, Y.C.; Chen, P.C.; Chiang, M.H.; Shih, H.N.; Chang, P.J.; Lee, C.W. Particulate matter exposure aggravates osteoarthritis severity. Clin. Sci. 2019, 133, 2171–2187. [Google Scholar] [CrossRef]
- Siebelt, M.; Groen, H.C.; Koelewijn, S.J.; de Blois, E.; Sandker, M.; Waarsing, J.H.; Müller, C.; van Osch, G.J.; de Jong, M.; Weinans, H. Increased physical activity severely induces osteoarthritic changes in knee joints with papain induced sulfate-glycosaminoglycan depleted cartilage. Arthritis Res. Ther. 2014, 16, R32. [Google Scholar] [CrossRef]
- Siebelt, M.; Jahr, H.; Groen, H.C.; Sandker, M.; Waarsing, J.H.; Kops, N.; Müller, C.; van Eden, W.; de Jong, M.; Weinans, H. Hsp90 inhibition protects against biomechanically induced osteoarthritis in rats. Arthritis Rheum. 2013, 65, 2102–2112. [Google Scholar] [CrossRef]
- Son, K.M.; Jung, H.A.; Hong, J.I.; Park, I.Y.; Kim, H.A. Development of a mouse model of knee osteoarthritis based on obesity and bipedal walking. J. Orthop. Res. 2019, 37, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Szychlinska, M.A.; Castrogiovanni, P.; Trovato, F.M.; Nsir, H.; Zarrouk, M.; Lo Furno, D.; Di Rosa, M.; Imbesi, R.; Musumeci, G. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 2019, 58, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Takada, S.; Nakamura, E.; Sabanai, K.; Tsukamoto, M.; Otomo, H.; Kanoh, S.; Murai, T.; Fukuda, H.; Okada, Y.; Uchida, S.; et al. Attenuation of post-traumatic osteoarthritis after anterior cruciate ligament injury via inhibition of hedgehog signaling. J. Orthop. Res. 2020, 38, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Gou, J.; Zhang, H.; Lu, J.; Jin, Z.; Jia, S.; Bai, L. The anti-inflammatory effects of 15-hete on osteoarthritis during treadmill exercise. Life Sci. 2021, 273, 119260. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Leal-Junior, E.C.; Frigo, L.; Pallotta, R.C.; Teixeira, S.; de Almeida, P.; Bjordal, J.M.; Lopes-Martins, R. Isolated and combined effects of photobiomodulation therapy, topical nonsteroidal anti-inflammatory drugs, and physical activity in the treatment of osteoarthritis induced by papain. J. Biomed. Opt. 2016, 21, 108001. [Google Scholar] [CrossRef]
- Tomazoni, S.S.; Leal-Junior, E.C.; Pallotta, R.C.; Teixeira, S.; de Almeida, P.; Lopes-Martins, R. Effects of photobiomodulation therapy, pharmacological therapy, and physical exercise as single and/or combined treatment on the inflammatory response induced by experimental osteoarthritis. Lasers Med. Sci. 2017, 32, 101–108. [Google Scholar] [CrossRef]
- Tsai, L.C.; Cooper, E.S.; Hetzendorfer, K.M.; Warren, G.L.; Chang, Y.H.; Willett, N.J. Effects of treadmill running and limb immobilization on knee cartilage degeneration and locomotor joint kinematics in rats following knee meniscal transection. Osteoarthr. Cartil. 2019, 27, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, Y.; Kong, Y.; Zhang, X.; Bai, L. The effects of different frequency treadmill exercise on lipoxin a4 and articular cartilage degeneration in an experimental model of monosodium iodoacetate-induced osteoarthritis in rats. PLoS ONE 2017, 12, e0179162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Y.; Li, X.; Zhang, H.; Gang, Y.; Bai, L. Alterations of autophagy in knee cartilage by treatment with treadmill exercise in a rat osteoarthritis model. Int. J. Mol. Med. 2019, 43, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Bricca, A.; Juhl, C.B.; Steultjens, M.; Roos, E.M. Impact of daily exercise dose on knee joint cartilage thickness in healthy animals—A meta-analysis of published randomized control trials. Osteoarthr. Cartil. 2016, 24, S404. [Google Scholar] [CrossRef]
- Bricca, A.; Juhl, C.B.; Grodzinsky, A.J.; Roos, E.M. Impact of a daily exercise dose on knee joint cartilage—A systematic review and meta-analysis of randomized controlled trials in healthy animals. Osteoarthr. Cartil. 2017, 25, 1223–1237. [Google Scholar] [CrossRef]
- Mazor, M.; Best, T.M.; Cesaro, A.; Lespessailles, E.; Toumi, H. Osteoarthritis biomarker responses and cartilage adaptation to exercise: A review of animal and human models. Scand. J. Med. Sci. Sports 2019, 29, 1072–1082. [Google Scholar] [CrossRef]
- Andrea Vargasa, C.; Sandoval, K.A.; Salvo, C.A.; del Sol, M.; Ernesto Ottone, N. Exercise in rats osteoarthritis. Morphological aspects and literature review. Int. J. Morphol. 2020, 38, 481–491. [Google Scholar]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique. Med. J. Aust. 1960, 1, 500. [Google Scholar] [CrossRef]
- Gabbai-Armelin, P.R.; Wilian Kido, H.; Fernandes, K.R.; Fortulan, C.A.; Muniz Renno, A.C. Effects of bio-inspired bioglass/collagen/magnesium composites on bone repair. J. Biomater. Appl. 2019, 34, 261–272. [Google Scholar] [CrossRef]
- Yamada, E.F.; Salgueiro, A.F.; Goulart, A.d.S.; Mendes, V.P.; Anjos, B.L.; Folmer, V.; da Silva, M.D. Evaluation of monosodium iodoacetate dosage to induce knee osteoarthritis: Relation with oxidative stress and pain. Int. J. Rheum. Dis. 2019, 22, 399–410. [Google Scholar] [CrossRef]
- Cifuentes, D.J.; Rocha, L.G.; Silva, L.A.; Brito, A.C.; Rueff-Barroso, C.R.; Porto, L.C.; Pinho, R.A. Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthr. Cartil. 2010, 18, 1088–1095. [Google Scholar] [CrossRef]
- Al-Hashem, F.; El Karib, A.O.; Bin-Jaliah, I.; Dallak, M.; Sakr, H.F.; Eid, R.A.; Zaki, M.S.A.; Al-Shamsi, M.; Haidara, M.A.; Al-Ani, B. Exercise protects against insulin-dependent diabetes-induced osteoarthritis in rats: A scanning electron microscopy study. Ultrastruct. Pathol. 2017, 41, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Assis, L.; Tim, C.; Magri, A.; Fernandes, K.R.; Vassão, P.G.; Renno, A.C.M. Interleukin-10 and collagen type II immunoexpression are modulated by photobiomodulation associated to aerobic and aquatic exercises in an experimental model of osteoarthritis. Lasers Med. Sci. 2018, 33, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Boudenot, A.; Presle, N.; Uzbekov, R.; Toumi, H.; Pallu, S.; Lespessailles, E. Effect of interval-training exercise on subchondral bone in a chemically-induced osteoarthritis model. Osteoarthr. Cartil. 2014, 22, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lou, Y.; Pan, Z.; Cao, X.; Zhang, L.; Zhu, C.; Liang, J. Treadmill and wheel exercise protect against JNK/NF-κB induced inflammation in experimental models of knee osteoarthritis. Biochem. Biophys. Res. Commun. 2020, 523, 117–122. [Google Scholar] [CrossRef]
- Cormier, J.; Cone, K.; Lanpher, J.; Kinens, A.; Henderson, T.; Liaw, L.; Bilsky, E.J.; King, T.; Rosen, C.J.; Stevenson, G.W. Exercise reverses pain-related weight asymmetry and differentially modulates trabecular bone microarchitecture in a rat model of osteoarthritis. Life Sci. 2017, 180, 51–59. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Bamsey, I.; Bate, S.T.; Berdoy, M.; Clark, R.A.; Cuthill, I.; Fry, D.; Karp, N.A.; Macleod, M.; Moon, L.; et al. The Experimental Design Assistant. PLoS Biol. 2017, 15, e2003779. [Google Scholar] [CrossRef]
- Srere, P.A. [1] citrate synthase: [ec 4.1.3.7. Citrate oxaloacetate-lyase (coa-acetylating)]. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1969; Volume 13, pp. 3–11. [Google Scholar]
- Fischer, J.C.; Ruitenbeek, W.; Berden, J.A.; Trijbels, J.M.; Veerkamp, J.H.; Stadhouders, A.M.; Sengers, R.C.; Janssen, A.J. Differential investigation of the capacity of succinate oxidation in human skeletal muscle. Clin. Chim. Acta 1985, 153, 23–36. [Google Scholar] [CrossRef]
- Mack, M. Inflammation and fibrosis. Matrix Biol. 2018, 68–69, 106–121. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol. Med. 1999, 27, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Lee, M.; Kim, S.W.; Bae, Y.H. Protection of insulin secreting cells from nitric oxide induced cellular damage by crosslinked hemoglobin. Biomaterials 2004, 25, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Bannister, J.V.; Calabrese, L. Assays for superoxide dismutase. Methods Biochem. Anal. 1987, 32, 279–312. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. A fluorometric method for determination of oxidized and reduced glutathione in tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Aksenov, M.Y.; Markesbery, W.R. Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci. Lett. 2001, 302, 141–145. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Leandro, C.G.; Levada, A.C.; Hirabara, S.M.; Manhães-de-Castro, R.; De-Castro, C.B.; Curi, R.; Pithon-Curi, T.C. A program of moderate physical training for wistar rats based on maximal oxygen consumption. J. Strength Cond. Res. 2007, 21, 751–756. [Google Scholar] [CrossRef]
- Pereira, B.; Curi, R.; Kokubun, E.; Bechara, E.J. 5-aminolevulinic acid-induced alterations of oxidative metabolism in sedentary and exercise-trained rats. J. Appl. Physiol. 1992, 72, 226–230. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Aster, J.C. Robbins & Cotran Patologia—Bases Patológicas das Doenças; Elsevier: Amsterdam, The Netherlands, 2013; Volume 9. [Google Scholar]
- Glezer, I.; Simard, A.R.; Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 2007, 147, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Tromm, C.B.; Rosa, G.L.d.; Bom, K.; Mariano, I.; Pozzi, B.; Tuon, T.; Silva, L.A.D.; Pinho, R.A.D. Efeito de diferentes frequências semanais de treinamento sobre parâmetros de estresse oxidativo. Rev. Bras. Cineantropometria Desempenho Hum. 2012, 14, 52–60. [Google Scholar] [CrossRef]
- Zahan, O.M.; Serban, O.; Gherman, C.; Fodor, D. The evaluation of oxidative stress in osteoarthritis. Med. Pharm. Rep. 2020, 93, 12–22. [Google Scholar] [CrossRef]
- Sen, C.K.; Packer, L.; Hänninen, O.O. Handbook of Oxidants and Antioxidants in Exercise; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- de Sire, A.; Marotta, N.; Marinaro, C.; Curci, C.; Invernizzi, M.; Ammendolia, A. Role of physical exercise and nutraceuticals in modulating molecular pathways of osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5722. [Google Scholar] [CrossRef]
- Blaney Davidson, E.N.; van Caam, A.P.; van der Kraan, P.M. Osteoarthritis year in review 2016: Biology. Osteoarthr. Cartil. 2017, 25, 175–180. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef]
- Woodell-May, J.E.; Sommerfeld, S.D. Role of inflammation and the immune system in the progression of osteoarthritis. J. Orthop. Res. 2020, 38, 253–257. [Google Scholar] [CrossRef]
- Magliulo, L.; Bondi, D.; Pini, N.; Marramiero, L.; Di Filippo, E.S. The wonder exerkines-novel insights: A critical state-of-the-art review. Mol. Cell. Biochem. 2022, 477, 105–113. [Google Scholar] [CrossRef]
- Metsios, G.S.; Moe, R.H.; Kitas, G.D. Exercise and inflammation. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101504. [Google Scholar] [CrossRef]
- Benatti, F.B.; Pedersen, B.K. Exercise as an anti-inflammatory therapy for rheumatic diseases-myokine regulation. Nat. Rev. Rheumatol. 2015, 11, 86–97. [Google Scholar] [CrossRef]
- Febbraio, M.A.; Pedersen, B.K. Muscle-derived interleukin-6: Mechanisms for activation and possible biological roles. FASEB J. 2002, 16, 1335–1347. [Google Scholar] [CrossRef]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Thielen, N.G.M.; van der Kraan, P.M.; van Caam, A.P.M. Tgfβ/bmp signaling pathway in cartilage homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.P.; Chen, W.P.; Wu, L.D. Lubricin: A novel potential biotherapeutic approaches for the treatment of osteoarthritis. Mol. Biol. Rep. 2011, 38, 2879–2885. [Google Scholar] [CrossRef]
- Alquraini, A.; Garguilo, S.; D’Souza, G.; Zhang, L.X.; Schmidt, T.A.; Jay, G.D.; Elsaid, K.A. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: An anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res. Ther. 2015, 17, 353. [Google Scholar] [CrossRef]
- Ni, G.X.; Lei, L.; Zhou, Y.Z. Intensity-dependent effect of treadmill running on lubricin metabolism of rat articular cartilage. Arthritis Res. Ther. 2012, 14, R256. [Google Scholar] [CrossRef]












| Week | Frequency (Weekly) | Time (min) | Speed (m/min) |
|---|---|---|---|
| Adaptation | 4× | 10 to 25 | 10 |
| 1 | 3× or 5× | 30 | 13 |
| 2 | 3× or 5× | 30 | 13 |
| 3 | 3× or 5× | 30 | 13 |
| 4 | 3× or 5× | 30 | 13 |
| 5 | 3× or 5× | 30 | 16 |
| 6 | 3× or 5× | 30 | 16 |
| 7 | 3× or 5× | 30 | 16 |
| 8 | 3× or 5× | 30 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colares, M.C.; Thirupathi, A.; da Silva, L.A.; Haupenthal, D.P.d.S.; Casagrande, L.d.R.; Venturini, L.M.; Gu, Y.; da Costa, C.; Lima, I.R.; da Silva, V.O.S.; et al. Exercise as Osteoarthritis Treatment in Wistar Rats Promotes Frequency-Dependent Benefits. Biology 2025, 14, 1537. https://doi.org/10.3390/biology14111537
Colares MC, Thirupathi A, da Silva LA, Haupenthal DPdS, Casagrande LdR, Venturini LM, Gu Y, da Costa C, Lima IR, da Silva VOS, et al. Exercise as Osteoarthritis Treatment in Wistar Rats Promotes Frequency-Dependent Benefits. Biology. 2025; 14(11):1537. https://doi.org/10.3390/biology14111537
Chicago/Turabian StyleColares, Mateus Cardoso, Anand Thirupathi, Leandro Almeida da Silva, Daniela Pacheco dos Santos Haupenthal, Laura de Roch Casagrande, Ligia Milanez Venturini, Yaodong Gu, Camila da Costa, Igor Ramos Lima, Vitória Oliveira Silva da Silva, and et al. 2025. "Exercise as Osteoarthritis Treatment in Wistar Rats Promotes Frequency-Dependent Benefits" Biology 14, no. 11: 1537. https://doi.org/10.3390/biology14111537
APA StyleColares, M. C., Thirupathi, A., da Silva, L. A., Haupenthal, D. P. d. S., Casagrande, L. d. R., Venturini, L. M., Gu, Y., da Costa, C., Lima, I. R., da Silva, V. O. S., da Silva, L. A., Lass, A. D., Pinho, R. A., & Silveira, P. C. L. (2025). Exercise as Osteoarthritis Treatment in Wistar Rats Promotes Frequency-Dependent Benefits. Biology, 14(11), 1537. https://doi.org/10.3390/biology14111537











