Climate-Driven Shifts in Bat Distributions Reveal Functional Reorganization and Spatial Mismatch Across Agroecosystems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
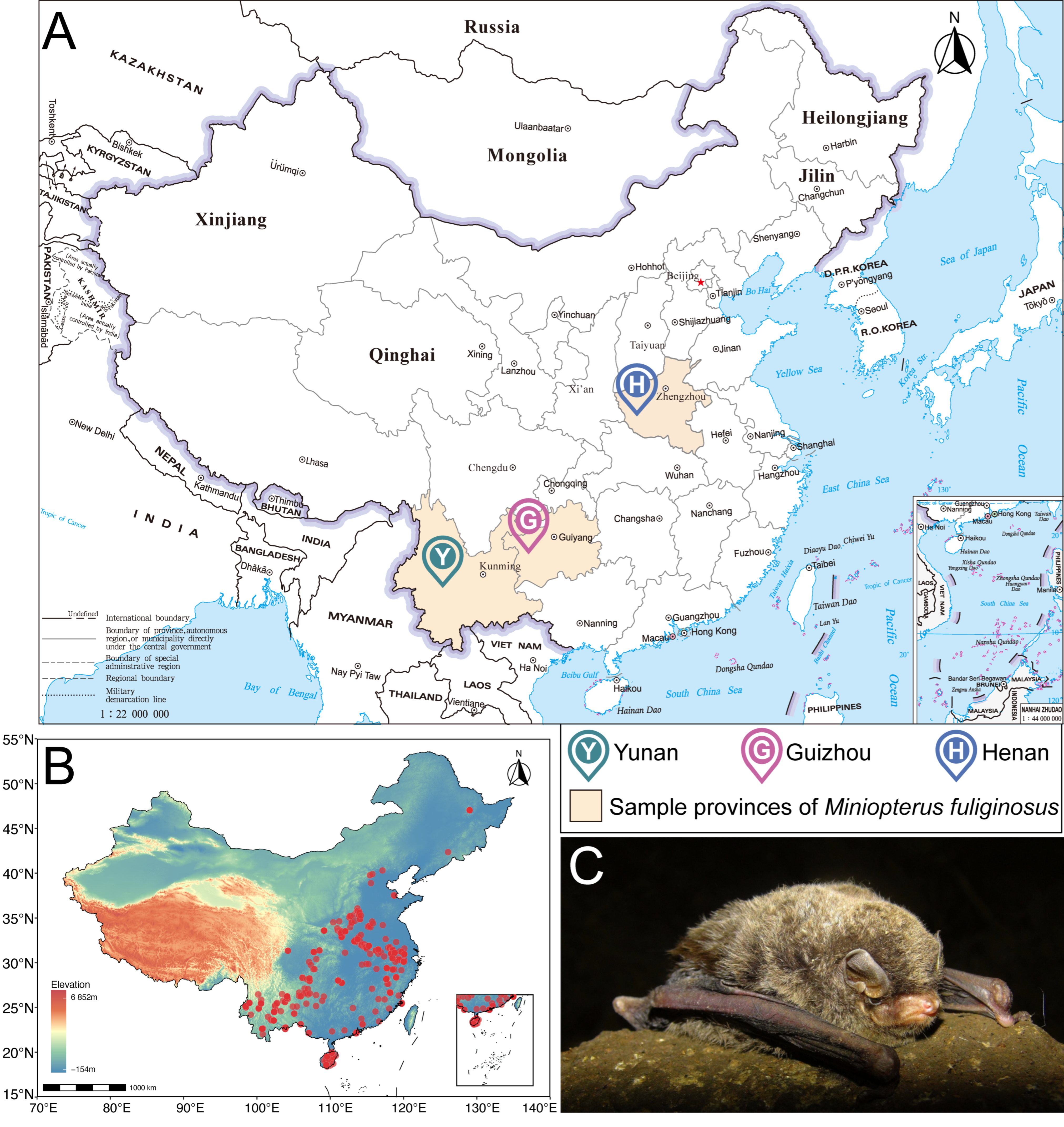
2.1. Study Sites and Sample Collection
2.2. DNA Extraction and PCR Amplification
2.3. Bioinformatic Analysis and Taxonomic Assignment
2.4. Species Occurrence Data and Environmental Predictors
2.5. Species Distribution Modeling and Projections
3. Results
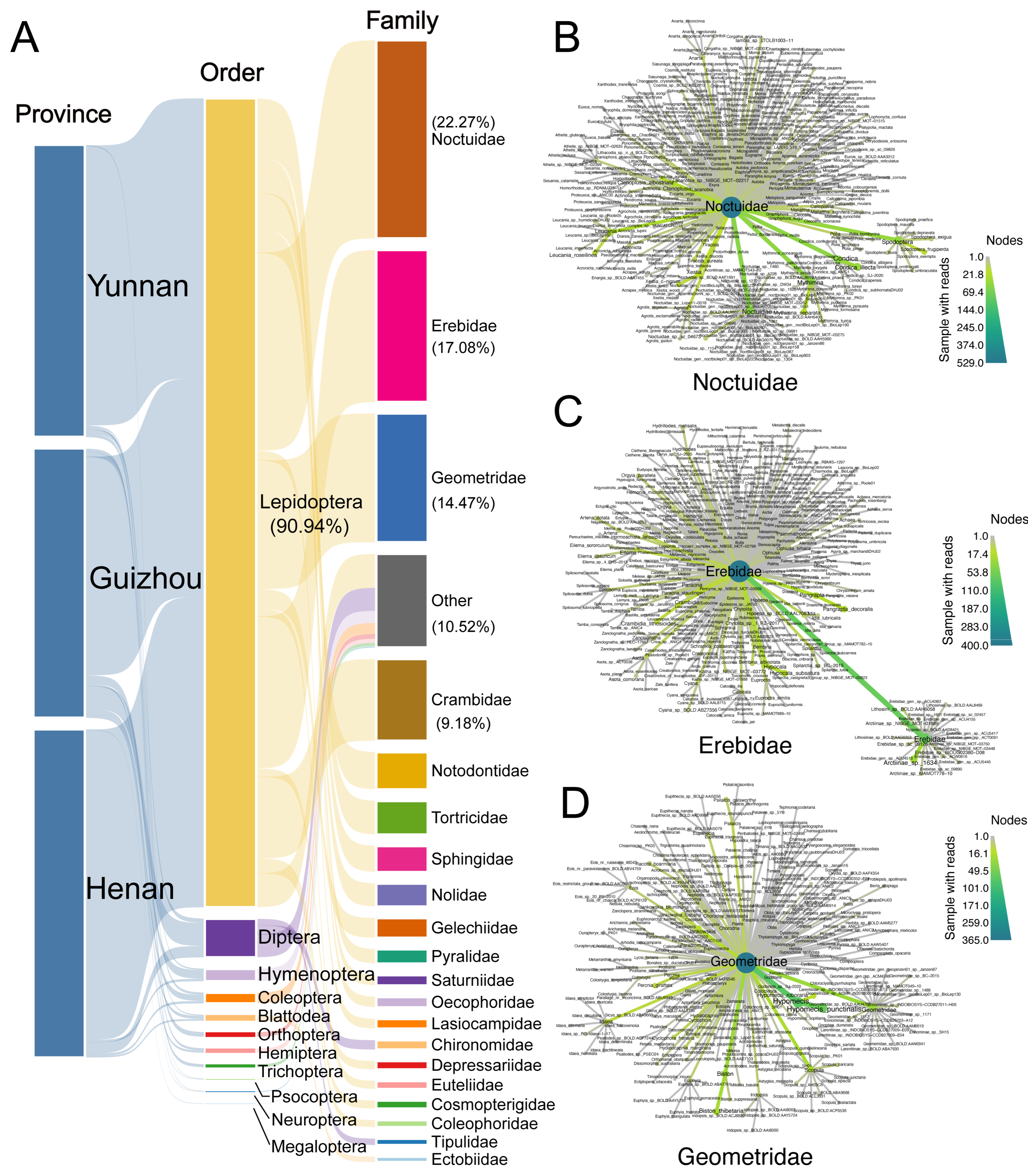
3.1. Dietary Composition Reveals Strong Specialization on Lepidoptera and Agricultural Pests
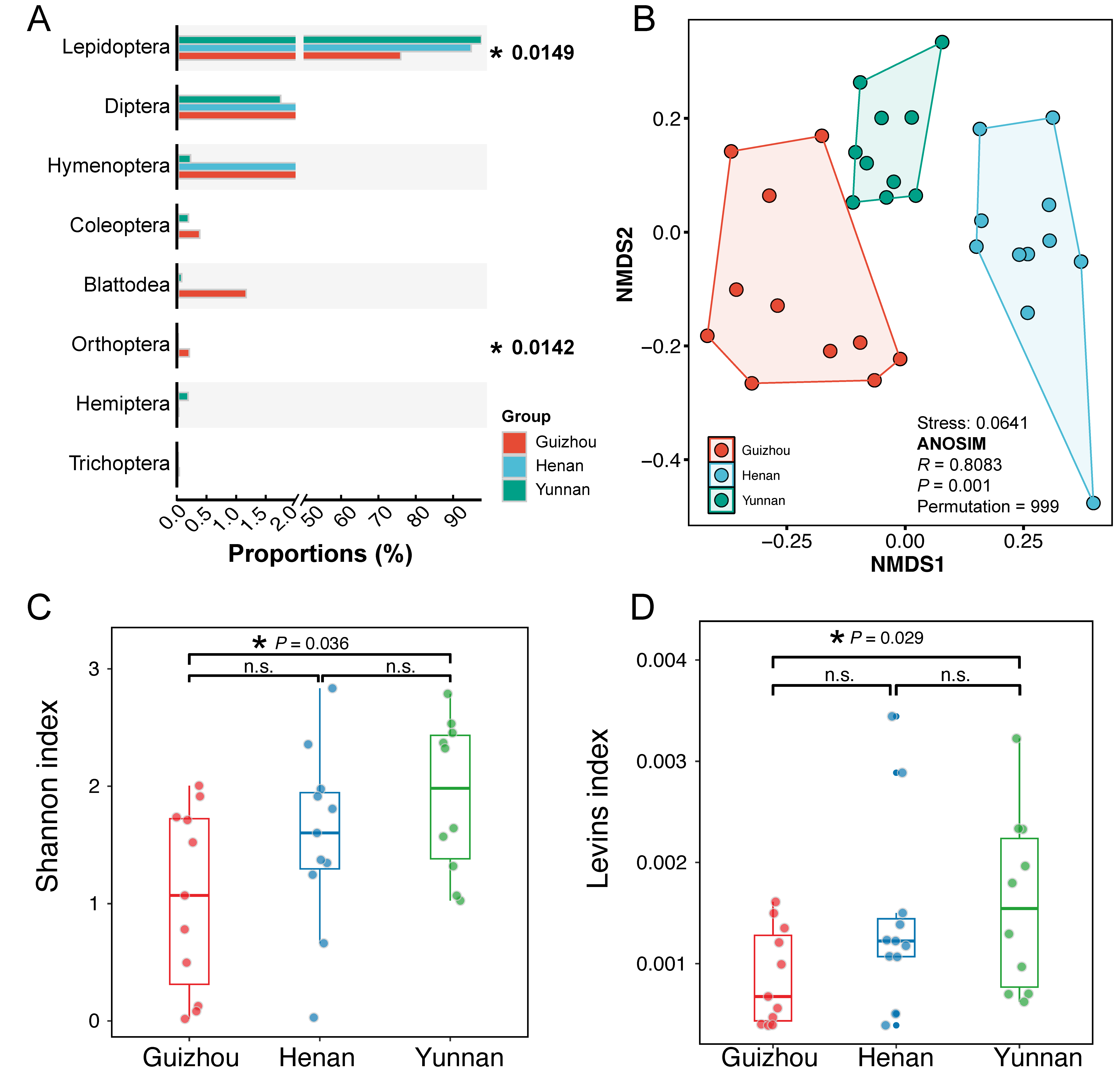
3.2. Regional Differences in Dietary Composition and Niche Metrics
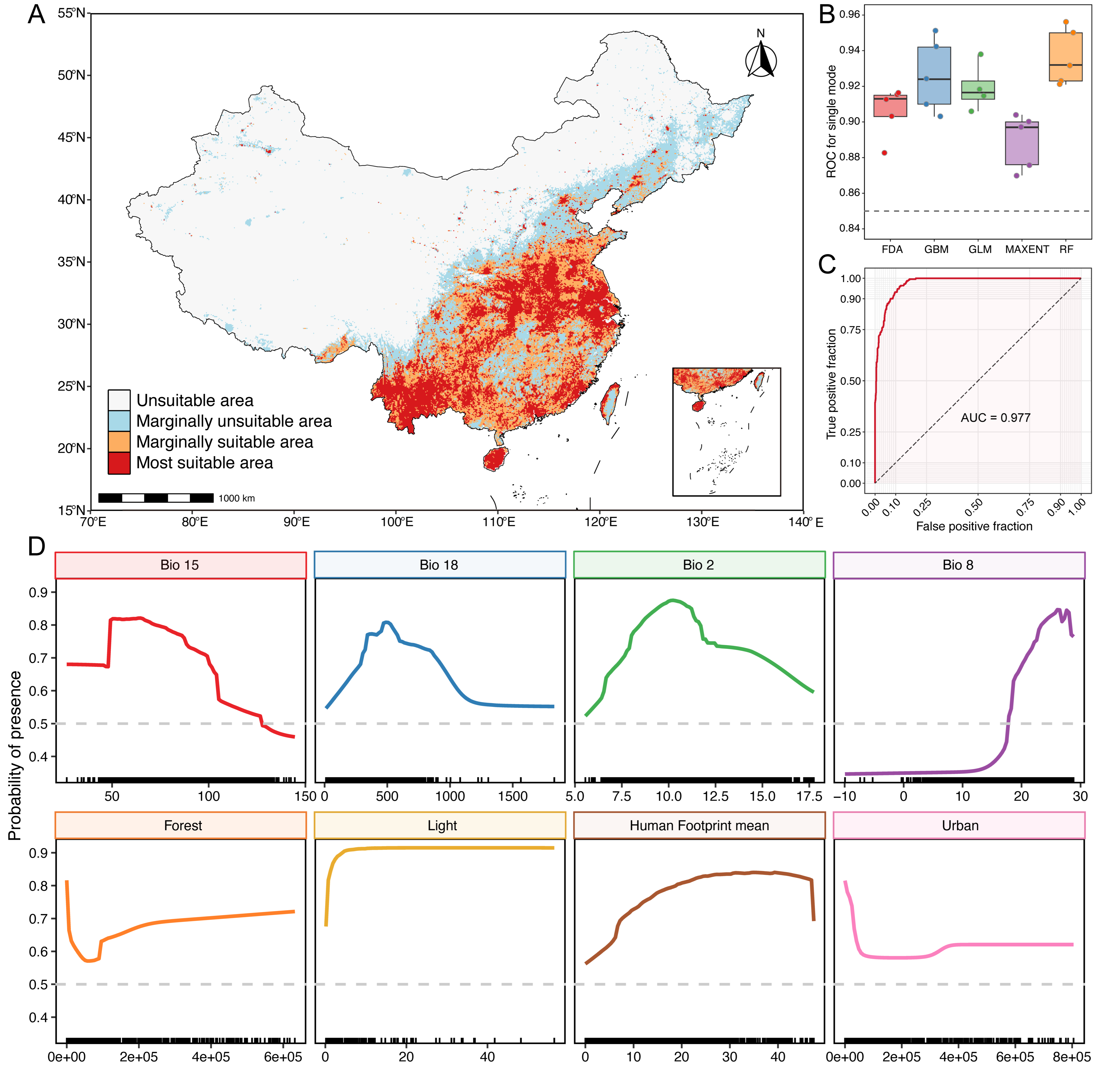
3.3. Model Evaluation, Variable Importance, and Current Habitat Suitability of Miniopterus fuliginosus
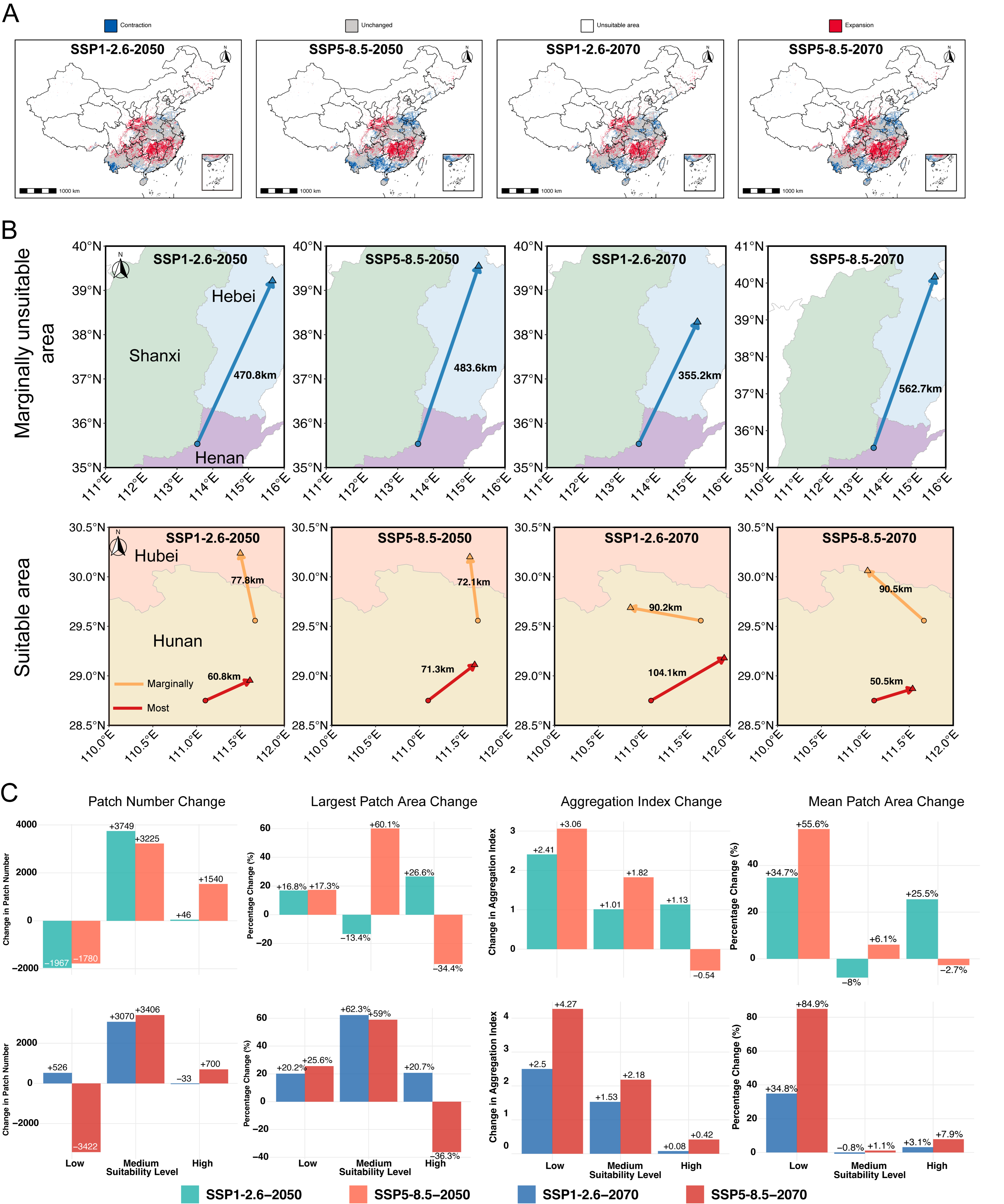
3.4. Climate-Driven Redistribution and Fragmentation of Suitable Habitat Across Future Scenarios
3.5. Structural Responses of Habitat Configuration Under Future Climate Scenarios
4. Discussion
4.1. Functional Trophic Specialization: Ecological and Evolutionary Underpinnings
4.2. Climate-Driven Range Shifts and Spatial Decoupling of Ecological Function
4.3. Habitat Configuration, Connectivity, and Functional Integrity Under Climate Change
4.4. Toward Sustainable Bat-Mediated Ecosystem Services Under Climate Change
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taylor, P.J.; Kearney, T.C.; Clark, V.R.; Howard, A.; Mdluli, M.V.; Markotter, W.; Geldenhuys, M.; Richards, L.R.; Rakotoarivelo, A.R.; Watson, J. Southern Africa’s Great Escarpment as an amphitheater of climate-driven diversification and a buffer against future climate change in bats. Glob. Change Biol. 2024, 30, e17344. [Google Scholar] [CrossRef]
- Lenoir, J.; Svenning, J.C. Climate-related range shifts—A global multidimensional synthesis and new research directions. Ecography 2015, 38, 15–28. [Google Scholar] [CrossRef]
- Devictor, V.; Mouillot, D.; Meynard, C.; Jiguet, F.; Thuiller, W.; Mouquet, N. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: The need for integrative conservation strategies in a changing world. Ecol. Lett. 2010, 13, 1030–1040. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Tscharntke, T.; Bommarco, R.; Clough, Y.; Crist, T.O.; Kleijn, D.; Rand, T.A.; Tylianakis, J.M.; van Nouhuys, S.; Vidal, S. Conservation biological control and enemy diversity on a landscape scale. Biol. Control 2008, 45, 238–253. [Google Scholar] [CrossRef]
- Bouarakia, O.; Linden, V.M.; Joubert, E.; Weier, S.M.; Grass, I.; Tscharntke, T.; Foord, S.H.; Taylor, P.J. Bats and birds control tortricid pest moths in South African macadamia orchards. Agric. Ecosyst. Environ. 2023, 352, 108527. [Google Scholar] [CrossRef]
- Sherwin, H.A.; Montgomery, W.I.; Lundy, M.G. The impact and implications of climate change for bats. Mammal Rev. 2013, 43, 171–182. [Google Scholar] [CrossRef]
- Fialas, P.C.; Santini, L.; Russo, D.; Amorim, F.; Rebelo, H.; Novella-Fernandez, R.; Marques, F.; Domer, A.; Vella, A.; Martinoli, A. Changes in community composition and functional diversity of European bats under climate change. Conserv. Biol. 2025, 39, e70025. [Google Scholar] [CrossRef] [PubMed]
- Kunz, T.H.; de Torrez, E.B.; Bauer, D.; Lobova, T.; Fleming, T.H. Ecosystem services provided by bats. Year Ecol. Conserv. Biol. 2011, 1223, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Maas, B.; Karp, D.S.; Bumrungsri, S.; Darras, K.; Gonthier, D.; Huang, J.C.C.; Lindell, C.A.; Maine, J.J.; Mestre, L.; Michel, N.L.; et al. Bird and bat predation services in tropical forests and agroforestry landscapes. Biol. Rev. 2016, 91, 1081–1101. [Google Scholar] [CrossRef]
- Festa, F.; Ancillotto, L.; Santini, L.; Pacifici, M.; Rocha, R.; Toshkova, N.; Amorim, F.; Benitez-Lopez, A.; Domer, A.; Hamidovic, D.; et al. Bat responses to climate change: A systematic review. Biol. Rev. 2023, 98, 19–33. [Google Scholar] [CrossRef]
- Zhang, C.M.; Jiang, T.L.; Lu, G.J.; Lin, A.Q.; Sun, K.P.; Liu, S.; Feng, J. Geographical variation in the echolocation calls of bent-winged bats, Miniopterus fuliginosus. Zoology 2018, 131, 36–44. [Google Scholar] [CrossRef]
- Li, S.; Sun, K.; Lu, G.; Lin, A.; Jiang, T.; Jin, L.; Hoyt, J.R.; Feng, J.J. Mitochondrial genetic differentiation and morphological difference of Miniopterus fuliginosus and Miniopterus magnater in China and Vietnam. Ecol. Evol. 2015, 5, 1214–1223. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, Y.; Huang, Z.; Feng, J.; Jiang, T. Pest suppression services and dietary niche differentiation of bats in Chinese smallholder farming systems: Implications for integrated pest management. J. Pest Sci. 2024, 97, 1587–1603. [Google Scholar] [CrossRef]
- Boyles, J.G.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Economic Importance of Bats in Agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Cohen, Y.; Bar-David, S.; Nielsen, M.; Bohmann, K.; Korine, C. An appetite for pests: Synanthropic insectivorous bats exploit cotton pest irruptions and consume various deleterious arthropods. Mol. Ecol. 2020, 29, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Coissac, E.; Pompanon, F.; Brochmann, C.; Willerslev, E. Towards next-generation biodiversity assessment using DNA metabarcoding. Mol. Ecol. 2012, 21, 2045–2050. [Google Scholar] [CrossRef]
- Araujo, M.B.; New, M. Ensemble forecasting of species distributions. Trends Ecol. Evol. 2007, 22, 42–47. [Google Scholar] [CrossRef]
- O’neill, B.C.; Oppenheimer, M.; Warren, R.; Hallegatte, S.; Kopp, R.E.; Pörtner, H.O.; Scholes, R.; Birkmann, J.; Foden, W.; Licker, R. IPCC reasons for concern regarding climate change risks. Nat. Clim. Change 2017, 7, 28–37. [Google Scholar] [CrossRef]
- Zhu, D.; Liu, Y.; Gong, L.; Si, M.; Wang, Q.; Feng, J.; Jiang, T. The Consumption and Diversity Variation Responses of Agricultural Pests and Their Dietary Niche Differentiation in Insectivorous Bats. Animals 2024, 14, 815. [Google Scholar] [CrossRef]
- Liu, Y.; Si, M.; Huang, Z.; Feng, J.; Jiang, T. Bats are sentinels for invasive pest surveillance based on DNA metabarcoding. Ecol. Indic. 2023, 152, 110354. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Alberdi, A.; Aizpurua, O.; Bohmann, K.; Gopalakrishnan, S.; Lynggaard, C.; Nielsen, M.; Gilbert, M.T.P. Promises and pitfalls of using high-throughput sequencing for diet analysis. Mol. Ecol. Resour. 2019, 19, 327–348. [Google Scholar] [CrossRef]
- Boyer, F.; Mercier, C.; Bonin, A.; Le Bras, Y.; Taberlet, P.; Coissac, E. obitools: A unix-inspired software package for DNA metabarcoding. Mol. Ecol. Resour. 2016, 16, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.; Beiko, R.G. 16S rRNA gene analysis with QIIME2. In Microbiome Analysis: Methods and Protocols; Springer: New York, NY, USA, 2018; pp. 113–129. [Google Scholar]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef] [PubMed]
- Siegenthaler, A.; Wangensteen, O.S.; Benvenuto, C.; Campos, J.; Mariani, S. DNA metabarcoding unveils multiscale trophic variation in a widespread coastal opportunist. Mol. Ecol. 2019, 28, 232–249. [Google Scholar] [CrossRef]
- Madden, T. The BLAST sequence analysis tool. NCBI Handb. 2013, 2, 425–436. [Google Scholar]
- Alberdi, A.; Aizpurua, O.; Gilbert, M.T.P.; Bohmann, K. Scrutinizing key steps for reliable metabarcoding of environmental samples. Methods Ecol. Evol. 2018, 9, 134–147. [Google Scholar] [CrossRef]
- Yang, D.; Wang, M.; Zhu, Y.; Zhang, L. Insect Fauna of Henan; Science Press: Beijing, China, 2010. [Google Scholar]
- Shen, X.; Ren, Y.; Niu, Y.; Yin, X.; Liu, X.; Liu, X.; Sun, H.; Ma, X. Insect Fauna of Henan; Science Press: Beijing, China, 2014. [Google Scholar]
- Li, H.; Ren, Y.; Zhang, D.; Du, X.; Li, W.; You, P. Insect Fauna of Henan; Science Press: Beijing, China, 2009. [Google Scholar]
- Wu, C.; Fang, C. Insect Fauna of Henan; Science Press: Beijing, China, 2010. [Google Scholar]
- Yang, X. Insect Fauna of the Qinling Mountains (12 Volumes); World Publishing Corporation: Beijing, China, 2018. (In Chinese) [Google Scholar]
- Gong, L.; Gu, H.; Chang, Y.; Wang, Z.; Shi, B.; Lin, A.; Wu, H.; Feng, J.; Jiang, T. Seasonal variation of population and individual dietary niche in the avivorous bat, Ia io. Oecologia 2023, 201, 733–747. [Google Scholar] [CrossRef]
- Barwell, L.J.; Isaac, N.J.; Kunin, W.E. Measuring β-diversity with species abundance data. J. Anim. Ecol. 2015, 84, 1112–1122. [Google Scholar] [CrossRef]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.j26pv2 (accessed on 9 April 2025).
- Aiello-Lammens, M.E.; Boria, R.A.; Radosavljevic, A.; Vilela, B.; Anderson, R.P. spThin: An R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 2015, 38, 541–545. [Google Scholar] [CrossRef]
- Mestre-Tomás, J. GeoThinneR: An R Package for Efficient Spatial Thinning of Species Occurrences and Point Data. arXiv 2025, arXiv:2505.07867. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Sulla-Menashe, D.; Friedl, M.A. User Guide to Collection 6 MODIS Land Cover (MCD12Q1 and MCD12C1) Product; Usgs: Reston, VA, USA, 2018; Volume 1, p. 18. [Google Scholar]
- Sanderson, E.W.; Jaiteh, M.; Levy, M.A.; Redford, K.H.; Wannebo, A.V.; Woolmer, G. The human footprint and the last of the wild: The human footprint is a global map of human influence on the land surface, which suggests that human beings are stewards of nature, whether we like it or not. BioScience 2002, 52, 891–904. [Google Scholar] [CrossRef]
- Akinwande, M.O.; Dikko, H.G.; Samson, A. Variance inflation factor: As a condition for the inclusion of suppressor variable (s) in regression analysis. Open J. Stat. 2015, 5, 754. [Google Scholar] [CrossRef]
- Naimi, B. Package ‘usdm’. 2017. Available online: https://cran.r-project.org/web/packages/usdm/index.html (accessed on 27 October 2025).
- Mi, C.; Ma, L.; Yang, M.; Li, X.; Meiri, S.; Roll, U.; Oskyrko, O.; Pincheira-Donoso, D.; Harvey, L.P.; Jablonski, D. Global protected areas as refuges for amphibians and reptiles under climate change. Nat. Commun. 2023, 14, 1389. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2013. [Google Scholar]
- Hijmans, R.J.; Bivand, R.; Forner, K.; Ooms, J.; Pebesma, E.; Sumner, M.D. Package ‘terra’. 2022. Available online: https://cran.r-project.org/web/packages/terra/index.html (accessed on 27 October 2025).
- Hijmans, R.J.; Van Etten, J.; Cheng, J.; Mattiuzzi, M.; Sumner, M.; Greenberg, J.A.; Lamigueiro, O.P.; Bevan, A.; Racine, E.B.; Shortridge, A.; et al. Package ‘raster’. 2015. Available online: https://cran.r-project.org/package=raster (accessed on 27 October 2025).
- Thuiller, W.; Lafourcade, B.; Engler, R.; Araújo, M.B. BIOMOD—A platform for ensemble forecasting of species distributions. Ecography 2009, 32, 369–373. [Google Scholar] [CrossRef]
- Vignali, S.; Barras, A.G.; Arlettaz, R.; Braunisch, V. SDMtune: An R package to tune and evaluate species distribution models. Ecol. Evol. 2020, 10, 11488–11506. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Duan, R.-Y.; Kong, X.-Q.; Huang, M.-Y.; Fan, W.-Y.; Wang, Z.-G. The predictive performance and stability of six species distribution models. PLoS ONE 2014, 9, e112764. [Google Scholar] [CrossRef]
- Braunisch, V.; Suchant, R. Predicting species distributions based on incomplete survey data: The trade-off between precision and scale. Ecography 2010, 33, 826–840. [Google Scholar] [CrossRef]
- Zhao, G.; Cui, X.; Sun, J.; Li, T.; Wang, Q.; Ye, X.; Fan, B. Analysis of the distribution pattern of Chinese Ziziphus jujuba under climate change based on optimized biomod2 and MaxEnt models. Ecol. Indic. 2021, 132, 108256. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Tebaldi, C.; Van Vuuren, D.P.; Eyring, V.; Friedlingstein, P.; Hurtt, G.; Knutti, R.; Kriegler, E.; Lamarque, J.-F.; Lowe, J. The scenario model intercomparison project (ScenarioMIP) for CMIP6. Geosci. Model Dev. 2016, 9, 3461–3482. [Google Scholar] [CrossRef]
- Zelinka, M.D.; Myers, T.A.; McCoy, D.T.; Po-Chedley, S.; Caldwell, P.M.; Ceppi, P.; Klein, S.A.; Taylor, K.E. Causes of higher climate sensitivity in CMIP6 models. Geophys. Res. Lett. 2020, 47, e2019GL085782. [Google Scholar] [CrossRef]
- Alberdi, A.; Razgour, O.; Aizpurua, O.; Novella-Fernandez, R.; Aihartza, J.; Budinski, I.; Garin, I.; Ibanez, C.; Izagirre, E.; Rebelo, H.; et al. DNA metabarcoding and spatial modelling link diet diversification with distribution homogeneity in European bats. Nat. Commun. 2020, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Hase, K.; Miyamoto, T.; Kobayasi, K.I.; Hiryu, S. Rapid frequency control of sonar sounds by the FM bat, Miniopterus fuliginosus, in response to spectral overlap. Behav. Process. 2016, 128, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Barber, J.R.; Plotkin, D.; Rubin, J.J.; Homziak, N.T.; Leavell, B.C.; Houlihan, P.R.; Miner, K.A.; Breinholt, J.W.; Quirk-Royal, B.; Padrón, P.S. Anti-bat ultrasound production in moths is globally and phylogenetically widespread. Proc. Natl. Acad. Sci. USA 2022, 119, e2117485119. [Google Scholar] [CrossRef]
- Razgour, O.; Clare, E.L.; Zeale, M.R.K.; Hanmer, J.; Schnell, I.B.; Rasmussen, M.; Gilbert, T.P.; Jones, G. High-throughput sequencing offers insight into mechanisms of resource partitioning in cryptic bat species. Ecol. Evol. 2011, 1, 556–570. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua, O.; Budinski, I.; Georgiakakis, P.; Gopalakrishnan, S.; Ibanez, C.; Mata, V.; Rebelo, H.; Russo, D.; Szodoray-Paradi, F.; Zhelyazkova, V.; et al. Agriculture shapes the trophic niche of a bat preying on multiple pest arthropods across Europe: Evidence from DNA metabarcoding. Mol. Ecol. 2018, 27, 815–825. [Google Scholar] [CrossRef]
- Tylianakis, J.M.; Didham, R.K.; Bascompte, J.; Wardle, D.A. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 2008, 11, 1351–1363. [Google Scholar] [CrossRef]
- Oliver, T.H.; Morecroft, M.D. Interactions between climate change and land use change on biodiversity: Attribution problems, risks, and opportunities. Wiley Interdiscip. Rev. Clim. Change 2014, 5, 317–335. [Google Scholar] [CrossRef]
- Pacifici, M.; Visconti, P.; Butchart, S.H.; Watson, J.E.; Cassola, F.M.; Rondinini, C. Species’ traits influenced their response to recent climate change. Nat. Clim. Change 2017, 7, 205–208. [Google Scholar] [CrossRef]
- Razgour, O.; Forester, B.; Taggart, J.B.; Bekaert, M.; Juste, J.; Ibáñez, C.; Puechmaille, S.J.; Novella-Fernandez, R.; Alberdi, A.; Manel, S. Considering adaptive genetic variation in climate change vulnerability assessment reduces species range loss projections. Proc. Natl. Acad. Sci. USA 2019, 116, 10418–10423. [Google Scholar] [CrossRef]
- Mokany, K.; Prasad, S.; Westcott, D.A. Loss of frugivore seed dispersal services under climate change. Nat. Commun. 2014, 5, 3971. [Google Scholar] [CrossRef]
- Oliver, T.H.; Heard, M.S.; Isaac, N.J.; Roy, D.B.; Procter, D.; Eigenbrod, F.; Freckleton, R.; Hector, A.; Orme, C.D.L.; Petchey, O.L. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 2015, 30, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Haddad, N.M.; Brudvig, L.A.; Clobert, J.; Davies, K.F.; Gonzalez, A.; Holt, R.D.; Lovejoy, T.E.; Sexton, J.O.; Austin, M.P.; Collins, C.D. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 2015, 1, e1500052. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.E.; Evans, T.; Venter, O.; Williams, B.; Tulloch, A.; Stewart, C.; Thompson, I.; Ray, J.C.; Murray, K.; Salazar, A.J.N.e.; et al. The exceptional value of intact forest ecosystems. Nat. Ecol. Evol. 2018, 2, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 487–515. [Google Scholar] [CrossRef]
- Russo, D.; Ancillotto, L. Sensitivity of bats to urbanization: A review. Mamm. Biol. 2015, 80, 205–212. [Google Scholar] [CrossRef]
- Weier, S.M.; Linden, V.M.; Hammer, A.; Grass, I.; Tscharntke, T.; Taylor, P.J. Bat guilds respond differently to habitat loss and fragmentation at different scales in macadamia orchards in South Africa. Agric. Ecosyst. Environ. 2021, 320, 107588. [Google Scholar] [CrossRef]
- Svenning, J.C.; Skov, F. Limited filling of the potential range in European tree species. Ecol. Lett. 2004, 7, 565–573. [Google Scholar] [CrossRef]
- Keeley, A.T.; Beier, P.; Jenness, J.S. Connectivity metrics for conservation planning and monitoring. Biol. Conserv. 2021, 255, 109008. [Google Scholar] [CrossRef]
- Costanza, J.K.; Terando, A.J. Landscape connectivity planning for adaptation to future climate and land-use change. Curr. Landsc. Ecol. Rep. 2019, 4, 1–13. [Google Scholar] [CrossRef]
- Morelli, T.L.; Maher, S.P.; Lim, M.C.; Kastely, C.; Eastman, L.M.; Flint, L.E.; Flint, A.L.; Beissinger, S.R.; Moritz, C. Climate change refugia and habitat connectivity promote species persistence. Clim. Change Responses 2017, 4, 8. [Google Scholar] [CrossRef]
- Maas, B.; Fabian, Y.; Kross, S.M.; Richter, A.J. Divergent farmer and scientist perceptions of agricultural biodiversity, ecosystem services and decision-making. Biol. Conserv. 2021, 256, 109065. [Google Scholar] [CrossRef]
- Minang, P.A.; van Noordwijk, M.; Freeman, O.E.; Mbow, C.; de Leeuw, J.; Catacutan, D. Climate-Smart Landscapes: Multifunctionality in Practice; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2015. [Google Scholar]
- Schneider, F.D.; Brose, U.; Rall, B.C.; Guill, C. Animal diversity and ecosystem functioning in dynamic food webs. Nat. Commun. 2016, 7, 12718. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Geng, Y.; Pan, Y.; Zeng, H.; Huang, Z.; Taylor, P.J.; Jiang, T. Climate-Driven Shifts in Bat Distributions Reveal Functional Reorganization and Spatial Mismatch Across Agroecosystems. Biology 2025, 14, 1528. https://doi.org/10.3390/biology14111528
Liu Y, Geng Y, Pan Y, Zeng H, Huang Z, Taylor PJ, Jiang T. Climate-Driven Shifts in Bat Distributions Reveal Functional Reorganization and Spatial Mismatch Across Agroecosystems. Biology. 2025; 14(11):1528. https://doi.org/10.3390/biology14111528
Chicago/Turabian StyleLiu, Yingying, Yang Geng, Yushi Pan, Hao Zeng, Zhenglanyi Huang, Peter John Taylor, and Tinglei Jiang. 2025. "Climate-Driven Shifts in Bat Distributions Reveal Functional Reorganization and Spatial Mismatch Across Agroecosystems" Biology 14, no. 11: 1528. https://doi.org/10.3390/biology14111528
APA StyleLiu, Y., Geng, Y., Pan, Y., Zeng, H., Huang, Z., Taylor, P. J., & Jiang, T. (2025). Climate-Driven Shifts in Bat Distributions Reveal Functional Reorganization and Spatial Mismatch Across Agroecosystems. Biology, 14(11), 1528. https://doi.org/10.3390/biology14111528




