Zebrafish (Danio rerio) Embryo–Larvae as a Biosensor for Water Quality Assessment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animal Maintenance
2.3. Experimental Design
2.4. Progeny Evaluation
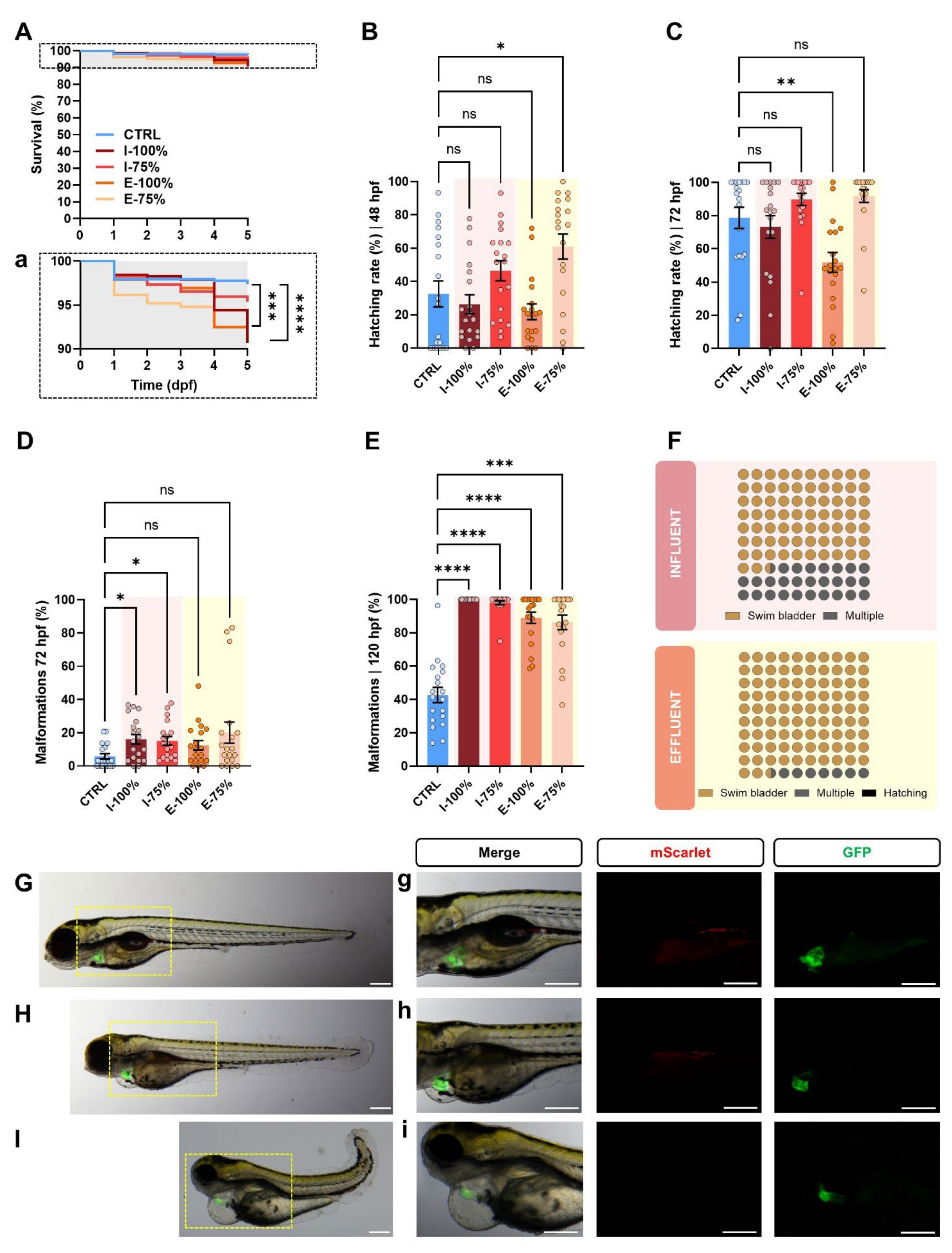
2.4.1. Survival, Hatching, and Malformation Evaluation
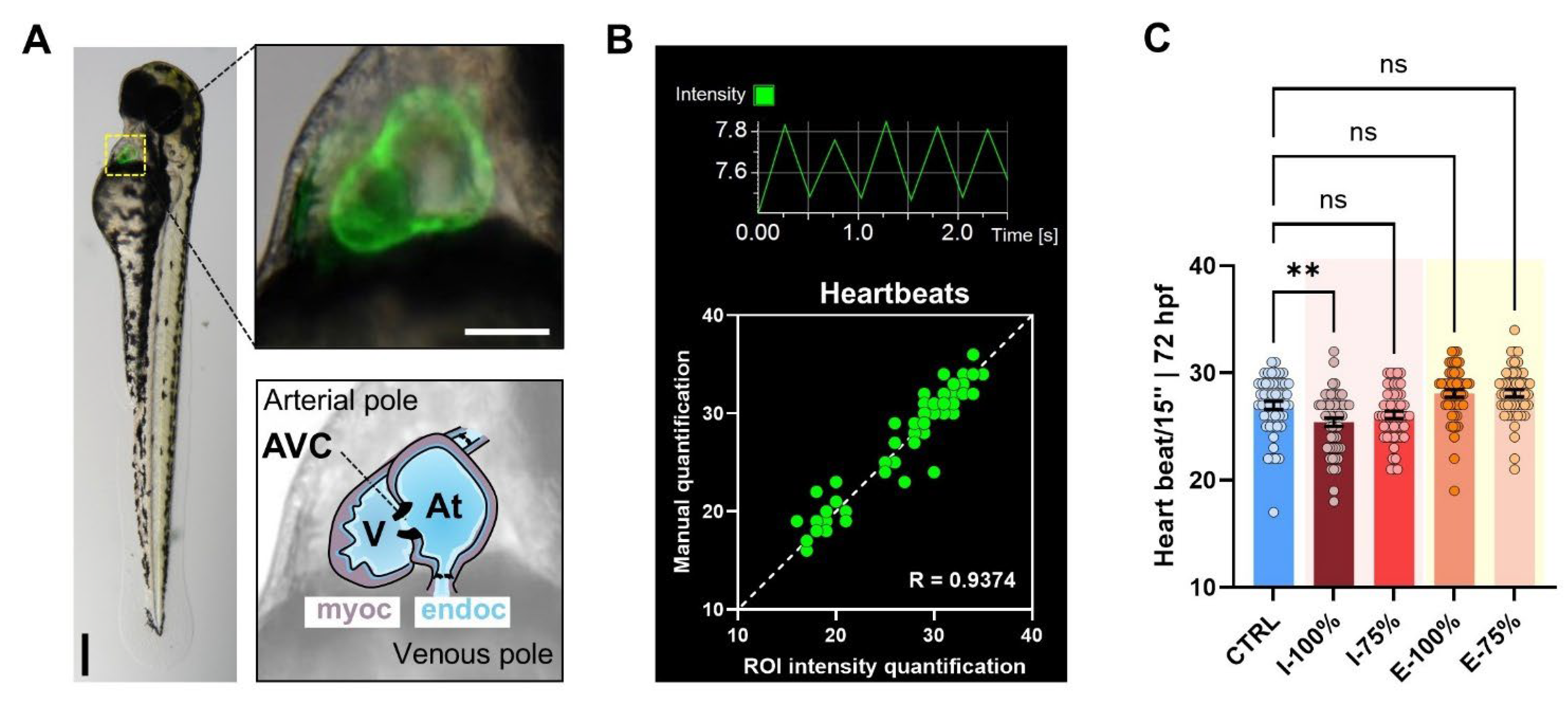
2.4.2. Heartbeat Analysis
2.4.3. PGC Number and Migration

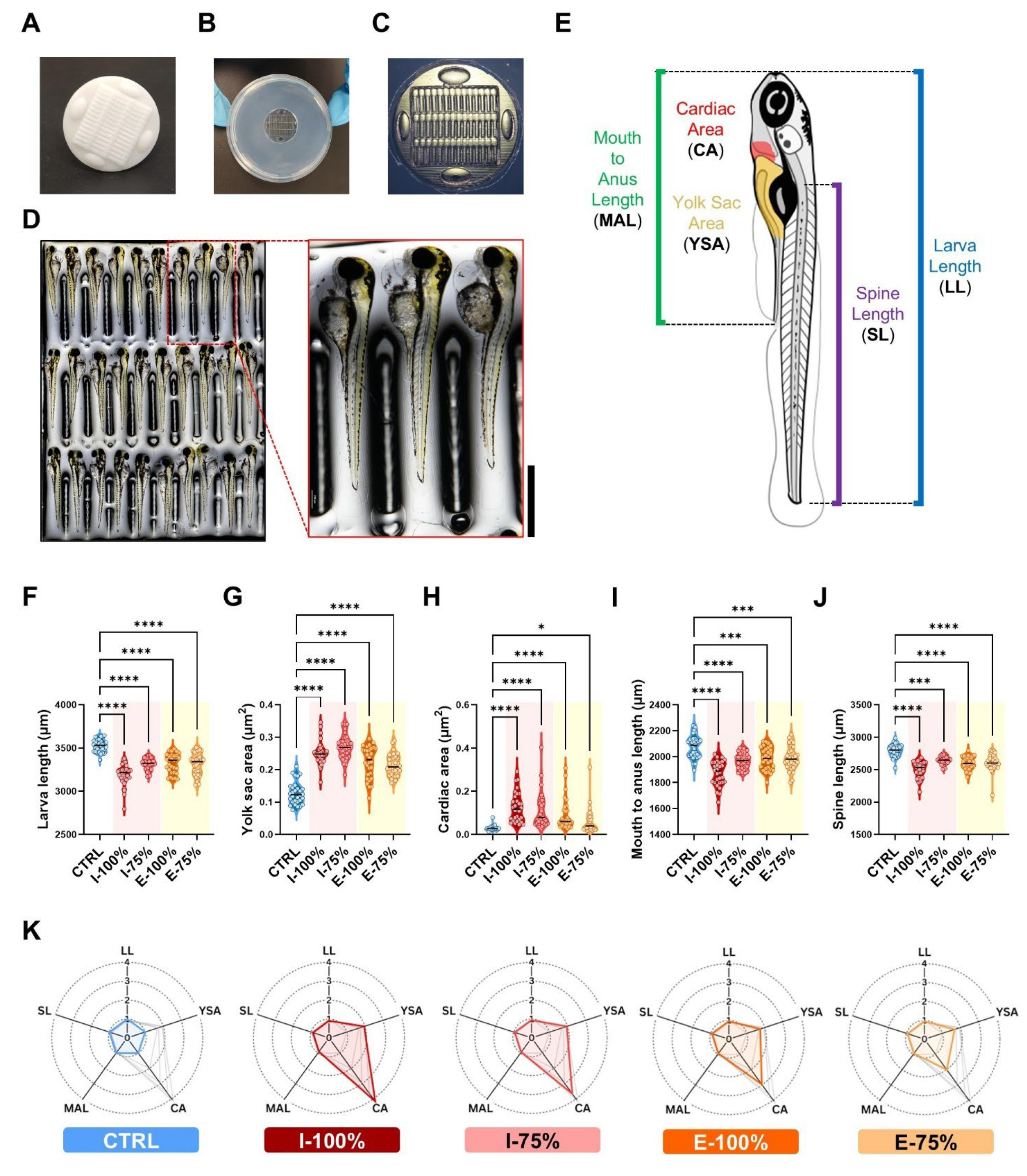
2.4.4. Biometry Analysis
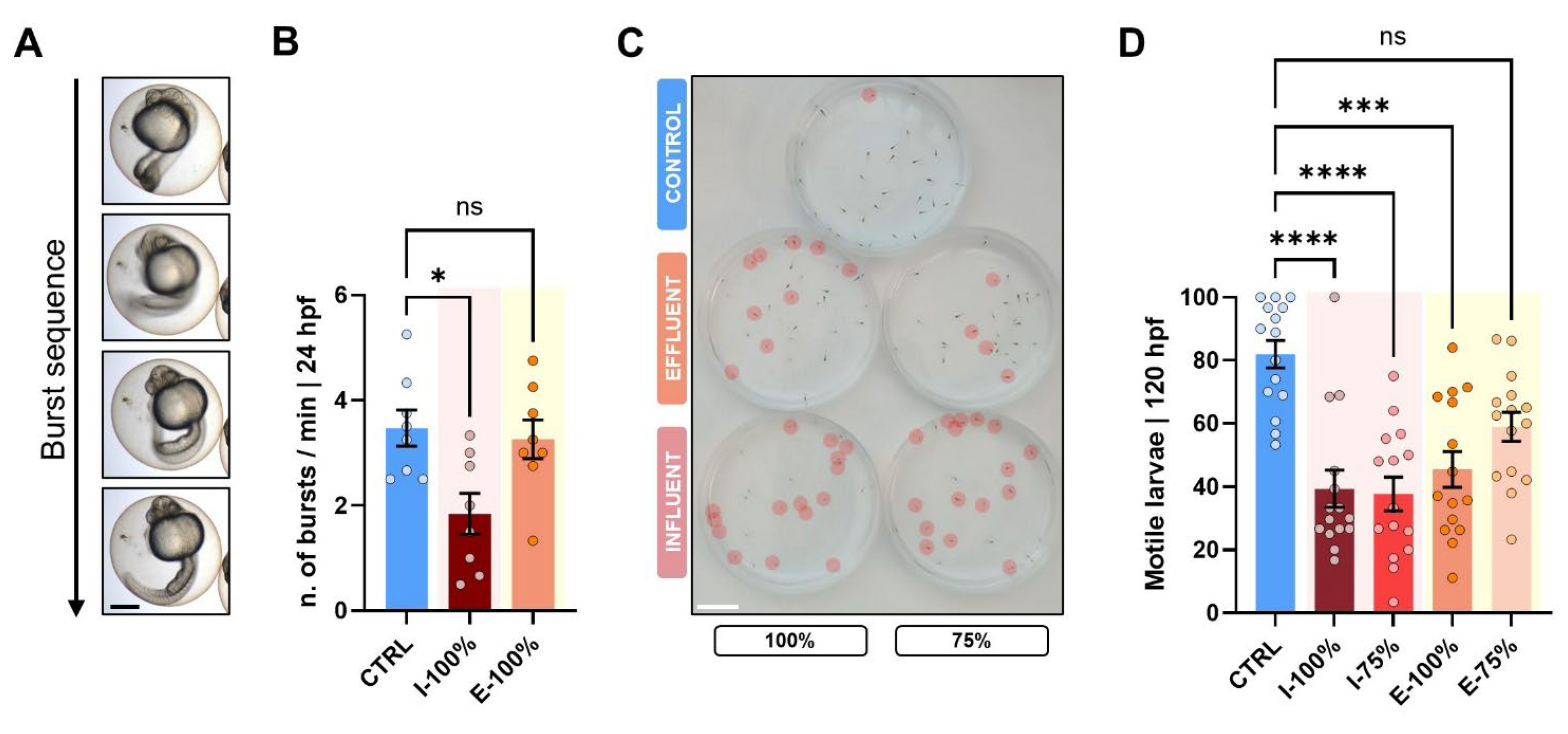
2.5. Behavioral Analysis
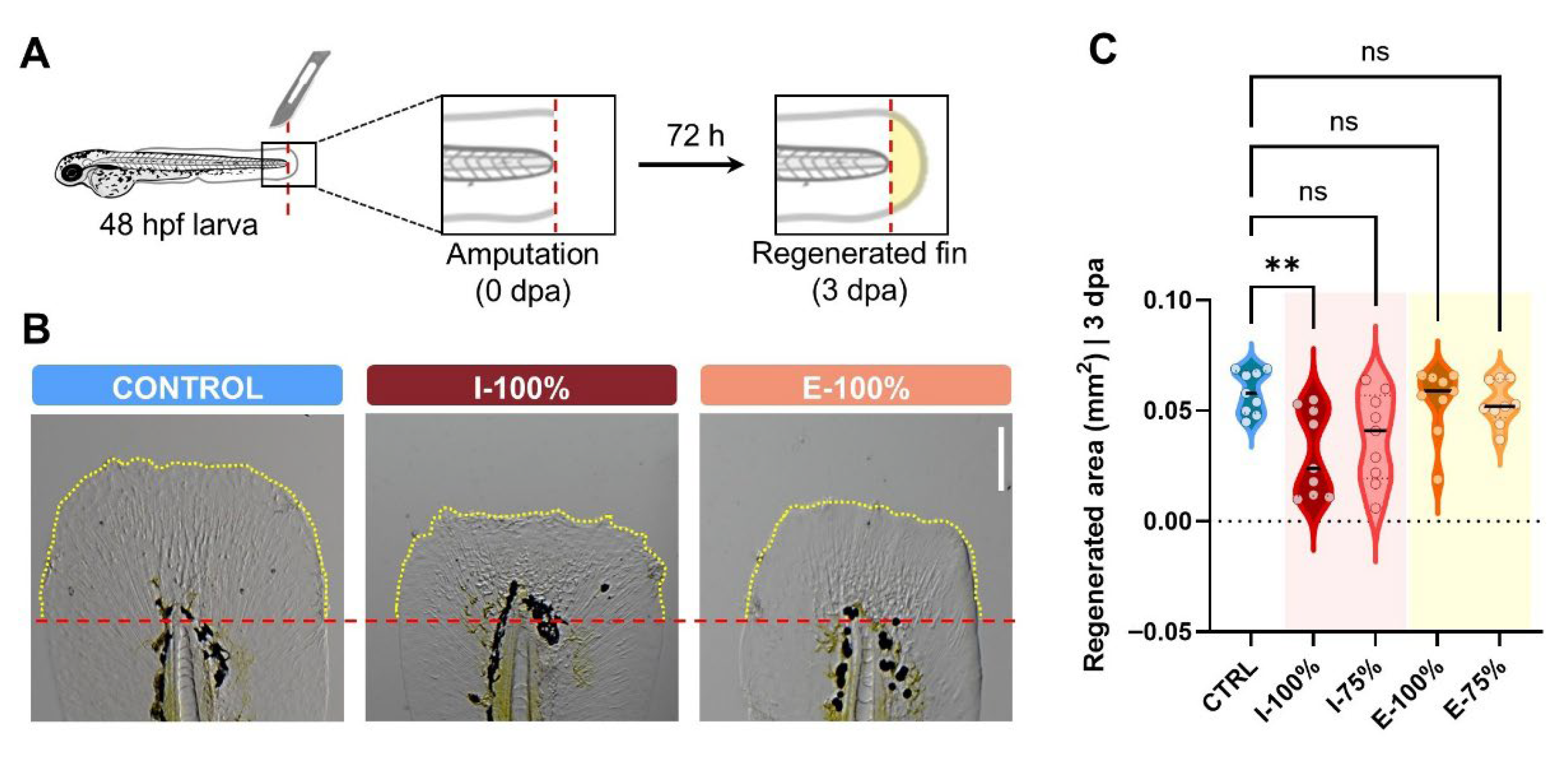
2.6. Fin Regeneration Analysis
2.7. Molecular Studies
2.7.1. Sample Collection
2.7.2. RNA Extraction
2.7.3. Retrotranscription
2.7.4. Quantitative PCR
2.8. Dose–Effect Analysis
2.9. Statistical Analysis
3. Results
3.1. Progeny Evaluation
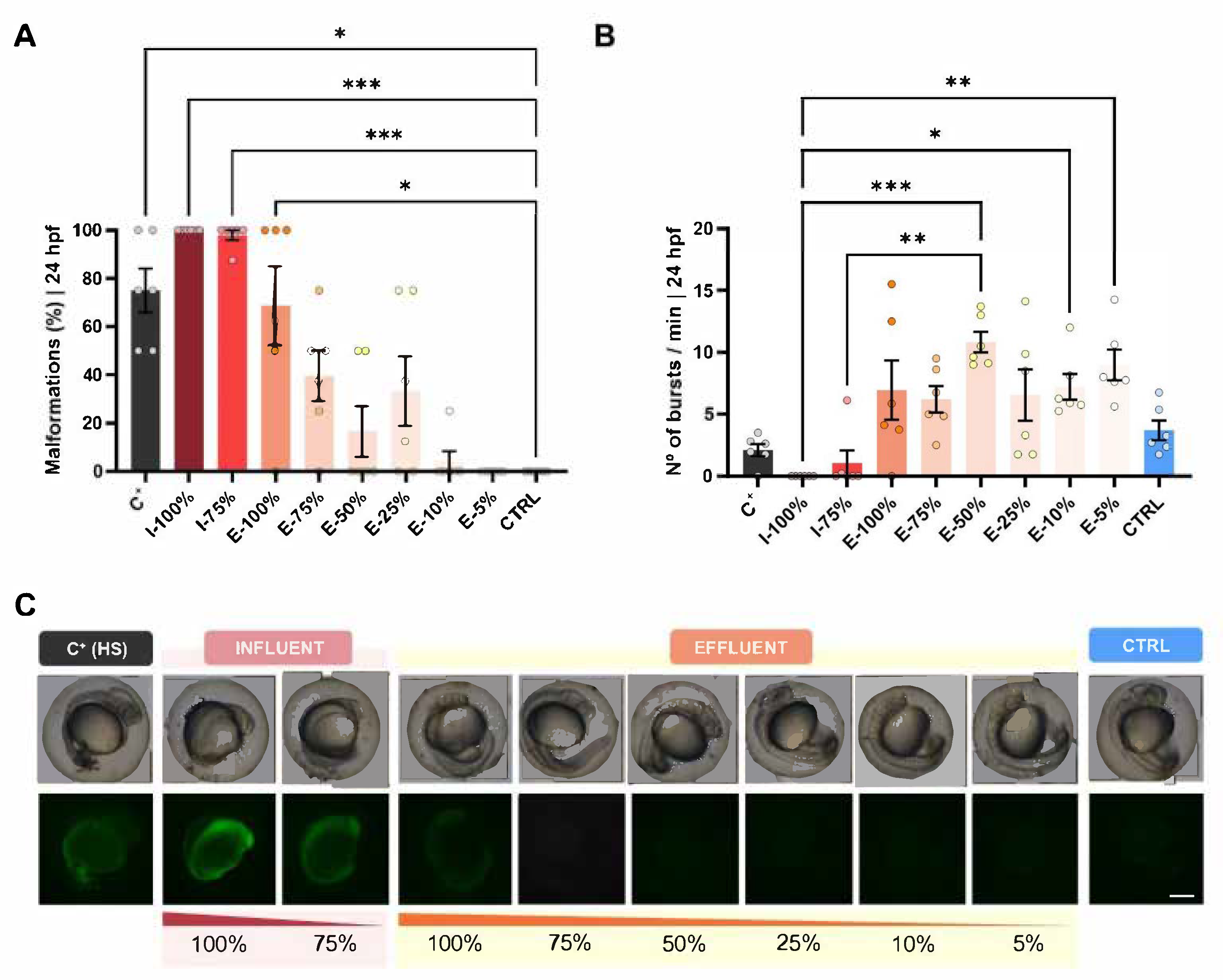
3.1.1. Survival, Hatching, and Malformation Evaluation
3.1.2. Heartbeat Analysis
3.1.3. PGC Migration to the Genital Ridge
3.1.4. Biometry Analysis
3.2. Behavioral Analysis
3.3. Fin Regeneration Evaluation
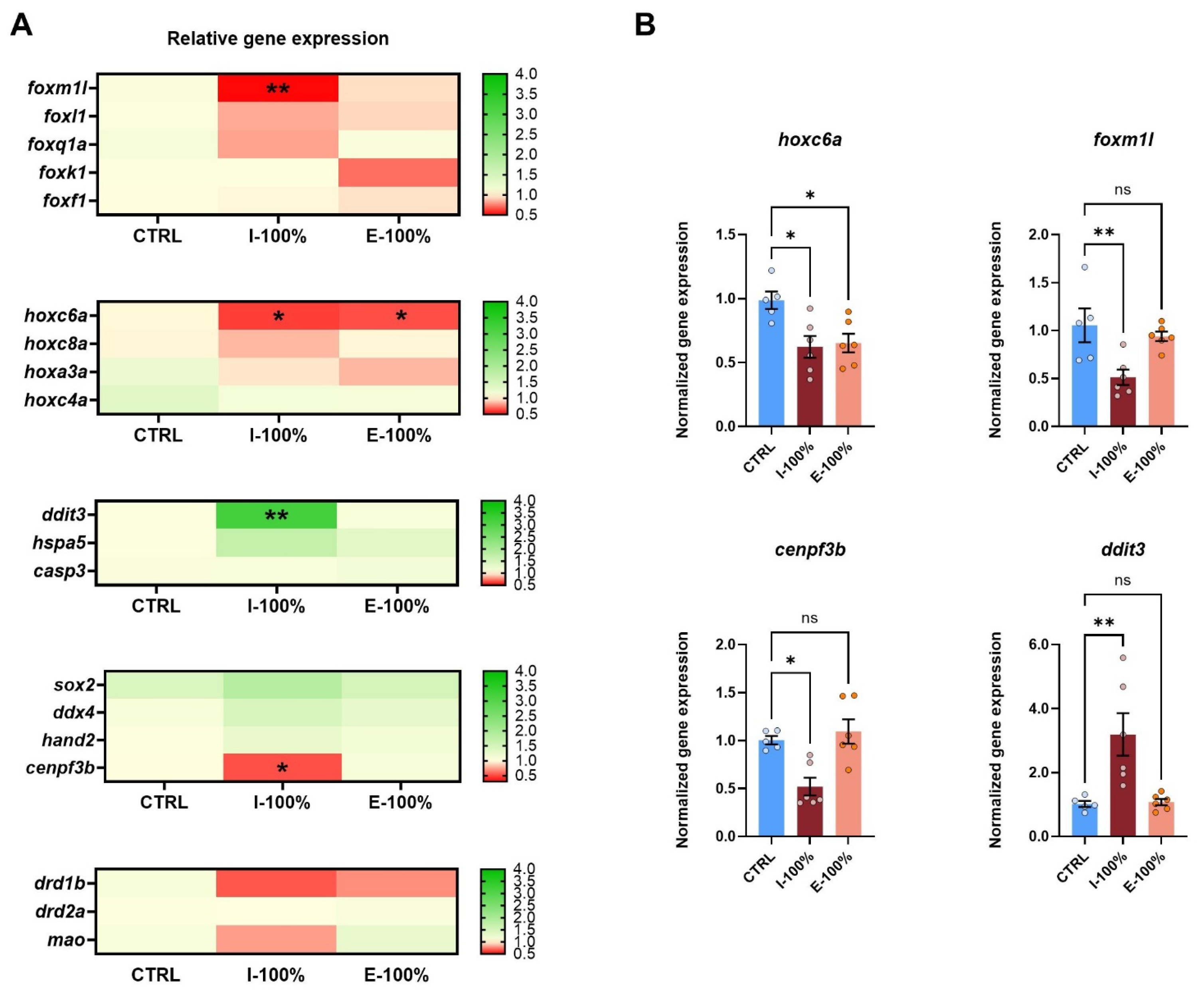
3.4. Molecular Studies
Gene Expression
3.5. Additional Analysis
3.6. Dose–Effect Analysis
4. Discussion
4.1. Progeny Evaluation
4.1.1. Survival, Hatching, and Malformation Evaluation
4.1.2. Heartbeat Analysis
4.1.3. PGC Number and Migration
4.1.4. Biometry Analysis
4.2. Behavioral Analysis
4.3. Fin Regeneration Analysis
4.4. Molecular Studies
4.5. Dose–Effect Analysis
5. Conclusions
Specific Conclusions
- Zebrafish embryos and larvae exposed to both influent and effluent waters exhibited high overall survival rates (>90%). However, statistically significant differences were detected, suggesting subtle effects on viability. Delays in hatching were observed at 48 hpf in embryos exposed to 75% effluent and at 72 hpf in those exposed to 100% effluent.
- Exposure to both influent and effluent waters resulted in significant morphological alterations, particularly affecting biometry as well as malformed swim bladder, poor yolk sac reabsorption, yolk sac and pericardial edema, and heart malformations. Notably, the heart rate was significantly altered only in embryos exposed to 100% influent. Behavioral analysis revealed a significant reduction in the number of bursts in embryos exposed to 100% influent and in larvae motility in all wastewater-exposed groups, except in the 75% effluent condition, which was comparable to the control.
- Exposure to 100% influent (I-100%) significantly increased the number of delocalized PGCs, while no differences were observed in cluster length. In contrast, effluent exposure did not produce significant alterations in PGC migration or localization.
- The regenerative capacity of the caudal fin was only affected in larvae exposed to 100% influent water.
- Exposure to influent water caused a significant downregulation of foxm1l and cenpf3b, while hoxc6a was downregulated in both influent and effluent conditions. In contrast, ddit3 was overexpressed. This suggests that influent water exerts a negative impact on key genes involved in axial and cardiac development, potentially explaining the observed malformations and reduced heart rate, and in genes related to ER stress and apoptosis.
- The use of the hsp70 transgenic reporter line indicated that malformations observed at 24 hpf were no longer present when embryos were exposed to diluted effluent. Embryos exposed to effluent water showed increased spontaneous movement within the chorion compared to controls.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| At | Atrium |
| AVC | Atrioventricular canal |
| BOD5 | Biochemical Oxygen Demand over five days |
| CA | Cardiac area |
| cenpf | Centromere protein F |
| Cmlc | Cardiac myosin light chain |
| COD | Chemical Oxygen Demand |
| C+ | Positive control |
| CTRL | Control |
| ddit3 | DNA Damage Inducible Transcript 3 |
| Dpa | Days post-amputation |
| Dpf | Days post-fertilization |
| DSS | Decision Support Systems |
| E | Effluent water from the secondary treatment in the León WWTP (different concentrations of effluent water are named E-100% and E-75%) |
| EM | Embryo medium |
| Endoc | Endocardium |
| ER | Endoplasmic reticulum |
| foxm1 | forkhead box M1 |
| hoxc6 | homeobox C6 |
| Hpf | Hours post-fertilization |
| Hsp70 | Tg(hsp70l:dn-fgfr1a-EGFP) |
| I | Influent water to the secondary treatment in the León WWTP (different concentrations of influent water are named I-100% and I-75%) |
| Kop | Tg(kop:mScarlet-I-nos 3′UTR-cmlc:GFP) |
| LL | Larvae length |
| MAL | Mouth-to-anus length |
| Myoc | Myocardium |
| PBS | Phosphate-buffered saline |
| PGCs | Primordial germ cells |
| ROI | Region of interest |
| SEM | Standard error of the mean |
| SL | Spine length |
| V | Ventricle |
| WWTP | Wastewater treatment plant |
| YSA | Yolk sac area |
References
- Ullah, A.; Hussain, S.; Wasim, A.; Jahanzaib, M. Development of a Decision Support System for the Selection of Wastewater Treatment Technologies. Sci. Total Environ. 2020, 731, 139158. [Google Scholar] [CrossRef] [PubMed]
- Samer, M. Biological and Chemical Wastewater Treatment Processes. In Wastewater Treatment Engineering; IntechOpen: London, UK, 2015; pp. 1–50. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Kato, S.; Kansha, Y. Comprehensive Review of Industrial Wastewater Treatment Techniques. Environ. Sci. Pollut. Res. Int. 2024, 31, 51064. [Google Scholar] [CrossRef] [PubMed]
- Quach-Cu, J.; Herrera-Lynch, B.; Marciniak, C.; Adams, S.; Simmerman, A.; Reinke, R.A. The Effect of Primary, Secondary, and Tertiary Wastewater Treatment Processes on Antibiotic Resistance Gene (ARG) Concentrations in Solid and Dissolved Wastewater Fractions. Water 2018, 10, 37. [Google Scholar] [CrossRef]
- Naidoo, S.; Olaniran, A.O. Treated Wastewater Effluent as a Source of Microbial Pollution of Surface Water Resources. Int. J. Environ. Res. Public Health 2014, 11, 249–270. [Google Scholar] [CrossRef]
- Ponce-Robles, L.; Mena, E.; Diaz, S.; Pagán-Muñoz, A.; Lara-Guillén, A.J.; Fellahi, I.; Alarcón, J.J. Integrated Full-Scale Solar CPC/UV-LED–Filtration System as a Tertiary Treatment in a Conventional WWTP for Agricultural Reuse Purposes. Photochem. Photobiol. Sci. 2023, 22, 641–654. [Google Scholar] [CrossRef]
- Nagpal, M.; Siddique, M.A.; Sharma, K.; Sharma, N.; Mittal, A. Optimizing Wastewater Treatment through Artificial Intelligence: Recent Advances and Future Prospects. Water Sci. Technol. 2024, 90, 731–757. [Google Scholar] [CrossRef]
- Nikel, K.E.; Tetreault, G.R.; Marjan, P.; Hicks, K.A.; Fuzzen, M.L.M.; Srikanthan, N.; McCann, E.K.; Dhiyebi, H.; Bragg, L.M.; Law, P.; et al. Wild Fish Responses to Wastewater Treatment Plant Upgrades in the Grand River, Ontario. Aquat. Toxicol. 2023, 255, 106375. [Google Scholar] [CrossRef]
- Monsinjon, T.; Knigge, T. Endocrine Disrupters Affect the Immune System of Fish: The Example of the European Seabass. Fish Shellfish Immunol. 2025, 162, 110303. [Google Scholar] [CrossRef]
- Di Prinzio, C.Y.; Andrade-Muñoz, A.S.; Assef, Y.A.; Dromaz, W.M.; Quinteros, P.; Miserendino, M.L. Impact of Treated Effluent Discharges on Fish Communities: Evaluating the Effects of Pollution on Fish Distribution, Abundance and Environmental Integrity. Sci. Total Environ. 2024, 917, 170237. [Google Scholar] [CrossRef]
- Saaristo, M.; Sharp, S.; McKenzie, R.; Hinwood, A. Pharmaceuticals in Biota: The Impact of Wastewater Treatment Plant Effluents on Fish in Australia. Environ. Pollut. 2024, 359, 124695. [Google Scholar] [CrossRef]
- Li, X.; He, M.; Sun, G.; Ma, C.; Li, Y.; Li, L.; Li, B.; Yang, M.; Zhang, Y. Toxicological Evaluation of Industrial Effluents Using Zebrafish: Efficacy of Tertiary Treatment of Coking Wastewater. Environ. Technol. Innov. 2023, 30, 103067. [Google Scholar] [CrossRef]
- Lai, K.P.; Gong, Z.; Tse, W.K.F. Zebrafish as the Toxicant Screening Model: Transgenic and Omics Approaches. Aquat. Toxicol. 2021, 234, 105813. [Google Scholar] [CrossRef] [PubMed]
- Silva Brito, R.; Canedo, A.; Farias, D.; Rocha, T.L. Transgenic Zebrafish (Danio Rerio) as an Emerging Model System in Ecotoxicology and Toxicology: Historical Review, Recent Advances, and Trends. Sci. Total Environ. 2022, 848, 157665. [Google Scholar] [CrossRef] [PubMed]
- Firdous, S.M.; Pal, S.; Khanam, S.; Zakir, F. Behavioral Neuroscience in Zebrafish: Unravelling the Complexity of Brain-Behavior Relationships. Naunyn-Schmiedebergs Arch. Pharmacol. 2024, 397, 9295–9313. [Google Scholar] [CrossRef]
- Choe, S.K.; Kim, C.H. Zebrafish: A Powerful Model for Genetics and Genomics. Int. J. Mol. Sci. 2023, 24, 8169. [Google Scholar] [CrossRef]
- Schenk, H.; Drummond, I.A. Kidney Development, Injury and Regeneration—Zebrafish. Curr. Top. Dev. Biol. 2025, 163, 307–321. [Google Scholar] [CrossRef]
- Bashirzade, A.A.; Zabegalov, K.N.; Volgin, A.D.; Belova, A.S.; Demin, K.A.; de Abreu, M.S.; Babchenko, V.Y.; Bashirzade, K.A.; Yenkoyan, K.B.; Tikhonova, M.A.; et al. Modeling Neurodegenerative Disorders in Zebrafish. Neurosci. Biobehav. Rev. 2022, 138, 104679. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Kimmel, C.B. Genetics and Early Development of Zebrafish. Trends Genet. 1989, 5, 283–288. [Google Scholar] [CrossRef]
- Wong, T.T.; Collodi, P. Inducible Sterilization of Zebrafish by Disruption of Primordial Germ Cell Migration. PLoS ONE 2013, 8, e68455. [Google Scholar] [CrossRef]
- Blechinger, S.R.; Warren, J.T.; Kuwada, J.Y.; Krone, P.H. Developmental Toxicology of Cadmium in Living Embryos of a Stable Transgenic Zebrafish Line. Environ. Health Perspect. 2002, 110, 1041. [Google Scholar] [CrossRef]
- Halloran, M.C.; Sato-Maeda, M.; Warren, J.T.; Su, F.; Lele, Z.; Krone, P.H.; Kuwada, J.Y.; Shoji, W. Laser-Induced Gene Expression in Specific Cells of Transgenic Zebrafish. Development 2000, 127, 1953–1960. [Google Scholar] [CrossRef] [PubMed]
- Aalto, A.; Olguin-Olguin, A.; Raz, E. Zebrafish Primordial Germ Cell Migration. Front. Cell Dev. Biol. 2021, 9, 684460. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.N.Y.; De Jong-Curtain, T.A.; Mawdsley, D.J.; White, S.J.; Shin, J.; Appel, B.; Dong, P.D.S.; Stainier, D.Y.R.; Heath, J.K. Formation of the Digestive System in Zebrafish: III. Intestinal Epithelium Morphogenesis. Dev. Biol. 2005, 286, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book. In A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, OR, USA, 2000; ISBN 978-999-486-057-9. [Google Scholar]
- Tarbashevich, K.; Ermlich, L.; Wegner, J.; Pfeiffer, J.; Raz, E. The Mitochondrial Protein Sod2 Is Important for the Migration, Maintenance, and Fitness of Germ Cells. Front. Cell Dev. Biol. 2023, 11, 1250643. [Google Scholar] [CrossRef]
- Kleinhans, D.S.; Lecaudey, V. Standardized Mounting Method of (Zebrafish) Embryos Using a 3D-Printed Stamp for High-Content, Semi-Automated Confocal Imaging. BMC Biotechnol. 2019, 19, 68. [Google Scholar] [CrossRef]
- Pase, L.; Nowell, C.J.; Lieschke, G.J. In Vivo Real-Time Visualization of Leukocytes and Intracellular Hydrogen Peroxide Levels During a Zebrafish Acute Inflammation Assay. Methods Enzym. 2012, 506, 135–156. [Google Scholar] [CrossRef]
- Riesco, M.F.; Robles, V. Cryopreservation Causes Genetic and Epigenetic Changes in Zebrafish Genital Ridges. PLoS ONE 2013, 8, 67614. [Google Scholar] [CrossRef]
- Divisato, G.; Formicola, D.; Esposito, T.; Merlotti, D.; Pazzaglia, L.; Del Fattore, A.; Siris, E.; Orcel, P.; Brown, J.P.; Nuti, R.; et al. ZNF687 Mutations in Severe Paget Disease of Bone Associated with Giant Cell Tumor. Am. J. Hum. Genet. 2016, 98, 275–286. [Google Scholar] [CrossRef]
- Robinson, B.L.; Dumas, M.; Cuevas, E.; Gu, Q.; Paule, M.G.; Ali, S.F.; Kanungo, J. Distinct Effects of Ketamine and Acetyl L-Carnitine on the Dopamine System in Zebrafish. Neurotoxicol Teratol. 2016, 54, 52–60. [Google Scholar] [CrossRef]
- Thörnqvist, P.O.; McCarrick, S.; Ericsson, M.; Roman, E.; Winberg, S. Bold Zebrafish (Danio Rerio) Express Higher Levels of Delta Opioid and Dopamine D2 Receptors in the Brain Compared to Shy Fish. Behav. Brain Res. 2019, 359, 927–934. [Google Scholar] [CrossRef]
- Valcarce, D.G.; Riesco, M.F.; Cuesta-Martín, L.; Esteve-Codina, A.; Martínez-Vázquez, J.M.; Robles, V. Stress Decreases Spermatozoa Quality and Induces Molecular Alterations in Zebrafish Progeny. BMC Biol. 2023, 21, 70. [Google Scholar] [CrossRef]
- Matsumoto, H.; Miyagi, H.; Nakamura, N.; Shiga, Y.; Ohta, T.; Fujiwara, S.; Tsuzuki, M. Carbonic Anhydrase Inhibitor Induces Otic Hair Cell Apoptosis via an Intrinsic Pathway and ER Stress in Zebrafish Larvae. Toxicol. Rep. 2021, 8, 1937–1947. [Google Scholar] [CrossRef]
- Santos-Villadangos, M.; Sellés-Egea, A.; Robles, V.; Valcarce, D.G. Temperature and Photoperiod Stress in Zebrafish Larvae: Impacts on Development, Gene Regulation and PGC Migration. Fish Physiol. Biochem. 2025, 51, 156. [Google Scholar] [CrossRef]
- Flourish. Available online: https://flourish.studio/ (accessed on 20 May 2025).
- Babić, S.; Barišić, J.; Višić, H.; Sauerborn Klobučar, R.; Topić Popović, N.; Strunjak-Perović, I.; Čož-Rakovac, R.; Klobučar, G. Embryotoxic and Genotoxic Effects of Sewage Effluents in Zebrafish Embryo Using Multiple Endpoint Testing. Water Res. 2017, 115, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, R.X.; da Silva Brito, R.; Pereira, A.C.; Monteiro, K.B.e.S.; Gonçalves, B.B.; Rocha, T.L. Ecotoxicological Assessment of Effluents from Brazilian Wastewater Treatment Plants Using Zebrafish Embryotoxicity Test: A Multi-Biomarker Approach. Sci. Total Environ. 2020, 735, 139036. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, F.; Li, H.; Tong, Y.; Li, W.; Xiong, J.; You, J. Bioassay-Based Identification and Removal of Target and Suspect Toxicants in Municipal Wastewater: Impacts of Chemical Properties and Transformation. J. Hazard. Mater. 2022, 437, 129426. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, Q.; Zhang, X.; Snyder, S.A.; Gong, Z.; Lam, S.H. An Integrated Approach with the Zebrafish Model for Biomonitoring of Municipal Wastewater Effluent and Receiving Waters. Water Res. 2018, 131, 33–44. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Matamoros, V.; Martín-Villacorta, J.; Bécares, E.; Bayona, J.M. Assessment of Full-Scale Natural Systems for the Removal of PPCPs from Wastewater in Small Communities. Water Res. 2010, 44, 1429–1439. [Google Scholar] [CrossRef]
- Van den Brandhof, E.J.; Montforts, M. Fish Embryo Toxicity of Carbamazepine, Diclofenac and Metoprolol. Ecotoxicol. Environ. Saf. 2010, 73, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Rawson, D.M.; Zhang, T.; Kalicharan, D.; Jongebloed, W.L. Field Emission Scanning Electron Microscopy and Transmission Electron Microscopy Studies of the Chorion, Plasma Membrane and Syncytial Layers of the Gastrula-Stage Embryo of the Zebrafish Brachydanio Rerio: A Consideration of the Structural and Functional Relationships with Respect to Cryoprotectant Penetration. Aquac. Res. 2000, 31, 325–336. [Google Scholar] [CrossRef]
- Tran, C.M.; Lee, H.; Lee, B.; Ra, J.S.; Kim, K.T. Effects of the Chorion on the Developmental Toxicity of Organophosphate Esters in Zebrafish Embryos. J. Hazard. Mater. 2021, 401, 123389. [Google Scholar] [CrossRef] [PubMed]
- Pelka, K.E.; Henn, K.; Keck, A.; Sapel, B.; Braunbeck, T. Size Does Matter—Determination of the Critical Molecular Size for the Uptake of Chemicals across the Chorion of Zebrafish (Danio rerio) Embryos. Aquat. Toxicol. 2017, 185, 1–10. [Google Scholar] [CrossRef]
- Kim, K.-T.; Tanguay, R.L. The Role of Chorion on Toxicity of Silver Nanoparticles in the Embryonic Zebrafish Assay. Environ. Health Toxicol. 2014, 29, e2014021. [Google Scholar] [CrossRef]
- Kais, B.; Schneider, K.E.; Keiter, S.; Henn, K.; Ackermann, C.; Braunbeck, T. DMSO Modifies the Permeability of the Zebrafish (Danio Rerio) Chorion-Implications for the Fish Embryo Test (FET). Aquat. Toxicol. 2013, 140–141, 229–238. [Google Scholar] [CrossRef]
- Teixidó, E.; Gómez-Catalán, J.; González-Linares, J.; Borràs, M.; Llobet, J.M. Evaluation of Teratogenic Effects Produced by Water Samples from Wastewater and Drinking Water Treatment Plants by Zebrafish Embryo Test. Reprod. Toxicol. 2009, 28, 133–134. [Google Scholar] [CrossRef]
- Saputra, F.; Kishida, M.; Hu, S.Y. Nitrate and Nitrite Exposure Induces Visual Impairments in Adult Zebrafish. Toxics 2024, 12, 518. [Google Scholar] [CrossRef]
- Cao, G.; Zhao, J.; Zhao, G.; Wan, D.; Wu, Z.; Li, R.; He, Q. Determination of the Acute and Chronic Toxicity of Sulfate from the Sulfur Autotrophic Denitrification Process to Juvenile Zebrafish (Danio Rerio). ACS Omega 2022, 7, 47165. [Google Scholar] [CrossRef]
- Haridevamuthu, B.; Raj, D.; Kesavan, D.; Muthuraman, S.; Kumar, R.S.; Mahboob, S.; Al-Ghanim, K.A.; Almutairi, B.O.; Arokiyaraj, S.; Gopinath, P.; et al. Trihydroxy Piperlongumine Protects Aluminium Induced Neurotoxicity in Zebrafish: Behavioral and Biochemical Approach. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 268, 109600. [Google Scholar] [CrossRef] [PubMed]
- Capriello, T.; Monteiro, S.M.; Félix, L.M.; Donizetti, A.; Aliperti, V.; Ferrandino, I. Apoptosis, Oxidative Stress and Genotoxicity in Developing Zebrafish after Aluminium Exposure. Aquat. Toxicol. 2021, 236, 105872. [Google Scholar] [CrossRef] [PubMed]
- Ferrandino, I.; Capriello, T.; Félix, L.M.; Di Meglio, G.; Santos, D.; Monteiro, S.M. Histological Alterations and Oxidative Stress in Adult Zebrafish Muscle after Aluminium Exposure. Environ. Toxicol. Pharmacol. 2022, 94, 103934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yuan, G.; Werdich, A.A.; Zhao, Y. Ibuprofen and Diclofenac Impair the Cardiovascular Development of Zebrafish (Danio Rerio) at Low Concentrations. Environ. Pollut. 2020, 258, 113613. [Google Scholar] [CrossRef]
- Weigt, S.; Huebler, N.; Strecker, R.; Braunbeck, T.; Broschard, T.H. Zebrafish (Danio rerio) Embryos as a Model for Testing Proteratogens. Toxicology 2011, 281, 25–36. [Google Scholar] [CrossRef]
- SINAC—Sistema de Informacion Nacional de Aguas de Consumo. Available online: https://sinac.sanidad.gob.es/CiudadanoWeb/ciudadano/informacionAbastecimientoActionDetalleRed.do (accessed on 15 June 2025).
- Hong, T.; Park, H.; An, G.; Song, G.; Lim, W. Ethalfluralin Induces Developmental Toxicity in Zebrafish via Oxidative Stress and Inflammation. Sci. Total Environ. 2023, 854, 158780. [Google Scholar] [CrossRef]
- Min, N.; Park, H.; Hong, T.; Song, J.; Song, G.; Lim, W. Terbutryn Causes Developmental Toxicity in Zebrafish (Danio rerio) via Apoptosis and Major Organ Malformation in the Early Stages of Embryogenesis. Sci. Total Environ. 2023, 893, 164839. [Google Scholar] [CrossRef]
- Rothe, L.E.; Botha, T.L.; Feld, C.K.; Weyand, M.; Zimmermann, S.; Smit, N.J.; Wepener, V.; Sures, B. Effects of Conventionally-Treated and Ozonated Wastewater on Mortality, Physiology, Body Length, and Behavior of Embryonic and Larval Zebrafish (Danio Rerio). Environ. Pollut. 2021, 286, 117241. [Google Scholar] [CrossRef]
- Hu, J.; Sun, S.; Guo, M.; Song, H. Use of Antagonists and Morpholinos in Loss-of-Function Analyses: Estrogen Receptor ESR2a Mediates the Effects of 17alpha-Ethinylestradiol on Primordial Germ Cell Distribution in Zebrafish. Reprod. Biol. Endocrinol. 2014, 12, 40. [Google Scholar] [CrossRef]
- Lombó, M.; Getino-álvarez, L.; Depincé, A.; Labbé, C.; Herráez, M.P. Embryonic Exposure to Bisphenol A Impairs Primordial Germ Cell Migration without Jeopardizing Male Breeding Capacity. Biomolecules 2019, 9, 307. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, Y.; Wang, F.; Dong, X.; Jiang, L.; Liu, C.; Zhao, Q.; Li, K. Generation of All-Male-like Sterile Zebrafish by Eliminating Primordial Germ Cells at Early Development. Sci. Rep. 2018, 8, 1834. [Google Scholar] [CrossRef]
- Petersen, A.M.; Earp, N.C.; Redmond, M.E.; Postlethwait, J.H.; Von Hippel, F.A.; Buck, C.L.; Cresko, W.A. Perchlorate Exposure Reduces Primordial Germ Cell Number in Female Threespine Stickleback. PLoS ONE 2016, 11, e0157792. [Google Scholar] [CrossRef]
- Wang, F.; Feng, Y.Y.; Wang, X.G.; Ou, M.; Zhang, X.C.; Zhao, J.; Chen, K.C.; Li, K.B. Production of All-Male Non-Transgenic Zebrafish by Conditional Primordial Germ Cell Ablation. Fish Physiol. Biochem. 2023, 49, 1215–1227. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Wang, Q.; Yuan, Z.; Li, R.; Wang, C.; Sun, Y. Effect of Long-Term Exposure to Dyeing Wastewater Treatment Plant Effluent on Growth and Gut Microbiota of Adult Zebrafish (Danio rerio). Environ. Sci. Pollut. Res. 2023, 30, 53674–53684. [Google Scholar] [CrossRef]
- Lin, E.; Ribeiro, A.; Ding, W.; Hove-Madsen, L.; Sarunic, M.V.; Faisal Beg, M.; Tibbits, G.F. Optical Mapping of the Electrical Activity of Isolated Adult Zebrafish Hearts: Acute Effects of Temperature. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, 823–836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gong, Z. Fluorescent Transgenic Zebrafish Tg(Nkx2.2a:MEGFP) Provides a Highly Sensitive Monitoring Tool for Neurotoxins. PLoS ONE 2013, 8, e55474. [Google Scholar] [CrossRef] [PubMed]
- Porretti, M.; Arrigo, F.; Di Bella, G.; Faggio, C. Impact of Pharmaceutical Products on Zebrafish: An Effective Tool to Assess Aquatic Pollution. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 261, 109439. [Google Scholar] [CrossRef] [PubMed]
- Rihel, J.; Prober, D.A.; Arvanites, A.; Lam, K.; Zimmerman, S.; Jang, S.; Haggarty, S.J.; Kokel, D.; Rubin, L.L.; Peterson, R.T.; et al. Zebrafish Behavioral Profiling Links Drugs to Biological Targets and Rest/Wake Regulation. Science 2010, 327, 348. [Google Scholar] [CrossRef]
- De Oliveira, A.A.S.; Brigante, T.A.V.; Oliveira, D.P. Tail Coiling Assay in Zebrafish (Danio Rerio) Embryos: Stage of Development, Promising Positive Control Candidates, and Selection of an Appropriate Organic Solvent for Screening of Developmental Neurotoxicity (DNT). Water 2021, 13, 119. [Google Scholar] [CrossRef]
- Martineau, P.R.; Mourrain, P. Tracking Zebrafish Larvae in Group—Status and Perspectives. Methods 2013, 62, 292–303. [Google Scholar] [CrossRef]
- Sehring, I.; Weidinger, G. Zebrafish Fin: Complex Molecular Interactions and Cellular Mechanisms Guiding Regeneration. Cold Spring Harb. Perspect. Biol. 2022, 14, a040758. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, Y.; Kawakami, A.; Kudo, A. Cellular and Molecular Processes of Regeneration, with Special Emphasis on Fish Fins. Dev. Growth Differ. 2007, 49, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart Regeneration in Zebrafish. Science (1979) 2002, 298, 2188–2190. [Google Scholar] [CrossRef] [PubMed]
- Alper, S.R.; Dorsky, R.I. Unique Advantages of Zebrafish Larvae as a Model for Spinal Cord Regeneration. Front. Mol. Neurosci. 2022, 15, 983336. [Google Scholar] [CrossRef]
- Kawakami, A.; Fukazawa, T.; Takeda, H. Early Fin Primordia of Zebrafish Larvae Regenerate by a Similar Growth Control Mechanism with Adult Regeneration. Dev. Dyn. 2004, 231, 693–699. [Google Scholar] [CrossRef]
- Pang, S.; Gao, Y.; Wang, F.; Wang, Y.; Cao, M.; Zhang, W.; Liang, Y.; Song, M.; Jiang, G. Toxicity of Silver Nanoparticles on Wound Healing: A Case Study of Zebrafish Fin Regeneration Model. Sci. Total Environ. 2020, 717, 137178. [Google Scholar] [CrossRef]
- Xia, C.; Tian, L.; Yu, J.; Lu, X.; Wang, H.; He, Z.; Qian, B.; Gu, L.; Wang, L.; Chen, J.; et al. Inhibitory Effects of Estrogenic Endocrine Disrupting Chemicals on Fin Regeneration in Zebrafish Are Dependent on Estrogen Receptors. Aquat. Toxicol. 2022, 247, 106156. [Google Scholar] [CrossRef]
- Ge, C.; Ye, Z.; Hu, W.; Tang, J.; Li, H.; Liu, F.; Liao, X.; Chen, J.; Zhang, S.; Cao, Z. Effects of Pyrazosulfuron-Ethyl on Caudal Fin Regeneration in Zebrafish Larvae. Ecotoxicol. Environ. Saf. 2025, 290, 117552. [Google Scholar] [CrossRef]
- Ghosh, P.; Sagerström, C.G. Developing Roles for Hox Proteins in Hindbrain Gene Regulatory Networks. Int. J. Dev. Biol. 2018, 62, 767–774. [Google Scholar] [CrossRef]
- Maeno, A.; Koita, R.; Nakazawa, H.; Fujii, R.; Yamada, K.; Oikawa, S.; Tani, T.; Ishizaka, M.; Satoh, K.; Ishizu, A.; et al. The Hox Code Responsible for the Patterning of the Anterior Vertebrae in Zebrafish. Development 2024, 151, dev202854. [Google Scholar] [CrossRef]
- Jackson, B.C.; Carpenter, C.; Nebert, D.W.; Vasiliou, V. Update of Human and Mouse Forkhead Box (Fox) Gene Families. Hum. Genom. 2010, 4, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Featherstone, M. A Zebrafish Hox Gene Acts before Gastrulation to Specify the Hemangioblast. Genesis 2020, 58, e23363. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Wang, Z.; Collins, J.E.; Andrews, R.M.; Stemple, D.; Gong, Z. Comparative Transcriptome Analyses Indicate Molecular Homology of Zebrafish Swimbladder and Mammalian Lung. PLoS ONE 2011, 6, e24019. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Maeno, A.; Adachi, U.; Ishizaka, M.; Yamada, K.; Koita, R.; Nakazawa, H.; Oikawa, S.; Fujii, R.; Furudate, H.; et al. Physical Constraints on the Positions and Dimensions of the Zebrafish Swim Bladder by Surrounding Bones. J. Anat. 2024, 246, 534–543. [Google Scholar] [CrossRef]
- Carlsson, P.; Mahlapuu, M. Forkhead Transcription Factors: Key Players in Development and Metabolism. Dev. Biol. 2002, 250, 1–23. [Google Scholar] [CrossRef]
- Kalin, T.V.; Wang, I.C.; Ackerson, T.J.; Major, M.L.; Detrisac, C.J.; Kalinichenko, V.V.; Lyubimov, A.; Costa, R.H. Increased Levels of the FoxM1 Transcription Factor Accelerate Development and Progression of Prostate Carcinomas in Both TRAMP and LADY Transgenic Mice. Cancer Res. 2006, 66, 1712. [Google Scholar] [CrossRef]
- Kim, I.M.; Ackerson, T.; Ramakrishna, S.; Tretiakova, M.; Wang, I.C.; Kalin, T.V.; Major, M.L.; Gusarova, G.A.; Yoder, H.M.; Costa, R.H.; et al. The Forkhead Box M1 Transcription Factor Stimulates the Proliferation of Tumor Cells during Development of Lung Cancer. Cancer Res. 2006, 66, 2153–2161. [Google Scholar] [CrossRef]
- Zuppo, D.A.; Missinato, M.A.; Santana-Santos, L.; Li, G.; Benos, P.V.; Tsang, M. Foxm1 Regulates Cardiomyocyte Proliferation in Adult Zebrafish after Cardiac Injury. Development 2023, 150, dev201163. [Google Scholar] [CrossRef]
- Cao, S.; Dong, Z.; Dong, X.; Jia, W.; Zhou, F.; Zhao, Q. Zebrafish Sox2 Is Required for the Swim Bladder Inflation by Controlling the Swim-Up Behavior. Zebrafish 2023, 20, 10. [Google Scholar] [CrossRef]
- Hartung, O.; Forbes, M.M.; Marlow, F.L. Zebrafish Vasa Is Required for Germ-Cell Differentiation and Maintenance. Mol. Reprod. Dev. 2014, 81, 946. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, X.; Dai, J.; Bai, Y.; Liu, F.; Luo, D. Deletion of Ddx4 Ovary-Specific Transcript Causes Dysfunction of Meiosis and Derepress of DNA Transposons in Zebrafish Ovaries. Biology 2024, 13, 1055. [Google Scholar] [CrossRef]
- Stainier, D.Y.R. Zebrafish Genetics and Vertebrate Heart Formation. Nat. Rev. Genet. 2001, 2, 39–48. [Google Scholar] [CrossRef]
- Laoukili, J.; Kooistra, M.R.H.; Brás, A.; Kauw, J.; Kerkhoven, R.M.; Morrison, A.; Clevers, H.; Medema, R.H. FoxM1 Is Required for Execution of the Mitotic Programme and Chromosome Stability. Nat. Cell Biol. 2005, 7, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Bolte, C.; Zhang, Y.; Wang, I.C.; Kalin, T.V.; Molkentin, J.D.; Kalinichenko, V.V. Expression of Foxm1 Transcription Factor in Cardiomyocytes Is Required for Myocardial Development. PLoS ONE 2011, 6, e22217. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.V.; Vergnolle, M.A.S.; Hussein, D.; Wozniak, M.J.; Allan, V.J.; Taylor, S.S. Silencing Cenp-F Weakens Centromeric Cohesion, Prevents Chromosome Alignment and Activates the Spindle Checkpoint. J. Cell Sci. 2005, 118, 4889–4900. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Winkfein, R.J.; Mack, G.; Rattner, J.B.; Yen, T.J. CENP-F Is a Protein of the Nuclear Matrix That Assembles onto Kinetochores at Late G2 and Is Rapidly Degraded after Mitosis. J. Cell Biol. 1995, 130, 507. [Google Scholar] [CrossRef]
- Lee, Y.S.; Lee, D.H.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Ferroptosis-Induced Endoplasmic Reticulum Stress: Cross-Talk between Ferroptosis and Apoptosis. Mol. Cancer Res. 2018, 16, 1073–1076. [Google Scholar] [CrossRef]
- Gu, X.; Li, K.; Laybutt, D.R.; He, M.L.; Zhao, H.L.; Chan, J.C.N.; Xu, G. Bip Overexpression, but Not CHOP Inhibition, Attenuates Fatty-Acid-Induced Endoplasmic Reticulum Stress and Apoptosis in HepG2 Liver Cells. Life Sci. 2010, 87, 724–732. [Google Scholar] [CrossRef]
- Eskandari, E.; Eaves, C.J. Paradoxical Roles of Caspase-3 in Regulating Cell Survival, Proliferation, and Tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Yang, W.; Xiu, Z.; He, Y.; Huang, W.; Li, Y.; Sun, T. Bip Inhibition in Glioma Stem Cells Promotes Radiation-Induced Immunogenic Cell Death. Cell Death Dis. 2020, 11, 786. [Google Scholar] [CrossRef]
- Tang, Y.; Fan, Z.; Yang, M.; Zhang, S.; Li, M.; Fang, Y.; Li, J.; Feng, X. Low Concentrations of the Antidepressant Venlafaxine Affect Courtship Behaviour and Alter Serotonin and Dopamine Systems in Zebrafish (Danio rerio). Aquat. Toxicol. 2022, 244, 106082. [Google Scholar] [CrossRef]


| Gene Name | Accession Number | Oligo | Sequence (5′ to 3′) | Product Size (bp) | Melting Temperature (°C) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|
| actb2 | NM_181601.5 | F | CGTGCTGTCTTCCCATCCA | 86 | 60 | 108.62 | [32] |
| R | TCACCAACGTAGCTGTCTTTCTG | ||||||
| rps18 | NM_173234.1 | F | AACACGAACATTGATGGAAGACG | 255 | 60 | 96.16 | [33] |
| R | ATTAGCAAGGACCTGGCTGTATTT | ||||||
| mao | NM_212827.3 | F | ACCAACTCAAAACCGCATTC | 151 | 60 | 97.21 | [34] |
| R | GTAGGCAAAAGGGTTCCACA | ||||||
| hand2 | BC083365.1 | F | ATGAGTTTAGTTGGAGGGTTTC | 324 | 62 | 108.56 | [13] |
| R | GCTGTTGATGCTCTGGGT | ||||||
| drd2a | NM_183068.1 | F | TGTGATTGCGAATCCTGCCT | 194 | 60 | 104.83 | [35] |
| R | CGGGATGGGTGCATTTCTTT | ||||||
| cenpf3b | XM_002665215.7 | F | AAACGGCACTGACAAGTTGG | 380 | 60 | 103.29 | [36] |
| R | GCCCACCTTCTGCCATAGTT | ||||||
| casp3 | NM_131877.3 | F | GGCAGATTTCCTCTATGCATACTC | 72 | 60 | 98.30 | [37] |
| R | CATGAGCCGGTCATTGTG | ||||||
| hspa5 | NM_213058.1 | F | AAGAGGCCGAAGAGAAGGAC | 133 | 60 | 104.63 | |
| R | AGCAGCAGAAGCCTCGAAATA | ||||||
| ddit3 | NM_001082825.1 | F | AAGGAAAGTGTAGGAGCTGA | 197 | 60 | 94.39 | |
| R | TCACGCTCTCCACAAGAAGA | ||||||
| sox2 | NM_213118.1 | F | ACTCCATGACCAACTCGCAG | 159 | 60 | 97.86 | [38] |
| R | AATGAGACGACGACGTGACC | ||||||
| ddx4 | NM_131057.1 | F | ATGGCATTCCCATCATTTCAG | 74 | 60 | 107.50 | |
| R | GGCCGCCGTTTTTCCT | ||||||
| drd1b | NM_001135976.2 | F | ACGCTGTCCATCCTTATCTC | 135 | 60 | 105.50 | This work |
| R | TGTCCGATTAAGGCTGGAG | ||||||
| foxf1 | NM_001080186.1 | F | TGCACGGGATCATCAGGGAC | 113 | 60 | 90.47 | |
| R | GCCGAGGCCGTGCTAGAATA | ||||||
| foxk1 | NM_199902.1 | F | TGAACCAGGAAGCCAGCGAA | 173 | 60 | 90.39 | |
| R | ACATTCGATCAGGTGCCCGT | ||||||
| foxl1 | NM_200984.1 | F | GTCTCCCTCCCGAGATGCAC | 115 | 60 | 90.64 | |
| R | CACTCTTTACGGGCACACGC | ||||||
| foxm1l | NM_201097.1 | F | CGACCAGAAGCAAACCGCTG | 84 | 60 | 99.71 | |
| R | GATCTGAGGGCAAGTGGGGG | ||||||
| foxq1a | NM_001243344.1 | F | GATCCTTCGAGACCGTGGGG | 187 | 60 | 106.65 | |
| R | TCGAAGGAGGCGTAGCGATG | ||||||
| hoxa3a | NM_131534.2 | F | GGCCAGCTCTTGGTTTACCC | 169 | 60 | 101.84 | |
| R | TGTAAATTGCCGAGCCGTCG | ||||||
| hoxc4a | NM_131122.2 | F | AGCTCAGCCTCTGCCAAACA | 97 | 60 | 92.87 | |
| R | GCTTGGGTTCCGCTCCATTG | ||||||
| hoxc6a | NM_131123.1 | F | CCACGTTGCCCAGGAGTACA | 114 | 60 | 90.60 | |
| R | ACTCCGCTGTGCGAGTTCAT | ||||||
| hoxc8a | NM_001005771.1 | F | GGCGGCGAAACATTAGAGCC | 197 | 60 | 107.82 | |
| R | GCCAATGCACAGGGGTTCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Villadangos, M.; Robles, V.; Valcarce, D.G. Zebrafish (Danio rerio) Embryo–Larvae as a Biosensor for Water Quality Assessment. Biology 2025, 14, 1533. https://doi.org/10.3390/biology14111533
Santos-Villadangos M, Robles V, Valcarce DG. Zebrafish (Danio rerio) Embryo–Larvae as a Biosensor for Water Quality Assessment. Biology. 2025; 14(11):1533. https://doi.org/10.3390/biology14111533
Chicago/Turabian StyleSantos-Villadangos, María, Vanesa Robles, and David G. Valcarce. 2025. "Zebrafish (Danio rerio) Embryo–Larvae as a Biosensor for Water Quality Assessment" Biology 14, no. 11: 1533. https://doi.org/10.3390/biology14111533
APA StyleSantos-Villadangos, M., Robles, V., & Valcarce, D. G. (2025). Zebrafish (Danio rerio) Embryo–Larvae as a Biosensor for Water Quality Assessment. Biology, 14(11), 1533. https://doi.org/10.3390/biology14111533






