Adulticidal Activity of the Insect Growth Regulators Methoprene and Cyromazine in House Flies (Musca domestica L.): Evidence from Feeding Trials
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. House Flies
2.2. Adulticidal Effect Tests
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations. World Population Prospects 2024. Available online: https://population.un.org/wpp/downloads (accessed on 31 July 2025).
- Çamurcu, H. Dünya Nüfus Artışı ve Getirdiği Sorunlar. Balıkesir Univ. Sos. Bilim. Enst. Derg. 2005, 8, 87–105. [Google Scholar]
- Anoopkumar, A.N.; Aneesh, E.M. A Critical Assessment of Mosquito Control and the Influence of Climate Change on Mosquito-Borne Disease Epidemics. Environ. Dev. Sustain. 2022, 24, 8900–8929. [Google Scholar] [CrossRef]
- Vonesch, N.; D’Ovidio, M.C.; Melis, P.; Remoli, M.E.; Ciufolini, M.G.; Tomao, P. Climate Change, Vector-Borne Diseases and Working Population. Ann. Ist. Super. Sanita. 2016, 52, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Koçak, Ö. Zararlı Savaşımı; Hacettepe Üniversitesi Teknolojiler Uygulama ve Araştırma Merkezi: Ankara, Türkiye, 1998. [Google Scholar]
- Çetin, H. Kent Zararlıları, Biyoloji, Ekoloji ve Mücadele Yöntemleri (Vektörler ve Diğerleri); Yıldız Ofset: Antalya, Türkiye, 2016; p. 203. [Google Scholar]
- Wang, J.N.; Hou, J.; Wu, Y.Y.; Guo, S.; Liu, Q.M.; Li, T.Q.; Gong, Z.Y. Resistance of House Fly, Musca domestica L. to Five Insecticides in Zhejiang Province, China: The Situation in 2017. Can. J. Infect. Dis. Med. Microbiol. 2019, 2019, 4851914. [Google Scholar] [CrossRef]
- Cheng, T.H.; Kesler, E.M. A three-year study on the effect of fly control on milk production by selected and randomized dairy herds. J. Econ. Entomol. 1961, 54, 751–757. [Google Scholar] [CrossRef]
- Kaufman, P.E.; Scott, J.G.; Rutz, D.A. Monitoring Insecticide Resistance in House Flies from New York Dairies. Pest. Manag. Sci. 2001, 57, 514–521. [Google Scholar] [CrossRef]
- Abbas, N.; Khan, H.A.A.; Shad, S.A. Resistance of the House Fly Musca domestica to Lambda-Cyhalothrin: Mode of Inheritance, Realized Heritability, and Cross-Resistance. Ecotoxicology 2014, 23, 791–801. [Google Scholar] [CrossRef]
- Erdoğan, G.; Çetin, H. Survey of Deltamethrin Resistance in House Flies (Musca domestica L.) collected from Kumluca the most important greenhouse production area of Turkey. Fresenius Environ. Bull. 2020, 29, 10252–10256. [Google Scholar]
- Çetin, H.; Yanikoğlu, A.; Akarsu, E.; Civril, M.; Odabaş, E.; Koç, S.; Polat, B. Monitoring of thiamethoxam resistance in Turkish house fly strains, Musca domestica (Diptera: Muscidae). J. Arthropod-Borne Dis. 2023, 17, 206. [Google Scholar] [CrossRef]
- Öz, E. Evaluation of Cyfluthrin and Etofenprox Resistance in House Fly Musca domestica Populations in Antalya, Türkiye. Biology 2024, 13, 767. [Google Scholar] [CrossRef]
- Khan, H.A.A. Resistance risk assessment, cross-resistance potential and realized heritability of resistance to methomyl in Musca domestica Linnaeus. Ecotoxicology 2024, 33, 226–234. [Google Scholar] [CrossRef]
- Ser, Ö.; Çetin, H. Pestisitlerin vektör mücadelesinde kullanımları. Turk. Klin. Vet. Sci. Pharmacol. Toxicol. 2016, 2, 26–34. [Google Scholar]
- Zhu, J.; Qu, R.; Wang, Y.; Ni, R.; Tian, K.; Yang, C.; Qiu, X. Up-Regulation of CYP6G4 Mediated by a CncC/maf Binding-Site-Containing Insertion Confers Resistance to Multiple Classes of Insecticides in the House Fly Musca domestica. Int. J. Biol. Macromol. 2023, 253, 127024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zhao, N.; Lun, X.; Zhao, C.; Liu, Q.; Meng, F. Long-term trends in housefly (Musca domestica L.) insecticide resistance in China. Pestic. Biochem. Physiol. 2024, 201, 105880. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Hirano, M.; Takimoto, Y.; Hatakoshi, M. Insect growth regulators for insect control, with emphasis on juvenile hormone analogs: Present and future prospects. In Pest Control with Enhanced Environmental Safety; Duke, S.O., Menn, J.J., Plimmer, J.R., Eds.; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1993; Volume 524, pp. 144–168. [Google Scholar]
- Tunaz, H.; Uygun, N. Insect growth regulators for insect pest control. Turk. J. Agric. For. 2004, 28, 377–387. [Google Scholar]
- Retnakaran, A.; Granett, J.; Ennis, T. Insect growth regulators. In Comprehensive Insect Physiology, Biochemistry and Pharmacology; Kerkut, G.A., Gilbert, L.I., Eds.; Pergamon Press: Oxford, UK, 1985; Volume 12, pp. 529–601. [Google Scholar]
- Li, Y.; He, X.; Sun, B.; Hu, N.; Li, J.; You, R.; Zhai, Q. Combined Exposure of Beta-Cypermethrin and Emamectin Benzoate Interferes with the HPO Axis through Oxidative Stress, Causing an Imbalance of Hormone Homeostasis in Female Rats. Reprod. Toxicol. 2024, 123, 108502. [Google Scholar] [CrossRef]
- Dhadialla, T.S.; Retnakaran, A.; Smagghe, G. Insect Growth and Development Disrupting Insecticides. In Insect Control: Biological and Synthetic Agents; Gilbert, L.I., Gill, S.S., Eds.; Academic Press: London, UK, 2010; pp. 121–181. [Google Scholar]
- Erdoğan, G.; Çetin, H. Insect growth regulators as chemosterilants: A study on house fly, Musca domestica L., 1758 (Diptera: Muscidae) populations in Türkiye. Turk. J. Entomol. 2025, 49, 159–174. [Google Scholar] [CrossRef]
- Bel, Y.; Wiesner, P.; Kayser, H. Candidate target mechanisms of the growth inhibitor cyromazine: Studies of phenylalanine hydroxylase, puparial amino acids, and dihydrofolate reductase in dipteran insects. Arch. Insect Biochem. Physiol. 2000, 45, 69–78. [Google Scholar] [CrossRef]
- Binnington, K.; Retnakaran, A. Epidermis-a biologically active target for metabolic inhibitors. In Physiology of the Insect Epidermis; Commonwealth Scientific and Industrial Research Organisation: East Melbourne, Australia, 1991; pp. 307–334. [Google Scholar]
- Henrick, C.A.; Staal, G.B.; Siddall, J.B. Alkyl 3,7,11-trimethyl-2,4-dodecadienoates, a new class of potent insect growth regulators with juvenile hormone activity. J. Agric. Food Chem. 1973, 21, 354–359. [Google Scholar] [CrossRef]
- Oberlander, H.; Silhacek, D.L. Insect growth regulators. In Alternatives to Pesticides in Stored-Product IPM; Springer: Boston, MA, USA, 2000; pp. 147–163. [Google Scholar]
- McGregor, H.E.; Kramer, K.J. Activity of insect growth regulators, hydroprene and methoprene, on wheat and corn against several stored-grain insects. J. Econ. Entomol. 1975, 68, 668–670. [Google Scholar] [CrossRef]
- Loschiavo, S.R. Effects of the synthetic insect growth regulators methoprene and hydroprene on survival, development or reproduction of six species of stored-products insects. J. Econ. Entomol. 1976, 69, 395–399. [Google Scholar] [CrossRef]
- Stockel, J.; Edwards, J.P. Susceptibility of Sitotroga cerealella (Oliv.) (Lepidoptera: Gelechiidae) to two insect juvenile hormone analogues. J. Stored Prod. Res. 1981, 17, 137–141. [Google Scholar] [CrossRef]
- Manzelli, M.A. Management of stored-tobacco pests, the cigarette beetle (Coleoptera: Anobiidae) and tobacco moth (Lepidoptera: Pyralidae), with methoprene. J. Econ. Entomol. 1982, 75, 721–723. [Google Scholar] [CrossRef]
- Edwards, J.P.; Short, J.E.; Rowlands, D.G. Metabolism and insect juvenile hormone activity of methoprene and three major metabolites in Tribolium castaneum (Herbst) pupae. J. Stored Prod. Res. 1988, 24, 165–172. [Google Scholar] [CrossRef]
- Samson, P.R.; Parker, R.J.; Hall, E.A. Efficacy of the insect growth regulators methoprene, fenoxycarb and diflubenzuron against Rhyzopertha dominica (F.) on maize and paddy rice. J. Stored Prod. Res. 1990, 26, 215–221. [Google Scholar] [CrossRef]
- Nayar, J.K.; Ali, A.; Zaim, M. Effectiveness and residual activity comparison of granular formulations of insect growth regulators pyriproxyfen and s-methoprene against Florida mosquitoes. J. Am. Mosq. Control Assoc. 2002, 18, 196–201. [Google Scholar]
- Jenson, E.A.; Arthur, F.H.; Nechols, J.R. Efficacy of methoprene applied at different temperatures and rates on surface substrates to control eggs and fifth instars of Plodia interpunctella. J. Econ. Entomol. 2009, 102, 1992–2002. [Google Scholar] [CrossRef]
- Jenson, E.A.; Arthur, F.H.; Nechols, J.R. Methoprene and synergized pyrethrins as aerosol treatments to control Plodia interpunctella. J. Stored Prod. Res. 2010, 46, 103–110. [Google Scholar] [CrossRef]
- Jenson, E.A.; Arthur, F.H.; Nechols, J.R. Efficacy of an esfenvalerate plus methoprene aerosol for the control of eggs and fifth instars of Plodia interpunctella. Insect Sci. 2010, 17, 21–28. [Google Scholar] [CrossRef]
- Wijayaratne, L.K.W.; Fields, P.G. Effect of methoprene on the heat tolerance and cold tolerance of Tribolium castaneum. J. Stored Prod. Res. 2010, 46, 166–173. [Google Scholar] [CrossRef]
- Wu, Z.W.; Ye, C.Y.; Ye, Z.T.; Zhang, X.X.; Zhang, Q.Y.; Zhang, Y.; Qian, X. Discovery of Enantiopure (S)-Methoprene Derivatives as Potent Biochemical Pesticide Candidates. J. Agric. Food Chem. 2024, 72, 24979–24988. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.M.; Campbell, J.; Arthur, F.; Zhu, K.Y. Effects of methoprene and synergized pyrethrin aerosol applications on Tribolium castaneum (Herbst) populations. J. Stored Prod. Res. 2015, 64, 168–174. [Google Scholar] [CrossRef]
- Bell, H.A.; Robinson, K.A.; Weaver, R.J. First Report of Cyromazine Resistance in a Population of UK House Fly (Musca domestica) Associated with Intensive Livestock Production. Pest Manag. Sci. 2010, 66, 693–695. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.; Wall, R. The use of triflumuron on sugar-baited targets for autosterilization of the house fly, Musca domestica. Entomol. Exp. Appl. 1995, 77, 159–165. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem. National Institutes of Health: Bethesda, MD, USA. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 31 July 2025).
- Blümel, S.; Gross, H. Effect of pesticide mixtures on the predatory mite Phytoseiulus persimilis A.-H. (Acarina: Phytoseiidae) in the laboratory. J. Appl. Entomol. 2001, 125, 201–205. [Google Scholar] [CrossRef]
- Ogden, N.H.; Lindsay, L.R. Effects of climate and climate change on vectors and vector-borne diseases: Ticks are different. Trends Parasitol. 2016, 32, 646–656. [Google Scholar] [CrossRef]
- Oter, K.; Gunay, F.; Tuzer, E.; Linton, Y.M.; Bellini, R.; Alten, B. First record of Stegomyia albopicta in Turkey determined by active ovitrap surveillance and DNA barcoding. Vector-Borne Zoonotic Dis. 2013, 13, 753–761. [Google Scholar] [CrossRef]
- Semenza, J.C.; Suk, J.E. Vector-borne diseases and climate change: A European perspective. FEMS Microbiol. Lett. 2018, 365, fnx244. [Google Scholar] [CrossRef]
- Parham, P.E.; Waldock, J.; Christophides, G.K.; Hemming, D.; Agusto, F.; Evans, K.J.; Michael, E. Climate, environmental and socio-economic change: Weighing up the balance in vector-borne disease transmission. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20130551. [Google Scholar] [CrossRef]
- Polat, N. Biyoçeşitlilik ve Önemi. In Terme’nin Biyoçeşitlilik ve Doğal Ortam Özellikleri; Serander: Trabzon, Türkiye, 2017; pp. 3–14. [Google Scholar]
- Şişli, M.; Boşgelmez, A.; Kocak, O.; Porsuk, H. The effects of malathion, fenitrothion and propoxur on the house fly, Musca domestica L. (Diptera: Muscidae), populations. Microbiol. Bull. 1983, 17, 49–62. [Google Scholar]
- Çağlar, S.S. The investigation on resistance level to tetramethrin of housefly, Musca domestica L. (Diptera: Muscidae) and life table studies. Turk. J. Zool. 1991, 15, 91–97. [Google Scholar]
- Akıner, M.M.; Çağlar, S.S. Monitoring of Five Different Insecticide Resistance Status in Turkish House Fly Musca domestica L. (Diptera: Muscidae) Populations and the Relationship between Resistance and Insecticide Usage Profile. Turk. Parazitol. Derg. 2012, 36, 87–91. [Google Scholar] [CrossRef]
- Koc, S.; Oz, E.; Erdogan, G.; Yanıkoğlu, A.; Çetin, H. Synthetic Pyrethroid Resistance in House Fly, Musca domestica L. (Diptera: Muscidae), from the Solid Waste Collection Facility of Varsak, Antalya, Turkey. Fresenius Environ. Bull. 2012, 21, 3424–3426. [Google Scholar]
- Çakır, D.; Çetin, H. Determination of Resistance Levels against Thiamethoxam in House Fly (Musca domestica L.) Populations in Antalya. Turk. Parazitol. Derg. 2021, 45, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.A. Monitoring Resistance to Methomyl and Synergism in the Non-Target Musca domestica from Cotton Fields of Punjab and Sindh Provinces, Pakistan. Sci. Rep. 2023, 13, 7074. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, M.; Li, X.; Ullah, F.; Zhang, Z.; Zhang, J.; Huang, J.; Chen, L.; Siddiqui, J.A.; Ren, X.; Zhou, S.; et al. Characterization of Indoxacarb Resistance in the Fall Armyworm: Selection, Inheritance, Cross-Resistance, Possible Biochemical Mechanisms, and Fitness Costs. Biology 2022, 11, 1718. [Google Scholar] [CrossRef]
- Huang, X.; Tang, X.; Liao, A.; Sun, W.; Lei, L.; Wu, J. Cyclopropane derivatives with triangular stability have shown promising applications in pesticide chemistry. J. Mol. Struct. 2025, 1326, 141171. [Google Scholar] [CrossRef]
- Rahman, A.U.; Khan, I.; Usman, A.; Khan, H. Evaluation of Insect Growth Regulators (IGRs) as Biological Pesticides for Control of Aedes aegypti Mosquitoes. J. Vector Borne Dis. 2024, 61, 129–135. [Google Scholar]
- Yanıkoğlu, A.; Çetin, H. Böcek Gelişim Düzenleyicileri. In Proceedings of the IV. Ulusal Vektör Mücadelesi Sempozyumu, Antalya, Türkiye, 1–4 November 2018; p. 52. [Google Scholar]
- Öz, E.; Polat, B.; Cengiz, A.; Kahraman, S.; Gültekin, Z.N.; Çalışkan, C.; Çetin, H. Effects of Solid and Aqueous Dietary Diflubenzuron Ingestion on Some Biological Parameters in Synthetic Pyrethroid-Resistant German Cockroach, Blattella germanica L. (Blattodea: Ectobiidae). Med. Vet. Entomol. 2024, 38, 172–178. [Google Scholar] [CrossRef]
- Wijayaratne, L.K.W.; Arthur, F.H.; Whyard, S. Methoprene and Control of Stored-Product Insects. J. Stored Prod. Res. 2018, 76, 161–169. [Google Scholar] [CrossRef]
- Ebeid, A.R.; Elbehery, H.H.; Farag, N.A.; Gesraha, M.A. Toxicity of Some Insecticides on the Hymenopteran Parasitoid, Bracon hebetor (Hymenoptera: Braconidae). Eur. J. Sustain. Dev. 2017, 6, 72. [Google Scholar] [CrossRef]
- Mansour, A.N.; Ghoneim, K.S.; Hamadah, K.S.; Elsoud, A.A.A. Demographic Toxicology of Insect Growth Regulators on the Nontarget Ectolarval Parasitoid Habrobracon hebetor. Sci. Rep. 2024, 14, 26189. [Google Scholar] [CrossRef]
- Sampson, B.J.; Werle, C.T.; Stringer, S.J.; Adamczyk, J.J. Ingestible Insecticides for Spotted Wing Drosophila Control: A Polyol, Erythritol, and an Insect Growth Regulator, Lufenuron. J. Appl. Entomol. 2017, 141, 8–18. [Google Scholar] [CrossRef]
- Fulcher, A.; Scott, J.M.; Qualls, W.A.; Müller, G.C.; Xue, R.D. Attractive Toxic Sugar Baits Mixed with Pyriproxyfen Sprayed on Plants against Adult and Larval Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2014, 51, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Chanbang, Y.; Arthur, F.H.; Wilde, G.E.; Throne, J.E. Efficacy of Diatomaceous Earth and Methoprene, Alone and in Combination, against Rhyzopertha dominica (F.) (Coleoptera: Bostrichidae) in Rough Rice. J. Stored Prod. Res. 2007, 43, 396–401. [Google Scholar] [CrossRef]
- Nisar, M.J.; Gogi, M.D.; Arif, M.J.; Sahi, S.T. Attraction and Retention-Period of Different Stuffs and Stuffing Techniques with Their Active Food Baits for the Management of Peach Fruit Fly, Bactrocera zonata (Diptera: Tephritidae). Int. J. Trop. Insect Sci. 2020, 40, 599–610. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; AbdElrahman, I.E.; Zedan, O.A.A.; Abdel-Rahman, Y.A.; Saba, R.M.; Abo Laban, G.F.; Ibrahim, I.S. Effect of Three Insect Growth Regulators on Certain Biological Aspects of the Lesser Grain Borer, Rhyzopertha dominica (Fabricius, 1792) (Coleoptera: Bostrichidae). Braz. J. Biol. 2023, 82, e267629. [Google Scholar] [CrossRef]
- Ali, M.; Iqbal, J.; Afzal, M.B.S.; Zia, K.; Khan, M.A.; Tariq, M.; Hameed, A.; Zafar, S.; Shakir, A.S. Evaluation of Some Insect Growth Regulators against Red Flour Beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae). Pak. J. Zool. 2016, 48, 1337–1342. [Google Scholar]
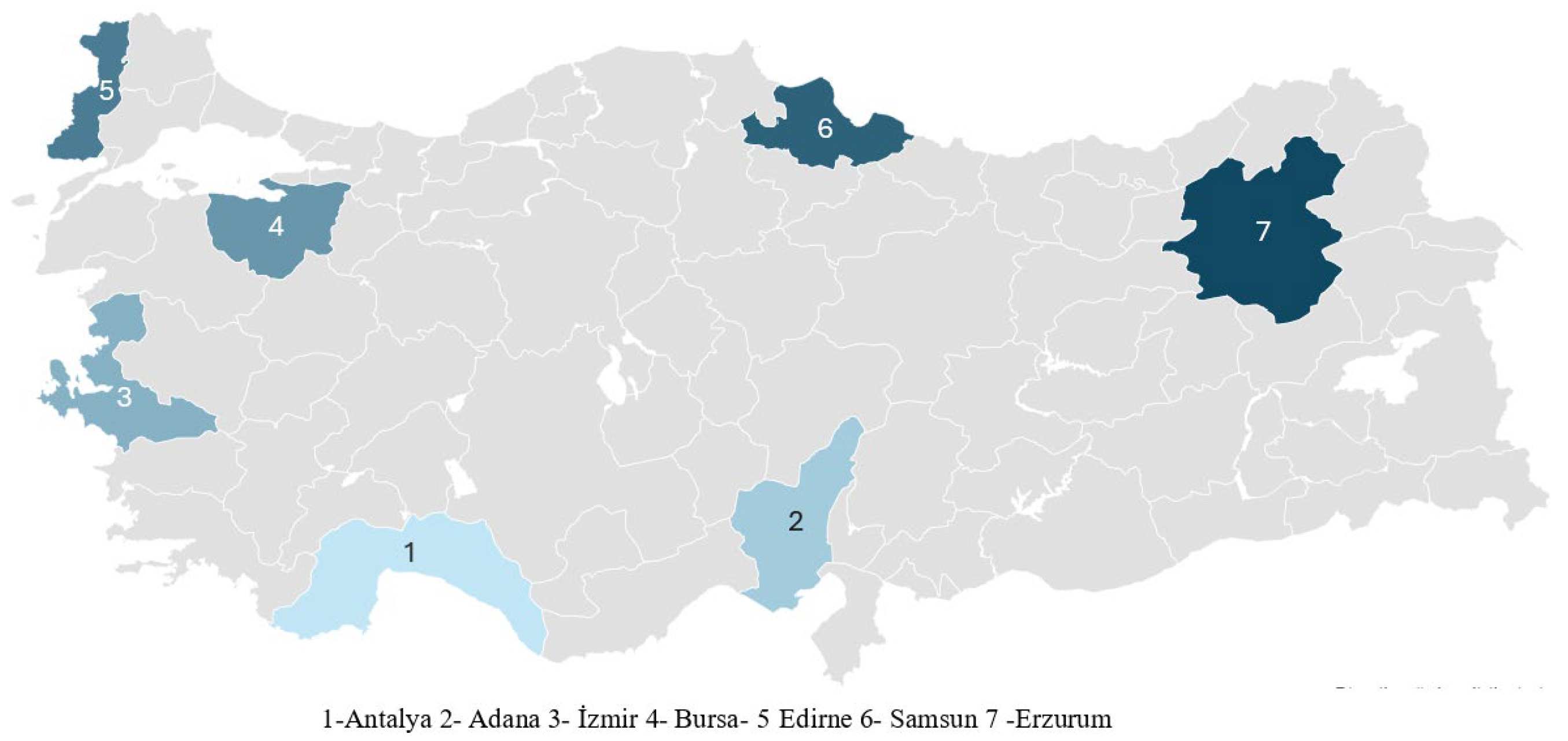
| Locality | District | Coordinates | Sampling Date |
|---|---|---|---|
| Adana | Ceyhan | 36.90413° N–35.94073° E | June 2021 |
| Antalya | Kepez | 36.99272° N–30.71738° E | June 2021 |
| Bursa | Nilüfer | 40.16571° N–28.72822° E | July 2021 |
| Edirne | Merkez | 41.62572° N–26.56585° E | July 2021 |
| Erzurum | Aşkale | 39.85275° N–40.60816° E | August 2021 |
| İzmir | Çeşme | 38.31391° N–26.47018° E | July 2021 |
| Samsun | İlkadım | 41.28796° N–36.20145° E | August 2021 |
| Name | Mode of Action | Cas Number | IUPAC Name |
|---|---|---|---|
| Cyromazine | CSI | 66215-27-8 | 2-N-cyclopropyl-1,3,5-triazine-2,4,6-triamine |
| Methoprene | JHA | 40596-69-8 | propan-2-yl (2E,4E)-11-methoxy-3,7,11-trimethyldodeca-2,4-dienoate |
| IGR | Antalya | Erzurum | Samsun | İzmir | Edirne | Bursa | Adana | WHO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
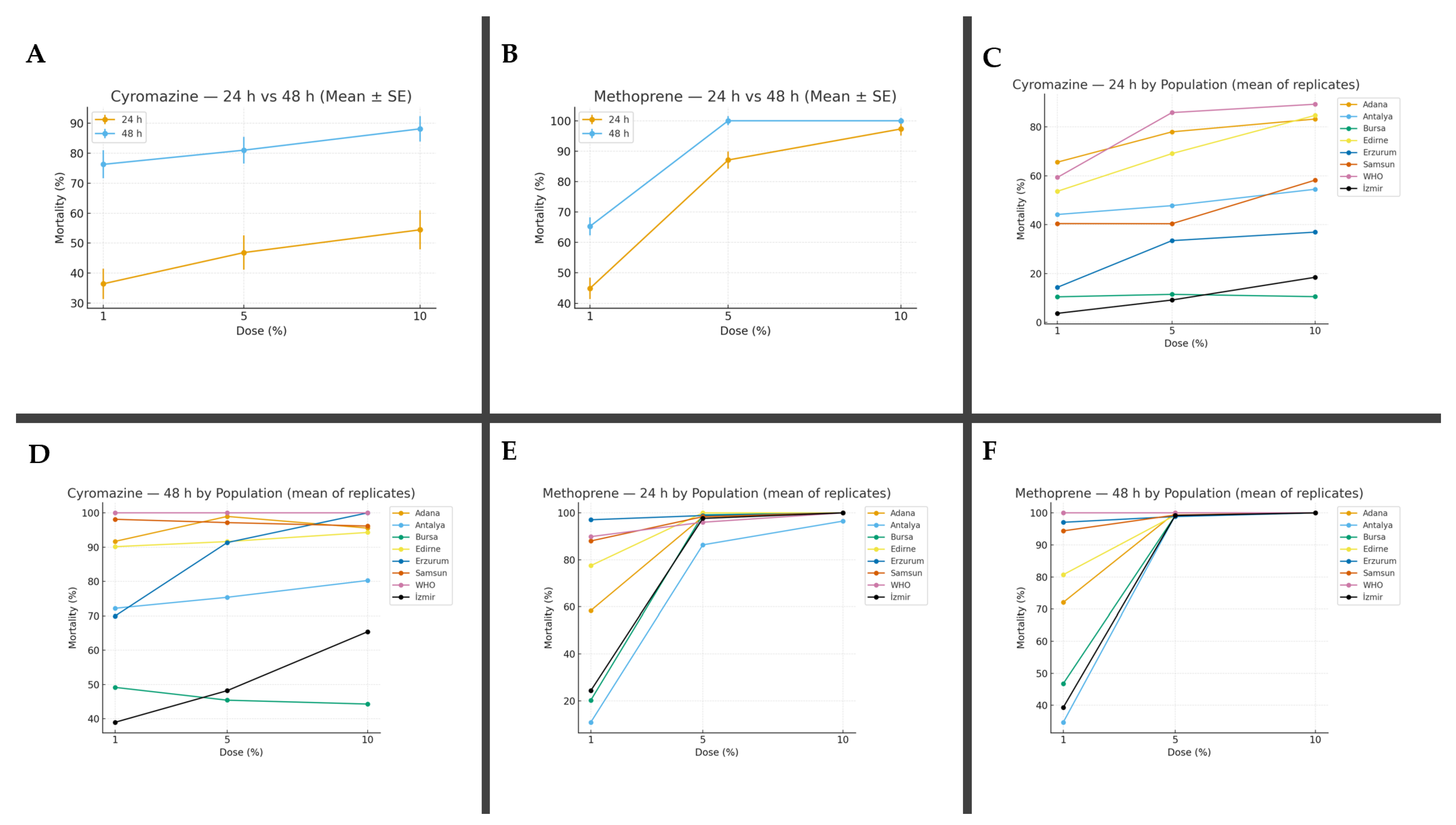
| Cyro %1 | 44.14 ± 7.16 B,b | 72.16 ± 7.54 C,b | 14.37 ± 0.83 B,b | 69.91 ± 4.80 C,b | 40.42 ± 7.48 B,b | 98.13 ± 1.80 C,b | 3.72 ± 0.79 A,a | 38.95 ± 4.08 B,b | 53.64 ± 3.06 B,b | 90.80 ± 2.28 C,b | 10.48 ± 1.54 A,b | 49.15 ± 8.85 B,b | 65.55 ± 4.93 B,b | 91.67 ± 4.74 C,b | 59.31 ± 21.33 B,b | 100 ± 0.00 C,b |
| Cyro %5 | 47.76 ± 1.51 B,b | 75.35 ± 5.30 C,b | 33.45 ± 3.49 B,c | 91.33 ± 4.67 C,bc | 40.38 ± 5.68 B,b | 97.16 ± 1.58 C,b | 9.17 ± 2.88 B,b | 48.20 ± 1.55 C,bc | 69.04 ± 3.33 B,c | 91.61 ± 2.27 C,b | 11.49 ± 4.99 B,b | 45.41 ± 2.29 C,b | 77.93 ± 3.62 B,bc | 98.96 ± 1.66 C,b | 85.81 ± 5.91 B,bc | 100 ± 0.00 C,b |
| Cyro%10 | 54.46 ± 1.69 B,b | 80.26 ± 2.15 B,b | 36.93 ± 8.41 B,c | 100 ± 0.00 C,c | 58.17± 19.80 B,b | 96.16 ± 1.00 B,b | 18.46 ± 1.54 A,c | 65.38 ± 9.88 B,c | 84.65 ± 2.48 B,d | 94.28 ± 0.36 C,b | 10.55 ± 5.96 A,b | 44.28 ± 9.04 B,b | 83.17 ± 6.39 B,c | 95.56 ± 4.81 C,b, | 89.23 ± 6.31 B,c | 100 ± 0.00 C,b |
| Control | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, |
| IGR | Antalya | Erzurum | Samsun | İzmir | Edirne | Bursa | Adana | WHO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Metho%1 | 10.81± 1.38 A.b | 34.69 ± 12.6 B,b | 97.06± 1.65 B,b | 97.06 ± 1.65 B,b | 88.01 ± 1.92 B,b | 94.37 ± 3.40 B,b | 24.30 ± 4.72 B,b | 39.33 ± 7.47 B,b | 77.49 ± 6.59 B,b | 80.69 ± 6.66 B,b | 20.20 ± 8.41 A,b | 46.76 ± 8.48 B,b | 58.37 ± 22.60 B,b | 72.06 ± 5.80 B,b | 89.86 ± 5.22 B,b | 100 ± 0.00 C,b |
| Metho %5 | 86.35 ± 2.28 B,c | 99.15± 0.83 C,c | 98.85 ± 1.11 B,b | 98.85 ± 1.11 B,b | 98.37 ± 1.57 B,c | 99.32 ± 0.70 B,b | 97.65 ± 1.42 B,c | 99.19 ± 0.82 C,c | 100 ± 0.00 B,c | 99.26 ± 0.72 B,c | 99.17 ± 0.83 B,c | 99.17 ± 0.83 B,c | 98.33 ± 2.50 B,c | 100 ± 0.00 B,c | 96.00 ± 5.77 B,c | 100 ± 0.00 B,b |
| Metho%10 | 96,47 ± 0.63 B,d | 100 ± 0.00 C,c | 100 ± 0.00 B,b | 100 ± 0.00 B,b | 100 ± 0.00 B,c | 100 ± 0.00 B,b | 100 ± 0.00 B,c | 100 ± 0.00 B,c | 100 ± 0.00 B,c | 100 ± 0.00 B,c | 100 ± 0.00 B,c | 100 ± 0.00 B,c | 100 ± 0.00 B,c | 100 ± 0.00 B,c | 100 ± 0.00 B,c | 100 ± 0.00 B,b |
| Control | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, | 0.00 ± 0.00 A,a, |
| Populations | Cyromazine %1 | Cyromazine %5 | Cyromazine %10 | Methoprene %1 | Methoprene %5 | Methoprene %10 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | 24 h | 48 h | |
| Control | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 0.00 ± 0.00 A |
| Antalya | 44.14 ± 7.16 CD | 72.16 ± 7.54 CD | 47.76 ± 1.51 CD | 75.35 ± 5.30 CD | 54.46 ± 1.69 BCDE | 80.26 ± 2.15 CD | 10.81± 1.38 AB | 34.69 ± 12.06 B | 86.35 ± 2.28 B | 99.15 ± 0.83 B | 96.47 ± 0.63 B | 100 ± 0.00 B |
| Erzurum | 14.37 ± 0.83 BC | 69.91 ± 4.80 CD | 33.45 ± 3.49 C | 91.33 ± 4.67 EF | 36.93 ± 8.41 BCD | 100 ± 0.00 E | 97.06± 1.65 E | 97.06 ± 1.65 DE | 98.85 ± 1.11 BC | 98.85 ± 1.11 B | 100 ± 0.00 C | 100 ± 0.00 B |
| Samsun | 40.42 ± 7.48 CD | 98.13 ± 1.80 EF | 40.38 ± 5.68 C | 97.16 ± 1.58 E | 58.17± 19.80 CDE | 96.16 ± 1.00 DE | 88.01 ± 1.92 DE | 94.37 ± 3.40 DE | 98.37 ± 1.57 BC | 99.32 ± 0.70 B | 100 ± 0.00 C | 100 ± 0.00 B |
| İzmir | 3.72 ± 0.79 AB | 38.95 ± 4.08 B | 9.17 ± 2.88 B | 48.20 ± 1.55 BC | 18.46 ± 1.54 ABC | 65.38 ± 9.88 BC | 24.30 ± 4.72 BC | 39.33 ± 7.47 BC | 97.65 ± 1.42 BC | 99.19 ± 0.82 B | 100 ± 0.00 C | 100 ± 0.00 B |
| Edirne | 53.64 ± 3.06 D | 90.80 ± 2.28 DE | 69.04 ± 3.33 DE | 91.61 ± 2.27 EF | 84.65 ± 2.48 DE | 94.28 ± 0.36 DE | 77.49 ± 6.59 DE | 80.69 ± 6.66 CDE | 100 ± 0.00 C | 99.26 ± 0.72 B | 100 ± 0.00 C | 100 ± 0.00 B |
| Bursa | 10.48 ± 1.54 AB | 49.15 ± 8.85 BC | 11.49 ± 4.99 B | 45.41 ± 2.29 B | 10.55 ± 5.96 AB | 44.28 ± 9.04 B | 20.20 ±8.41 B | 46.76 ± 8.48 BC | 99.17 ± 0.83 C | 99.17 ± 0.83 B | 100 ± 0.00 C | 100 ± 0.00 B |
| Adana | 65.55 ± 4.93 D | 91.67 ± 4.74 DEF | 77.93 ± 3.62 E | 98,96 ± 1.66 E | 83.17 ± 6.39 DE | 95.56 ± 4.81 E | 58.37 ± 22.60 CD | 72.06 ± 5.80 BCD | 98.33 ± 2.50 BC | 100 ± 0.00 B | 100 ± 0.00 C | 100 ± 0.00 B |
| WHO | 59.31 ± 21.33 D | 100 ± 0.00 E | 85.81 ± 5.91 E | 100 ± 0.00 E | 89.23 ± 6.31 E | 100 ± 0.00 E | 89.86 ± 5.22 DE | 100 ± 0.00 E | 96.00 ± 5.77 BC | 100 ± 0.00 B | 100 ± 0.00 C | 100 ± 0.00 B |
| F: 23.161 p: <0.001 | F: 67.309 p: <0.001 | F: 67.024 p: <0.001 | F: 74.776 p: <0.001 | F: 67.024 p: <0.001 | F: 13.759 p: <0.001 | F: 77.852 p: <0.001 | F: 31.586 p: <0.001 | F: 25.179 p: <0.001 | F: 62.386 p: <0.001 | F: 6730.51 p: <0.001 | F: - p: <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdoğan, G. Adulticidal Activity of the Insect Growth Regulators Methoprene and Cyromazine in House Flies (Musca domestica L.): Evidence from Feeding Trials. Biology 2025, 14, 1495. https://doi.org/10.3390/biology14111495
Erdoğan G. Adulticidal Activity of the Insect Growth Regulators Methoprene and Cyromazine in House Flies (Musca domestica L.): Evidence from Feeding Trials. Biology. 2025; 14(11):1495. https://doi.org/10.3390/biology14111495
Chicago/Turabian StyleErdoğan, Gökhan. 2025. "Adulticidal Activity of the Insect Growth Regulators Methoprene and Cyromazine in House Flies (Musca domestica L.): Evidence from Feeding Trials" Biology 14, no. 11: 1495. https://doi.org/10.3390/biology14111495
APA StyleErdoğan, G. (2025). Adulticidal Activity of the Insect Growth Regulators Methoprene and Cyromazine in House Flies (Musca domestica L.): Evidence from Feeding Trials. Biology, 14(11), 1495. https://doi.org/10.3390/biology14111495






