Isolation and Characterization of Chlorpyrifos-Degrading Gut Bacteria from Field-Collected Larvae of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Pesticides
2.3. Isolation of Chlorpyrifos-Degrading Gut Bacteria
2.4. Molecular Identification of Bacterial Isolates
2.5. Factors Affecting the Growth of Chlorpyrifos-Degrading Isolates
2.6. In Vitro Chlorpyrifos Biodegradation Assay
2.7. Screening of Biodegradation Capabilities by Chlorpyrifos-Degrading Isolates to Other Pesticides
2.8. In Vivo Chlorpyrifos Biodegradation Assay
2.8.1. Experimental Design
2.8.2. Bioassay of S. frugiperda 4th Instar Larvae
2.8.3. Biochemical Assays
Insect Treatment and Sample Preparation
Acetylcholine Esterase (AChE) Assay
Glutathione S-Transferase (GST) Assay
2.8.4. Quantification of Chlorpyrifos Residues in Larval Feces
2.8.5. Bacterial Count in Larval Feces
2.9. Data Analysis
3. Results
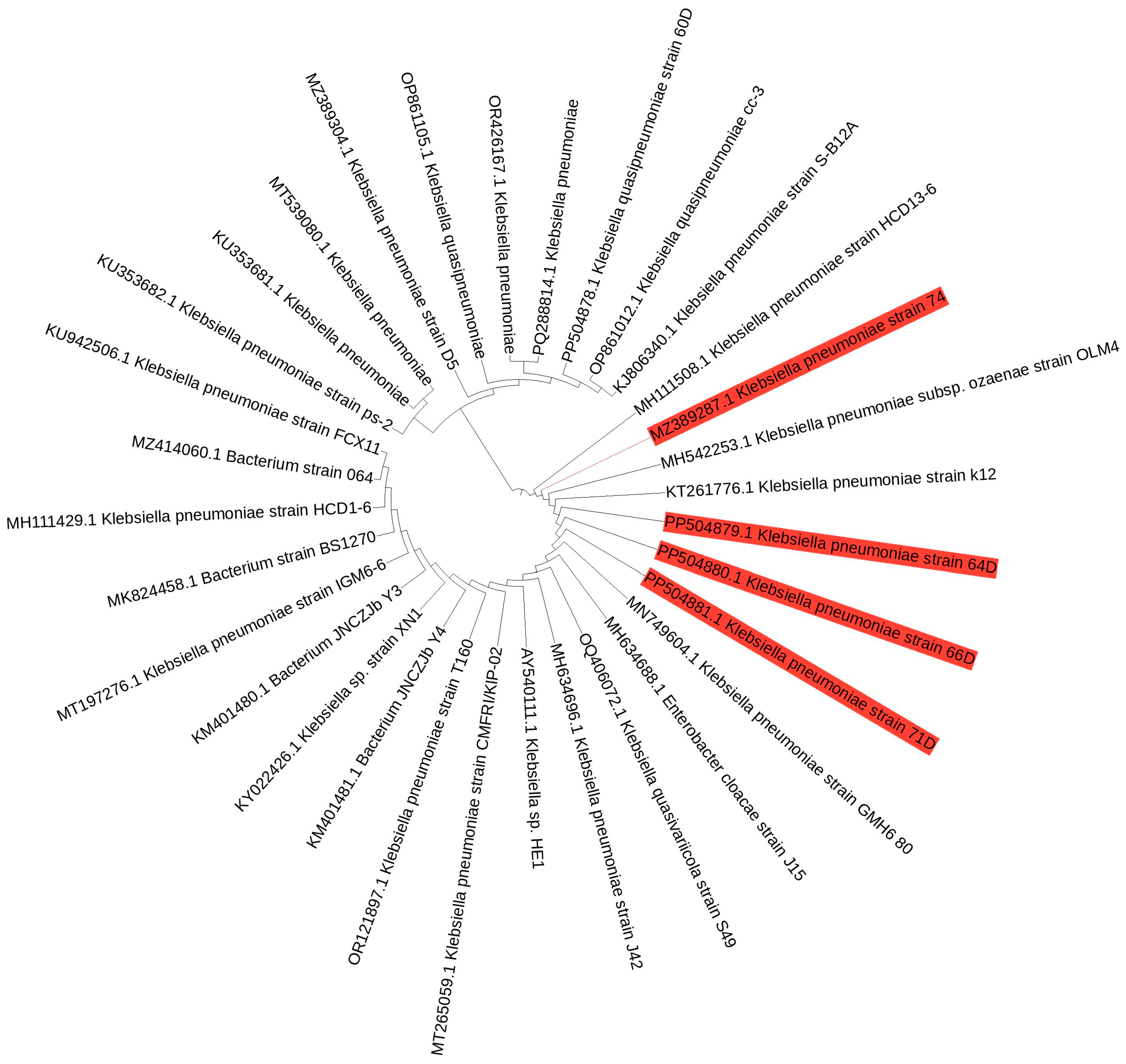
3.1. Isolation and Molecular Identification of Chlorpyrifos-Degrading Gut Bacteria
3.2. Factors Affecting the Growth of Chlorpyrifos-Degrading Isolates
3.2.1. Effect of Incubation Temperature
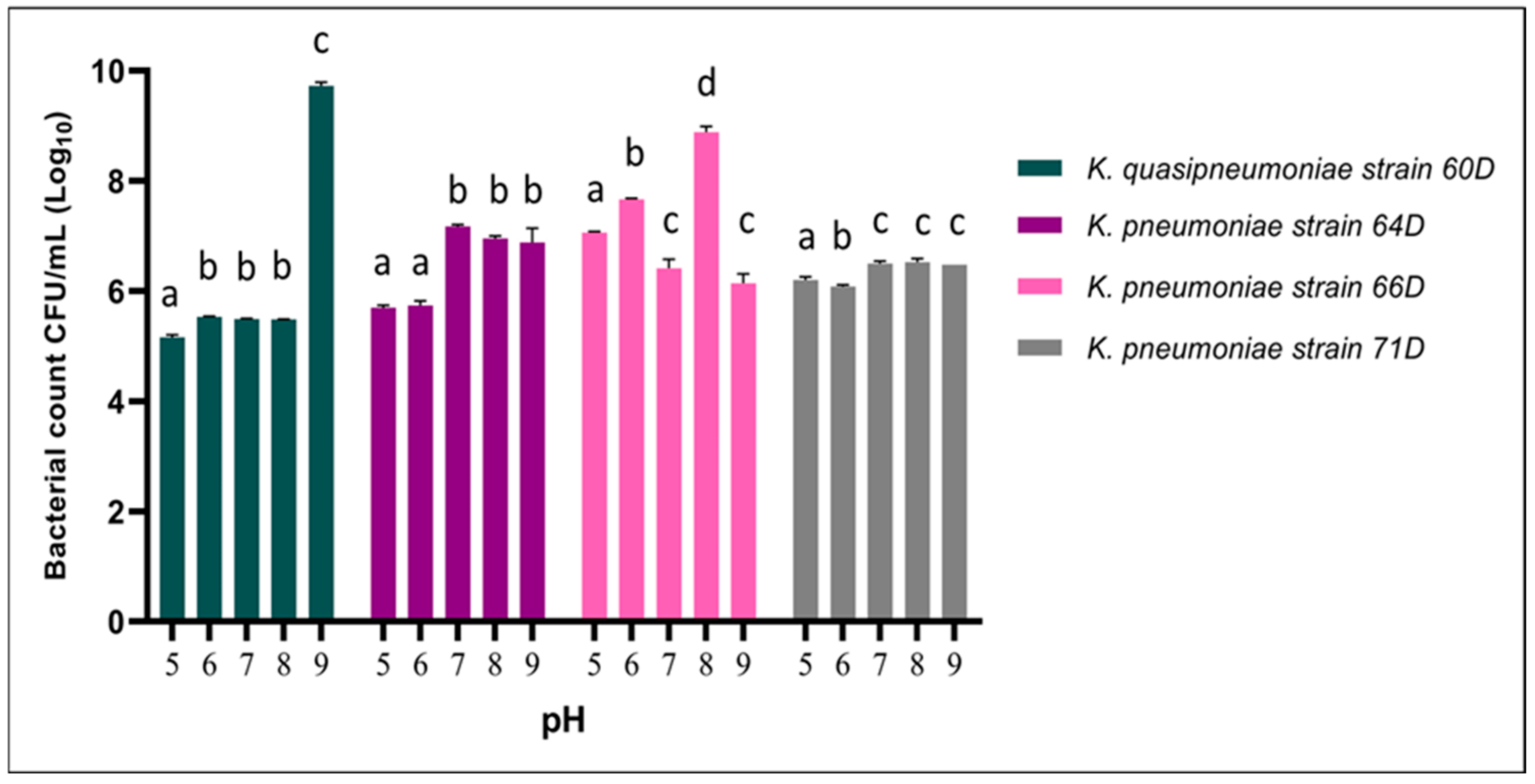
3.2.2. Effect of pH
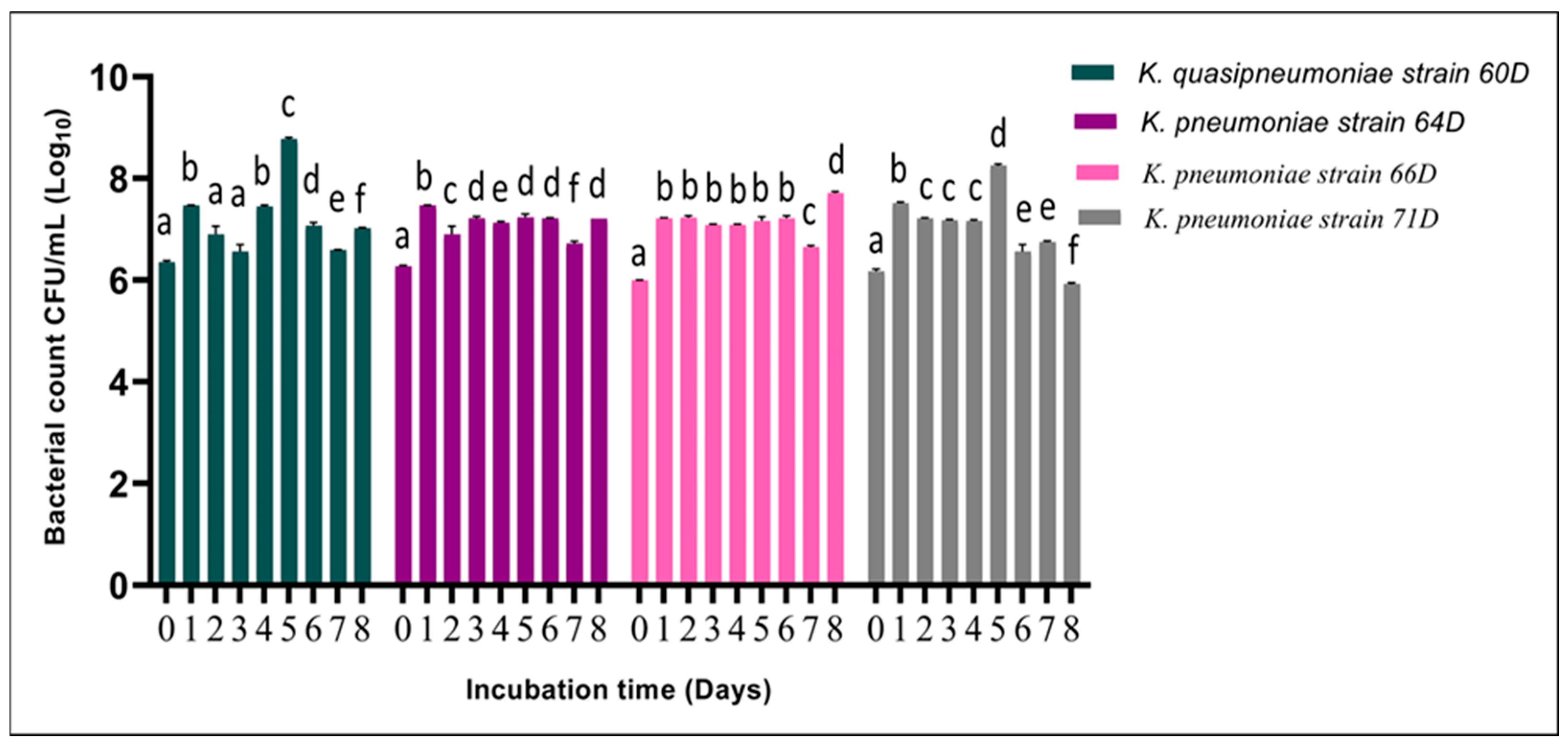
3.2.3. Effect of Incubation Time
3.3. In Vitro Insecticide Biodegradability of Isolated Gut Bacteria
3.4. Screening of Biodegradation Capabilities by Chlorpyrifos-Degrading Isolates to Other Pesticides
3.5. In Vivo Chlorpyrifos Biodegradation Assay
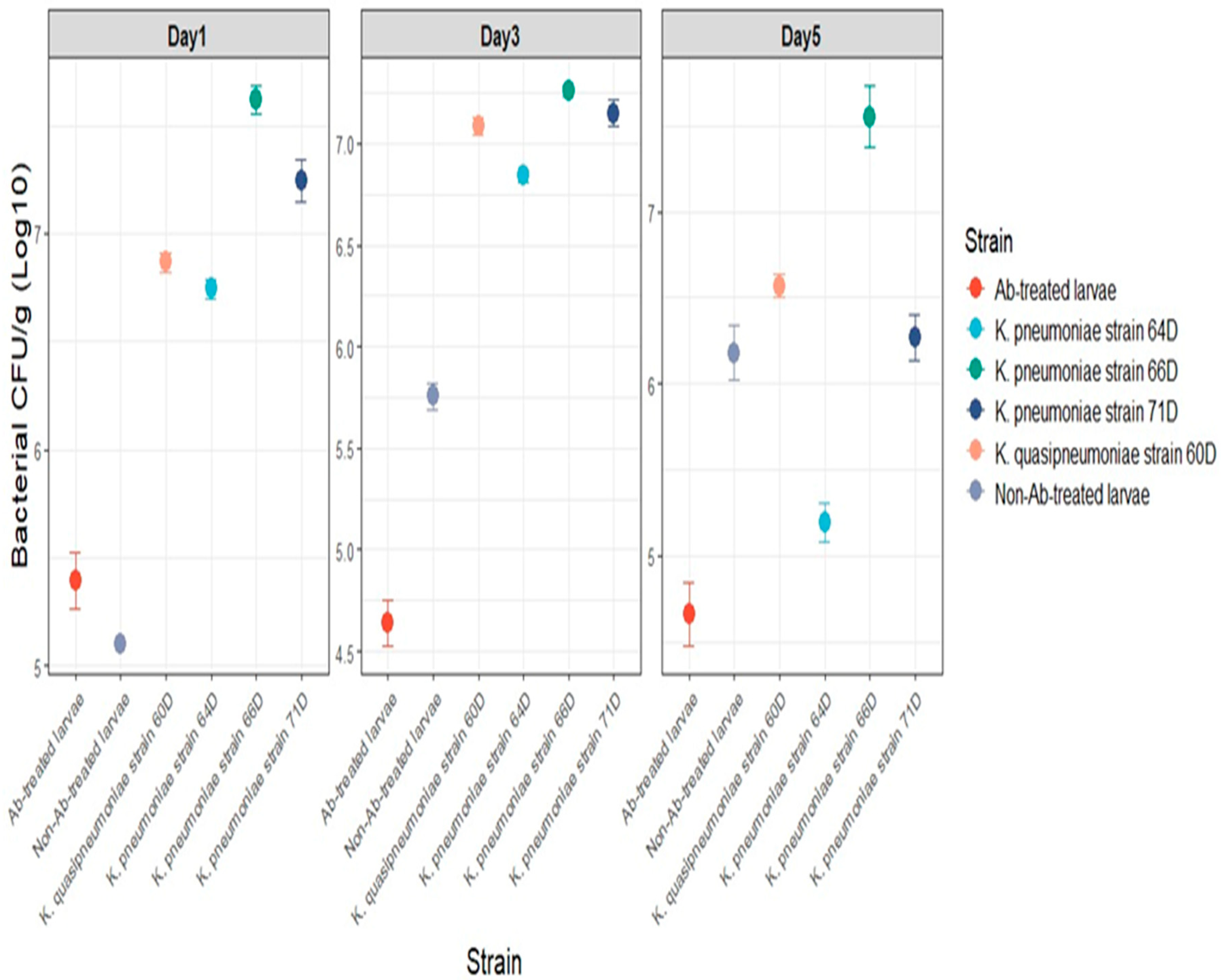
3.5.1. Bacterial Counting in Larval Gut
3.5.2. Larval Weight
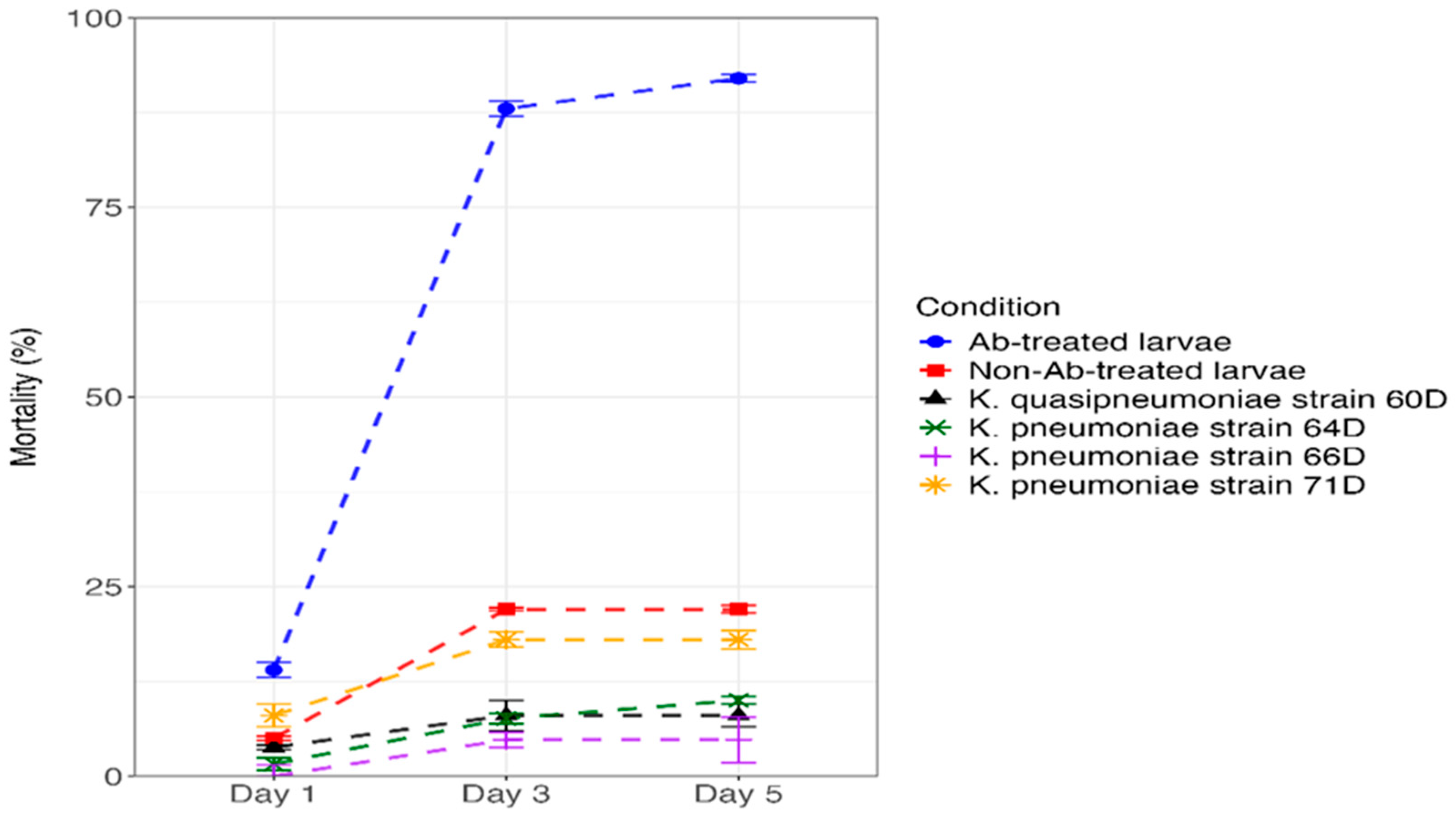
3.5.3. Mortality
3.5.4. Detoxification Enzymes Activity
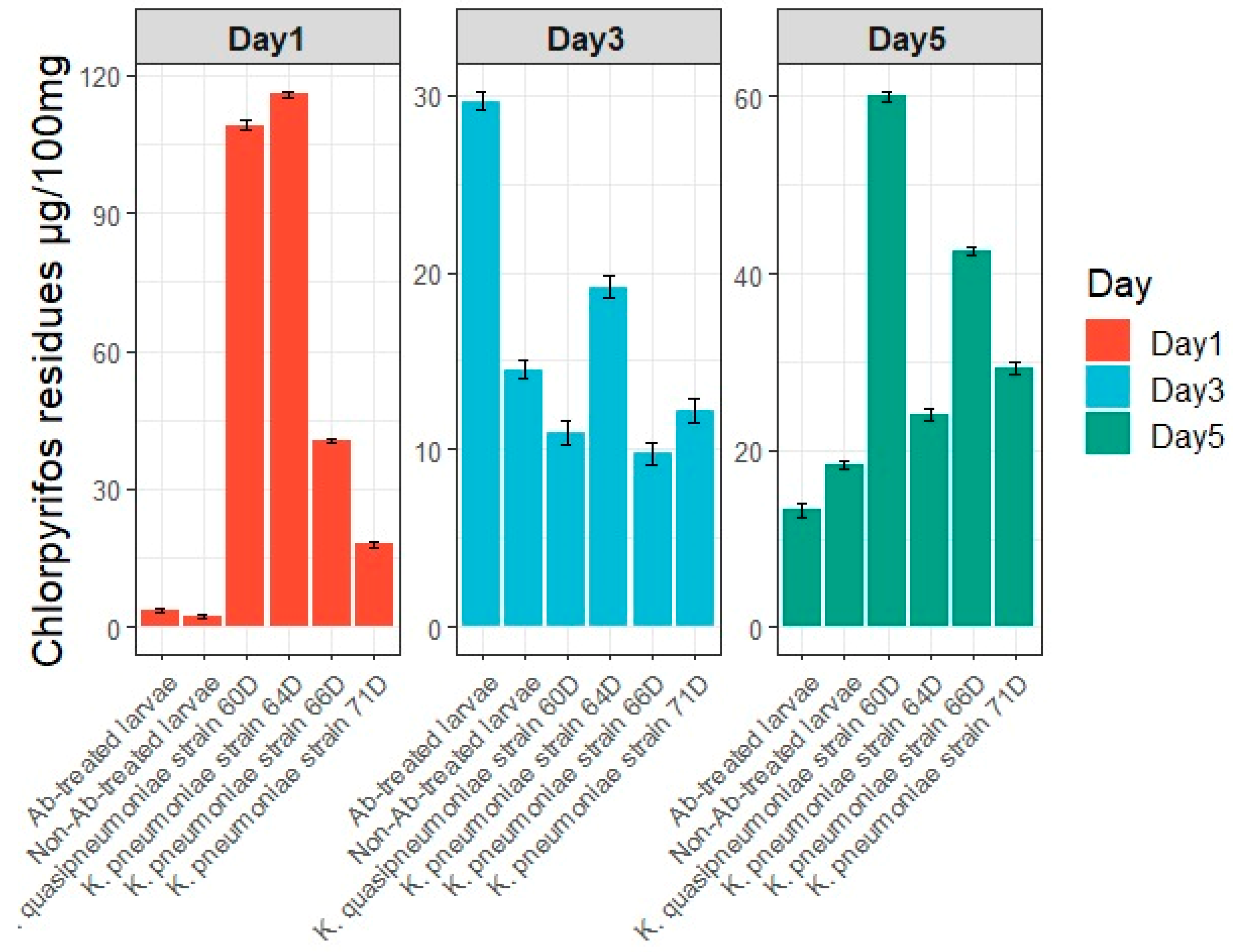
3.5.5. Pesticide Residues in Feces (Larval Frass)
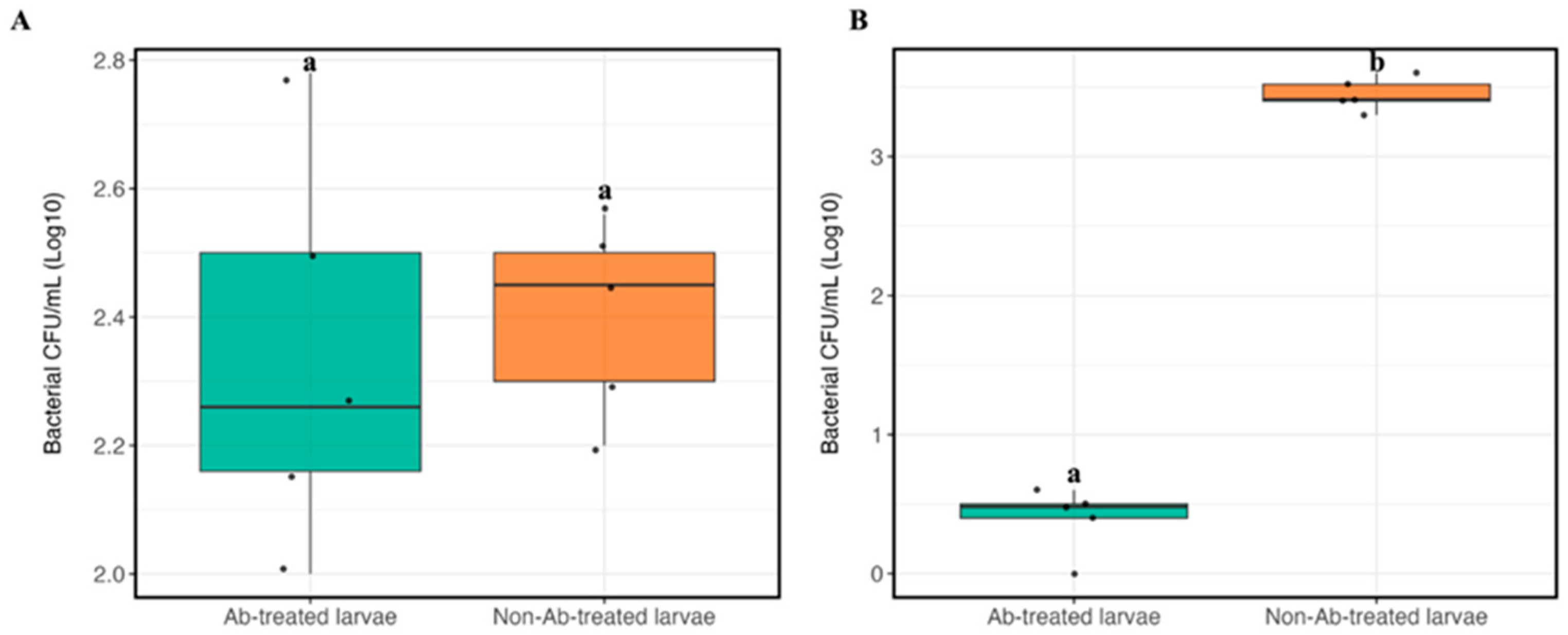
3.5.6. Bacteria Counting in Larval Feces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briefing Note on FAO Actions on Fall Armyworm. Available online: https://openknowledge.fao.org/items/a33e9a05-d913-4701-bf99-1158839be003 (accessed on 5 March 2019).
- Garlet, C.G.; Gubiani, P.d.S.; Palharini, R.B.; Moreira, R.P.; Godoy, D.N.; Farias, J.R.; Bernardi, O. Field-evolved resistance to chlorpyrifos by Spodoptera frugiperda (Lepidoptera: Noctuidae): Inheritance mode, cross-resistance patterns, and synergism. Pest Manag. Sci. 2021, 77, 5367–5374. [Google Scholar] [CrossRef]
- Smegal, D.C. Human Health Risk Assessment Chlorpyrifos; US Environmental Protection Agency, Office of Prevention, Pesticides and Toxic Substances, Office of Pesticide Programs, Health Effects Division, US Government Printing Office: Washington, DC, USA, 2000; pp. 1–131. [Google Scholar]
- Simma, E.A.; Dermauw, W.; Balabanidou, V.; Snoeck, S.; Bryon, A.; Clark, R.M.; Yewhalaw, D.; Vontas, J.; Duchateau, L.; Van Leeuwen, T. Genome-wide gene expression profiling reveals that cuticle alterations and P450 detoxification are associated with deltamethrin and DDT resistance in Anopheles arabiensis populations from Ethiopia. Pest Manag. Sci. 2019, 75, 1808–1818. [Google Scholar] [CrossRef]
- Khan, H.A.A. Characterization of permethrin resistance in a Musca domestica strain: Resistance development, cross-resistance potential and realized heritability. Pest Manag. Sci. 2019, 75, 2969–2974. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, T.; Venkatesan, T.; Prabhu, D.; Jeyakanthan, J.; Gracy, G.R.; Jalali, S.K.; Rai, A. Insecticide-resistance mechanism of Plutella xylostella (L.) associated with amino acid substitutions in acetylcholinesterase-1: A molecular docking and molecular dynamics investigation. Comput. Biol. Chem. 2018, 77, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Balabanidou, V.; Grigoraki, L.; Vontas, J. Insect cuticle: A critical determinant of insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 68–74. [Google Scholar] [CrossRef]
- Peterson, B.F. Microbiome toxicology—Bacterial activation and detoxification of insecticidal compounds. Curr. Opin. Insect Sci. 2024, 63, 101192. [Google Scholar] [CrossRef] [PubMed]
- Engel, P.; Moran, N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef]
- Xia, X.; Sun, B.; Gurr, G.M.; Vasseur, L.; Xue, M.; You, M. Gut microbiota mediate insecticide resistance in the diamondback moth, Plutella xylostella (L.). Front. Microbiol. 2018, 9, 25. [Google Scholar] [CrossRef]
- Malook Su Arora, A.K.; Wong, A.C.N. The role of microbiomes in shaping insecticide resistance: Current insights and emerging paradigms. Curr. Opin. Insect Sci. 2025, 69, 101346. [Google Scholar] [CrossRef] [PubMed]
- ElKraly, O.A.; Awad, M.; El-Saadany, H.M.; Hassanein, S.E.; Elrahman, T.A.; Elnagdy, S.M. Impact of gut microbiota composition on black cutworm, Agrotis ipsilon (hufnagel) metabolic indices and pesticide degradation. Anim. Microbiome 2023, 5, 44. [Google Scholar] [CrossRef]
- Gomes, A.F.F.; Omoto, C.; Cônsoli, F.L. Gut bacteria of field-collected larvae of Spodoptera frugiperda undergo selection and are more diverse and active in metabolizing multiple insecticides than laboratory-selected resistant strains. J. Pest Sci. 2020, 93, 833–851. [Google Scholar] [CrossRef]
- Chen, B.; Zhang, N.; Xie, S.; Zhang, X.; He, J.; Muhammad, A.; Sun, C.; Lu, X.; Shao, Y. Gut bacteria of the silkworm Bombyx mori facilitate host resistance against the toxic effects of organophosphate insecticides. Environ. Int. 2020, 143, 105886. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Hayatsu, M.; Hosokawa, T.; Nagayama, A.; Tago, K.; Fukatsu, T. Symbiont-mediated insecticide resistance. Proc. Natl. Acad. Sci. USA 2012, 109, 8618–8622. [Google Scholar] [CrossRef]
- Mason, C.J.; Peiffer, M.; Hoover, K.; Felton, G. Tomato chemical defenses intensify corn earworm (Helicoverpa zea) mortality from opportunistic bacterial pathogens. J. Chem. Ecol. 2023, 49, 313–324. [Google Scholar] [CrossRef]
- Mason, C.J.; Peiffer, M.; Felton, G.W.; Hoover, K. Host-Specific larval lepidopteran mortality to pathogenic Serratia mediated by poor diet. J. Invertebr. Pathol. 2022, 194, 107818. [Google Scholar] [CrossRef]
- Itoh, H.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Detoxifying symbiosis: Microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 2018, 35, 434–454. [Google Scholar] [CrossRef]
- Akami, M.; Njintang, N.Y.; Gbaye, O.A.; Andongma, A.A.; Rashid, M.A.; Niu, C.-Y.; Nukenine, E.N. Gut bacteria of the cowpea beetle mediate its resistance to dichlorvos and susceptibility to Lippia adoensis essential oil. Sci. Rep. 2019, 9, 6435. [Google Scholar] [CrossRef]
- Shen, S.; Dowd, P. Detoxification spectrum of the cigarette beetle symbiont Symbiotaphrina kochii in culture. Entomol. Exp. Appl. 1991, 60, 51–59. [Google Scholar] [CrossRef]
- Cheng, T.; Wu, J.; Wu, Y.; Chilukuri, R.V.; Huang, L.; Yamamoto, K.; Feng, L.; Li, W.; Chen, Z.; Guo, H. Genomic adaptation to polyphagy and insecticides in a major East Asian noctuid pest. Nat. Ecol. Evol. 2017, 1, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.G.D.; Moraes, L.A.B.D.; Trigo, J.R.; Omoto, C.; Consoli, F.L. The gut microbiota of insecticide-resistant insects houses insecticide-degrading bacteria: A potential source for biotechnological exploitation. PLoS ONE 2017, 12, e0174754. [Google Scholar] [CrossRef]
- Ramya, S.L.; Venkatesan, T.; Srinivasa Murthy, K.; Jalali, S.K.; Verghese, A. Detection of carboxylesterase and esterase activity in culturable gut bacterial flora isolated from diamondback moth, Plutella xylostella (Linnaeus), from India and its possible role in indoxacarb degradation. Braz. J. Microbiol. 2016, 47, 327–336. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Fan, R.; Liu, Y.; Syed, A.H.; Benlin, Y.; Chu, Q.; Ding, Z.; Ghani, M.I.; Liu, X.; Wakil, W.; et al. The larval gut of Spodoptera frugiperda harbours culturable bacteria with metabolic versatility after insecticide exposure. Insect Mol. Biol. 2025, 34, 452–469. [Google Scholar] [CrossRef]
- Adams, N.H.; Umar, R.; Ishaya, S.; Nweke, O.D.; Ilyasu, N.S.; Jagaba, A.H.; Usman, S.; Yakasai, H.M. Chlorpyrifos degradation by Bacillus sp. strain UPMB10 isolated from polluted environment: Analysis and characterization of the metabolite by GC-MS. Case Stud. Chem. Environ. Eng. 2024, 9, 100608. [Google Scholar] [CrossRef]
- Turner, S.; Pryer, K.M.; Miao, V.P.; Palmer, J.D. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis 1. J. Eukaryot. Microbiol. 1999, 46, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Lane, D. 16S/23S rRNA sequencing. In Nucleic acid Techniques in Bacterial Systematics; Wiley: Chichester, UK, 1991; pp. 125–175. [Google Scholar]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Abdel Ghani, S.B.; Hanafi, A.H. QuEChERS method combined with GC-MS for pesticide residues determination in water. J. Anal. Chem. 2016, 71, 508–512. [Google Scholar] [CrossRef]
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X.; Li, C. Evaluation of seven chemical pesticides by mixed microbial culture (PCS-1): Degradation ability, microbial community, and Medicago sativa phytotoxicity. J. Hazard. Mater. 2020, 389, 121834. [Google Scholar] [CrossRef]
- Moustafa, M.A.; Fouad, E.A.; Ibrahim, E.; Erdei, A.L.; Kárpáti, Z.; Fónagy, A. The comparative toxicity, biochemical and physiological impacts of chlorantraniliprole and indoxacarb on Mamestra brassicae (Lepidoptera: Noctuidae). Toxics 2023, 11, 212. [Google Scholar] [CrossRef]
- Moustafa, M.A.; Fouad, E.A.; Abdel-Mobdy, Y.; Hamow, K.Á.; Mikó, Z.; Molnár, B.P.; Fónagy, A. Toxicity and sublethal effects of chlorantraniliprole and indoxacarb on Spodoptera littoralis (Lepidoptera: Noctuidae). Appl. Entomol. Zool. 2021, 56, 115–124. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Moustafa, M.A.; El-Said, N.A.; Ahmed, F.S.; Amer, A.; Awad, M.; Alfuhaid, N.A. In vitro and silico exploration of the insecticidal properties of Lavandula multifida L. essential oil and its binary combinations with cyantraniliprole and emamectin benzoate on Spodoptera frugiperda (Lepidoptera: Noctuidae). Crop Prot. 2025, 187, 106969. [Google Scholar] [CrossRef]
- Awad, M.; El Kenawy, A.H.; Alfuhaid, N.A.; Ibrahim, E.-D.S.; Jósvai, J.K.; Fónagy, A.; Moustafa, M.A. Lethal and sublethal effects of cyantraniliprole on the Biology and Metabolic enzyme activities of two lepidopteran Pests, Spodoptera littoralis and Agrotis ipsilon, and A generalist predator, Chrysoperla carnea (Neuroptera: Chrysopidae). Insects 2024, 15, 450. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.; Pabst, M.J.; Jakoby, W. The first enzymatic step in mercapturic acid formation. Glutathione-S-transferase. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Lalander, C.; Senecal, J.; Calvo, M.G.; Ahrens, L.; Josefsson, S.; Wiberg, K.; Vinnerås, B. Fate of pharmaceuticals and pesticides in fly larvae composting. Sci. Total Environ. 2016, 565, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Schwantes, D.; Celso Gonçalves, A., Jr.; Conradi Junior, É.; Campagnolo, M.A.; Zimmermann, J. Determination of CHLORPYRIFOS by GC/ECD in water and its sorption mechanism study in a RHODIC FERRALSOL. J. Environ. Health Sci. Eng. 2020, 18, 149–162. [Google Scholar] [CrossRef]
- Wirsching, J.; Pagel, H.; Ditterich, F.; Uksa, M.; Werneburg, M.; Zwiener, C.; Berner, D.; Kandeler, E.; Poll, C. Biodegradation of pesticides at the limit: Kinetics and microbial substrate use at low concentrations. Front. Microbiol. 2020, 11, 2107. [Google Scholar] [CrossRef]
- Douglas, A.E. Multiorganismal insects: Diversity and function of resident microorganisms. Annu. Rev. Entomol. 2015, 60, 17–34. [Google Scholar] [CrossRef]
- Lü, D.; Dong, Y.; Yan, Z.; Liu, X.; Zhang, Y.; Yang, D.; He, K.; Wang, Z.; Wang, P.; Yuan, X. Dynamics of gut microflora across the life cycle of Spodoptera frugiperda and its effects on the feeding and growth of larvae. Pest Manag. Sci. 2023, 79, 173–182. [Google Scholar] [CrossRef]
- Diez-Rodríguez, G.I.; Omoto, C. Inheritance of lambda-cyhalothrin resistance in Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae). Neotrop. Entomol. 2001, 30, 311–316. [Google Scholar] [CrossRef]
- Yu, S.; Nguyen, S.; Abo-Elghar, G. Biochemical characteristics of insecticide resistance in the fall armyworm, Spodoptera frugiperda (JE Smith). Pestic. Biochem. Physiol. 2003, 77, 1–11. [Google Scholar] [CrossRef]
- Elshikh, M.S.; Alarjani, K.M.; Huessien, D.S.; Elnahas, H.A.; Esther, A.R. Enhanced Biodegradation of Chlorpyrifos by Bacillus cereus CP6 and Klebsiella pneumoniae CP19 from municipal waste water. Environ. Res. 2022, 205, 112438. [Google Scholar] [CrossRef]
- Maya, K.; Singh, R.; Upadhyay, S.; Dubey, S.K. Kinetic analysis reveals bacterial efficacy for biodegradation of chlorpyrifos and its hydrolyzing metabolite TCP. Process Biochem. 2011, 46, 2130–2136. [Google Scholar] [CrossRef]
- Vidya Lakshmi, C.; Kumar, M.; Khanna, S. Biodegradation of chlorpyrifos in soil by enriched cultures. Curr. Microbiol. 2009, 58, 35–38. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Lu, Y. Biodegradation of insecticides by gut bacteria isolated from stored grain beetles and its implication in host insecticide resistance. J. Stored Prod. Res. 2022, 96, 101943. [Google Scholar] [CrossRef]
- Akbar, S.; Sultan, S. Soil bacteria showing a potential of chlorpyrifos degradation and plant growth enhancement. Braz. J. Microbiol. 2016, 47, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Pietri, J.E.; Liang, D. The links between insect symbionts and insecticide resistance: Causal relationships and physiological tradeoffs. Ann. Entomol. Soc. Am. 2018, 111, 92–97. [Google Scholar] [CrossRef]
- Liu, X.-D.; Guo, H.-F. Importance of endosymbionts Wolbachia and Rickettsia in insect resistance development. Curr. Opin. Insect Sci. 2019, 33, 84–90. [Google Scholar] [CrossRef]
- Greer, J.B.; Magnuson, J.T.; Hester, K.; Giroux, M.; Pope, C.; Anderson, T.; Liu, J.; Dang, V.; Denslow, N.D.; Schlenk, D. Effects of chlorpyrifos on cholinesterase and serine lipase activities and lipid metabolism in brains of rainbow trout (Oncorhynchus mykiss). Toxicol. Sci. 2019, 172, 146–154. [Google Scholar] [CrossRef]
- Xiao, T.; Lu, K. Functional characterization of CYP6AE subfamily P450s associated with pyrethroid detoxification in Spodoptera litura. Int. J. Biol. Macromol. 2022, 219, 452–462. [Google Scholar] [CrossRef]
- Singh, B.K.; Walker, A. Microbial degradation of organophosphorus compounds. FEMS Microbiol. Rev. 2006, 30, 428–471. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cao, Y.; Rose, R.L.; Brimfield, A.A.; Dai, D.; Goldstein, J.A.; Hodgson, E. Metabolism of chlorpyrifos by human cytochrome P450 isoforms and human, mouse, and rat liver microsomes. Drug Metab. Dispos. 2001, 29, 1201–1204. [Google Scholar] [PubMed]
- Slotkin, T.A. Guidelines for developmental neurotoxicity and their impact on organophosphate pesticides: A personal view from an academic perspective. Neurotoxicology 2004, 25, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.; Ranjbar, A.; Shadnia, S.; Nikfar, S.; Rezaie, A. Pesticides and oxidative stress: A review. Med. Sci. Monit. 2004, 10, 141–147. [Google Scholar]


| Groups | 1 Day | 3 Days | 5 Days |
|---|---|---|---|
| Klebsiella quasipneumoniae strain 60D | 77.9 ± 0.91 b | 45.13 ± 1.94 b | 57.94 ± 1.81 ab |
| Klebsiella pneumoniae strain 64D | 80.38 ± 0.68 a | 69.8 ± 0.32 a | 25 ± 1.82 c |
| Klebsiella pneumoniae strain 66D | 51.3 ± 0.15 d | 46.93 ± 1.64 b | 61.14 ± 2.12 a |
| Klebsiella pneumoniae strain 71D | 65.52 ± 0.74 c | 50.15 ± 2.06 b | 53.08 ± 1.82 b |
| Strain/Pesticide | Chlorantraniliprole (Coragen) 0.6 ppm | Carbamate (Lannate) 0.5 ppm | Pyrethroid (Lambda-Cyhalothrin) 5 ppm |
|---|---|---|---|
| K. quasipneumoniae 60D | Positive | Positive | Positive |
| K. pneumoniae 64D | Positive | Positive | Negative |
| K. pneumoniae 66D | Positive | Positive | Negative |
| K. pneumoniae 71D | Positive | Positive | Negative |
| Antibiotic-Treated (mg) | Non-Antibiotic-Treated (mg) | t-Test | p-Value | |
|---|---|---|---|---|
| Larval weight (mg) | 8.57 ± 1.89 | 12.73 ± 0.47 | −3.02 | 0.039 |
| Enzyme | Groups | 1 Day | 3 Days | 5 Days |
|---|---|---|---|---|
| AchE(Mmole/mg of protein) | Ab-treated larva | 0.74 ± 0.12 a | 0.61 ± 0.02 a | 0.43 ± 0.05 a |
| Non-Ab-treated larva | 0.32 ± 0.05 cd | 0.43 ± 0.07 a | 0.36 ± 0.03 a | |
| Klebsiella quasipneumoniae strain 60D | 0.21 ± 0.06 d | 0.48 ± 0.06 a | 0.42 ± 0.01 a | |
| Klebsiella pneumoniae strain 64D | 0.25 ± 0.05 cd | 0.58 ± 0.08 a | 0.28 ± 0.08 a | |
| Klebsiella pneumoniae strain 66D | 0.58 ± 0.06 ab | 0.61 ± 0.03 a | 0.46 ± 0.09 a | |
| Klebsiella pneumoniae strain 71D | 0.41 ± 0.03 bcd | 0.66 ± 0.03 a | 0.26 ± 0.02 a | |
| P-Value | 0.001 | 0.171 | 0.020 | |
| F-Value(df) | 14.54(6) | 1.80(6) | 3.71(6) | |
| GST (Mmole/mg of protein) | Ab-treated larva | 0.44 ± 0.25 a | 0.38 ± 0.07 b | 0.87 ± 0.07 a |
| Non-Ab-treated larva | 0.3 ± 0.05 a | 0.79 ± 0.04 a | 0.46 ± 0.03 b | |
| Klebsiella quasipneumoniae strain 60D | 0.2 ± 0.03 a | 0.24 ± 0.08 b | 0.39 ± 0.03 b | |
| Klebsiella pneumoniae strain 64D | 0.28 ± 0.01 a | 0.45 ± 0.09 b | 0.47 ± 0.16 b | |
| Klebsiella pneumoniae strain 66D | 0.41 ± 0.14 a | 0.35 ± 0.05 b | 0.29 ± 0.13 b | |
| Klebsiella pneumoniae strain 71D | 0.35 ± 0.07 a | 0.8 ± 0.12 a | 0.3 ± 0.11 b | |
| P-Value | 0.479 | 0.001 | 0.001 | |
| F-Value(df) | 0.97(6) | 10.96(6) | 8.94(6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Zayat, A.S.; Ahmed, M.N.; Sofy, M.; El-Hefny, D.E.; Alfuhaid, N.A.; El-Sayed, D.; Fathy, H.M.; Awad, M. Isolation and Characterization of Chlorpyrifos-Degrading Gut Bacteria from Field-Collected Larvae of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Biology 2025, 14, 1468. https://doi.org/10.3390/biology14111468
El-Zayat AS, Ahmed MN, Sofy M, El-Hefny DE, Alfuhaid NA, El-Sayed D, Fathy HM, Awad M. Isolation and Characterization of Chlorpyrifos-Degrading Gut Bacteria from Field-Collected Larvae of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Biology. 2025; 14(11):1468. https://doi.org/10.3390/biology14111468
Chicago/Turabian StyleEl-Zayat, Ayatollah S., Marwa N. Ahmed, Manar Sofy, Dalia E. El-Hefny, Nawal A. Alfuhaid, Dina El-Sayed, Hayam M. Fathy, and Mona Awad. 2025. "Isolation and Characterization of Chlorpyrifos-Degrading Gut Bacteria from Field-Collected Larvae of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae)" Biology 14, no. 11: 1468. https://doi.org/10.3390/biology14111468
APA StyleEl-Zayat, A. S., Ahmed, M. N., Sofy, M., El-Hefny, D. E., Alfuhaid, N. A., El-Sayed, D., Fathy, H. M., & Awad, M. (2025). Isolation and Characterization of Chlorpyrifos-Degrading Gut Bacteria from Field-Collected Larvae of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Biology, 14(11), 1468. https://doi.org/10.3390/biology14111468





