Effects of Different Afforestation Measures on Biological Soil Crust Properties and Microbial Communities in an Alpine Sandy Land
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sample Site Selection and BSCs Sample Collection
2.3. Physicochemical Properties and Enzymatic Activities
2.4. 16S rDNA Extraction and Sequencing
2.5. Data Processing and Analysis
3. Results
3.1. Particle Composition of BSCs
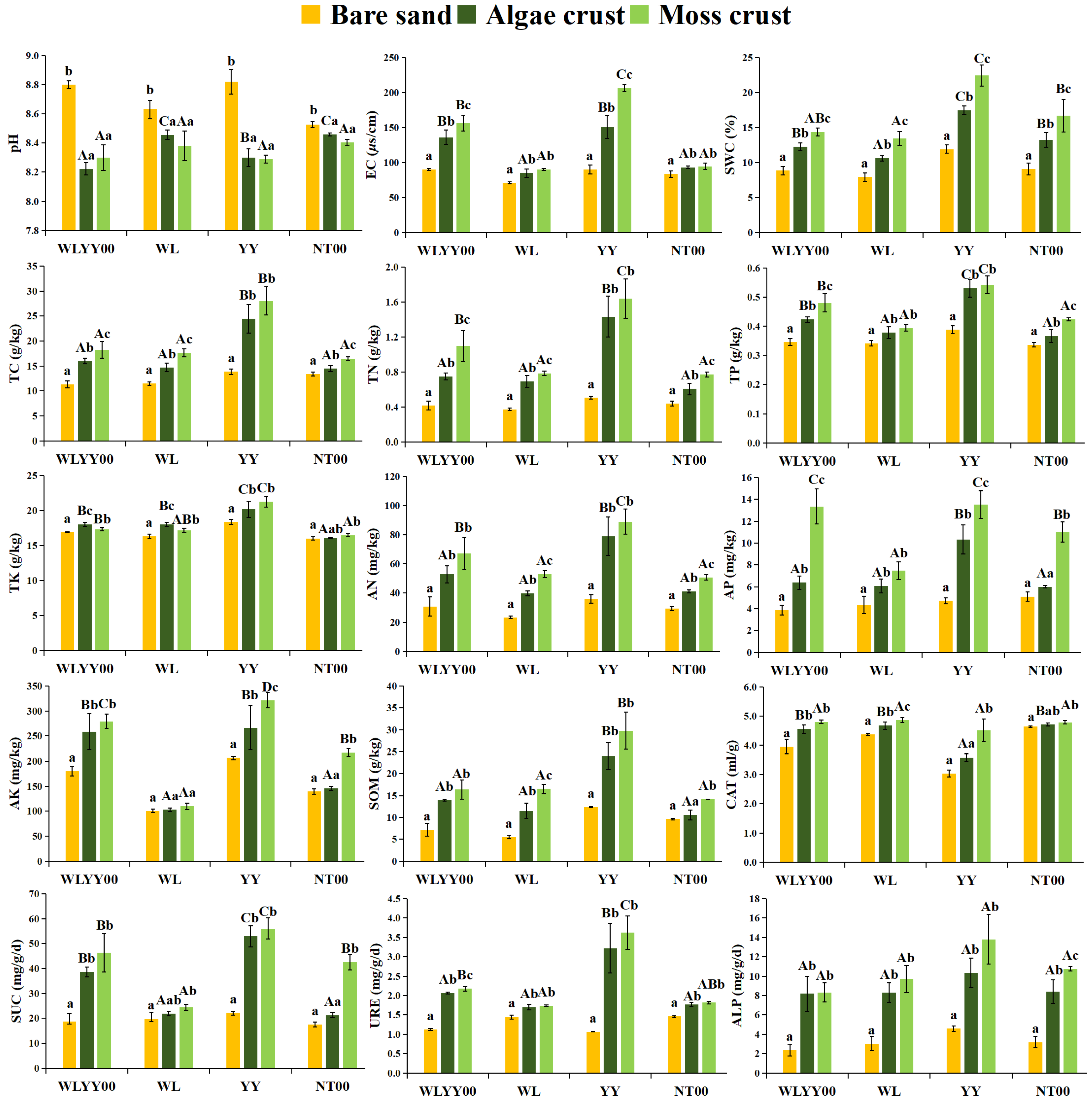
3.2. Physicochemical Properties and Enzymatic Activities of BSCs
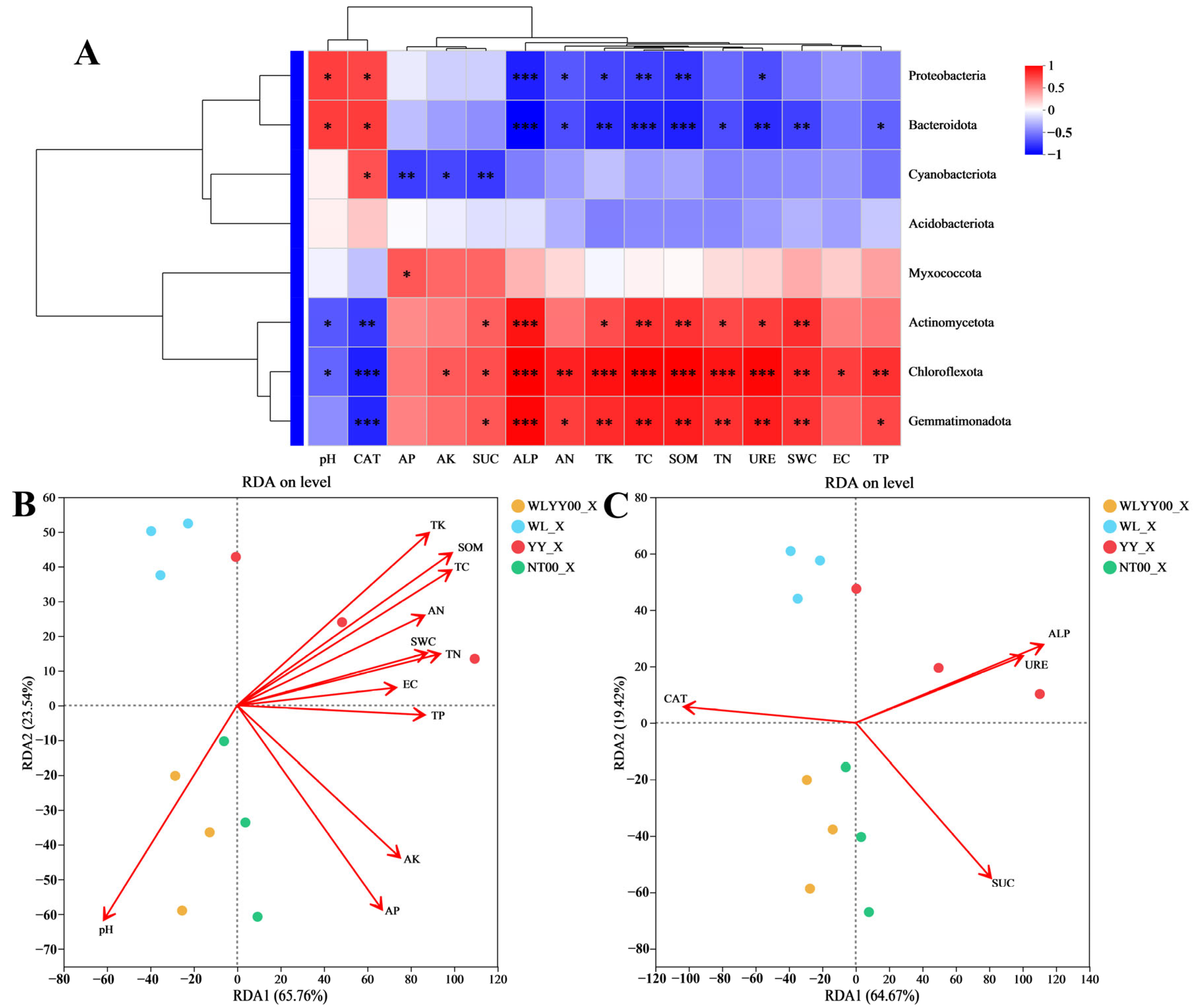
3.3. Correlation Analysis of Physicochemical Properties and Enzymatic Activities
3.4. Structural Characteristics of Bacterial Communities in BSCs
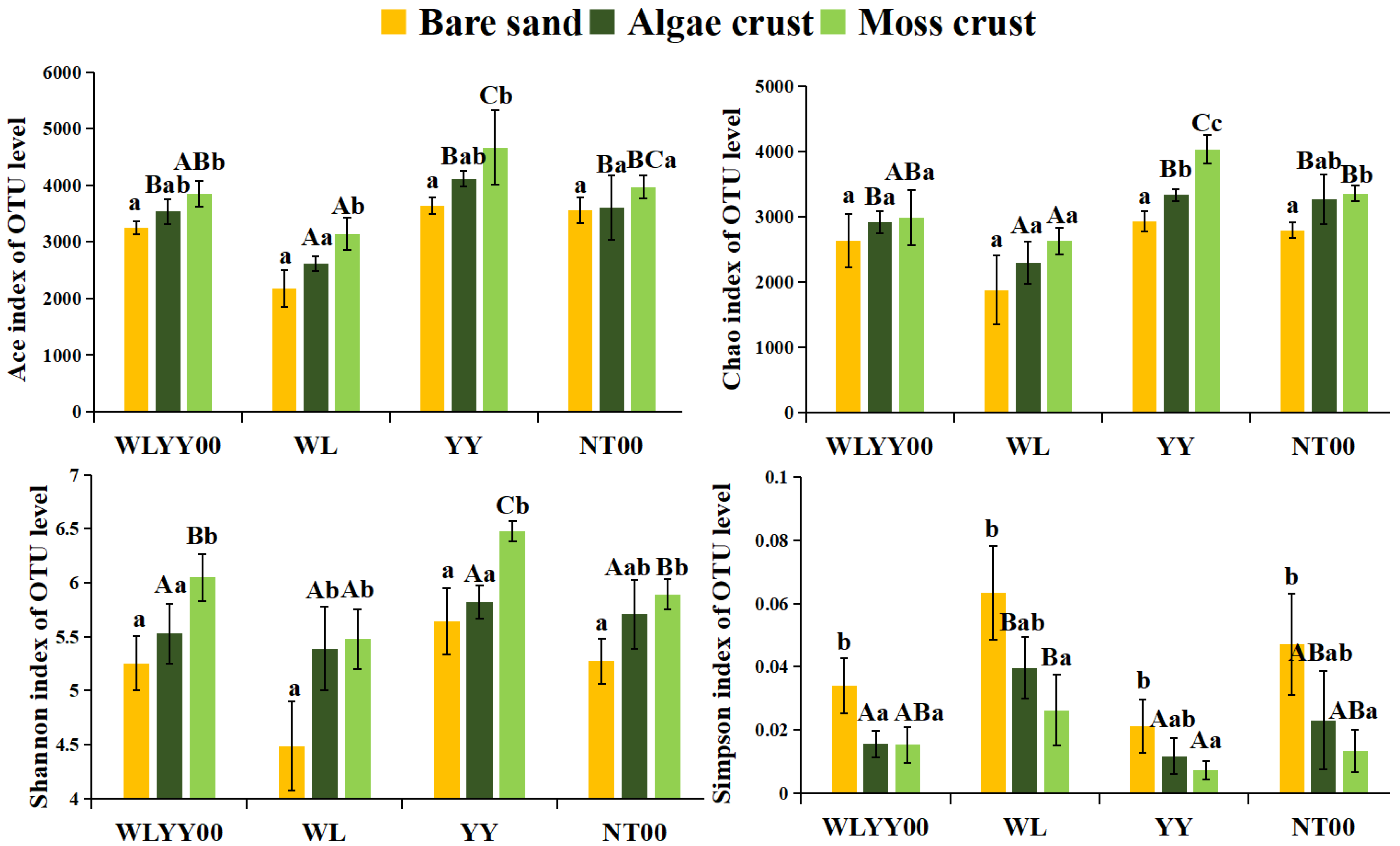
3.4.1. Statistical Analysis of OTUs
3.4.2. Alpha Diversity Indices
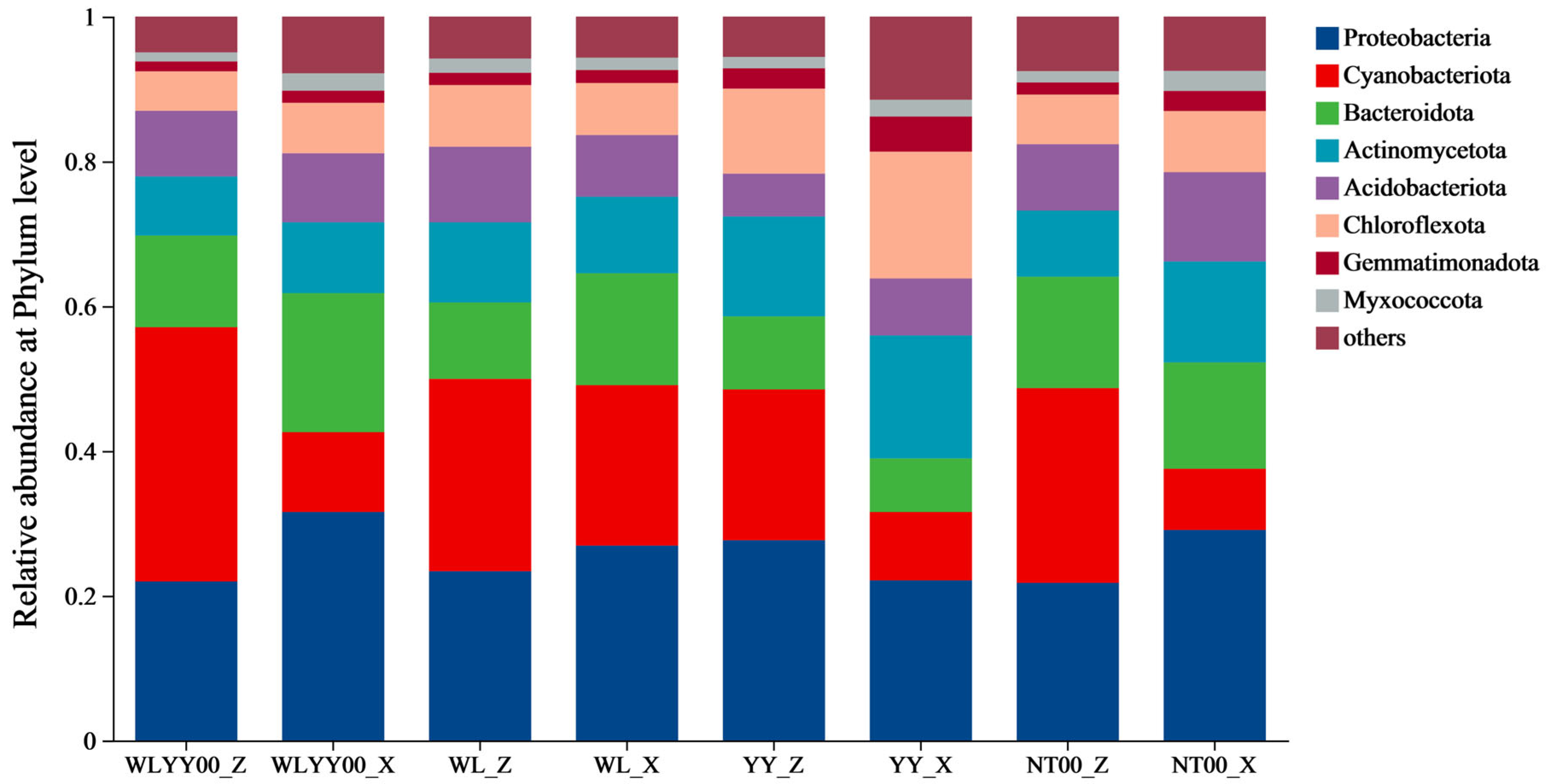
3.4.3. Bacterial Community Composition
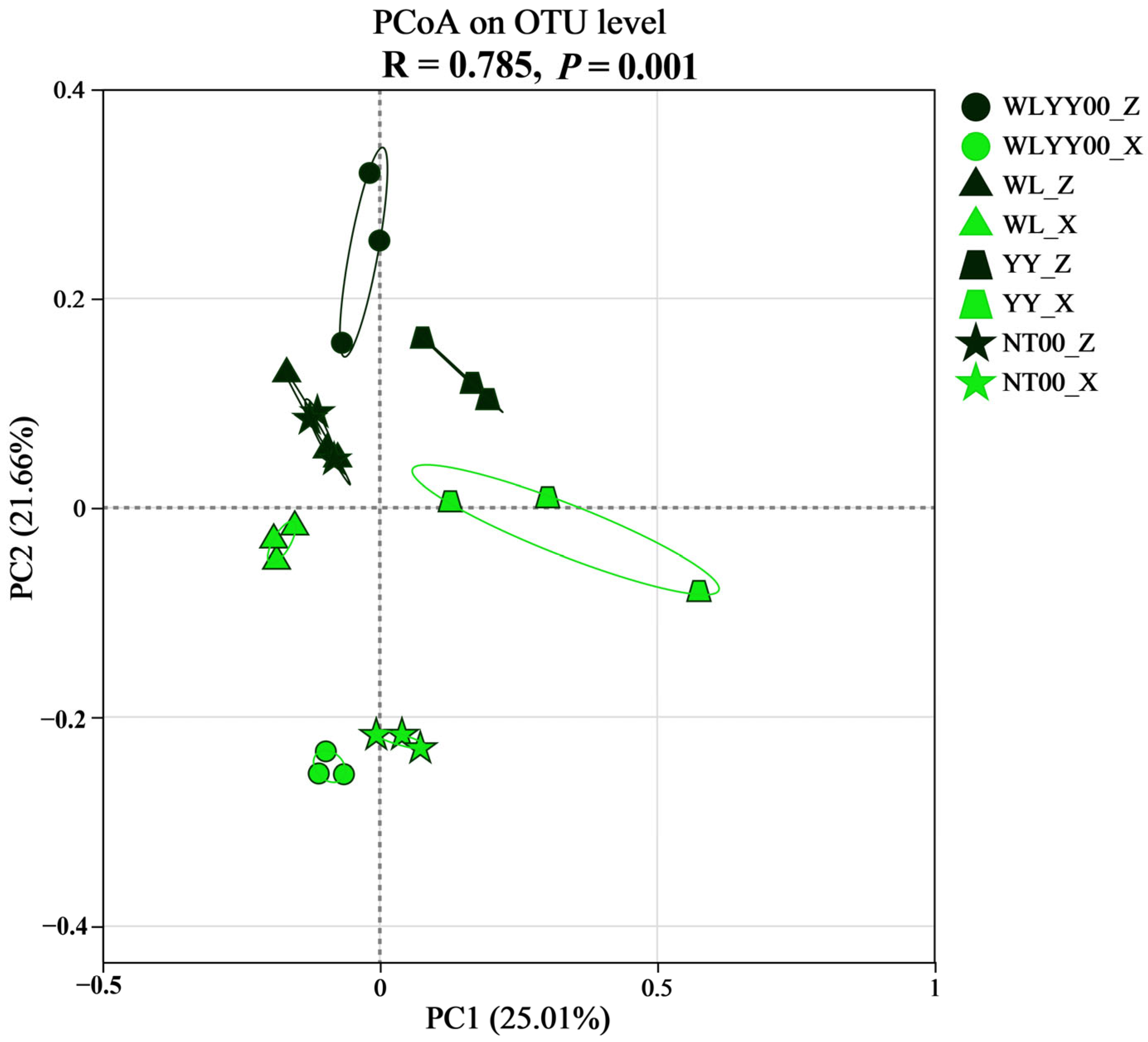
3.4.4. PCoA
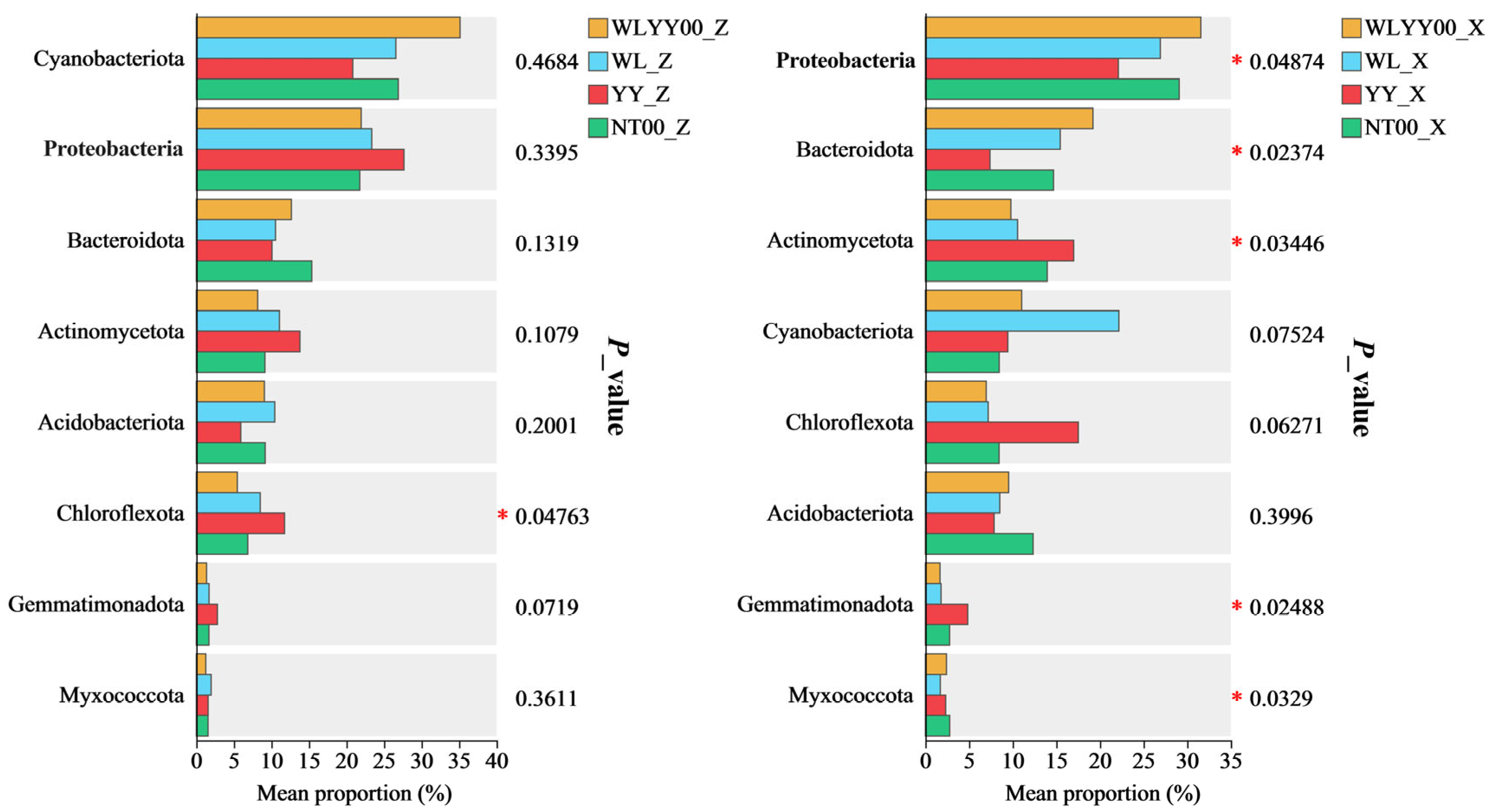
3.4.5. Significance Testing of Intergroup Differences Among Major Bacterial Phyla
3.5. Correlation Analysis of Physicochemical Properties, Enzymatic Activities, and Bacterial Community Structure
3.5.1. Algae Crust
3.5.2. Moss Crust
3.6. Functional Prediction of Bacterial Communities in BSCs of Different Afforested Sites
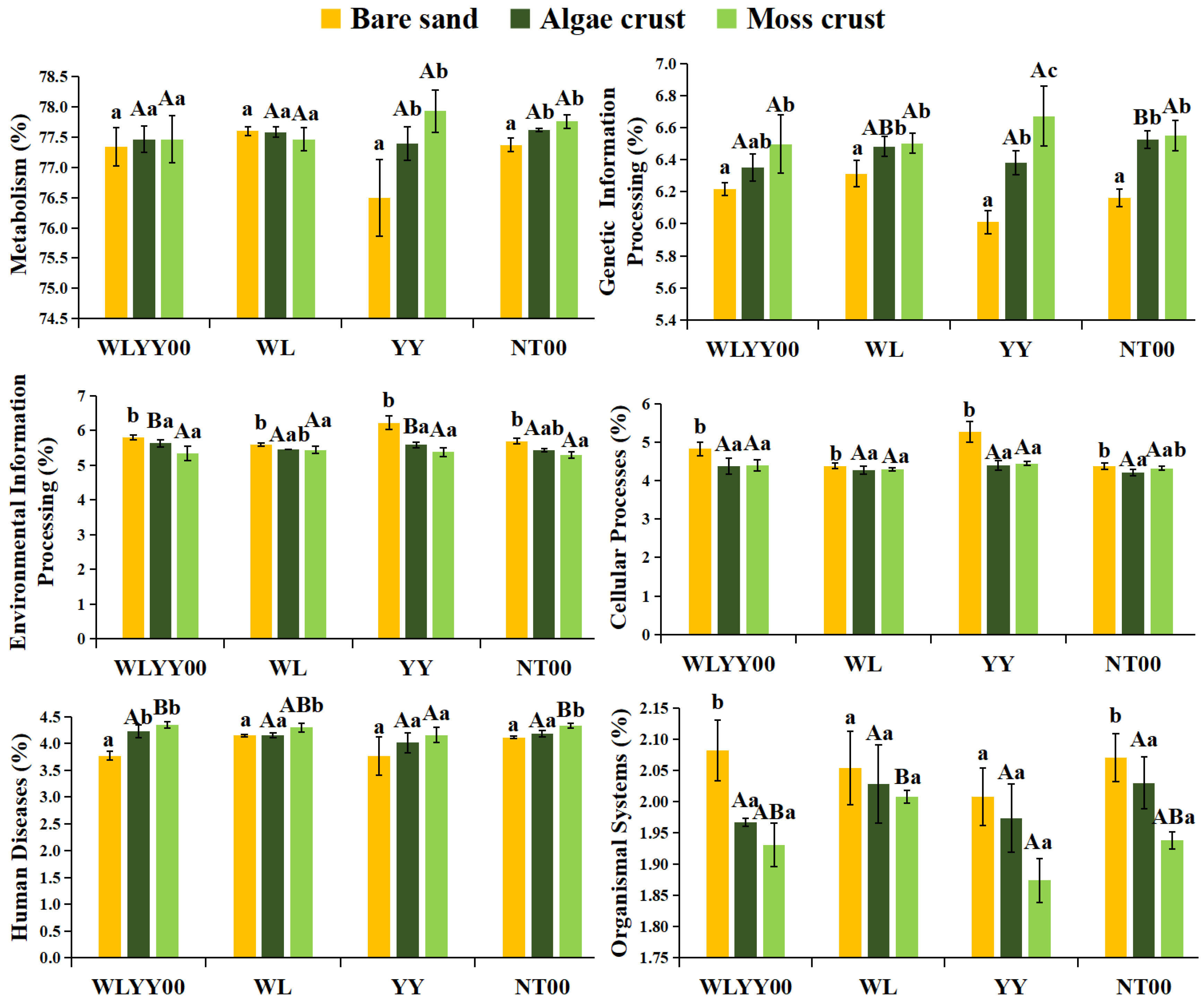
3.6.1. Prediction of Primary Functions
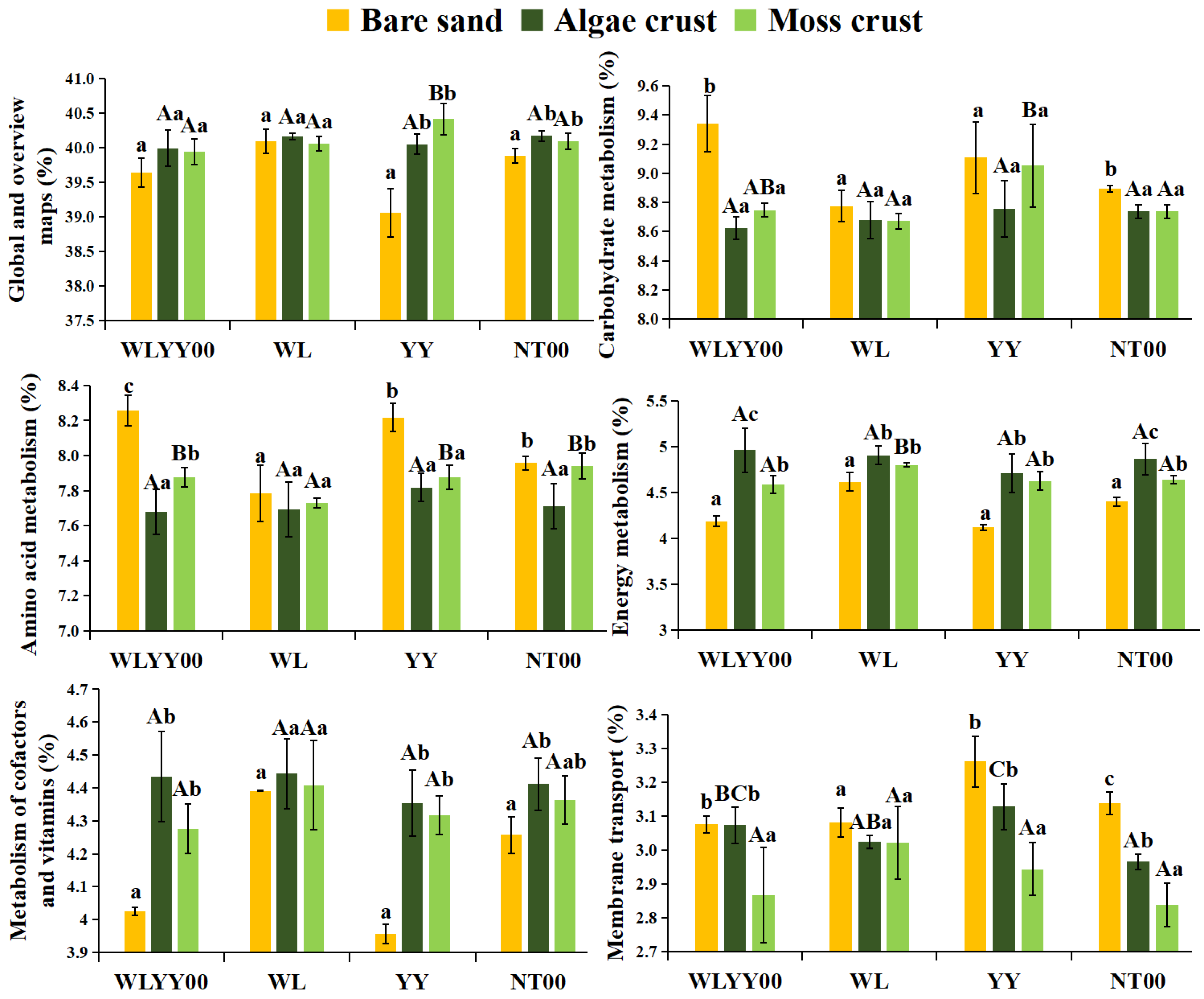
3.6.2. Prediction of Secondary Functions
4. Discussion
4.1. Characteristics of BSCs Particle Composition in Areas with Different Afforestation Measures
4.2. Characteristics of Physicochemical Properties and Enzymatic Activities of BSCs in Different Afforested Sites
4.3. Characteristics of Bacterial Community Structure and Functions of BSCs in Different Afforested Sites
5. Conclusions
6. Patents
- [1]
- Shaobo Du, Huichun Xie, Chongyi E., Tianyue Zhao, Shuang Ji, Zhiqiang Dong, Shaoxiong Zhang, Haokun Wu. A plant fixation device for desertification control in deserts. ZL202322873122.9, 26 July 2024.
- [2]
- Shaobo Du, Huichun Xie, Chongyi E., Shujing Qi, Haokun Wu, Shuang Ji, Tianyue Zhao, Zhiqiang Dong. An invention relates to a portable spraying device for desert algae biological control of sand. ZL202421424816.2, 28 March 2025.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BSCs | Biological soil crusts |
| WLYY00 | Salix cheilophila+ Populus simonii plantation |
| WL | Salix cheilophila plantation |
| YY | Populus simonii plantation |
| NT00 | Caragana korshinskii plantation |
References
- Xue, J.; Gui, D.; Lei, J.; Sun, H.; Zeng, F.; Mao, D.; Jin, Q.; Liu, Y. Oasification: An unable evasive process in fighting against desertification for the sustainable development of arid and semiarid regions of China. Catena 2019, 179, 197–209. [Google Scholar] [CrossRef]
- Lu, H. New trend of studying desert evolution and desertification. Sci. Sin. Terrae 2025, 55, 2089–2093. [Google Scholar] [CrossRef]
- Garcia-Pichel, F. The microbiology of biological soil crusts. Annu. Rev. Microbiol. 2023, 77, 149–171. [Google Scholar] [CrossRef]
- Zhao, J.; Zheng, Y.; Zhang, B.; Chen, Y.; Zhang, Y. Progress in the study of algae and mosses in biological soil crusts. Front. Biol. China 2009, 4, 143–150. [Google Scholar] [CrossRef]
- Zhang, Q.; Lv, J.; Ma, Y.; Li, E.; Shen, C.; Chen, J. Microbial community structure and potential function of algal crusts in different regions of Gurbantunggut Desert, Xinjiang, China. Acta Ecol. Sin. 2024, 44, 6317–6330. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, K.; Yang, Y.; Bu, C. Field Artificial Cultivation Technology of Moss Dominated Crust in Mu Us Sandland. J. Soil Water Conserv. 2016, 36, 165–170. [Google Scholar] [CrossRef]
- Su, Y.G.; Li, X.R.; Chen, Y.W.; Zhang, Z.S.; Yan, L. Carbon fixation of cyanobacterial-algal crusts after desert fixation and its implication to soil organic carbon accumulation in desert. Land Degrad. Dev. 2013, 24, 342–349. [Google Scholar] [CrossRef]
- Lu, Q.; Xiao, Y.; Lu, Y. Employment of algae-based biological soil crust to control desertification for the sustainable development: A mini-review. Algal Res. 2022, 65, 102747. [Google Scholar] [CrossRef]
- Elbert, W.; Weber, B.; Burrows, S.; Steinkamp, J.; Büdel, B.; Andreae, M.O.; Pöschl, U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat. Geosci. 2012, 5, 459–462. [Google Scholar] [CrossRef]
- Jiao, B.; Zhang, B.; Zhao, K.; Yan, L.; Wu, Z. Promoting effect of biological soil crusts succession on soil nitrogen transformation and microbial activity in water-wind erosion crisscross region of Loess Plateau. J. Desert Res. 2023, 43, 191–199. [Google Scholar] [CrossRef]
- He, H.; Liu, W.; Chang, Z.; Hou, C.; Sun, L.; Chi, X. Effects of biological soil crust succession on soil nutrients, microbial community composition in desert regions. Arid Land Geogr. 2024, 47, 1724–1734. [Google Scholar] [CrossRef]
- Cui, Y.; Lv, Y.; Li, B. Physicochemical properties of soil microbiotic crusts on Erdos Plateau. Soils 2004, 36, 197–202. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, B.; Gao, Y.; Cheng, L.; Jia, X.; Pang, Y.; Zhao, H. Composition and influencing factors of the biological soil crust bacterial communities in the Sabina vulgaris community in Mu Us Sandy Land. J. Desert Res. 2020, 40, 130–141. [Google Scholar] [CrossRef]
- Li, X.; Yao, Z.; Dong, Z. Driving mechanism of aeolian desertification in Gonghe Basin of Qinghai Province. Bull. Soil Water Conserv. 2018, 38, 337–344. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Z.; He, L.; Zhao, X.; Yang, X. The allocation and cycling characteristics of main nutrients for Caragana intermedia with different stand age on alpine sandy land. For. Res. 2023, 36, 119–128. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, Y. Effects of soil crusts on physicochemical properties of shallow soil in alpine sandy area. J. Arid Land Resour. Environ. 2022, 36, 154–160. [Google Scholar] [CrossRef]
- Li, Q.; Yang, D.; Jia, Z.; Zhang, L.; Zhang, Y.; Feng, L.; He, L.; Yang, K.; Dai, J.; Chen, J.; et al. Changes in soil organic carbon and total nitrogen stocks along a chronosequence of Caragana intermedia plantations in alpine sandy land. Ecol. Eng. 2019, 133, 53–59. [Google Scholar] [CrossRef]
- Du, S.; Xie, H.; E, C.; Zhang, G.; Qiao, F.; Geng, G. Effectiveness of different sand control measures in improving the surface soil in alpine sandy land. Land Degrad. Dev. 2025, 36, 5269–5286. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Deng, L.; Zhou, H. Differences in the physical and chemical properties of biological soil crusts in different shrub communities in the Gonghe Basin. Arid Zone Res. 2023, 40, 1797–1805. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, L.; Jia, X.; Wu, B.; Li, Y.; Yue, Y.; Zhou, H.; Zhao, X. The dynamics of soil carbon release covered with biological soil crusts in Alpine sand area. Acta Ecol. Sin. 2020, 40, 6396–6404. [Google Scholar] [CrossRef]
- Yoshihara, Y.; Tatsuno, Y.; Miyasaka, K.; Sasaki, T. Can complementarity in water use help explain diversity-productivity relationships in semi-arid grasslands? J. Arid Environ. 2020, 173, 103994. [Google Scholar] [CrossRef]
- Bao, S. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Zhang, N.; He, X.D.; Gao, Y.B.; Li, Y.H.; Wang, H.T.; Ma, D.; Zhang, R.; Yang, S. Pedogenic carbonate and soil dehydrogenase activity in response to soil organic matter in Artemisia ordosica community. Pedosphere 2010, 20, 229–235. [Google Scholar] [CrossRef]
- Li, M.; Nie, H.; Zhang, S.; Zhou, F.; Han, D.; Zhan, L.; Tian, Y.; Shi, M.; Zhang, E. Correlation between FDA enzyme activity and soil fertility under combining application organic and nitrogen fertilizer in facility vegetable field. Acta Hortic. Sin. 2016, 43, 907–917. [Google Scholar] [CrossRef]
- Hu, B.; Liang, D.; Liu, J.; Lei, L.; Yu, D. Transformation of heavy metal fractions on soil urease and nitrate reductase activities in copper and selenium co-contaminated soil. Ecotoxicol. Environ. Saf. 2014, 110, 41–48. [Google Scholar] [CrossRef]
- Wang, L.; Mei, W.; Chen, X.; Fang, H.; Liu, X.; Yang, D.; Wang, W. Determination of water-soluble sugar in rice by 3,5-dinitrosalicylic acid colorimetric method. J. Chin. Cereals Oils Assoc. 2020, 35, 168–173. [Google Scholar]
- Du, S.; Xie, H.; Zhang, G.; Qiao, F.; Geng, G.; E, C. Improvement effects of different afforestation measures on the surface soil of alpine sandy land. Biology 2025, 14, 144. [Google Scholar] [CrossRef]
- Zuo, X.A.; Zhao, X.Y.; Zhao, H.L.; Li, Y.Q.; Guo, Y.R.; Zhao, Y.P. Changes of species diversity and productivity in relation to soil properties in sandy grassland in Horqin sand land. Environ. Sci. 2007, 28, 945–951. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhao, H.L.; Yi, X.Y.; Zuo, X.A.; Chen, Y.P. Dynamics of carbon and nitrogen storages in plant-soil system during desertification process in Horqin sandy land. Environ. Sci. 2006, 27, 635–640. [Google Scholar] [CrossRef]
- Su, Y.; Zhao, H. Influences of grazing and exclosure on carbon sequestration in degraded sandy Grassland, Inner Mongolia, North China. Environ. Sci. 2003, 27, 23–28. [Google Scholar] [CrossRef]
- Du, J.; Li, Y.; Yang, X.; Li, Y.; Ma, X. Effects of biological soil crusts types on soil physicochemical properties in the southeast fringe of the Tengger Desert. J. Desert Res. 2018, 38, 111–116. [Google Scholar] [CrossRef]
- Li, Y.; Brandle, J.; Awada, T.; Chen, Y.; Han, J.; Zhang, F.; Luo, Y. Accumulation of carbon and nitrogen in the plant-soil system after afforestation of active sand dunes in China’s Horqin Sandy Land. Agric. Ecosyst. Environ. 2013, 177, 75–84. [Google Scholar] [CrossRef]
- Du, S.; Xie, H.; Zhang, G.; Qiao, F.; Geng, G.; E, C. Response characteristics of biological soil crusts under different afforestation measures in alpine sandy land. Biology 2025, 14, 532. [Google Scholar] [CrossRef]
- Yin, R.; Wu, Y.; Zhang, X.; Hasi, R.J.; Tian, X.; Wang, J.; Li, Z.; Miao, H. Study on physicochemical properties of biological crusts and subsurface sediments in southern edge of Mu Us desert. J. Soil Water Conserv. 2013, 27, 120–124. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, C.; Zhu, X.; Zhou, D.; Tian, J.; Gu, F.; Huang, S.; Li, Z.; Zhao, Z.; Wang, X. Comprehensive assessment and evolution analysis of soil salinization in artificial oasis in arid desert area. China Environ. Sci. 2022, 42, 367–379. [Google Scholar] [CrossRef]
- Yan, P.; Qu, J.; Wang, L.; Xiao, J.; Zhang, Y.; Wang, X.; Guo, S. Effect of sand barrier fixation on the formation and development of biological soil crust. Arid Zone Res. 2023, 40, 1931–1937. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Xiao, B.; Wang, F. Evaporation characteristics of soil covered with moss crust in the wind-water erosion crisscross region of the Loess Plateau. J. Soil Water Conserv. 2020, 34, 208–215. [Google Scholar] [CrossRef]
- Tian, S.; Liu, X.; Jin, B.; Chen, Y.; Wang, Y.; Tian, X.; Zhao, X. Effects of litter on soil organic carbon fixation in Reaumuria soongorica communities in the Sangong River basin. Acta Ecol. Sin. 2019, 39, 5339–5347. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.; Guo, X.; Lu, Y.; Guo, H.; Zhang, Y.; Zhou, X. Vertical distribution characteristics and influencing factors of soil organic carbon under biological soil crusts in the Gurbantunggut Desert. Acta Ecol. Sin. 2024, 44, 2946–2954. [Google Scholar] [CrossRef]
- Belnap, J.; Büdel, B.; Lange, O.L. Biological soil crusts: Characteristics and distribution. In Biological Soil Crusts: Structure, Function, and Management; Belnap, J., Lange, O.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 3–30. [Google Scholar] [CrossRef]
- Li, X.R.; Zhang, P.; Su, Y.G.; Jia, R.L. Carbon fixation by biological soil crusts following revegetation of sand dunes in arid desert regions of China: A four-year field study. Catena 2012, 97, 119–126. [Google Scholar] [CrossRef]
- Miralles, I.; Ladrón de Guevara, M.; Chamizo, S.; Rodríguez-Caballero, E.; Ortega, R.; van Wesemael, B.; Cantón, Y. Soil CO2 exchange controlled by the interaction of biocrust successional stage and environmental variables in two semiarid ecosystems. Soil Biol. Biochem. 2018, 124, 11–23. [Google Scholar] [CrossRef]
- Si, S.; Li, Y.; Hui, R.; Liu, L.; Xie, M.; Wang, Y. Effects of snow on the photosynthetic physiological characteristics of biological soil crusts in A desert region. J. Desert Res. 2018, 38, 560–567. [Google Scholar] [CrossRef]
- Shi, K.; Jia, Z.; Zhang, H.; Zhang, Y.; Li, Q.; Feng, L.; He, L. Root distribution characteristics of typical sand-fixing plants in Gonghe Basin of Qinghai Province. Sci. Soil Water Conserv. 2016, 14, 78–85. [Google Scholar] [CrossRef]
- Bao, J.; Sun, J.; Wang, J. A review on microbial community assembly in biological soil crusts. J. Desert Res. 2022, 42, 33–43. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Ouyang, H.; Lan, S.; Hu, C. Pyrosequencing reveals significant changes in microbial communities along the ecological succession of biological soil crusts in the Tengger Desert of China. Pedosphere 2018, 28, 350–362. [Google Scholar] [CrossRef]
- Li, J.; Yang, R.; Duan, X.; Liu, J.; Liu, B. Effects of different biological soil crust types on microbial community structure and composition in Baijitan, China. J. Ecol. Rural Environ. 2023, 39, 97–106. [Google Scholar] [CrossRef]
- Li, K.; Zhang, B.; Zhao, K.; Zhang, Y.; Liu, Y. Succession of carbon-fixing microbial community in different stages of biological soil crusts in the Mu Us Sandy Land. Acta Ecol. Sin. 2024, 44, 1177–1190. [Google Scholar] [CrossRef]
- Fan, J.; Li, S.; Wang, R.; Yu, H.; Huang, J. Influence of biocrusts succession process on bacterial community structure of biocrustal layer and subsoils in desert steppe. Chin. J. Ecol. 2021, 40, 2033–2044. [Google Scholar] [CrossRef]
- Ma, L.H.; Meng, X.C.; Wang, G.Q.; Ma, Z.F.; Li, Y.K.; Li, Y.M.; Zhou, H.K.; Zhang, F.W.; Lin, L. Effects of moss crust inoculation on soil properties and microbial communities in alpine meadow in Sanjiangyuan, China. Chin. J. Plant Ecol. 2025, 49, 173–188. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Zhang, P.; Song, G.; Hui, R.; Wang, Z.; Wang, J. Development of bacterial communities in biological soil crusts along a revegetation chronosequence in the Tengger Desert, northwest China. Biogeosciences 2017, 14, 3801–3814. [Google Scholar] [CrossRef]
- Zhang, J.; He, J.; Wei, J.; Huang, C.; Wang, W.; Cai, Y. Effects of application period of microbial inoculants on rhizosphere soil bacterial diversity, enzyme activity and yield and quality of flue-cured tobacco. Crops 2025, 2, 162–171. [Google Scholar] [CrossRef]
- Du, Y.; Gao, G.; Chen, L.; Ding, G.; Zhang, Y.; Cao, H. Soil bacteria community structure and function prediction in the Hulun Buir Sandy Area. China Environ. Sci. 2019, 39, 4840–4848. [Google Scholar] [CrossRef]
- Yang, J.; Liu, F.; Han, X.; Lin, X.; Zhang, M.; Nie, X. Diversity and functional prediction of soil bacterial communities across different elevations in tropical rainforest of Diaoluo Mountain. Southwest China J. Agric. Sci. 2024, 37, 2270–2279. [Google Scholar] [CrossRef]
- Yuan, Z.; Jiang, J.; Hu, L.; Duan, T.; Yang, J.; Zhu, H.; Zhang, H.; Zhang, X.; Zhu, L.; Fu, Q. Analysis of bacterial community structure and functional prediction in citrus rhizosphere soils from different regions. Till. Cultiv. 2025, 45, 8–18+36. [Google Scholar] [CrossRef]




| Sample Site | Longitude | Latitude | Elevation (m) | Area (m2) | Coverage | Crust Type | Crust Thickness/mm | Crust Coverage/% |
|---|---|---|---|---|---|---|---|---|
| WLYY00 | 100°14′27.23” E | 36°15′32.432” N | 2828 | 133,333 | 67% | Bare sand | nd | 31.33 ± 11.47 |
| Algae crust | 7.12 ± 1.06 | 41.62 ± 14.38 | ||||||
| Moss crust | 13.11 ± 2.11 | 27.04 ± 4.31 | ||||||
| YY | 100°13′259.1” E | 36°15′32.713” N | 2827 | 46,666 | 81% | Bare sand | nd | 13.01 ± 2.74 |
| Algae crust | 4.74 ± 0.37 | 22.73 ± 4.13 | ||||||
| Moss crust | 9.22 ± 1.43 | 64.25 ± 11.74 | ||||||
| WL | 100°14′16.54” E | 36°14′50.678” N | 2830 | 40,000 | 64% | Bare sand | nd | 31.12 ± 9.48 |
| Algae crust | 5.16 ± 0.85 | 27.18 ± 3.04 | ||||||
| Moss crust | 8.45 ± 1.39 | 41.71 ± 8.45 | ||||||
| NT00 | 100°14′36.26” E | 36°15′5.3167” N | 2825 | 133,333 | 91% | Bare sand | nd | 17.23 ± 7.51 |
| Algae crust | 4.16 ± 0.77 | 21.08 ± 6.36 | ||||||
| Moss crust | 8.68 ± 1.46 | 61.70 ± 14.54 |
| Indicator | Method | Reference |
|---|---|---|
| Soil particle composition | Laser method | [21] |
| pH | Potentiometric method (water/soil ratio of 2.5:1) | [22] |
| Electrical conductivity (EC) | Conductometric method (water/soil ratio of 5:1) | [22] |
| Soil water content (SWC) | Drying method | [22] |
| Alkaline-dissolved nitrogen (AN) | Alkaline dissolution and diffusion method | [22] |
| Available phosphorus (AP) | Sodium bicarbonate leaching and molybdenum antimony colorimetric method | [22] |
| Total phosphorus (TP) | Sodium hydroxide fusion and molybdenum antimony colorimetric method | [22] |
| Soil organic matter (SOM) | Potassium dichromate–concentrated sulfuric acid external heating method | [22] |
| Total carbon (TC) and total nitrogen (TN) | Combustion method | [22] |
| Total potassium (TK) and available potassium (AK) | Flame photometry | [22] |
| Urease (URE) | Indophenolic acid colorimetric method | [23] |
| Alkaline phosphatase (ALP) | Disodium phenyl phosphate colorimetric method | [24] |
| Sucrase (SUC) | 3,5-Dinitrosalicylic acid colorimetric method | [25] |
| Catalase (CAT) | Potassium permanganate titration method | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, S.; Xie, H.; Zhang, G.; Qiao, F.; Geng, G.; E, C. Effects of Different Afforestation Measures on Biological Soil Crust Properties and Microbial Communities in an Alpine Sandy Land. Biology 2025, 14, 1530. https://doi.org/10.3390/biology14111530
Du S, Xie H, Zhang G, Qiao F, Geng G, E C. Effects of Different Afforestation Measures on Biological Soil Crust Properties and Microbial Communities in an Alpine Sandy Land. Biology. 2025; 14(11):1530. https://doi.org/10.3390/biology14111530
Chicago/Turabian StyleDu, Shaobo, Huichun Xie, Gaosen Zhang, Feng Qiao, Guigong Geng, and Chongyi E. 2025. "Effects of Different Afforestation Measures on Biological Soil Crust Properties and Microbial Communities in an Alpine Sandy Land" Biology 14, no. 11: 1530. https://doi.org/10.3390/biology14111530
APA StyleDu, S., Xie, H., Zhang, G., Qiao, F., Geng, G., & E, C. (2025). Effects of Different Afforestation Measures on Biological Soil Crust Properties and Microbial Communities in an Alpine Sandy Land. Biology, 14(11), 1530. https://doi.org/10.3390/biology14111530







