Hypoxia Supports LPS-Driven Tolerance and Functional Activation in BV-2 Microglial Cells
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. BV-2 Cell Culture
2.2. Stimulation Procedure and Hypoxia Induction in Vitro
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Antibodies
2.5. Protein Extraction and SDS-PAGE Western Blotting
2.6. Measurement of Reactive Oxygen Species (ROS)
2.7. Lactate Measurements
2.8. In Vitro Transmigration Assay
2.9. In Vitro Phagocytosis Assay
2.10. Analysis of Cell Viability and Cytotoxicity
2.11. RNA Isolation and Real-Time qPCR
2.12. Statistical Analysis
3. Results
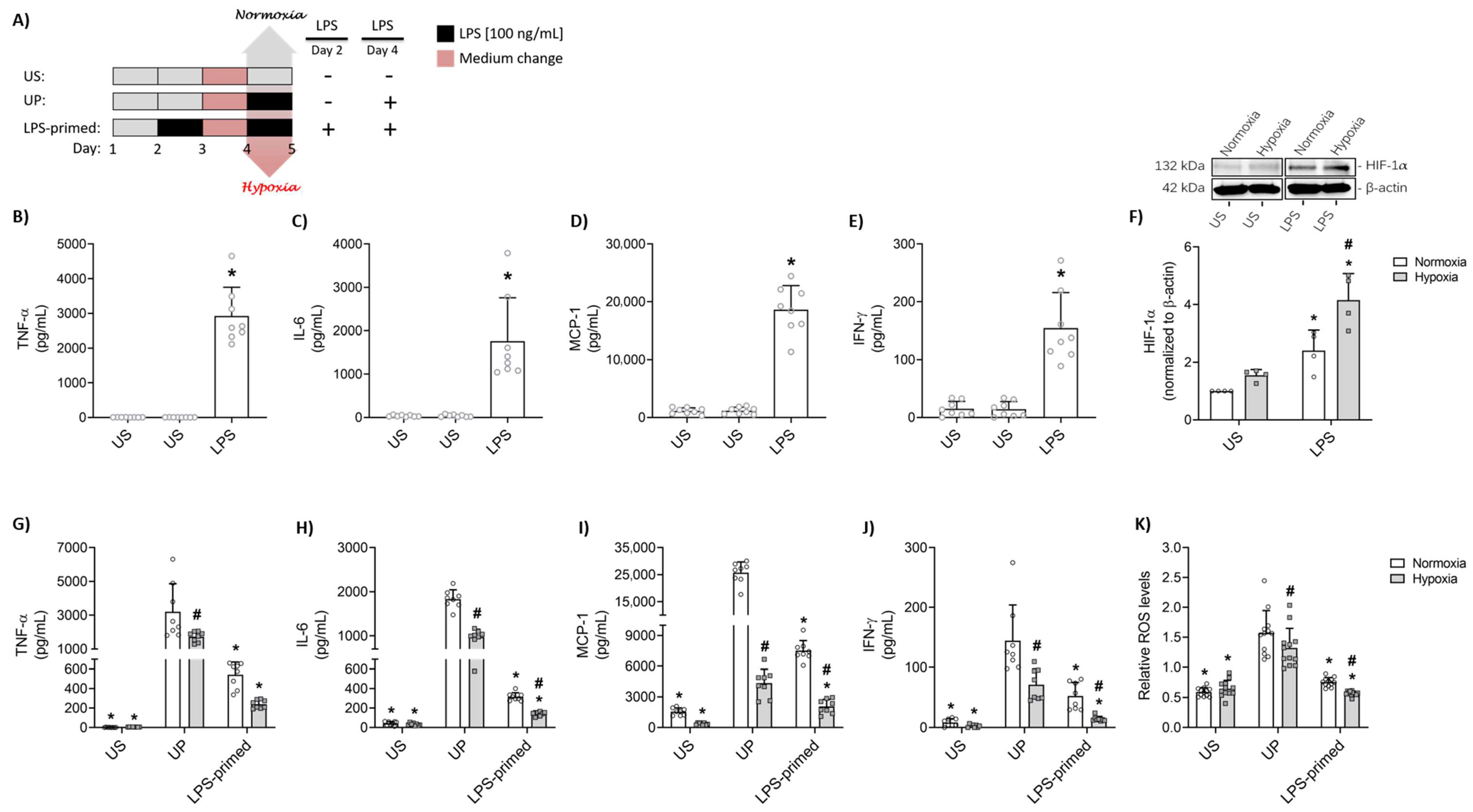
3.1. Hypoxia Potentiates LPS-Mediated Tolerance in BV-2 Microglial Cells
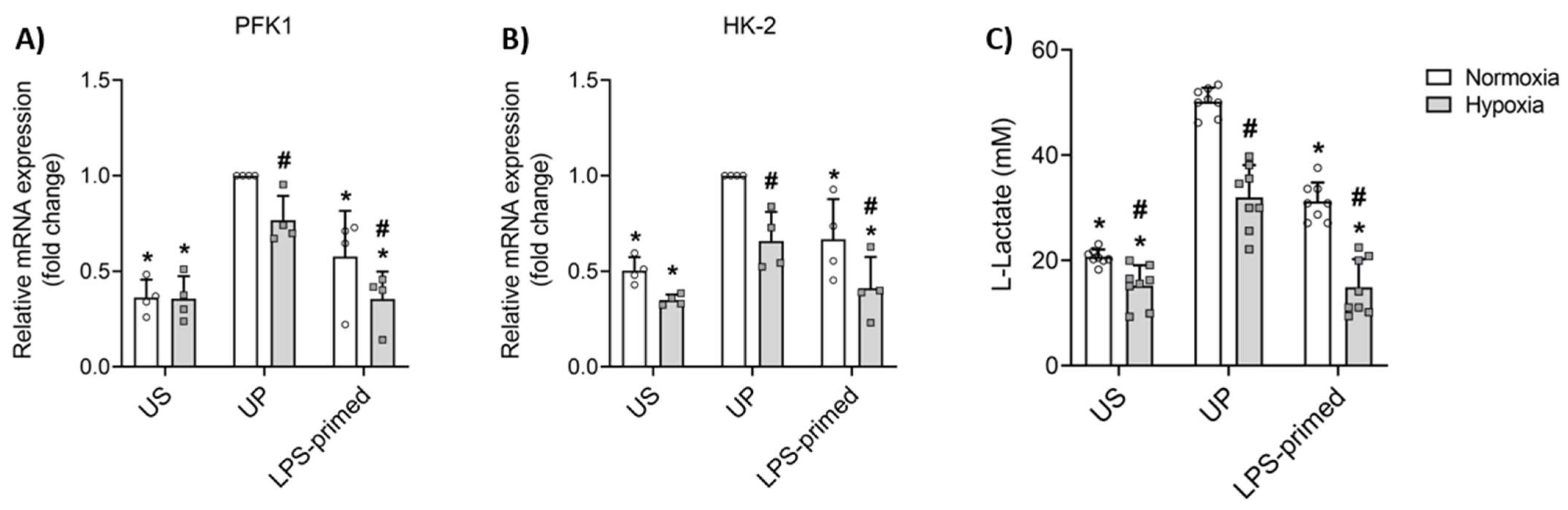
3.2. Glycolysis Mediates LPS-Induced Tolerance in BV-2 Microglia
3.3. MyD88-Dependent Suppression of NFκB-p65 Supports LPS-Induced Tolerance Under Normoxic and Hypoxic Conditions
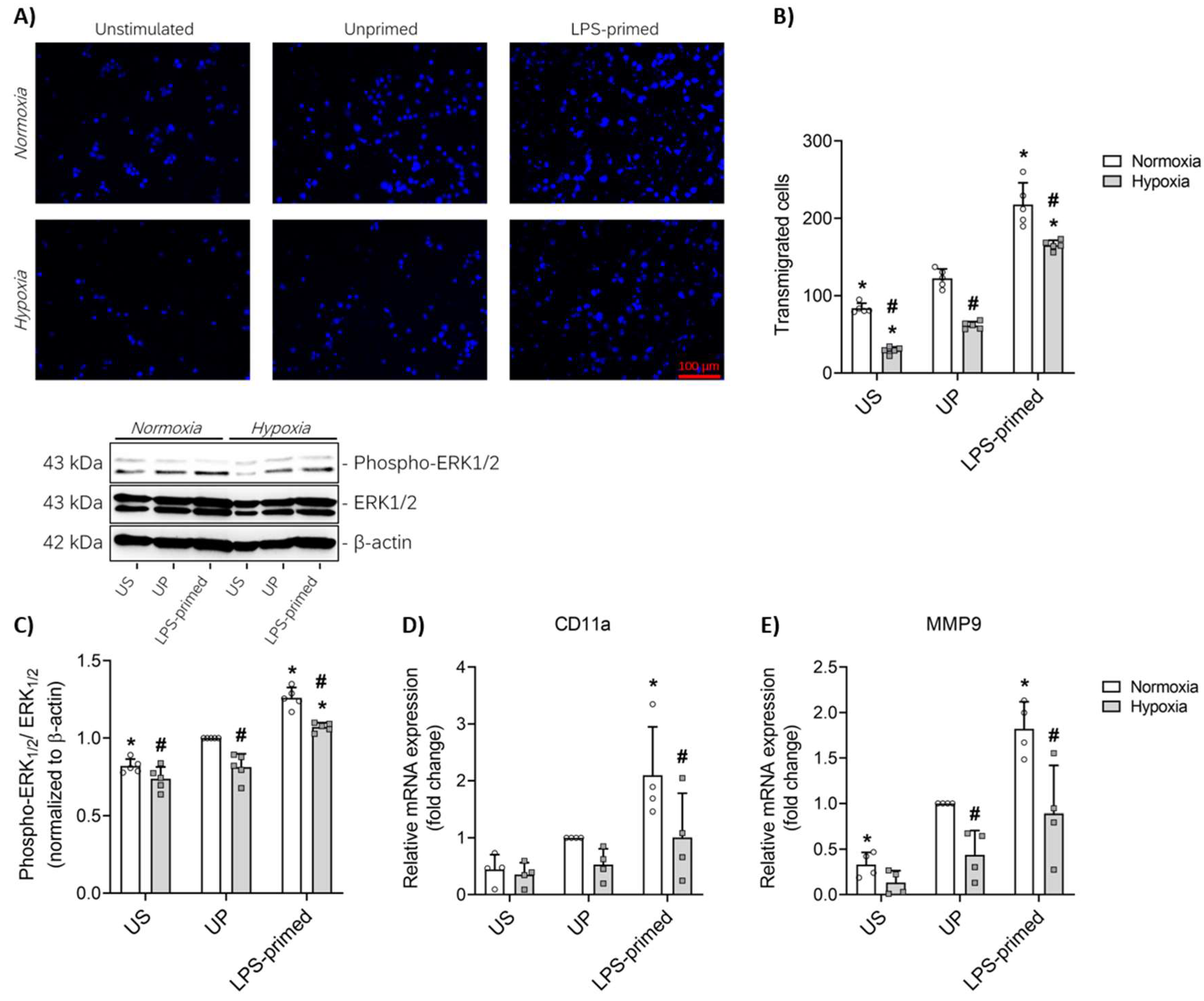
3.4. LPS-Induced Tolerance Promotes Increased Migration of BV-2 Microglia Mediated by ERK1-2 Pathway
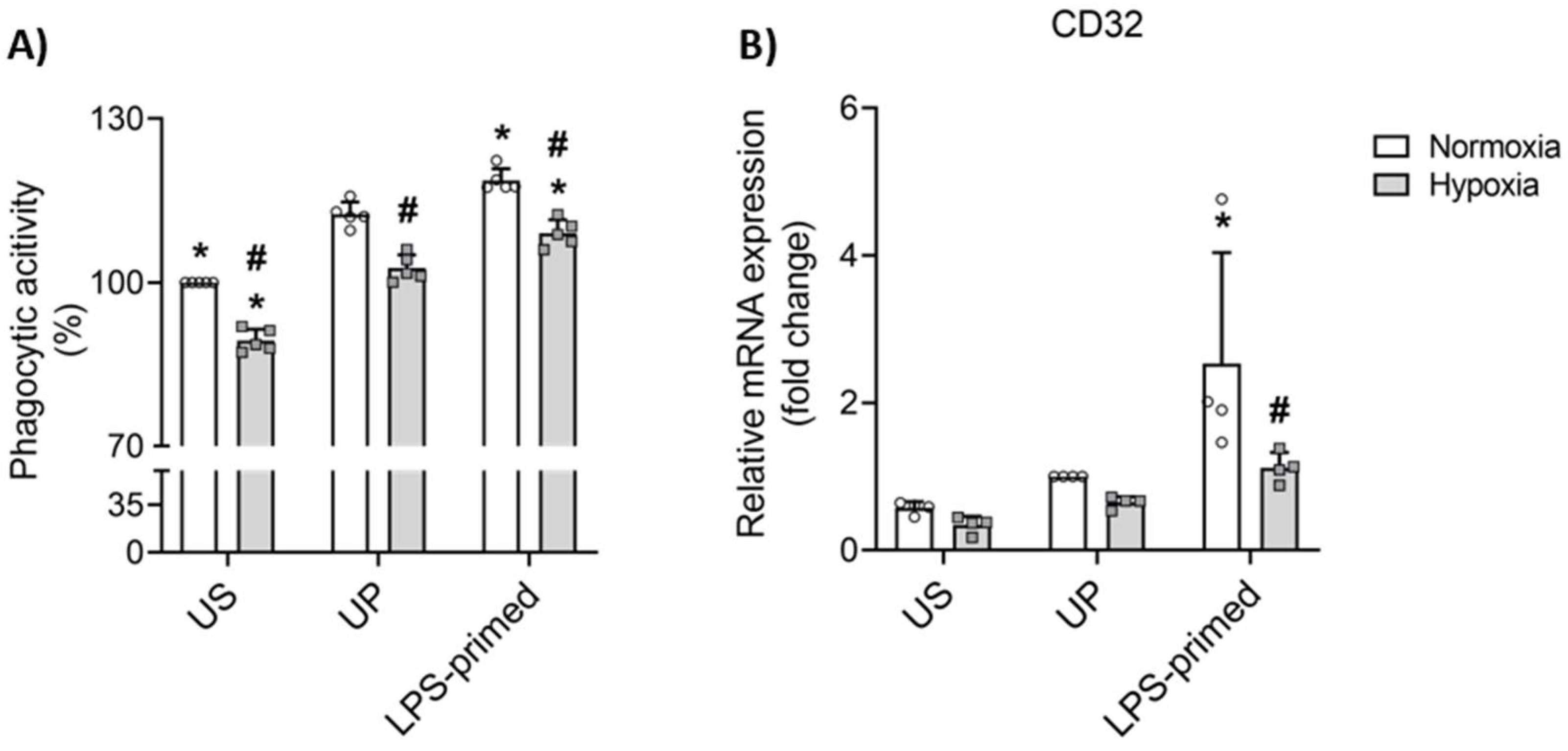
3.5. LPS-Induced Tolerance Triggers Increased Phagocytic Activity Under Normoxic and Hypoxic Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | Adenosine triphosphate |
| CD11a | Integrin alpha L |
| CD32 | Cluster of differentiation 32 |
| CNS | Central nervous system |
| DAMP | Damage-associated molecular pattern |
| ECAR | Extracellular acidification rate |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HIF-1α | Hypoxia-inducible factor 1 alpha |
| HK-2 | Hexokinase 2 |
| HMC3 | Human microglial clone 3 |
| IFN-γ | Interferon gamma |
| IH | Intermittent hypoxia |
| IKK | IkappaB kinase |
| IL-6 | Interleukin 6 |
| LPS | Lipopolysaccharide |
| MAPK | Mitogen-activated protein kinase |
| MCP-1 | Monocyte chemoattractant protein-1 |
| miR | MicroRNA |
| MMP9 | Matrix metalloproteinase 9 |
| MyD88 | Myeloid differentiation primary response gene 88 |
| NF-κB | Nuclear factor kappa B |
| OGD | Oxygen-glucose deprivation |
| PAMP | Pathogen-associated molecular pattern |
| PaO2 | Oxygen partial pressure |
| PFK1 | Phosphofructokinase 1 |
| PRRs | Pattern recognition receptors |
| ROS | Reactive oxygen species |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor alpha |
| UP | Unprimed |
| US | Unstimulated |
References
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Low, D.; Ginhoux, F. Recent Advances in the Understanding of Microglial Development and Homeostasis. Cell. Immunol. 2018, 330, 68–78. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Nimmerjahn, A.; Kirchhoff, F.; Helmchen, F. Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo. Science 2005, 308, 1314–1318. [Google Scholar] [CrossRef]
- Hanisch, U.-K.; Kettenmann, H. Microglia: Active Sensor and Versatile Effector Cells in the Normal and Pathologic Brain. Nat. Neurosci. 2007, 10, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Tay, T.L.; Savage, J.C.; Hui, C.W.; Bisht, K.; Tremblay, M.È. Microglia across the Lifespan: From Origin to Function in Brain Development, Plasticity and Cognition. J. Physiol. 2017, 595, 1929–1945. [Google Scholar] [CrossRef]
- Pallarés-Moratalla, C.; Bergers, G. The Ins and Outs of Microglial Cells in Brain Health and Disease. Front. Immunol. 2024, 15, 1305087. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M.; Cardona, A.E. The Myeloid Cells of the Central Nervous System Parenchyma. Nature 2010, 468, 253–262. [Google Scholar] [CrossRef]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia Emerge from Erythromyeloid Precursors via Pu.1- and Irf8-Dependent Pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.-E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia States and Nomenclature: A Field at Its Crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef]
- Lajqi, T.; Stojiljkovic, M.; Williams, D.L.; Hudalla, H.; Bauer, M.; Witte, O.W.; Wetzker, R.; Bauer, R.; Schmeer, C. Memory-Like Responses of Brain Microglia Are Controlled by Developmental State and Pathogen Dose. Front. Immunol. 2020, 11, 546415. [Google Scholar] [CrossRef]
- Lajqi, T.; Lang, G.-P.; Haas, F.; Williams, D.L.; Hudalla, H.; Bauer, M.; Groth, M.; Wetzker, R.; Bauer, R. Memory-Like Inflammatory Responses of Microglia to Rising Doses of LPS: Key Role of PI3Kγ. Front. Immunol. 2019, 10, 2492. [Google Scholar] [CrossRef]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, R.; Xu, Z.; Ke, Y.; Sun, R.; Yang, H.; Zhang, X.; Zhen, X.; Zheng, L.-T. Early Glycolytic Reprogramming Controls Microglial Inflammatory Activation. J. Neuroinflamm. 2021, 18, 129. [Google Scholar] [CrossRef] [PubMed]
- Song, W.M.; Colonna, M. The Identity and Function of Microglia in Neurodegeneration. Nat. Immunol. 2018, 19, 1048–1058. [Google Scholar] [CrossRef] [PubMed]
- De Schepper, S.; Crowley, G.; Hong, S. Understanding Microglial Diversity and Implications for Neuronal Function in Health and Disease. Dev. Neurobiol. 2021, 81, 507–523. [Google Scholar] [CrossRef]
- Park, B.S.; Lee, J.O. Recognition of Lipopolysaccharide Pattern by TLR4 Complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Yeh, W.-C.; Ohashi, P.S. LPS/TLR4 Signal Transduction Pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin Tolerance: New Mechanisms, Molecules and Clinical Significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Lajqi, T.; Köstlin-Gille, N.; Bauer, R.; Zarogiannis, S.G.; Lajqi, E.; Ajeti, V.; Dietz, S.; Kranig, S.A.; Rühle, J.; Demaj, A.; et al. Training vs. Tolerance: The Yin/Yang of the Innate Immune System. Biomedicines 2023, 11, 766. [Google Scholar] [CrossRef]
- Trowsdale, J.; Betz, A.G. Mother’s Little Helpers: Mechanisms of Maternal-Fetal Tolerance. Nat. Immunol. 2006, 7, 241–246. [Google Scholar] [CrossRef]
- Boly, T.J.; Bermick, J.R. Maternal-Fetal Tolerance: Not Just a Uterine Affair. J. Leukoc. Biol. 2022, 111, 515–517. [Google Scholar] [CrossRef]
- Dietz, S.; Hebel, J.; Rühle, J.; Huff, A.; Eltzschig, H.K.; Lajqi, T.; Poets, C.F.; Gille, C.; Köstlin-Gille, N. Impact of the Adenosine Receptor A2BR Expressed on Myeloid Cells on Immune Regulation during Pregnancy. Eur. J. Immunol. 2024, 54, e2451149. [Google Scholar] [CrossRef]
- Alippe, Y.; Hatterschide, J.; Coyne, C.B.; Diamond, M.S. Innate Immune Responses to Pathogens at the Maternal-Fetal Interface. Nat. Rev. Immunol. 2025. [Google Scholar] [CrossRef]
- Fu, B.; Li, X.; Sun, R.; Tong, X.; Ling, B.; Tian, Z.; Wei, H. Natural Killer Cells Promote Immune Tolerance by Regulating Inflammatory TH17 Cells at the Human Maternal-Fetal Interface. Proc. Natl. Acad. Sci. USA 2013, 110, E231–E240. [Google Scholar] [CrossRef] [PubMed]
- Rühle, J.; Ginzel, M.; Dietz, S.; Schwarz, J.; Lajqi, T.; Beer-Hammer, S.; Poets, C.F.; Gille, C.; Köstlin-Gille, N. Depletion of Ly6G-Expressing Neutrophilic Cells Leads to Altered Peripheral T-Cell Homeostasis and Thymic Development in Neonatal Mice. Int. J. Mol. Sci. 2023, 24, 7763. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; Patterson, P.H. Placental Regulation of Maternal-Fetal Interactions and Brain Development. Dev. Neurobiol. 2012, 72, 1317–1326. [Google Scholar] [CrossRef] [PubMed]
- Schaafsma, W.; Basterra, L.B.; Jacobs, S.; Brouwer, N.; Meerlo, P.; Schaafsma, A.; Boddeke, E.W.G.M.; Eggen, B.J.L. Maternal Inflammation Induces Immune Activation of Fetal Microglia and Leads to Disrupted Microglia Immune Responses, Behavior, and Learning Performance in Adulthood. Neurobiol. Dis. 2017, 106, 291–300. [Google Scholar] [CrossRef]
- Prins, J.R.; Eskandar, S.; Eggen, B.J.L.; Scherjon, S.A. Microglia, the Missing Link in Maternal Immune Activation and Fetal Neurodevelopment; and a Possible Link in Preeclampsia and Disturbed Neurodevelopment? J. Reprod. Immunol. 2018, 126, 18–22. [Google Scholar] [CrossRef]
- Baburamani, A.A.; Ek, C.J.; Walker, D.W.; Castillo-Melendez, M. Vulnerability of the Developing Brain to Hypoxic-Ischemic Damage: Contribution of the Cerebral Vasculature to Injury and Repair? Front. Physiol. 2012, 3, 424. [Google Scholar] [CrossRef]
- Wu, L.; Chang, E.; Zhao, H.; Ma, D. Regulated Cell Death in Hypoxic-Ischaemic Encephalopathy: Recent Development and Mechanistic Overview. Cell Death Discov. 2024, 10, 277. [Google Scholar] [CrossRef]
- Torres-Cuevas, I.; Parra-Llorca, A.; Sánchez-Illana, A.; Nuñez-Ramiro, A.; Kuligowski, J.; Cháfer-Pericás, C.; Cernada, M.; Escobar, J.; Vento, M. Oxygen and Oxidative Stress in the Perinatal Period. Redox Biol. 2017, 12, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Vento, M. Oxygen Supplementation in the Neonatal Period: Changing the Paradigm. Neonatology 2014, 105, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.; Rühle, J.; Stephan, K.; Dietz, S.; Geißert, J.; Schoppmeier, U.; Frick, J.S.; Hudalla, H.; Lajqi, T.; Poets, C.F.; et al. HIF-1α Targeted Deletion in Myeloid Cells Decreases MDSC Accumulation and Alters Microbiome in Neonatal Mice. Eur. J. Immunol. 2023, 53, e2250144. [Google Scholar] [CrossRef] [PubMed]
- Lajqi, T.; Köstlin-Gille, N.; Gille, C. Trained Innate Immunity in Pediatric Infectious Diseases. Pediatr. Infect. Dis. J. 2024, 43, e57–e59. [Google Scholar] [CrossRef]
- Filippi, L.; Pascarella, F.; Pini, A.; Cammalleri, M.; Bagnoli, P.; Morganti, R.; Innocenti, F.; Castagnini, N.; Melosi, A.; Scaramuzzo, R.T. Fetal Oxygenation from the 23rd to the 36th Week of Gestation Evaluated through the Umbilical Cord Blood Gas Analysis. Int. J. Mol. Sci. 2023, 24, 12487. [Google Scholar] [CrossRef]
- Burton, G.J.; Cindrova-Davies, T.; Yung, H.W.; Jauniaux, E. Hypoxia and Reproductive Health: Oxygen and Development of the Human Placenta. Reproduction 2021, 161, F53–F65. [Google Scholar] [CrossRef]
- Schmidt, C.; Schneble, N.; Wetzker, R. The Fifth Dimension of Innate Immunity. J. Cell Commun. Signal. 2014, 8, 363–367. [Google Scholar] [CrossRef]
- Schneble, N.; Schmidt, C.; Bauer, R.; Müller, J.P.; Monajembashi, S.; Wetzker, R. Phosphoinositide 3-Kinase γ Ties Chemoattractant- and Adrenergic Control of Microglial Motility. Mol. Cell. Neurosci. 2017, 78, 1–8. [Google Scholar] [CrossRef]
- Schmidt, C.; Schneble, N.; Müller, J.P.; Bauer, R.; Perino, A.; Marone, R.; Rybalkin, S.D.; Wymann, M.P.; Hirsch, E.; Wetzker, R. Phosphoinositide 3-Kinase γ Mediates Microglial Phagocytosis via Lipid Kinase-Independent Control of CAMP. Neuroscience 2013, 233, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Lajqi, T.; Frommhold, D.; Braun, M.; Alexander Kranig, S.; Pöschl, J.; Hudalla, H. Gram-Positive Staphylococcus Aureus LTA Promotes Distinct Memory-like Effects in Murine Bone Marrow Neutrophils. Cell. Immunol. 2022, 376, 104535. [Google Scholar] [CrossRef]
- Lajqi, T.; Braun, M.; Kranig, S.A.; Frommhold, D.; Johannes, P.; Hudalla, H. LPS Induces Opposing Memory-like Inflammatory Responses in Mouse Bone Marrow Neutrophils. Int. J. Mol. Sci. 2021, 22, 9803. [Google Scholar] [CrossRef]
- Lajqi, T.; Köstlin-Gille, N.; Hillmer, S.; Braun, M.; Kranig, S.A.; Dietz, S.; Krause, C.; Rühle, J.; Frommhold, D.; Pöschl, J.; et al. Gut Microbiota-Derived Small Extracellular Vesicles Endorse Memory-like Inflammatory Responses in Murine Neutrophils. Biomedicines 2022, 10, 442. [Google Scholar] [CrossRef]
- Gille, C.; Jungwirth, M.; Pezer, S.; Dietz-Ziegler, S.; Köstlin-Gille, N.; Lajqi, T. 4-Phenyl Butyric Acid (4-PBA) Suppresses Neutrophil Recruitment in a Murine Model of Acute Perinatal Inflammation. J. Immunol. Res. 2025, 2025, 2438058. [Google Scholar] [CrossRef]
- Lajqi, T.; Marx, C.; Hudalla, H.; Haas, F.; Große, S.; Wang, Z.Q.; Heller, R.; Bauer, M.; Wetzker, R.; Bauer, R. The Role of the Pathogen Dose and PI3Kγ in Immunometabolic Reprogramming of Microglia for Innate Immune Memory. Int. J. Mol. Sci. 2021, 22, 2578. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Marie, M.A.; Sanderlin, E.J.; Yang, L.V. Transwell In Vitro Cell Migration and Invasion Assays. Methods Mol. Biol. 2023, 2644, 349–359. [Google Scholar] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Gollwitzer, E.S.; Marsland, B.J. Impact of Early-Life Exposures on Immune Maturation and Susceptibility to Disease. Trends Immunol. 2015, 36, 684–696. [Google Scholar] [CrossRef]
- Henn, A.; Lund, S.; Hedtjarn, M.; Hedtjärn, M.; Schrattenholz, A.; Pörzgen, P.; Leist, M. The Suitability of BV2 Cells as Alternative Model System for Primary Microglia Cultures or for Animal Experiments Examining Brain Inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef]
- Racki, V.; Marcelic, M.; Stimac, I.; Petric, D.; Kucic, N. Effects of Haloperidol, Risperidone, and Aripiprazole on the Immunometabolic Properties of BV-2 Microglial Cells. Int. J. Mol. Sci. 2021, 22, 4399. [Google Scholar] [CrossRef]
- Chhor, V.; Le Charpentier, T.; Lebon, S.; Oré, M.V.; Celador, I.L.; Josserand, J.; Degos, V.; Jacotot, E.; Hagberg, H.; Sävman, K.; et al. Characterization of Phenotype Markers and Neuronotoxic Potential of Polarised Primary Microglia In Vitro. Brain. Behav. Immun. 2013, 32, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Monga, S.; Weizman, A.; Gavish, M. The Efficacy of the Novel TSPO Ligands 2-Cl-MGV-1 and 2,4-Di-Cl-MGV-1 Compared to the Classical TSPO Ligand PK 11195 to Counteract the Release of Chemokines from LPS-Stimulated BV-2 Microglial Cells. Biology 2020, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Ko, Y.-H.; Lee, Y.; Lee, S.-Y.; Jang, C.-G. Antineuroinflammatory Effects of 7,3′,4′-Trihydroxyisoflavone in Lipopolysaccharide-Stimulated BV2 Microglial Cells through MAPK and NF-ΚB Signaling Suppression. Biomol. Ther. 2021, 29, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Fraga, D.; Raborn, E.S.; Ferreira, G.A.; Cabral, G.A. Cannabinoids Inhibit Migration of Microglial-like Cells to the HIV Protein Tat. J. Neuroimmune Pharmacol. 2011, 6, 566–577. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The Role of Pattern-Recognition Receptors in Innate Immunity: Update on Toll-like Receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zou, J.; Chen, J.; Zhong, X.; Kang, R.; Tang, D. Pattern Recognition Receptors: Function, Regulation and Therapeutic Potential. Signal Transduct. Target. Ther. 2025, 10, 216. [Google Scholar] [CrossRef]
- Lively, S.; Schlichter, L.C. Microglia Responses to Pro-Inflammatory Stimuli (LPS, IFNγ + TNFα) and Reprogramming by Resolving Cytokines (IL-4, IL-10). Front. Cell. Neurosci. 2018, 12, 215. [Google Scholar] [CrossRef]
- Ye, X.; Zhu, M.; Che, X.; Wang, H.; Liang, X.-J.; Wu, C.; Xue, X.; Yang, J. Lipopolysaccharide Induces Neuroinflammation in Microglia by Activating the MTOR Pathway and Downregulating Vps34 to Inhibit Autophagosome Formation. J. Neuroinflamm. 2020, 17, 18. [Google Scholar] [CrossRef]
- Weidemann, A.; Johnson, R.S. Biology of HIF-1alpha. Cell Death Differ. 2008, 15, 621–627. [Google Scholar] [CrossRef]
- Chen, S.; Sang, N. Hypoxia-Inducible Factor-1: A Critical Player in the Survival Strategy of Stressed Cells. J. Cell. Biochem. 2016, 117, 267–278. [Google Scholar] [CrossRef]
- Haase, V.H. Therapeutic Targeting of the HIF Oxygen-Sensing Pathway: Lessons Learned from Clinical Studies. Exp. Cell Res. 2017, 356, 160–165. [Google Scholar] [CrossRef]
- Jewell, U.R.; Kvietikova, I.; Scheid, A.; Bauer, C.; Wenger, R.H.; Gassmann, M. Induction of HIF-1alpha in Response to Hypoxia Is Instantaneous. FASEB J. 2001, 15, 1312–1314. [Google Scholar] [CrossRef]
- Jiang, B.H.; Semenza, G.L.; Bauer, C.; Marti, H.H. Hypoxia-Inducible Factor 1 Levels Vary Exponentially over a Physiologically Relevant Range of O2 Tension. Am. J. Physiol. 1996, 271, C1172–C1180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Huang, X.; Zhao, T.; Qiao, M.; Zhao, X.; Zhao, M.; Xu, L.; Zhao, Y.; Wu, L.; Wu, K.; et al. Hypoxia Augments LPS-Induced Inflammation and Triggers High Altitude Cerebral Edema in Mice. Brain Behav. Immun. 2017, 64, 266–275. [Google Scholar] [CrossRef]
- Martinello, K.A.; Meehan, C.; Avdic-Belltheus, A.; Lingam, I.; Ragab, S.; Hristova, M.; Tann, C.J.; Peebles, D.; Hagberg, H.; Wolfs, T.G.A.M.; et al. Acute LPS Sensitization and Continuous Infusion Exacerbates Hypoxic Brain Injury in a Piglet Model of Neonatal Encephalopathy. Sci. Rep. 2019, 9, 10184. [Google Scholar] [CrossRef]
- Cavaillon, J.M. The Nonspecific Nature of Endotoxin Tolerance. Trends Microbiol. 1995, 3, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Beeson, P.B. Tolerance to Bacterial Pyrogens: I. Factors Influencing Its Development. J. Exp. Med. 1947, 86, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.; Weis, S.; Netea, M.G.; Wetzker, R. Remembering Pathogen Dose: Long-Term Adaptation in Innate Immunity. Trends Immunol. 2018, 39, 438–445. [Google Scholar] [CrossRef]
- Lajqi, T.; Pöschl, J.; Frommhold, D.; Hudalla, H. The Role of Microbiota in Neutrophil Regulation and Adaptation in Newborns. Front. Immunol. 2020, 11, 568685. [Google Scholar] [CrossRef]
- Straley, M.E.; Togher, K.L.; Nolan, A.M.; Kenny, L.C.; O’Keeffe, G.W. LPS Alters Placental Inflammatory and Endocrine Mediators and Inhibits Fetal Neurite Growth in Affected Offspring during Late Gestation. Placenta 2014, 35, 533–538. [Google Scholar] [CrossRef]
- Brown, A.G.; Maubert, M.E.; Anton, L.; Heiser, L.M.; Elovitz, M.A. The Tracking of Lipopolysaccharide through the Feto-Maternal Compartment and the Involvement of Maternal TLR4 in Inflammation-Induced Fetal Brain Injury. Am. J. Reprod. Immunol. 2019, 82, e13189. [Google Scholar] [CrossRef]
- Menon, R. Fetal Inflammatory Response at the Fetomaternal Interface: A Requirement for Labor at Term and Preterm. Immunol. Rev. 2022, 308, 149–167. [Google Scholar] [CrossRef]
- Donina, Z.A. Preconditioning with Moderate Hypoxia Increases Tolerance to Subsequent Severe Hypoxia in Rats with LPS-Induced Endotoxemia. J. Evol. Biochem. Physiol. 2024, 60, 1213–1220. [Google Scholar] [CrossRef]
- Kiers, D.; Wielockx, B.; Peters, E.; van Eijk, L.T.; Gerretsen, J.; John, A.; Janssen, E.; Groeneveld, R.; Peters, M.; Damen, L.; et al. Short-Term Hypoxia Dampens Inflammation in Vivo via Enhanced Adenosine Release and Adenosine 2B Receptor Stimulation. eBioMedicine 2018, 33, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Yang, L.; Ikenoue, T.; Mallard, C.; Hagberg, H. Endotoxin-Induced Hypoxic-Ischemic Tolerance Is Mediated by up-Regulation of Corticosterone in Neonatal Rat. Pediatr. Res. 2006, 59, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Piao, W.; Song, C.; Chen, H.; Diaz, M.A.Q.; Wahl, L.M.; Fitzgerald, K.A.; Li, L.; Medvedev, A.E. Endotoxin Tolerance Dysregulates MyD88- and Toll/IL-1R Domain-Containing Adapter Inducing IFN-Beta-Dependent Pathways and Increases Expression of Negative Regulators of TLR Signaling. J. Leukoc. Biol. 2009, 86, 863–875. [Google Scholar] [CrossRef]
- Gaber, T.; Strehl, C.; Buttgereit, F. Metabolic Regulation of Inflammation. Nat. Rev. Rheumatol. 2017, 13, 267–279. [Google Scholar] [CrossRef]
- Ratter, J.M.; Rooijackers, H.M.M.; Hooiveld, G.J.; Hijmans, A.G.M.; de Galan, B.E.; Tack, C.J.; Stienstra, R. In Vitro and in Vivo Effects of Lactate on Metabolism and Cytokine Production of Human Primary PBMCs and Monocytes. Front. Immunol. 2018, 9, 2564. [Google Scholar] [CrossRef]
- Qin, Q.; Wang, D.; Qu, Y.; Li, J.; An, K.; Mao, Z.; Li, J.; Xiong, Y.; Min, Z.; Xue, Z. Enhanced Glycolysis-Derived Lactate Promotes Microglial Activation in Parkinson’s Disease via Histone Lactylation. npj Park. Dis. 2025, 11, 3. [Google Scholar] [CrossRef]
- Chun, B.J.; Aryal, S.P.; Varughese, P.; Sun, B.; Bruno, J.A.; Richards, C.I.; Bachstetter, A.D.; Kekenes-Huskey, P.M. Purinoreceptors and Ectonucleotidases Control ATP-Induced Calcium Waveforms and Calcium-Dependent Responses in Microglia: Roles of P2 Receptors and CD39 in ATP-Stimulated Microglia. Front. Physiol. 2022, 13, 1037417. [Google Scholar] [CrossRef]
- Rueschpler, L.; Schloer, S. Beyond the Surface: P2Y Receptor Downstream Pathways, TLR Crosstalk and Therapeutic Implications for Infection and Autoimmunity. Pharmacol. Res. 2025, 219, 107884. [Google Scholar] [CrossRef] [PubMed]
- Puchałowicz, K.; Tarnowski, M.; Baranowska-Bosiacka, I.; Chlubek, D.; Dziedziejko, V. P2X and P2Y Receptors—Role in the Pathophysiology of the Nervous System. Int. J. Mol. Sci. 2014, 15, 23672–23704. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Csóka, B.; Németh, Z.H.; Vizi, E.S.; Pacher, P. A(2B) Adenosine Receptors in Immunity and Inflammation. Trends Immunol. 2009, 30, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Eckle, T.; Faigle, M.; Grenz, A.; Laucher, S.; Thompson, L.F.; Eltzschig, H.K. A2B Adenosine Receptor Dampens Hypoxia-Induced Vascular Leak. Blood 2008, 111, 2024–2035. [Google Scholar] [CrossRef]
- Abebayehu, D.; Spence, A.J.; Qayum, A.A.; Taruselli, M.T.; McLeod, J.J.A.; Caslin, H.L.; Chumanevich, A.P.; Kolawole, E.M.; Paranjape, A.; Baker, B.; et al. Lactic Acid Suppresses IL-33-Mediated Mast Cell Inflammatory Responses via Hypoxia-Inducible Factor-1α-Dependent MiR-155 Suppression. J. Immunol. 2016, 197, 2909–2917. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, P.; Wang, K.; Zhang, Y.; Lv, Y.; Fan, P.; Yang, L.; Zhang, S.; Wang, T.; Zhao, J.; et al. Lactate Modulates Microglial Inflammatory Responses after Oxygen-Glucose Deprivation through HIF-1α-Mediated Inhibition of NF-ΚB. Brain Res. Bull. 2023, 195, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Shen, Q.; Xu, P.; Luo, J.J.; Tang, Y. Phagocytosis of Microglia in the Central Nervous System Diseases. Mol. Neurobiol. 2014, 49, 1422–1434. [Google Scholar] [CrossRef]
- Thomson, C.A.; McColl, A.; Graham, G.J.; Cavanagh, J. Sustained Exposure to Systemic Endotoxin Triggers Chemokine Induction in the Brain Followed by a Rapid Influx of Leukocytes. J. Neuroinflamm. 2020, 17, 94. [Google Scholar] [CrossRef]
- Ullrich, O.; Diestel, A.; Eyüpoglu, I.Y.; Nitsch, R. Regulation of Microglial Expression of Integrins by Poly(ADP-Ribose) Polymerase-1. Nat. Cell Biol. 2001, 3, 1035–1042. [Google Scholar] [CrossRef]
- Shukla, A.K.; McIntyre, L.L.; Marsh, S.E.; Schneider, C.A.; Hoover, E.M.; Walsh, C.M.; Lodoen, M.B.; Blurton-Jones, M.; Inlay, M.A. CD11a Expression Distinguishes Infiltrating Myeloid Cells from Plaque-Associated Microglia in Alzheimer’s Disease. Glia 2019, 67, 844–856. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, H.-H.; Li, D.; Li, H.-P. Neuroprotective Effects of Matrix Metalloproteinases in Cerebral Ischemic Rats by Promoting Activation and Migration of Astrocytes and Microglia. Brain Res. Bull. 2019, 146, 136–142. [Google Scholar] [CrossRef]
- Li, X.-Q.; Cao, X.-Z.; Wang, J.; Fang, B.; Tan, W.-F.; Ma, H. Sevoflurane Preconditioning Ameliorates Neuronal Deficits by Inhibiting Microglial MMP-9 Expression after Spinal Cord Ischemia/Reperfusion in Rats. Mol. Brain 2014, 7, 69. [Google Scholar] [CrossRef]
- Wendeln, A.-C.; Degenhardt, K.; Kaurani, L.; Gertig, M.; Ulas, T.; Jain, G.; Wagner, J.; Häsler, L.M.; Wild, K.; Skodras, A.; et al. Innate Immune Memory in the Brain Shapes Neurological Disease Hallmarks. Nature 2018, 556, 332–338. [Google Scholar] [CrossRef]
- Schaafsma, W.; Zhang, X.; van Zomeren, K.C.; Jacobs, S.; Georgieva, P.B.; Wolf, S.A.; Kettenmann, H.; Janova, H.; Saiepour, N.; Hanisch, U.K.; et al. Long-Lasting pro-Inflammatory Suppression of Microglia by LPS-Preconditioning Is Mediated by RelB-Dependent Epigenetic Silencing. Brain Behav. Immun. 2015, 48, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Chung, W.-S. Glial Phagocytosis for Synapse and Toxic Proteins in Neurodegenerative Diseases. Mol. Neurodegener. 2025, 20, 81. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.C.; Khan, A.M.; Mendoza, M.C. ERK Signaling for Cell Migration and Invasion. Front. Mol. Biosci. 2022, 9, 998475. [Google Scholar] [CrossRef]
- Fan, Y.; Xie, L.; Chung, C.Y. Signaling Pathways Controlling Microglia Chemotaxis. Mol. Cells 2017, 40, 163–168. [Google Scholar] [CrossRef]
- Jang, K.-B.; You, M.-J.; Yang, B.; Rim, C.; Kim, H.-J.; Kwon, M.-S. Persistent Acidic Environment Induces Impaired Phagocytosis via ERK in Microglia. Neurochem. Res. 2022, 47, 1341–1353. [Google Scholar] [CrossRef]
- Zhang, F.; Niu, L.; Li, S.; Le, W. Pathological Impacts of Chronic Hypoxia on Alzheimer’s Disease. ACS Chem. Neurosci. 2019, 10, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Life with Oxygen. Science 2007, 318, 62–64. [Google Scholar] [CrossRef]
- Navarrete-Opazo, A.; Mitchell, G.S. Therapeutic Potential of Intermittent Hypoxia: A Matter of Dose. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R1181–R1197. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, J.; Gu, Y.; Ji, X.; Nan, G. Intermittent Hypoxia Conditioning as a Potential Prevention and Treatment Strategy for Ischemic Stroke: Current Evidence and Future Directions. Front. Neurosci. 2022, 16, 1067411. [Google Scholar] [CrossRef] [PubMed]
- Dale, E.A.; Ben Mabrouk, F.; Mitchell, G.S. Unexpected Benefits of Intermittent Hypoxia: Enhanced Respiratory and Nonrespiratory Motor Function. Physiology 2014, 29, 39–48. [Google Scholar] [CrossRef]
- Li, G.; Guan, Y.; Gu, Y.; Guo, M.; Ma, W.; Shao, Q.; Liu, J.; Ji, X. Intermittent Hypoxic Conditioning Restores Neurological Dysfunction of Mice Induced by Long-Term Hypoxia. CNS Neurosci. Ther. 2023, 29, 202–215. [Google Scholar] [CrossRef]
- Burtscher, J.; Mallet, R.T.; Burtscher, M.; Millet, G.P. Hypoxia and Brain Aging: Neurodegeneration or Neuroprotection? Ageing Res. Rev. 2021, 68, 101343. [Google Scholar] [CrossRef]
- Wen, B.; Zheng, Z.; Wang, L.; Qian, X.; Wang, X.; Chen, Y.; Bao, J.; Jiang, Y.; Ji, K.; Liu, H. HIF-1α Is Essential for the Augmentation of Myometrial Contractility during Labor. Biol. Reprod. 2022, 107, 1540–1550. [Google Scholar] [CrossRef]
- Wray, S.; Alruwaili, M.; Prendergast, C. Hypoxia and Reproductive Health: Hypoxia and Labour. Reproduction 2021, 161, F67–F80. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.T.; Colgan, S.P. Regulation of Immunity and Inflammation by Hypoxia in Immunological Niches. Nat. Rev. Immunol. 2017, 17, 774–785. [Google Scholar] [CrossRef]
- Zhao, H.; Wong, R.J.; Stevenson, D.K. The Impact of Hypoxia in Early Pregnancy on Placental Cells. Int. J. Mol. Sci. 2021, 22, 9675. [Google Scholar] [CrossRef] [PubMed]
- Colamatteo, A.; Fusco, C.; Micillo, T.; D’Hooghe, T.; de Candia, P.; Alviggi, C.; Longobardi, S.; Matarese, G. Immunobiology of Pregnancy: From Basic Science to Translational Medicine. Trends Mol. Med. 2023, 29, 711–725. [Google Scholar] [CrossRef] [PubMed]

| Primer | Primer Sequences (5′–3′) | |
|---|---|---|
| PFK1 (Phosphofructokinase 1) | Forward: | TGACATGACCATTGGCACAG |
| Reverse: | TCTTGCTACTCAGGATTCGG | |
| HK-2 (Hexokinase 2) | Forward: | ATTGTCCAGTGCATCGCGGA |
| Reverse: | AGGTCAAACTCCTCTCGCCG | |
| p65 (Transcription factor p65) | Forward: | CTTCCTCAGCCATGGTACCTCT |
| Reverse: | CAAGTCTTCATCAGCATCAAACTG | |
| CD11a (Integrin αL) | Forward: | AGATCGAGTCCGGACCCACAG |
| Reverse: | GGCAGTGATAGAGGCCTCCCG | |
| MMP9 (Matrix metallopeptidase 9) | Forward: | ACCACTAAAGGTCGCTCGGATGG |
| Reverse: | AGTACTGCTTGCCCAGGAAGACG | |
| CD32 (Cluster of differentiation 32) | Forward: | AATCCTGCCGTTCCTACTGATC |
| Reverse: | GTGTCACCGTGTCTTCCTTGAG | |
| GAPDH (Glyceraldehyde-3-phosphate dehydrogenase) | Forward: | CATGGCCTTCCGTGTTTCCTA |
| Reverse: | CCTGCTTCACCACCTTCTTGAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavero Vargas, A.; Köstlin-Gille, N.; Bauer, R.; Dietz-Ziegler, S.; Lokaj, A.S.; Lutterbach, S.; Gille, C.; Lajqi, T. Hypoxia Supports LPS-Driven Tolerance and Functional Activation in BV-2 Microglial Cells. Biology 2025, 14, 1512. https://doi.org/10.3390/biology14111512
Chavero Vargas A, Köstlin-Gille N, Bauer R, Dietz-Ziegler S, Lokaj AS, Lutterbach S, Gille C, Lajqi T. Hypoxia Supports LPS-Driven Tolerance and Functional Activation in BV-2 Microglial Cells. Biology. 2025; 14(11):1512. https://doi.org/10.3390/biology14111512
Chicago/Turabian StyleChavero Vargas, Alicia, Natascha Köstlin-Gille, Reinhard Bauer, Stefanie Dietz-Ziegler, Anita S. Lokaj, Soumya Lutterbach, Christian Gille, and Trim Lajqi. 2025. "Hypoxia Supports LPS-Driven Tolerance and Functional Activation in BV-2 Microglial Cells" Biology 14, no. 11: 1512. https://doi.org/10.3390/biology14111512
APA StyleChavero Vargas, A., Köstlin-Gille, N., Bauer, R., Dietz-Ziegler, S., Lokaj, A. S., Lutterbach, S., Gille, C., & Lajqi, T. (2025). Hypoxia Supports LPS-Driven Tolerance and Functional Activation in BV-2 Microglial Cells. Biology, 14(11), 1512. https://doi.org/10.3390/biology14111512








