Bioaccumulation of Lanthanum by Two Strains of Marine Diatoms Nanofrustulum shiloi and Halamphora kolbei
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
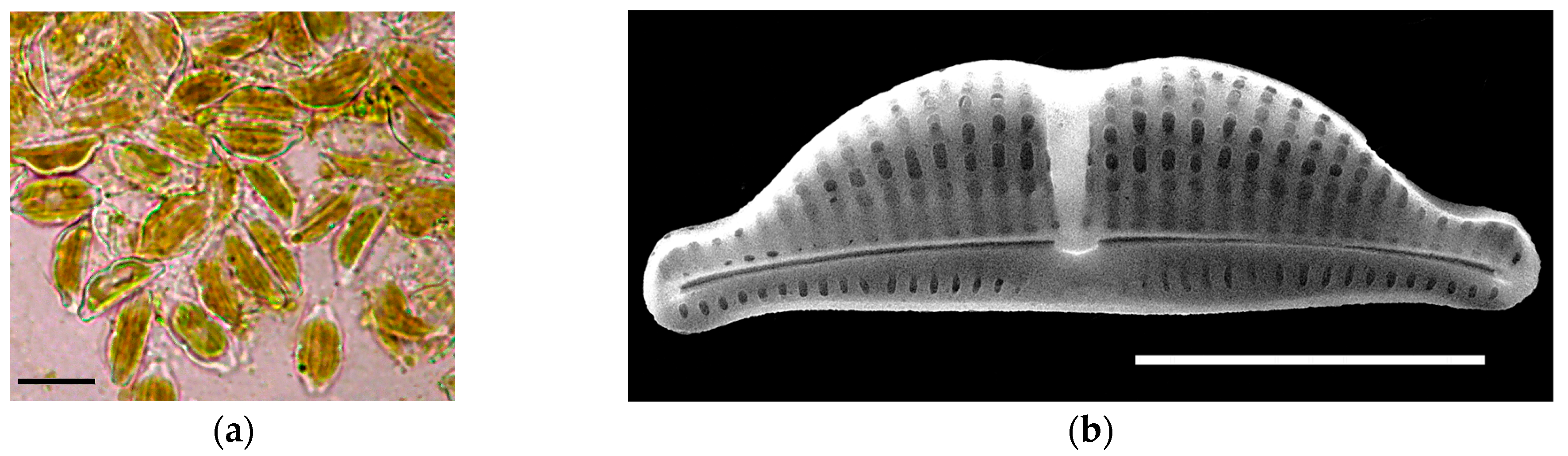
2.1. Isolation of Diatoms and Strain Characteristics
2.2. Cultivation of Diatoms in Lanthanum-Supplemented Medium
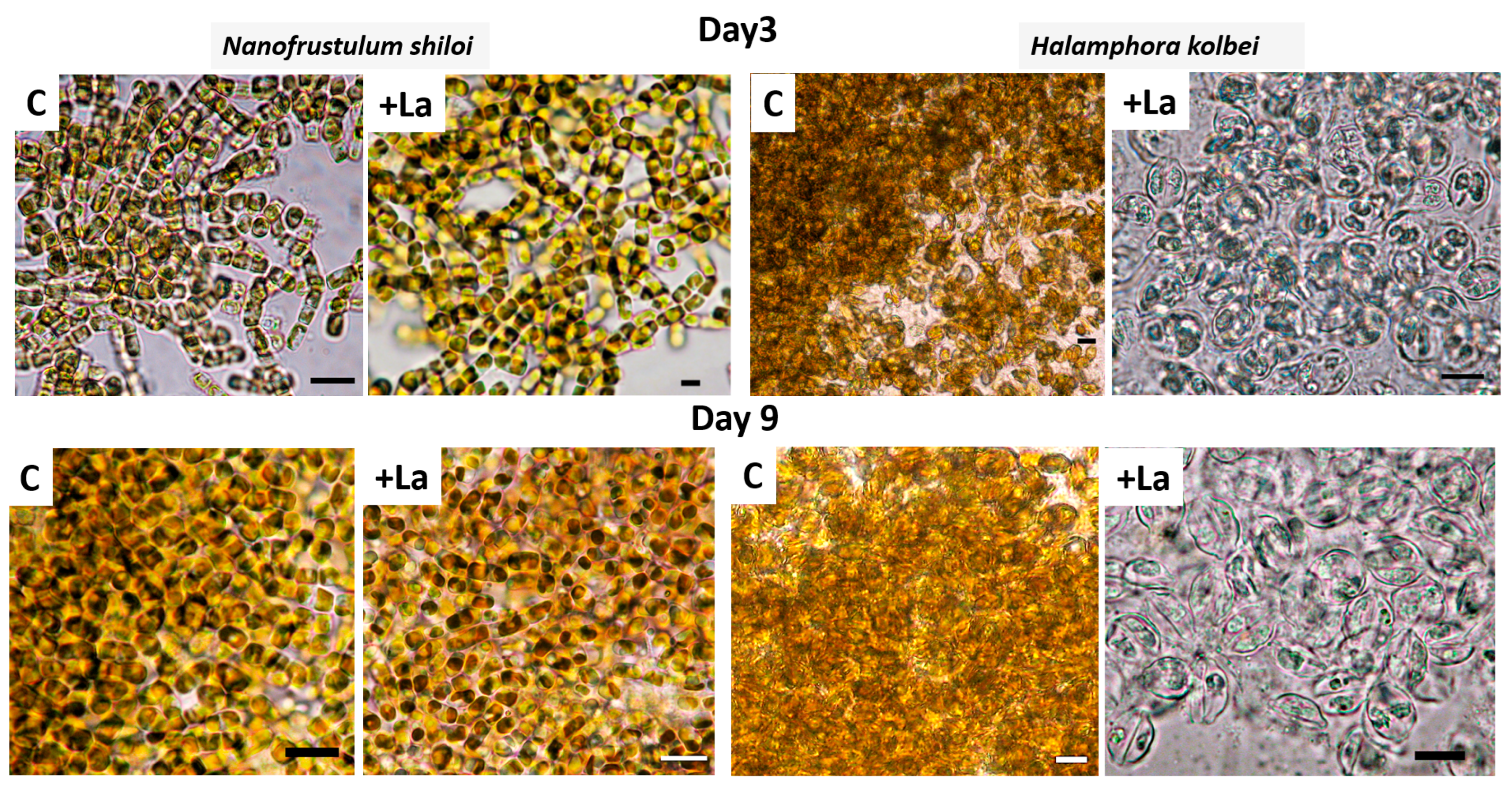
2.3. Sample Processing and Microscopy
2.4. Analytical Sample Preparation and Elemental Analysis in Diatom Biomass
2.5. Statistical Analysis
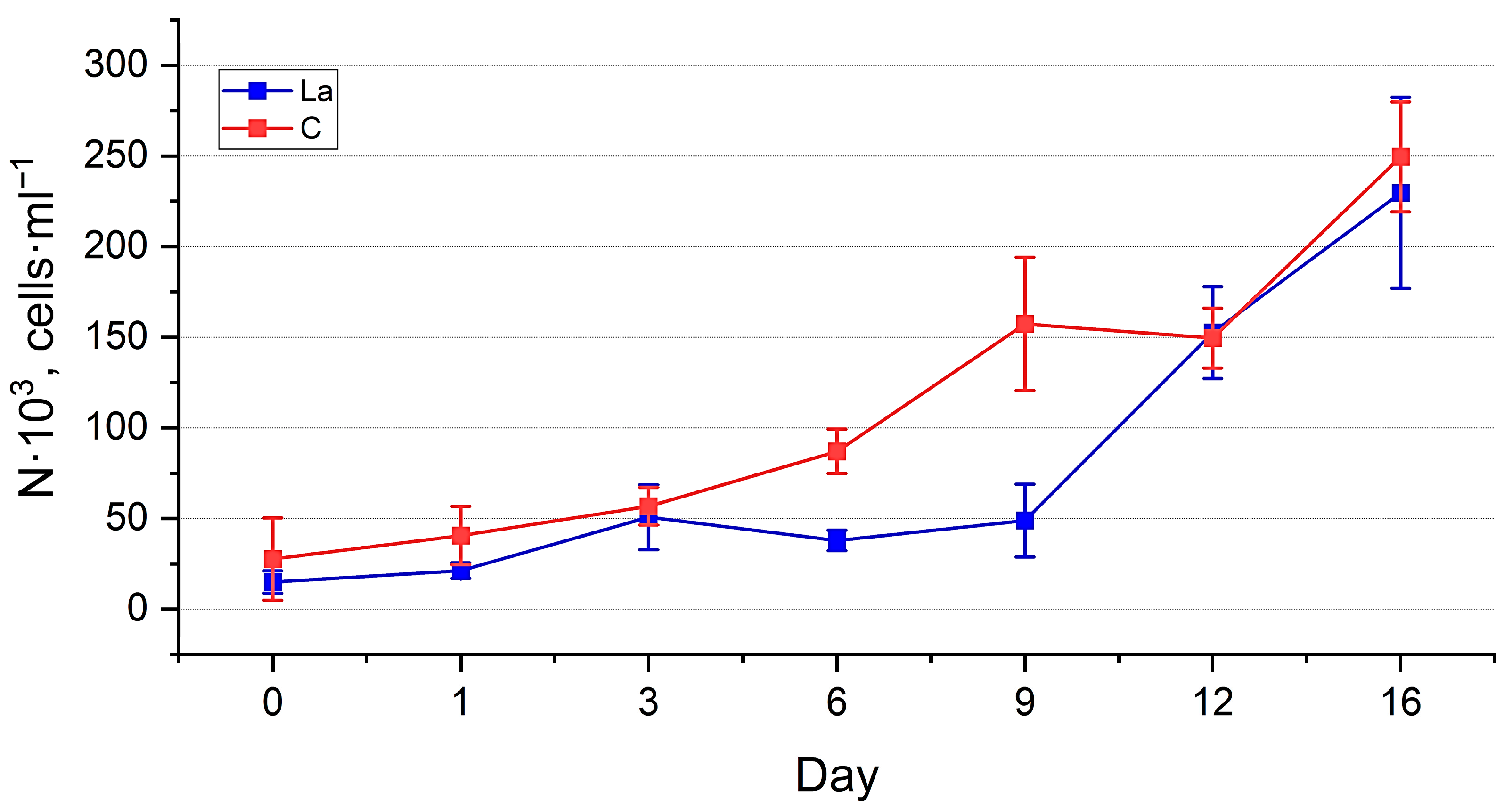
3. Results
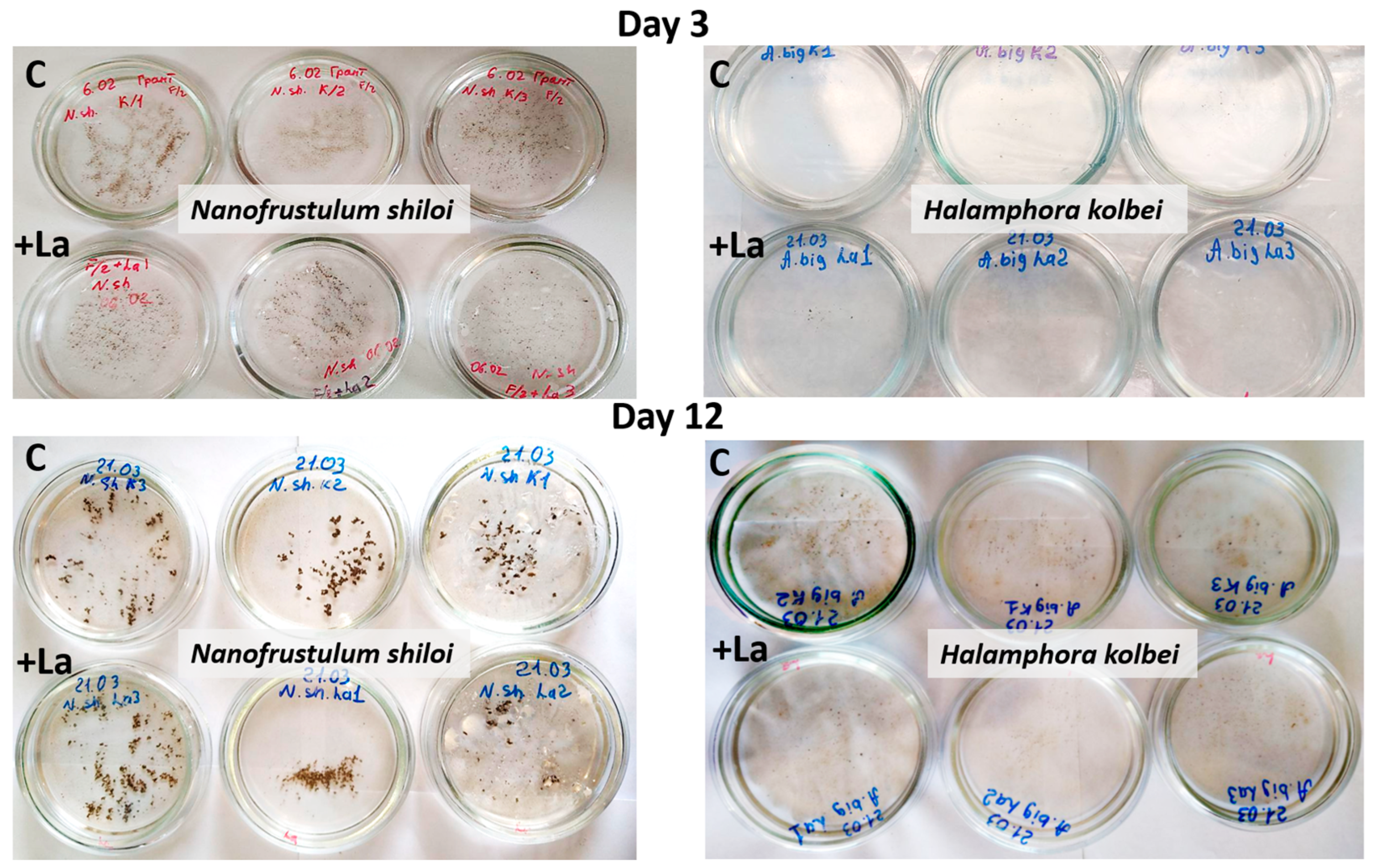
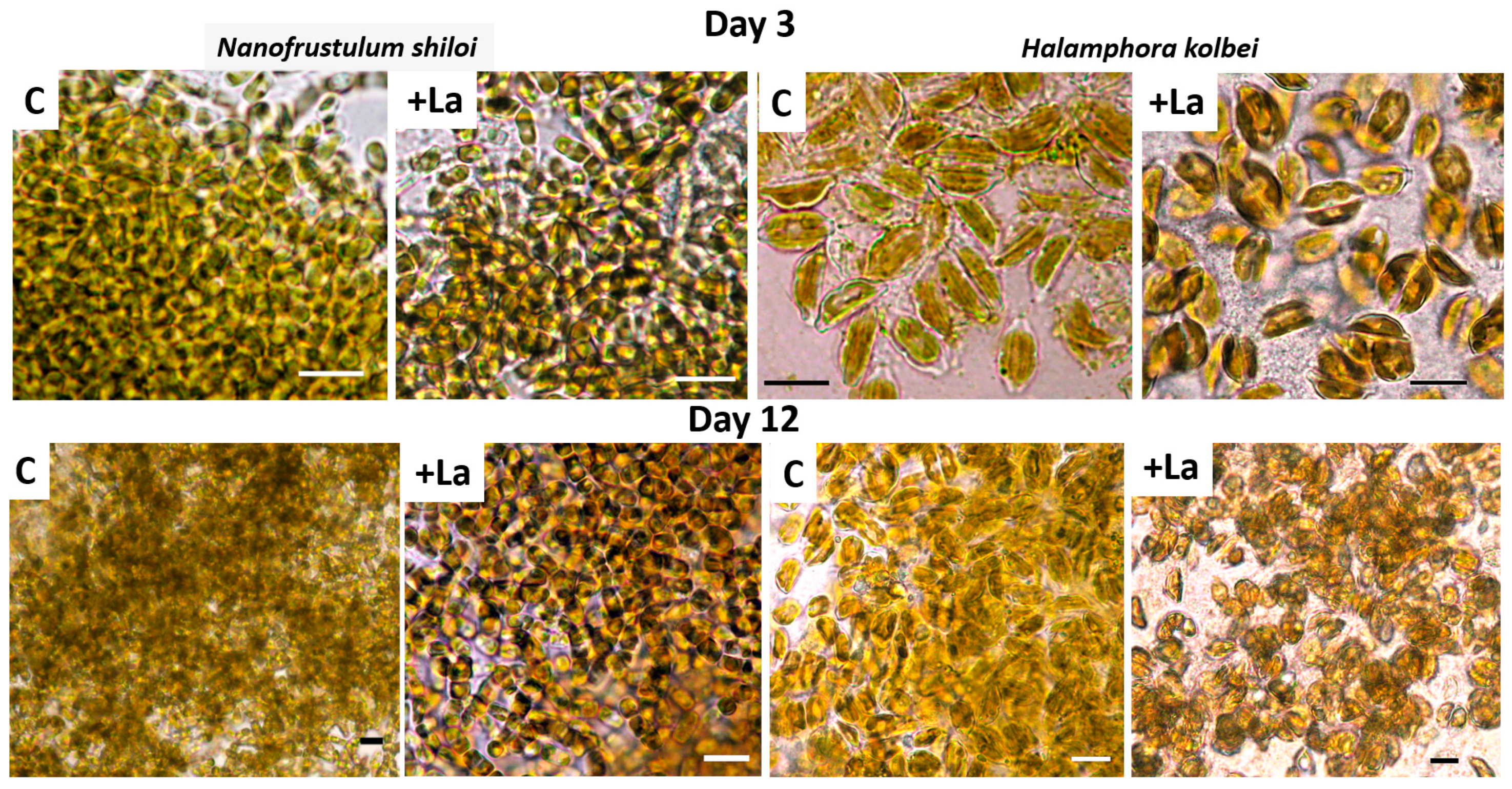
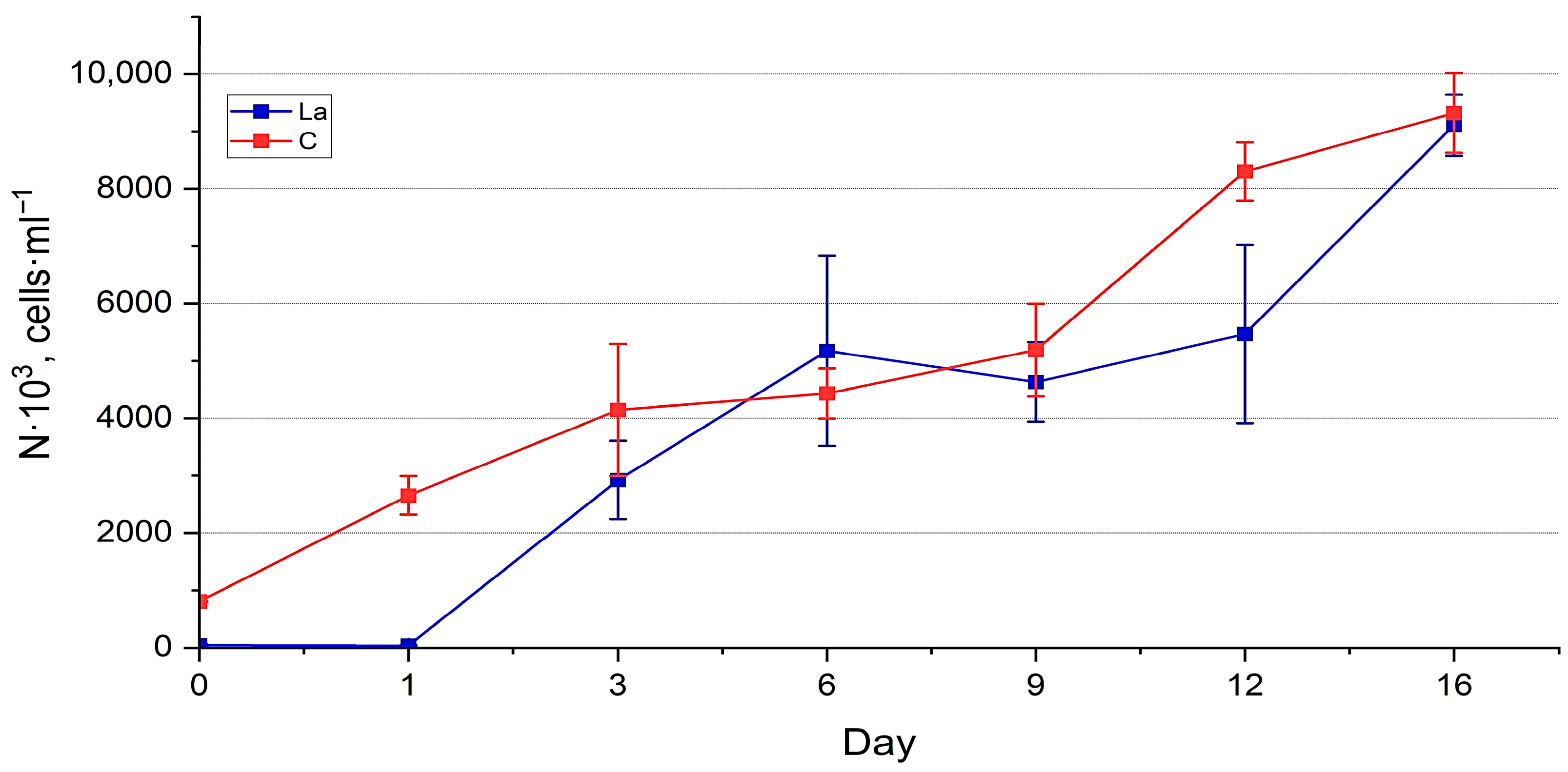
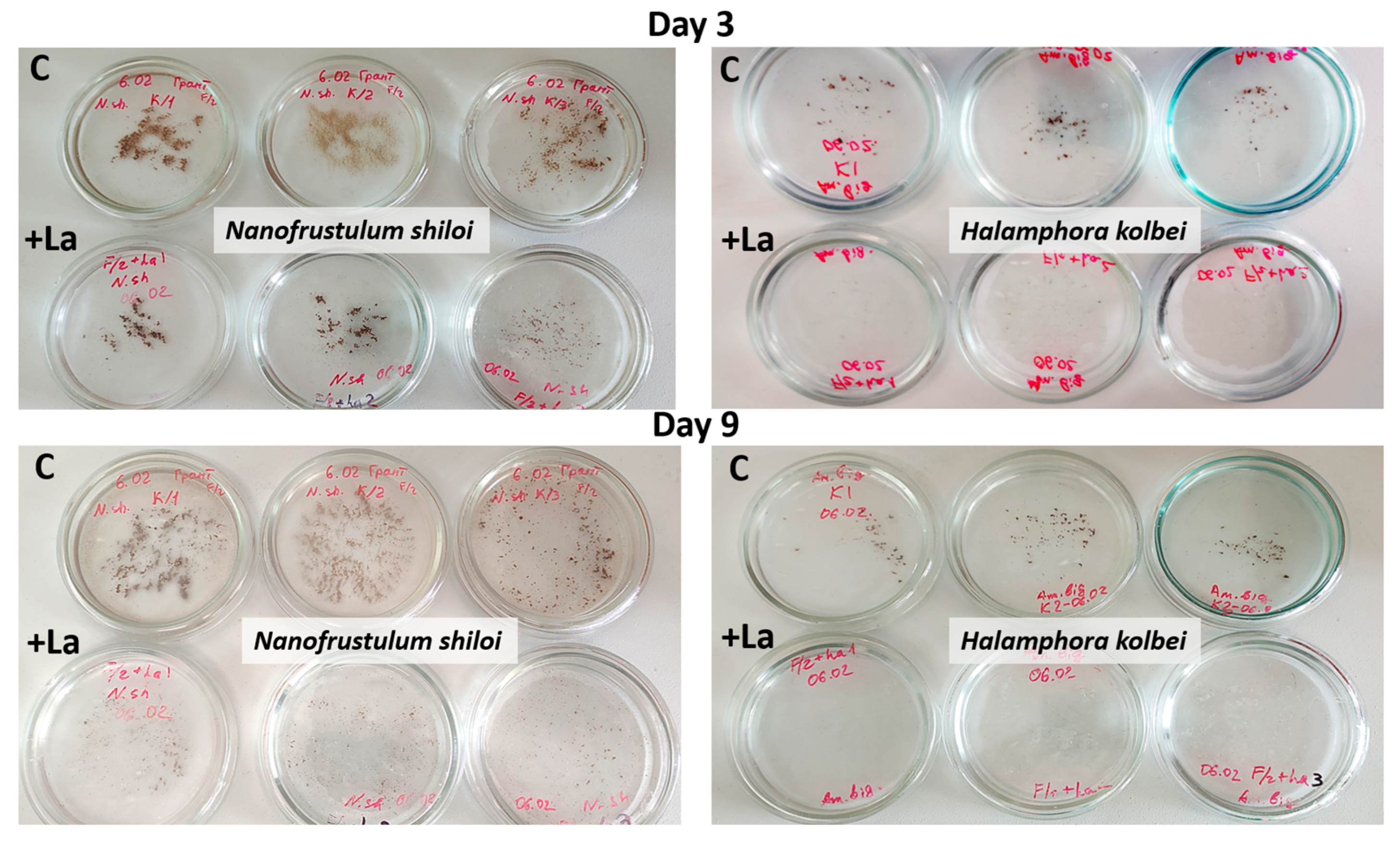
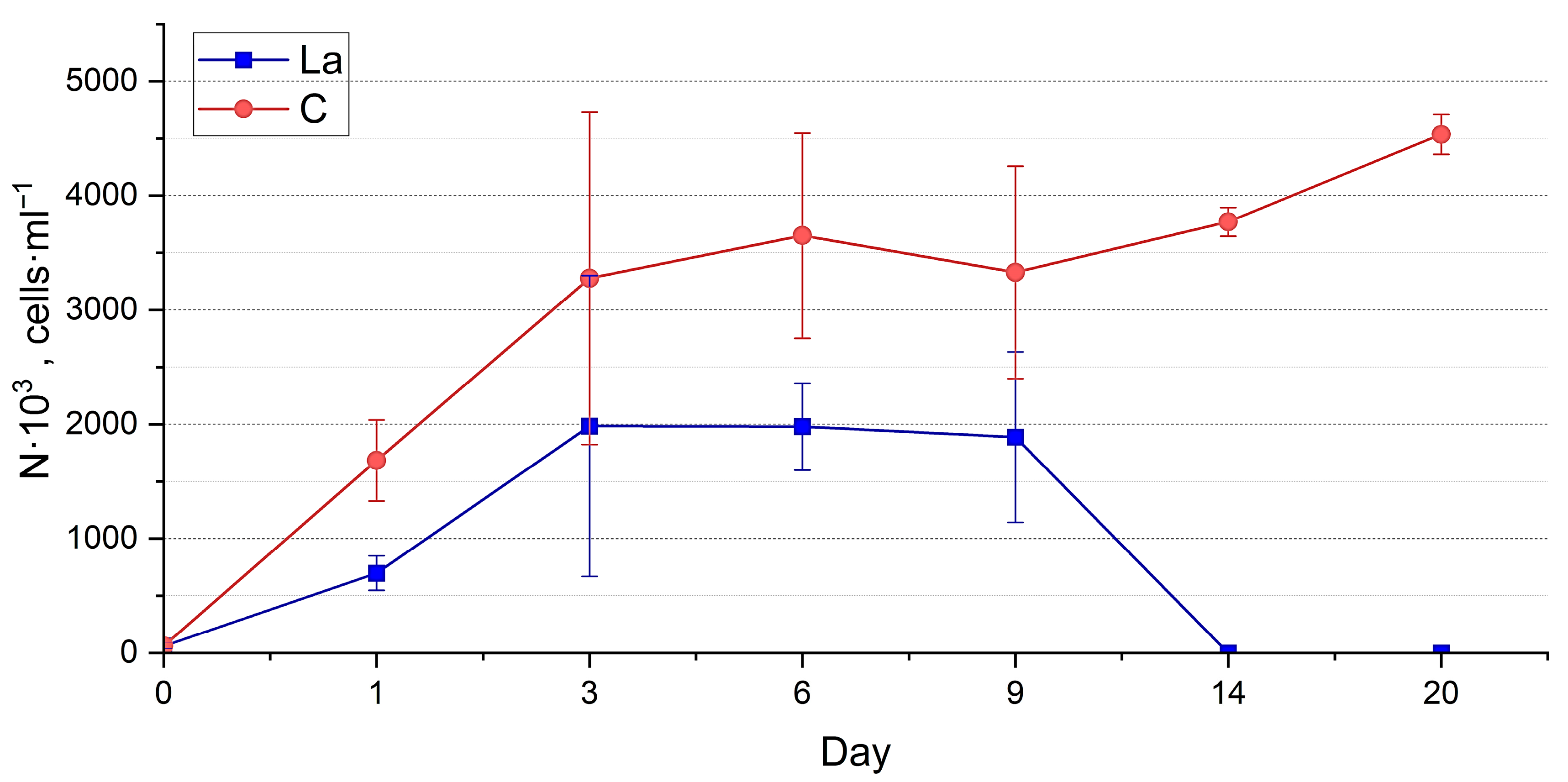
3.1. Nanofrustulum shiloi and Halamphora kolbei Cultivation in Media Supplemented with La
3.1.1. La Concentration 10 Mg·L−1
3.1.2. La Concentration 50 Mg·L−1
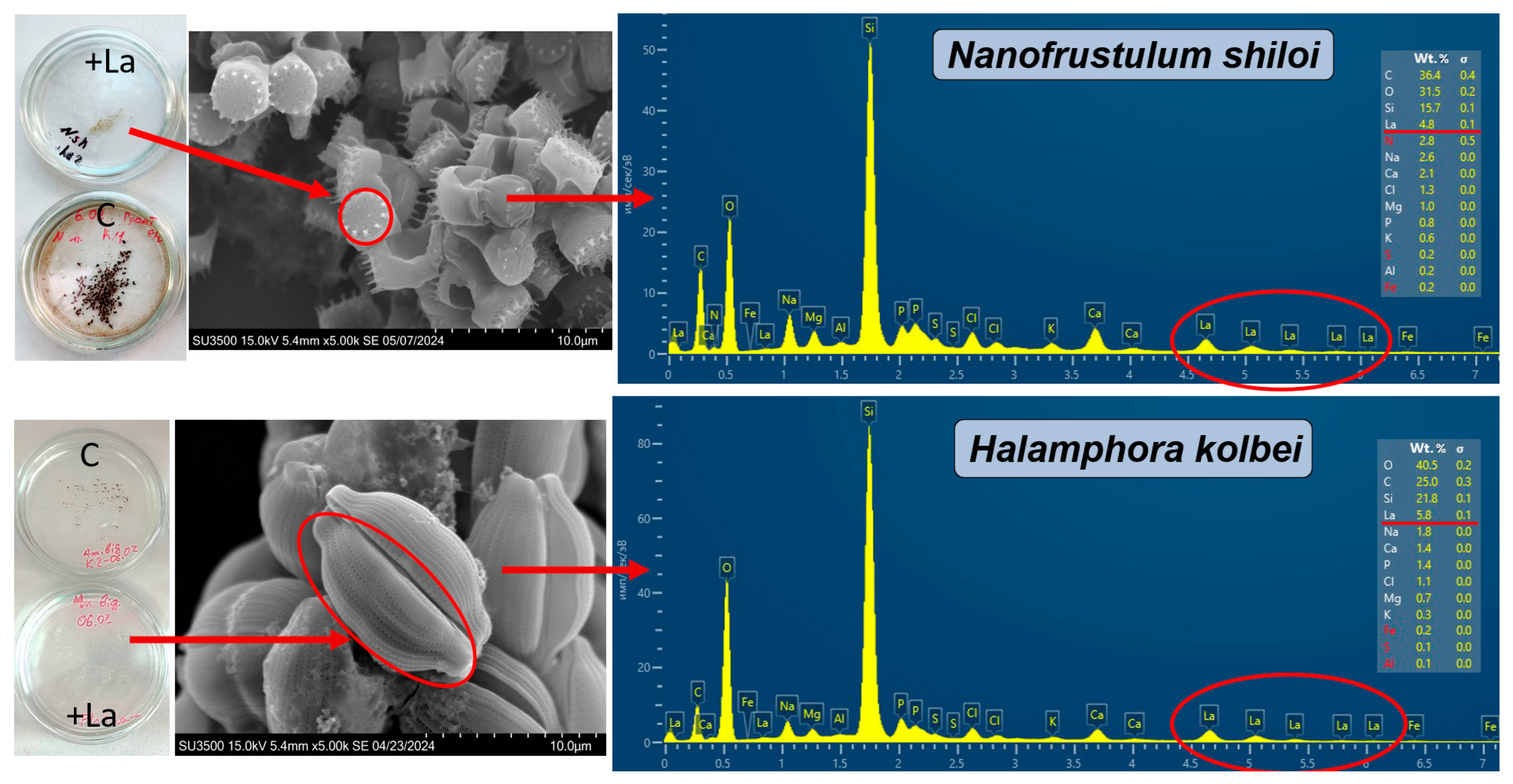
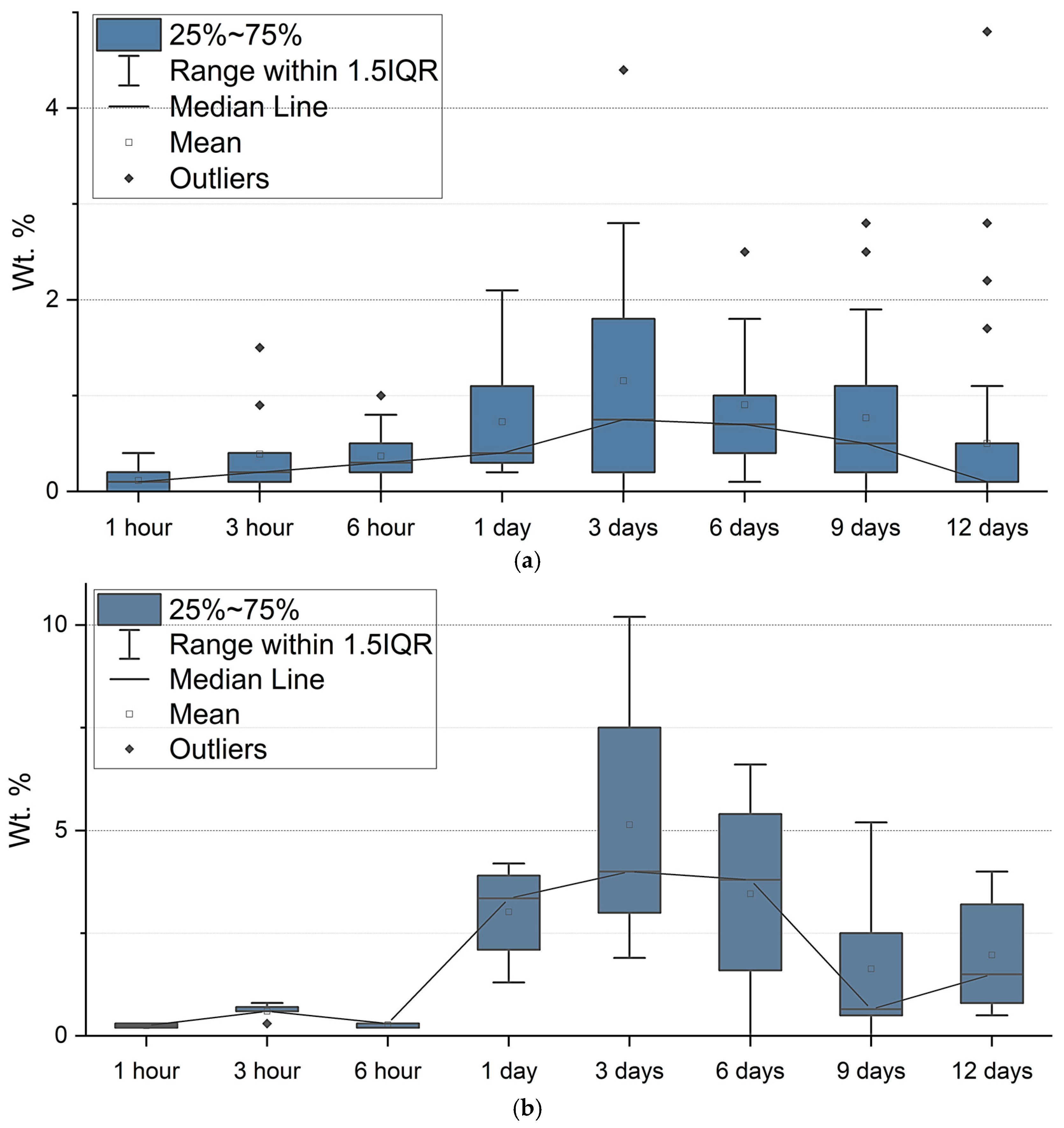
3.2. Elemental Composition of the Biomass of Nanofrustulum shiloi and Halamphora kolbei Cultivated in the 10 Mg·L−1 La-Supplemented and Control Media
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, D.G.; Crawford, R.M.; Round, F.E. Bacillariophyta. In Handbook of the Protists; Archibald, J., Simpson, A.G.B., Slamovits, C.H., Eds.; Springer: Cham, Switzerland, 2016; pp. 1–62. [Google Scholar] [CrossRef]
- Ryabushko, L.I. Microphytobenthos of the Black Sea; Gaevskaya, A.V., Ed.; EKOSI-Gidrofizica: Sevastopol, Ukraine, 2013; 416p. (In Russian) [Google Scholar]
- Baeüerlein, E. Biomineralization: From Biology to Biotechnology and Medical Application; Baeüerlein, E., Ed.; Angewandte Chemie International Edition: Weinheim, Germany, 2001; 337p. [Google Scholar]
- Falciatore, A.; Jaubert, M.; Bouly, J.-P.; Bailleul, B.; Mockc, T. Diatom molecular research comes of age: Model species for studying phytoplankton biology and diversity. Plant Cell 2020, 32, 547–572. [Google Scholar] [CrossRef]
- Gao, X.; Bowler, C.; Kazamia, E. Iron Metabolism Strategies in Diatoms. J. Exp. Bot. 2021, 72, 2165–2180. [Google Scholar] [CrossRef] [PubMed]
- Groussman, R.D.; Parker, M.S.; Armbrust, E.V. Diversity and evolutionary history of iron metabolism genes in diatoms. PLoS ONE 2015, 10, e0129081. [Google Scholar] [CrossRef] [PubMed]
- Torres, E.; Cid, R.; Herrero, C.; Abalde, J. Removal of cadmium ions by the marine diatom Phaeodactylum tricornutum Bohlin accumulation and long-term kinetics of uptake. Bioresour. Technol. 1998, 63, 213–220. [Google Scholar] [CrossRef]
- Hernández-Ávila, J.; Salinas-Rodríguez, E.; Cerecedo-Sáenz, E.; Reyes-Valderrama, M.I.; Arenas-Flores, A.; Román-Gutiérrez, A.D.; Rodríguez-Lugo, V. Diatoms and Their Capability for Heavy Metal Removal by Cationic Exchange. Metals 2017, 7, 169. [Google Scholar] [CrossRef]
- Kiran Marella, T.; Saxena, A.; Tiwari, A. Diatom Mediated Heavy Metal Remediation: A Review. Bioresour. Technol. 2020, 305, 123068. [Google Scholar] [CrossRef]
- Duong, T.T.; Feurtet-Mazel, A.; Coste, M.; Dagnino, A.; Boudou, A. Experimental toxicity and bioaccumulation of cadmium in freshwater periphytic diatoms in relation with biofilm maturity. Sci. Total Environ. 2010, 408, 552–562. [Google Scholar] [CrossRef]
- Sjahrul, M.; Arifin, A. Phytoremediation of Cd2+ by Marine Phytoplanktons, Tetraselmis chuii and Chaetoceros calcitrans. Int. J. Chem. 2012, 4, 69–74. [Google Scholar] [CrossRef]
- Şişman-Aydın, G.; Büyükışık, B.; Oral, R. Bioaccumulation of Cadmium in Marine Diatom: Thalassiosira allenii. Turk. J. Fish. Aquat. Sci. 2013, 13, 705–712. [Google Scholar] [CrossRef]
- Cherifi, O.; Sbihi, K.; Bertrand, M.; Cherifi, K. The Removal of Metals (Cd, Cu and Zn) from the Tensift River Using the Diatom Navicula subminuscula Manguin: A Laboratory Study. Int. J. Adv. Res. Biol. Sci. 2016, 3, 177–187. [Google Scholar]
- Schmitt, D.; Müller, A.; Csögör, Z.; Frimmel, F.H.; Posten, C. the adsorption kinetics of metal ions onto different microalgae and siliceous earth. Water Res. 2001, 35, 779–785. [Google Scholar] [CrossRef]
- Deng, G.F.; Zhang, T.W.; Yang, L.M.; Wang, Q.Q. Studies of biouptake and transformation of mercury by a typical unicellular diatom Phaeodactylum tricornutum. Chin. Sci. Bull. 2013, 58, 256–265. [Google Scholar] [CrossRef]
- Lang, Y.; Monte, F.; Rodriguez, B.; Dockery, P.; Finn, D.P.; Pandit, A. Integration of TiO2 into the diatom Thalassiosira weissflogii during frustule synthesis. Sci. Rep. 2013, 3, 3205. [Google Scholar] [CrossRef]
- Adl, M.; Nejadsattari, T.; Noroozi, M.; Asri, Y.; Saadatmand, S. Investigating the Ability of Chromium (VI) Adsorption by Nitzschia palea. Mod. Phytomorphol. 2020, 14, 64–69. [Google Scholar]
- Sternberg, E.; Tang, D.; Ho, C.-T.; Jeandel, C.; Morel, F.M.M. Barium uptake and adsorption in diatoms. Geochim. Cosmochim. Acta 2005, 69, 2745–2752. [Google Scholar] [CrossRef]
- Tian, Q.; Zhang, J.; Zhang, Y. Occurrence of structural aluminium (Al) in marine diatom biological silica: Visible evidence from microscopic analysis. Ocean Sci. 2022, 18, 321–329. [Google Scholar] [CrossRef]
- González, A.G.; Pokrovsky, O.S.; Ivanova, I.S. Interaction of freshwater diatom with gold nanoparticles: Adsorption, assimilation, and stabilization by cell exometabolites. Minerals 2018, 8, 99. [Google Scholar] [CrossRef]
- Wagner, M.; Hendy, I.L.; Lai, B. Characterizing Ag uptake and storage in the marine diatom Thalassiosira pseudonana: Implications for Ag biogeochemical cycling. Mar. Chem. 2022, 247, 104175. [Google Scholar] [CrossRef]
- Azam, F. Silicic-acid uptake in diatoms studied with [68Ge] germanic acid as tracer. Planta 1974, 121, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Kulaksız, S.; Bau, M. Rare Earth Elements in the Rhine River, Germany: First Case of Anthropogenic Lanthanum as a Dissolved Microcontaminant in the Hydrosphere. Environ. Int. 2011, 37, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Ryabinin, A.I.; Malchenko, Y.I.; Malchenko, Y.A.; Saltykova, L.V. Fields of concentrations of trace elements in coastal waters of the Black Sea near the western and southern coasts of the Crimea in 1990–2002. Mar. Hydrophys. J. 2008, 6, 38–52. [Google Scholar]
- Ryabinin, A.I.; Malchenko, Y.A.; Saltykova, L.V.; Danilova, E.A.; Bobrova, S.A. Variability of the fields of concentration of microelements and strontium in seawater near the South Coast of the Crimea in 2002–2007. Phys. Oceanogr. 2011, 21, 115–128. [Google Scholar] [CrossRef]
- Ryabinin, A.I.; Smirnova, L.L.; Bobrova, S.A.; Yerkushov, V.Y.; Danilova, Y.A.; Malchenko, Y.A.; Andreeva, N.A. State of Chemical and Microbiological Pollution of the Biosphere of the Sevastopol Region with Precipitation and Aerosols in the Period 2008–2010. Sci. Works Ukr. Sci. Res. Hydrometeorol. Inst. 2011, 260, 208–234. [Google Scholar]
- Ryabushko, V.I.; Gureeva, E.V.; Kapranov, S.V.; Bobko, N.I.; Prazukin, A.V.; Nekhoroshev, M.V. Rare earth elements in brown algae of the genus Cystoseira (Phaeophyceae) (Black Sea). Eur. J. Phycol. 2022, 57, 433–445. [Google Scholar] [CrossRef]
- Bingler, L.S.; Byrne, R.H.; Vargo, G.A.; Tomas, C.R. Rare earth element uptake by the marine diatom. Chem. Speciat. Bioavailab. 1989, 1, 103–110. [Google Scholar] [CrossRef]
- Akagi, T.; Fu, F.-F.; Hongo, Y.; Takahashi, K. Composition of rare earth elements in settling particles collected in the highly productive North Pacific Ocean and Bering Sea: Implications for siliceous-matter dissolution kinetics and formation of two REE-enriched phases. Geochim. Cosmochim. Acta 2011, 75, 4857–4876. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, W.; Li, L.; Long, Z.; Li, S.; Li, T.; Wang, Y.; Inganäs, O.; Tang, J. Living diatoms integrate polysaccharide-Eu3+ complex for UV downconversion. J. Mater. Res. Technol. 2022, 19, 2774–2780. [Google Scholar] [CrossRef]
- Tai, P.; Zhao, Q.; Su, D.; Li, P.; Stagnitti, F. Biological toxicity of lanthanide elements on algae. Chemosphere 2010, 80, 1031–1035. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zheng, X.; Liu, H.; Xie, W.; Liu, H.; Shen, Z.; Shang, C.; Liu, C.; Li, Y.; Zhu, J.; et al. In situ self-assembled La and Y nanoparticles based on biomineralization of diatom frustules to boost the electrochemical performance of SiO2 anodes for lithium-ion batteries. Adv. Sustain. Syst. 2025, 9, 2500068. [Google Scholar] [CrossRef]
- Simonsen, R. Untersuchungen zur Systematik und Ökologie der Bodendiatomeen der westlichen Ostsee. Int. Rev. Ges. I 1962, 1, 9–144. [Google Scholar]
- Blaginina, A.; Ryabushko, L.I. Finding of a rare species of diatom Nanofrustulum shiloi (Lee, Reimer et Mcenery) Round, Hallsteinsen & Paasche, 1999 in the periphyton of the coastal waters of the Black Sea. Int. J. Algae 2021, 23, 247–256. [Google Scholar] [CrossRef]
- Blaginina, A.A.; Zheleznova, S.N.; Miroshnichenko, E.S.; Gevorgiz, R.G.; Ryabushko, L.I. The diatom Nanofrustulum shiloi as a promising species in modern biotechnology. Appl. Biochem. Microbiol. 2024, 60, 483–495. [Google Scholar] [CrossRef]
- Levkov, Z. Amphora sensu lato. In Diatoms of Europe; Lange-Bertalot, H., Ed.; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2009; Volume 5, p. 916. [Google Scholar]
- Álvarez-Blanco, I.; Blanco, S. Benthic diatoms from Mediterranean coasts. Bibl. Diatomol. 2014, 60, 1–409. [Google Scholar]
- Zidarova, R.; Ivanov, P.; Hristova, O.; Dzhurova, B.; Hineva, E. The unexpected diversity in Amphora sensu lato (Bacillariophyta) at Sozopol Bay, the western Black Sea. Phytotaxa 2022, 544, 103–127. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Sun, S.; Zheng, X.; Liu, H.; Ma, X.; Liu, C.; Bai, J.; Liu, H.; Xia, J.; Wang, J. Toxicity Passivation of Rare Earth Elements via the Diatom-Mediated “Mineralization Self-Regulation” Mechanism: A Silicon Metabolic Feedback-Driven Pathway for Nanoparticle Formation. J. Environ. Chem. Eng. 2025, 13, 119504. [Google Scholar] [CrossRef]
- Siciliano, A.; Guida, M.; Serafini, S.; Micillo, M.; Galdiero, E.; Carfagna, S.; Salbitani, G.; Tommasi, F.; Lofrano, G.; Suarez, E.G.P.; et al. Long-Term Multi-Endpoint Exposure of the Microalga Raphidocelis subcapitata to Lanthanum and Cerium. Sci. Total Environ. 2021, 790, 148229. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education. Palaeontol. Electron. 2001, 4, 1–9. Available online: https://www.nhm.uio.no/english/research/resources/past/ (accessed on 31 July 2025).
- Li, W.; Zhang, R.; Xu, Z.; Chen, X.; Song, P. Effects of La3+ on growth, transformation, and gene expression of Escherichia coli. Biol. Trace Elem. Res. 2003, 94, 167–177. [Google Scholar] [CrossRef]
- Merroun, M.L.; Chekroun, K.B.; Arias, J.M.; González-Muñoz, M.T. Lanthanum fixation by Myxococcus xanthus: Cellular location and extracellular polysaccharide observation. Chemosphere 2003, 52, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.C.; Chu, Z.S.; Yan, F.; Zeng, Q.R. Effects of lanthanum (III) and EDTA on the growth and competition of Microcystis aeruginosa and Scenedesmus quadricauda. Limnologica 2009, 39, 86–93. [Google Scholar] [CrossRef]
- Balusamy, B.; Kandhasamy, Y.G.; Senthamizhan, A.; Chandrasekaran, G.; Subramanian, M.S.; Kumaravel, T.S. Characterization and bacterial toxicity of lanthanum oxide bulk and nanoparticles. J. Rare Earths 2012, 30, 1298–1302. [Google Scholar] [CrossRef]
- Aharchaou, I.; Beaubien, C.; Campbell, P.G.C.; Fortin, C. Lanthanum and cerium toxicity to the freshwater green alga Chlorella fusca: Applicability of the biotic ligand model. Environ. Toxicol. Chem. 2020, 39, 996–1005. [Google Scholar] [CrossRef]
- Koval, E.; Olkova, A. Determination of the sensitivity of cyanobacteria to rare earth elements La and Ce. Pol. J. Environ. Stud. 2022, 31, 985–988. [Google Scholar] [CrossRef]
- Liu, R.; Wei, Z.; Dong, W.; Wang, R.; Adams, J.M.; Yang, L.; Krause, S.M.B. Unraveling the impact of lanthanum on methane consuming microbial communities in rice field soils. Front. Microbiol. 2024, 15, 1298154. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.B.; Körsten, S.; Rösken, L.M.; Cappel, F.; Beresko, C.; Ankerhold, G.; Schönleber, A.; Geimer, S.; Eckerf, D.; Wehner, S. Cyanobacterial promoted enrichment of rare earth elements europium, samarium and neodymium and intracellular europium particle formation. RSC Adv. 2019, 9, 32581. [Google Scholar] [CrossRef] [PubMed]
- Paper, M.; Koch, M.; Jung, P.; Lakatos, M.; Nilges, T.; Brück, T.B. Rare earths stick to rare cyanobacteria: Future potential for bioremediation and recovery of rare earth elements. Front. Bioeng. Biotechnol. 2023, 11, 1130939. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Cepoi, L.; Rudi, L.; Chiriac, T.; Grozdov, D.; Pavlov, S.; Djur, S. Accumulation of dysprosium, samarium, terbium, lanthanum, neodymium and ytterbium by Arthrospira platensis and their effects on biomass biochemical composition. J. Rare Earths 2021, 39, 1133–1143. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Ren, H.; Wang, Z.; Ouyang, Y.; Wang, S.; Hussain, J.; Zeb, I.; Kong, Y.; Liu, S.; et al. Identification of potassium transport proteins in algae and determination of their role under salt and saline-alkaline stress. Algal Res. 2023, 69, 102923. [Google Scholar] [CrossRef]
- Goecke, F.; Jerez, C.G.; Zachleder, V.; Figueroa, F.L.; Bišová, K.; Řezanka, T.; Vítová, M. Use of lanthanides to alleviate the effects of metal ion-deficiency in Desmodesmus quadricauda (Sphaeropleales, Chlorophyta). Front. Microbiol. 2015, 6, 2. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Rahman, M.A.; Lamb, D.; Bolan, N.S.; Saggar, S.; Surapaneni, A.; Chen, C. Rare earth elements (REE) for the removal and recovery of phosphorus: A review. Chemosphere 2022, 286, 131661. [Google Scholar] [CrossRef]
- Osterholz, H.; Simon, H.; Beck, M.; Maerz, J.; Rackebrandt, S.; Brumsack, H.-J.; Feudel, U.; Simon, M. Impact of diatom growth on trace metal dynamics (Mn, Mo, V, U). J. Sea Res. 2014, 87, 35–45. [Google Scholar] [CrossRef]
- Gauthier, M.R.; Senhorinho, G.N.A.; Scott, J.A. Microalgae under environmental stress as a source of antioxidants. Algal Res. 2020, 52, 102104. [Google Scholar] [CrossRef]
- Twining, B.S.; Baines, S.B.; Vogt, S.; Nelson, D.M. Role of diatoms in nickel biogeochemistry in the ocean. Glob. Biogeochem. Cycles 2012, 26, GB4001. [Google Scholar] [CrossRef]
- Harrison, P.J.; Yu, P.W.; Thompson, P.A.; Price, N.M.; Phillips, D.J. Survey of selenium requirements in marine phytoplankton. Mar. Ecol. Prog. Ser. 1988, 47, 89–96. [Google Scholar] [CrossRef]
- Price, N.M.; Thompson, P.A.; Harrison, P.J. Selenium: An essential element for growth of the coastal marine diatom Thalassiosira pseudonana (Bacillariophyceae). J. Phycol. 1987, 23, 1–9. [Google Scholar] [CrossRef]
- Pytlik, N.; Kaden, J.; Finger, M.; Naumann, J.; Wanke, S.; Machill, S.; Brunner, E. Biological synthesis of diatoms in synthesis of targeted novel bioparticles and biomonitoring gold nanoparticles by the diatom Stephanopyxis turris and in vivo SERS analyses. Algal Res. 2017, 28, 9–15. [Google Scholar] [CrossRef]
- Brzozowska, W.; Wojtczak, I.; Sprynskyy, M. The Use of Diatoms in the Synthesis of New 3D Micro-Nanostructured Composites (SiO2/CaCO3/Corg/NdVO4NPs and SiO2/CaO/Corg/NdVO4NPs) Exhibiting an Intense Anti-Stokes Photoluminescence. Materials 2024, 17, 490. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balycheva, D.S.; Blaginina, A.A.; Lishaev, V.N.; Kapranov, S.V.; Miroshnichenko, E.S.; Zheleznova, S.N.; Simokon, M.V.; Ryabushko, V.I. Bioaccumulation of Lanthanum by Two Strains of Marine Diatoms Nanofrustulum shiloi and Halamphora kolbei. Biology 2025, 14, 1489. https://doi.org/10.3390/biology14111489
Balycheva DS, Blaginina AA, Lishaev VN, Kapranov SV, Miroshnichenko ES, Zheleznova SN, Simokon MV, Ryabushko VI. Bioaccumulation of Lanthanum by Two Strains of Marine Diatoms Nanofrustulum shiloi and Halamphora kolbei. Biology. 2025; 14(11):1489. https://doi.org/10.3390/biology14111489
Chicago/Turabian StyleBalycheva, Daria Sergeevna, Anastasiia Andreevna Blaginina, Vyacheslav Nikolaevich Lishaev, Sergey Victorovich Kapranov, Ekaterina Sergeevna Miroshnichenko, Svetlana Nikolaevna Zheleznova, Mikhail Vitalievich Simokon, and Vitaliy Ivanovich Ryabushko. 2025. "Bioaccumulation of Lanthanum by Two Strains of Marine Diatoms Nanofrustulum shiloi and Halamphora kolbei" Biology 14, no. 11: 1489. https://doi.org/10.3390/biology14111489
APA StyleBalycheva, D. S., Blaginina, A. A., Lishaev, V. N., Kapranov, S. V., Miroshnichenko, E. S., Zheleznova, S. N., Simokon, M. V., & Ryabushko, V. I. (2025). Bioaccumulation of Lanthanum by Two Strains of Marine Diatoms Nanofrustulum shiloi and Halamphora kolbei. Biology, 14(11), 1489. https://doi.org/10.3390/biology14111489





