Effects of Biochar and PGPR Application on the Physicochemical Properties and Humus Components of Soil Used for Planting Fruit Mulberry Seedlings Under Salt Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Selection and Identification
2.2. Basic Soil and Plant Materials
2.3. Experimental Design
2.4. Data Collection and Determinations
2.5. Statistical Analyses
3. Results
3.1. Soil Physical Properties
3.2. Basic Chemical Properties in Rhizosphere Soil
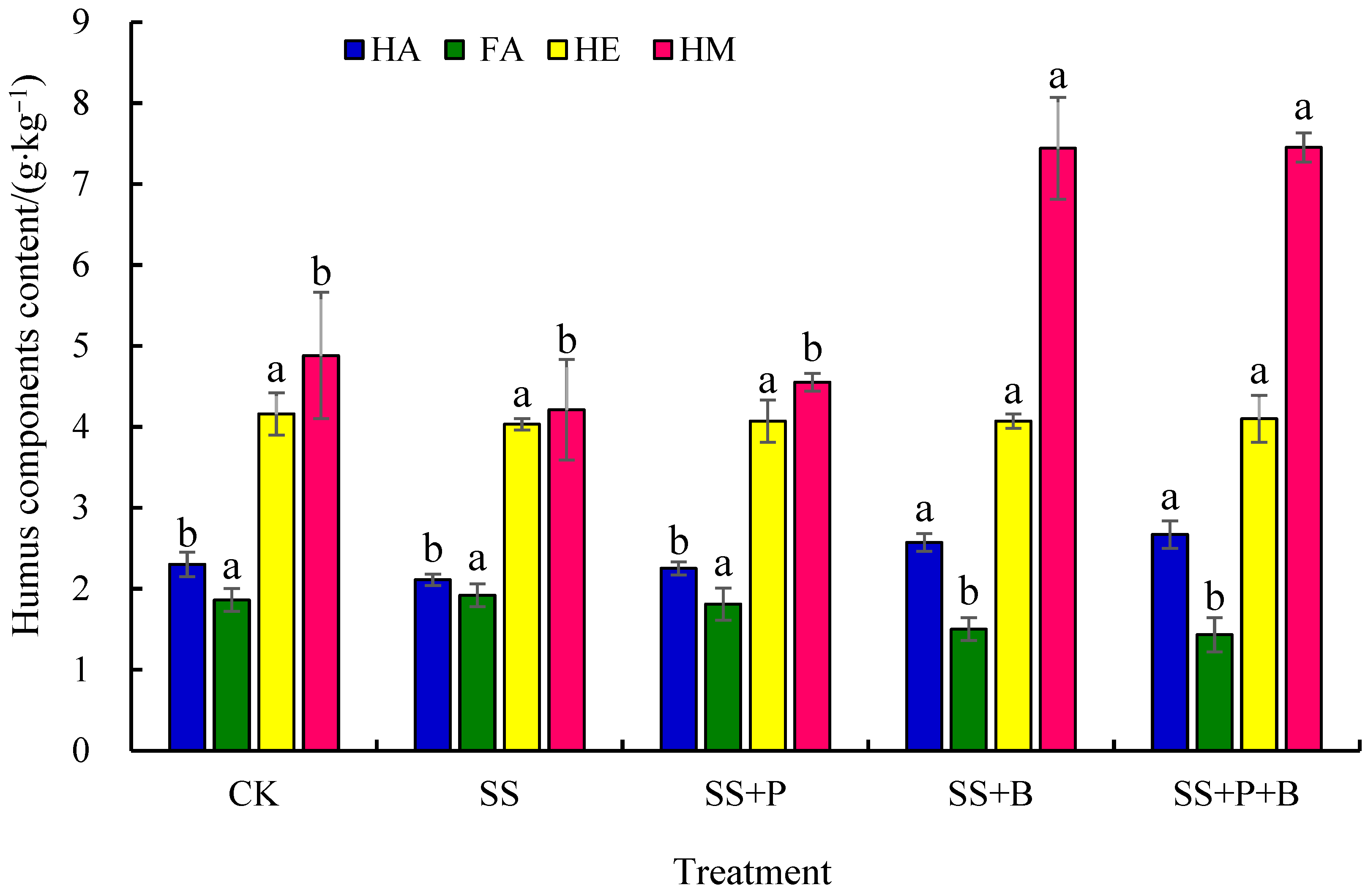
3.3. Humus Components in Rhizosphere Soil
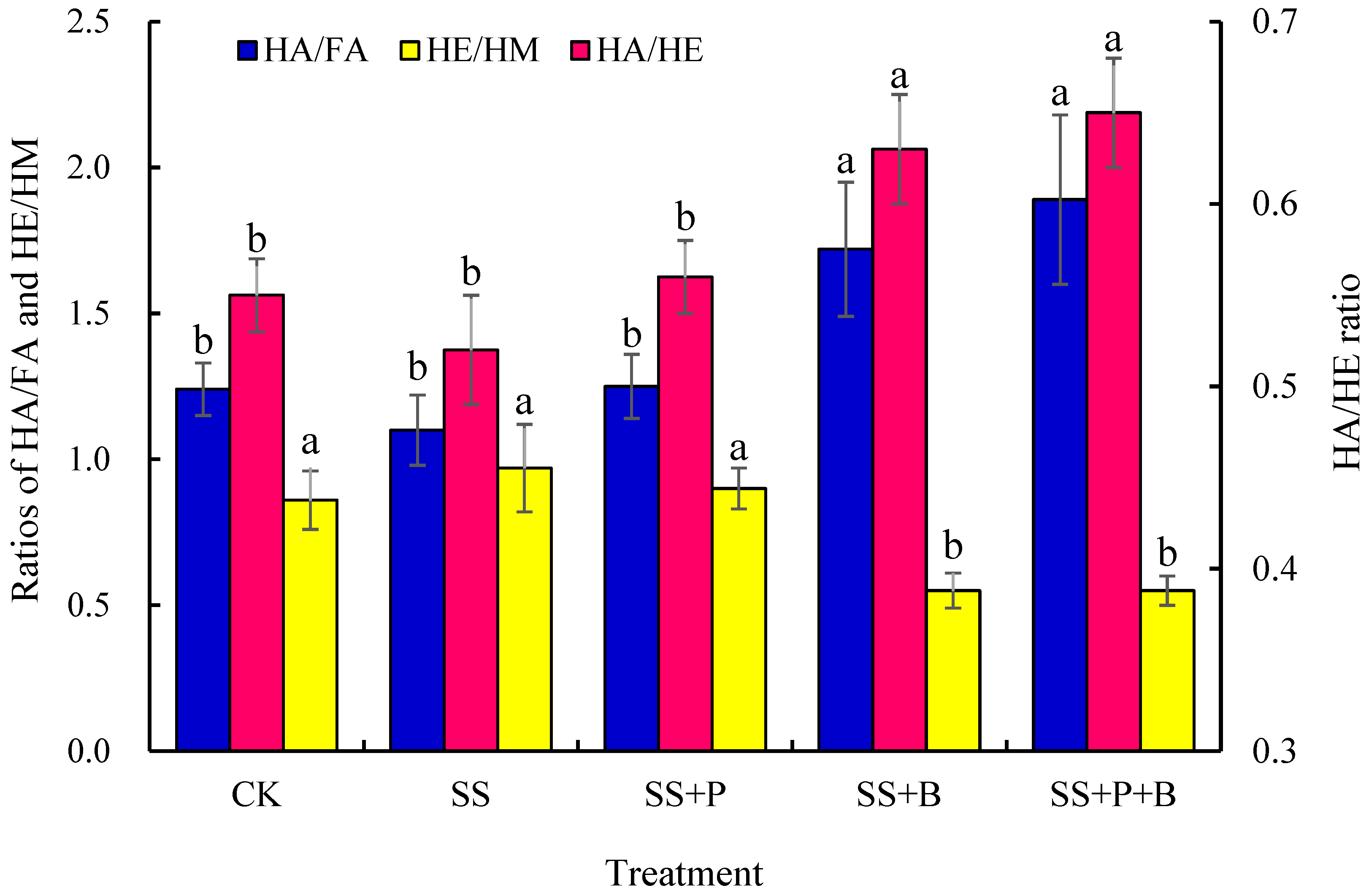
3.4. Humus Stability in Rhizosphere Soil
3.5. Rhizosphere Soil Enzyme Activity
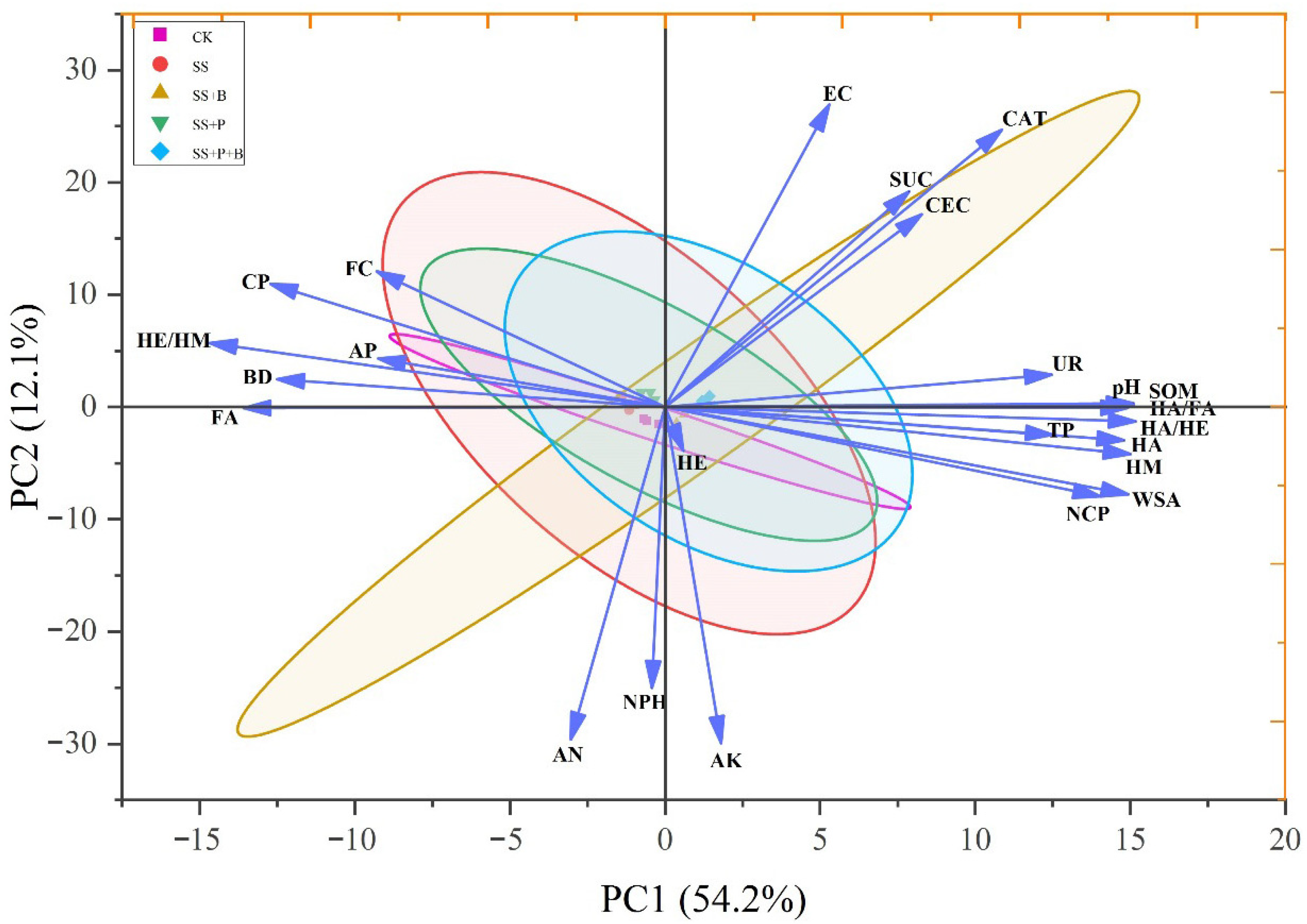
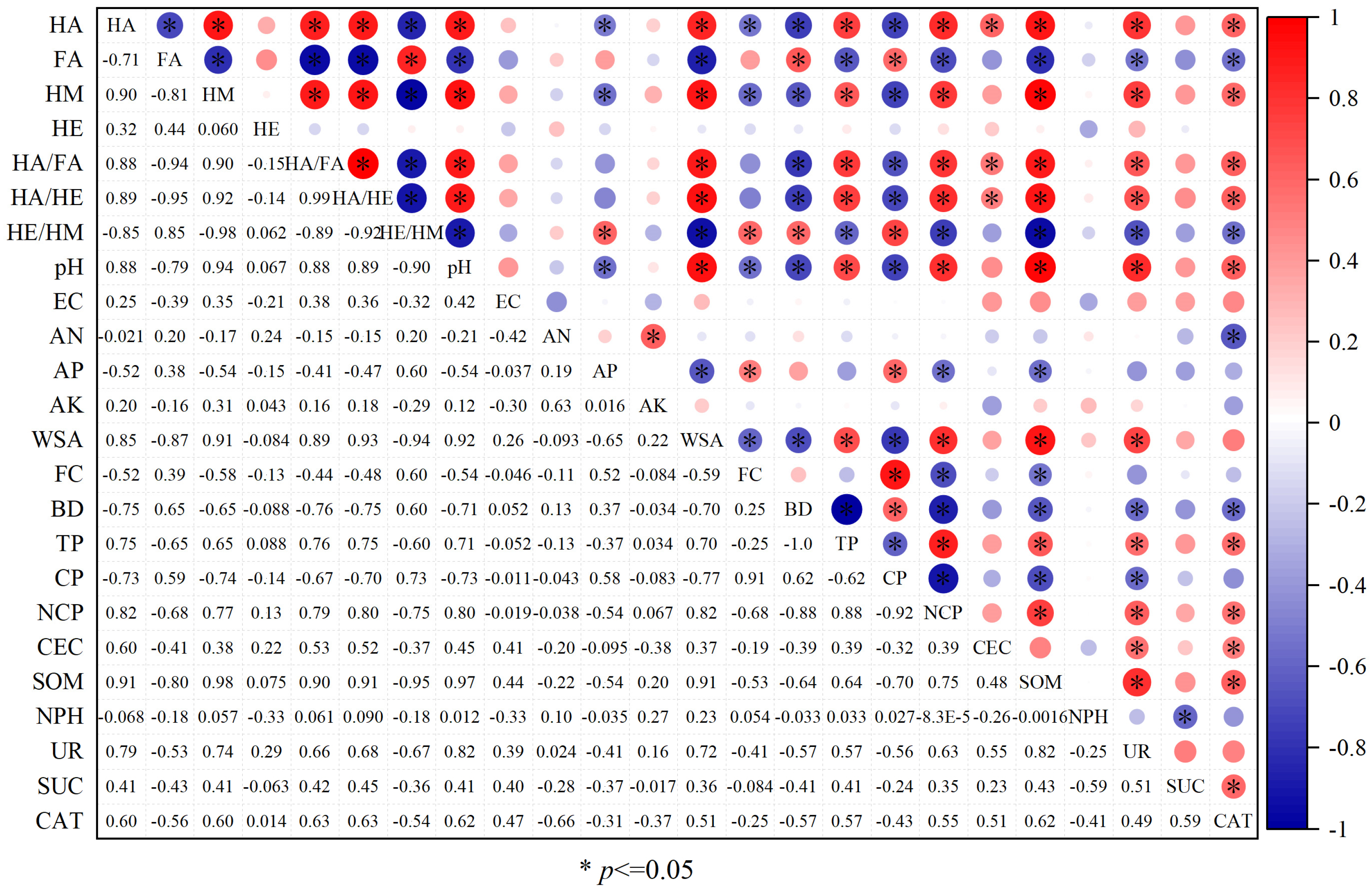
3.6. Relationship Between Soil Physicochemical Properties and Humus Components
4. Discussion
4.1. Soil Physical and Chemical Properties
4.2. Humus Components and Stability in Rhizosphere Soil
4.3. Enzyme Activity in Rhizosphere Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, J.S. Development and prospect of the research on salt-affected soils in China. Acta Pedol. Sin. 2008, 45, 837–845. [Google Scholar]
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent Food Agric. 2016, 2, 1127500. [Google Scholar] [CrossRef]
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Lavakush, S.; Singh, V. Impact of plant growth promoting rhizobacteria on crop production. Int. J. Agric. Res. 2010, 5, 954–983. [Google Scholar] [CrossRef]
- Kong, Z.Y.; Liu, H.G. Modification of rhizosphere microbial communities: A possible mechanism of plant growth promoting rhizobacteria enhancing plant growth and fitness. Front. Plant Sci. 2022, 13, 920813. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.Y.; Suo, S.Z.; Shao, K.Z.; Zhao, Q.; Yao, D.; Li, H.P.; Zhang, J.L.; Rensing, C. Biofertilizers with beneficial rhizobacteria improved plant growth and yield in chili (Capsicum annuum L.). World J. Microbiol. Biotechnol. 2020, 36, 86. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent advances in bacterial amelioration of plant drought and salt stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- El-Amriti, F.A.; Ouf, S.A.; Abu-Elghait, M.; Desouky, S.E.; Mohamed, M.S.M. Alleviation of salt stress on Zea mays L. plant by PGPR isolates as an effective sustainable strategy. Biocatal. Agric. Biotechnol. 2024, 61, 103346. [Google Scholar] [CrossRef]
- Ullah, S.; Ikram, M.; Sarfaraz, S.; Haq, I.U.; Khan, A.; Murad, Z.; Munsif, F. Influence of plant growth promoting rhizobacteria (PGPR) on the growth and yield of sunflower (Helianthus annus L.) under salt stress. J. Crop Health 2024, 76, 1221–1234. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.Q.; Du, B.H.; Li, H.H. Effect of biochar applied with plant growth-promoting rhizobacteria (PGPR) on soil microbial community composition and nitrogen utilization in tomato. Pedosphere 2021, 31, 872–881. [Google Scholar] [CrossRef]
- Albareda, M.; Rodríguez-Navarro, D.N.; Camacho, M.; Temprano, F.J. Alternatives to peat as a carrier for rhizobia inoculants: Solid and liquid formulations. Soil Biol. Biochem. 2008, 40, 2771–2779. [Google Scholar] [CrossRef]
- Chen, W.F.; Meng, J.; Han, X.R.; Lan, Y.; Zhang, W.M. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Sheng, Y.Q.; Zhu, L.Z. Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci. Total Environ. 2018, 622–623, 1391–1399. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Lu, Q.Y.; Zhang, F.; Wang, W.; Wu, C.Y. Effects of biochar on the yield of Melon and the diversity of rhizosphere soil microbial communities under saline-alkali stress. Plants 2025, 14, 1423. [Google Scholar] [CrossRef] [PubMed]
- Eghlima, G.; Mohammadi, M.; Aghamir, F. Biochar application improved soil properties, growth performances, essential oil, and rosmarinic acid content of Thymus vulgaris L. under salt stress. Plant Physiol. Biochem. 2025, 222, 109698. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, K.R.; Yang, J.E.; Ok, Y.S.; Owens, G.; Nehls, T.; Wessolek, G.; Kim, K.H. Effect of biochar on reclaimed tidal land soil properties and maize (Zea mays L.) response. Chemosphere 2016, 142, 153–159. [Google Scholar] [CrossRef]
- Guo, X.X.; Wu, S.B.; Wang, X.Q.; Liu, H.T. Impact of biochar addition on three-dimensional structural changes in aggregates associated with humus during swine manure composting. J. Clean. Prod. 2021, 280, 124380. [Google Scholar] [CrossRef]
- Wei, Z.M.; Ahmed Mohamed, T.; Zhao, L.; Zhu, Z.C.; Zhao, Y.; Wu, J.Q. Microhabitat drive microbial anabolism to promote carbon sequestration during composting. Bioresour. Technol. 2022, 346, 126577. [Google Scholar] [CrossRef]
- Paul, E.A. The nature and dynamics of soil organic matter: Plant inputs, microbial transformations, and organic matter stabilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Jones, D.L.; Rousk, J.; Edwards-Jones, G.; Deluca, T.H.; Murphy, D.V. Biochar-mediated changes in soil quality and plant growth in a three year field trial. Soil Biol. Biochem. 2012, 45, 113–124. [Google Scholar] [CrossRef]
- Liu, Q.M.; He, X.Y.; Wang, K.L.; Li, D.J. Biochar drives humus formation during composting by regulating the specialized metabolic features of microbiome. Chem. Eng. J. 2023, 458, 141380. [Google Scholar] [CrossRef]
- Song, N.N.; Jia, C.Z.; Wang, J.C.; Zheng, D.; Wang, X.X.; Wang, F.L. The decrease of heavy metal accumulation in wheat: The impact of biochar on soil humus composition and cadmium bioavailability. Fresenius Environ. Bull. 2021, 30, 922–930. [Google Scholar]
- Amoakwah, E.; Arthur, E.; Frimpong, K.A.; Parikh, S.J.; Islam, R. Soil organic carbon storage and quality are impacted by corn cob biochar application on a tropical sandy loam. J. Soils Sediments 2020, 20, 1960–1969. [Google Scholar] [CrossRef]
- Yi, S.S.; Chang, N.Y.; Imhoff, P.T. Predicting water retention of biochar-amended soil from independent measurements of biochar and soil properties. Adv. Water Resour. 2020, 142, 103638. [Google Scholar] [CrossRef]
- Schmidt, H.P.; Kammann, C.; Hagemann, N.; Leifeld, J.; Bucheli, T.D.; Monedero, M.A.S.; Cayuela, M.L. Biochar in agriculture-A systematic review of 26 global meta-analyses. Glob. Change Biol. Bioenergy 2021, 13, 1708–1730. [Google Scholar] [CrossRef]
- Zhang, H.H.; Li, X.; Zhang, S.B.; Yin, Z.P.; Zhu, W.X.; Li, J.B.; Meng, L.; Zhong, H.X.; Xu, N.; Wu, Y.N.; et al. Rootstock alleviates salt stress in grafted mulberry seedlings: Physiological and PSII function responses. Front. Plant Sci. 2018, 9, 1806. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, D.F.; Turgeon, R.; Chen, J.; Lin, T.B.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.K.; Lv, Z.Q. Physiological and proteomic responses of Mulberry trees (Morus alba. L.) to combined salt and drought stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, L. The Mulberry (Morus alba L.) fruit: A review of characteristic components and health benefits. J. Agric. Food Chem. 2017, 65, 10383–10394. [Google Scholar] [CrossRef]
- Kumar, S.G.; Madhusudhan, K.V.; Sreenivasulu, N.; Sudhakar, C. Stress responses in two genotypes of mulberry (Morus alba L.) under NaCl salinity. Indian J. Exp. Biol. 2000, 38, 192–195. [Google Scholar]
- Wang, Y.; Jiang, W.; Li, C.L.; Wang, Z.J.; Lu, C.; Cheng, J.S.; Wei, S.L.; Yang, J.S.; Yang, Q. Integrated transcriptomic and metabolomic analyses elucidate the mechanism of flavonoid biosynthesis in the regulation of mulberry seed germination under salt stress. BMC Plant Biol. 2024, 24, 132. [Google Scholar] [CrossRef]
- Holt, J.G.; Krieg, N.R.; Sneath, P.H.A.; Staley, J.T.; Williams, S.T. Bergey’s Manual of Determinative Bacteriology, 9th ed.; William & Wilkins: Baltimore, MD, USA, 1994. [Google Scholar]
- Wang, X.P.; Zabowski, D. Nutrient composition of Douglas-fir rhizosphere and bulk soil solutions. Plant Soil 1998, 200, 13–20. [Google Scholar] [CrossRef]
- National Agricultural Technology Extension and Service Center. Technical Specifications for Soil Analysis, 2nd ed.; China Agriculture Press: Beijing, China, 2006. [Google Scholar]
- Wang, Q.Y.; Cao, D.Y.; Wang, D.; Zhan, Z.Y.; He, W.Y.; Sun, Q.; Chen, W.F.; Lan, Y. Effects of long-term application of biochar on nutrients, fractions of humic in brown soil. Sci. Agric. Sin. 2024, 57, 2612–2622. [Google Scholar]
- Tan, Y.; Yang, K.J.; Qin, J.S.; Ni, L.K.; Liao, S.H.; Zeng, D.J.; Pan, H.B.; Gu, D.X. Soil hydrology characteristics among forest type, stand age and successive rotation in Eucalyptus plantations in southern China. Forests 2024, 15, 423. [Google Scholar] [CrossRef]
- Tang, J.; Pan, F.F.; Davy, A.J.; Yin, J.Z.; Wu, D.F.; Yang, Q.H. Responses of water-stable aggregates, their associated organic carbon and crop yield to the application of biogas slurry in a fluvo-aquic soil of the North China plain. Soil Use Manag. 2024, 40, e12969. [Google Scholar] [CrossRef]
- Yan, S.; Niu, Z.Y.; Zhang, A.G.; Yan, H.T.; Zhang, H.; He, K.X.; Xiao, X.Y.; Wang, N.L.; Guan, C.W.; Liu, G.S. Biochar application on paddy and purple soils in southern China: Soil carbon and biotic activity. R. Soc. Open Sci. 2019, 6, 181499. [Google Scholar] [CrossRef]
- Liu, Y.L.; Jiang, W.T.; Zhao, W.L.; Xu, L.X.; Wang, M.Q.; Jian, J.J.; Chen, X.W.; Wang, E.H.; Yan, J.X. Effects of biochar application on soil properties and the growth of Melissa officinalis L. under salt stress. Sci. Hortic. 2024, 338, 113704. [Google Scholar] [CrossRef]
- Luo, Y.J.; Lopez, J.B.G.; van Veelen, H.P.J.; Kok, D.J.D.; Postma, R.; Thijssen, D.; Sechi, V.; ter Heijne, A.; Bezemer, T.M.; Buisman, C.J.N. Effects of different soil organic amendments (OAs) on extracellular polymeric substances (EPS). Eur. J. Soil Biol. 2024, 121, 103624. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K. The amelioration effects of low temperature biochar generated from nine crop residues on an acidic Ultisol. Soil Use Manag. 2011, 27, 110–115. [Google Scholar] [CrossRef]
- Mendes, J.D.S.; Fernandes, J.D.; Garofalo Chaves, L.H.; Carvallo Guerra, H.O.; Tito, G.A.; Chaves, I.D.B. Chemical and physical changes of soil amended with biochar. Water Air Soil Pollut. 2021, 232, 338. [Google Scholar] [CrossRef]
- Cao, D.Y.; Lan, Y.; Chen, W.F.; Yang, X.; Wang, D.; Ge, S.H.; Yang, J.X.; Wang, Q.Y. Successive applications of fertilizers blended with biochar in the soil improve the availability of phosphorus and productivity of maize (Zea mays L.). Eur. J. Agron. 2021, 130, 126344. [Google Scholar] [CrossRef]
- Chen, J.H.; Yang, S.M.; Wang, Y.Y.; He, L.L.; Liu, Y.X.; Gan, Y.; Lü, H.H. Effects of biochar addition on perilla growth and the rhizosphere microbial community under salt stress. J. Agro-Environ. Sci. 2024, 43, 2947–2958. [Google Scholar]
- Huang, J.; Zhu, C.Q.; Kong, Y.L.; Cao, X.C.; Zhu, L.F.; Zhang, Y.C.; Ning, Y.W.; Tian, W.H.; Zhang, H.; Yu, Y.J.; et al. Biochar application alleviated rice salt stress via modifying soil properties and regulating soil bacterial abundance and community structure. Agronomy 2022, 12, 409. [Google Scholar] [CrossRef]
- Su, C.X.; Dong, X. Effects of growth-promoting rhizobacteria and biochar preparation on growth and development of pepper and soil improvement. Shandong Agric. Sci. 2021, 53, 109–117. [Google Scholar]
- Xiu, L.Q.; Gu, W.Q.; Sun, Y.Y.; Wu, D.; Wang, Y.N.; Zhang, H.G.; Zhang, W.M.; Chen, W.F. The fate and supply capacity of potassium in biochar used in agriculture. Sci. Total Environ. 2023, 902, 165969. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, K.; Hafez, E.M.; Elhawat, N.; Omara, A.E.D.; Rashwan, E.; Mohamed, H.H.; Alshaal, T.; Gadow, S.I. Revitalizing soybean plants in saline, Cd-polluted soil using Si-NPs, biochar, and PGPR. Plants 2024, 13, 3550. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, Y.Y.; Li, Y.; Tian, X.; Li, X.Y.; Wen, Y.J. Effects of the combination of biogas slurry and chemical fertilizer over three years on soil aggregate stability in Cambisols. J. Soils Sediments 2025, 25, 1201–1212. [Google Scholar] [CrossRef]
- Orlova, N.; Abakumov, E.; Orlova, E.; Yakkonen, K.; Shahnazarova, V. Soil organic matter alteration under biochar amendment: Study in the incubation experiment on the Podzol soils of the Leningrad region (Russia). J. Soils Sediments 2019, 19, 2708–2716. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, X.; Bao, Z.R.; Gao, J.; Meng, J.; Han, X.R.; Lan, Y.; Liu, Z.Q.; Chen, W.F. Responses of microbial necromass carbon and microbial community structure to straw- and straw-derived biochar in brown earth soil of Northeast China. Front. Microbiol. 2022, 13, 967746. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Yuan, J.; Yang, X.; Han, X.R.; Lan, Y.; Cao, D.Y.; Sun, Q.; Cui, X.; Meng, J.; Chen, W.F. Responses of soil respiration and C sequestration efficiency to biochar amendment in maize field of Northeast China. Soil Tillage Res. 2022, 223, 105442. [Google Scholar] [CrossRef]
- Wang, Q.; Zhu, Y.X.; Xu, L.Z.; Chen, B.Y.; Liu, C.Z.; Ma, X.F.; Meng, Q.F.; Liu, B.; Huang, Z.W.; Jiao, Y.S.; et al. Responses of soil humus composition and humic acid structural characteristics to the addition of different types of biochar in phaeozems. J. Soil Sci. Plant Nutr. 2023, 23, 1611–1618. [Google Scholar] [CrossRef]
- Ying, J.Y.; Zhang, X.; Wu, W.X.; Nan, Q.; Wang, G.R.; Dong, D. The effects of long-term rice straw and biochar return on soil humus composition and structure in paddy soil. Plant Soil Environ. 2024, 70, 772–782. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Y.Z.; Feng, L.; Xu, Y.R.; Du, Z.W.; Zhang, L.Q. Biochar prepared from maize straw and molasses fermentation wastewater: Application for soil improvement. RSC Adv. 2020, 10, 14510–14519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Dou, S.; Meng, F.R.; Yin, X.B.; Zhou, X. Transformation of biochar into extracted humic substances under short-term laboratory incubation conditions: Evidence from stable carbon isotopes. Soil Tillage Res. 2022, 215, 105189. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Z.J.; Bai, C.X.; Li, R.; Li, M.; Wang, Y.; Yu, W.T.; Liu, P.X.; Yin, R.S.; Wang, S. Effect of metal cations with different valences on the humus composition of dark-brown soil mixed with Tilia wood shavings. Agronomy 2023, 13, 2681. [Google Scholar] [CrossRef]
- Abd El-Rahim, M.G.M.; Abdelrhman, A.A.; Rekaby, S.A.; Thabit, F.N. Compost combined with peanut shells biochar: Enhanced for mation and structural characteristics of soil humic acids and yield of wheat crop in a clay loam soil. Egypt. J. Soil Sci. 2025, 65, 1077–1090. [Google Scholar] [CrossRef]
- Hou, L.K.; Wang, Y.C.; Wang, Z.P.; Gao, R.C.; Zhou, X.; Yang, S.Y.; Luo, X.; Jiang, Z.F.; Liu, Z.H. Effects of Biochar on Soil Quality in a Maize Soybean Rotation on Mollisols. Agronomy 2025, 15, 1226. [Google Scholar] [CrossRef]
- Kolb, S.E.; Fermanich, K.J.; Dornbush, M.E. Effect of charcoal quantity on microbial biomass and activity in temperate soils. Soil Sci. Soc. Am. J. 2009, 73, 1173–1181. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Ok, Y.S.; Palansooriya, K.N.; Kirkham, M.B.; Chowdhury, S.; Bolan, N. Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2020, 2, 379–420. [Google Scholar] [CrossRef]
- Lu, C.N.; Xu, Z.M.; Fei, B.Y.; Zhao, C.; Chen, G.; Li, J.Q. Effects of combined application of biochar and organic fertilizer on soil properties, enzyme activity and bacterial community structure. Soils 2025, 57, 333–341. [Google Scholar]
| Oxidase | Hydrolyzed Starch | V-P Test | D-Glucose | D-Mannitol | Methyl Red | Utilization of Nitrate | Motility | Sucrose |
|---|---|---|---|---|---|---|---|---|
| + a | − | − | − | − | + | + | + | − |
| pH | EC (μS∙cm−1) | Total C (%) | Total N (g∙kg−1) | Total P (g∙kg−1) | Total K (g∙kg−1) | CEC (cmol∙kg−1) |
|---|---|---|---|---|---|---|
| 8.98 | 1450 | 41.77 | 25.20 | 0.70 | 10.70 | 11.00 |
| Treatment | Bulk Density (g∙cm−3) | Field Water-Holding Capacity (g∙kg−1) | Total Porosity (%) | Capillary Porosity (%) | Non-Capillary Porosity (%) | Water Stable Macro-Aggregate (%) |
|---|---|---|---|---|---|---|
| CK | 1.33 ± 0.02 b | 195.33 ± 11.59 a | 49.81 ± 0.65 b | 25.99 ± 1.76 abc | 23.82 ± 2.26 b | 22.53 ± 0.67 b |
| SS | 1.41 ± 0.04 a | 209.00 ± 15.62 a | 46.67 ± 1.57 c | 29.51 ± 1.64 a | 17.16 ± 1.66 c | 20.73 ± 1.20 c |
| SS+P | 1.38 ± 0.03 ab | 199.67 ± 28.50 a | 48.05 ± 1.21 bc | 27.44 ± 3.43 ab | 20.61 ± 2.75 bc | 21.70 ± 1.22 bc |
| SS+B | 1.34 ± 0.04 b | 186.00 ± 3.61 a | 49.43 ± 1.51 b | 24.93 ± 1.00 bc | 24.51 ± 2.44 b | 25.33 ± 0.51 a |
| SS+P+B | 1.27 ± 0.04 c | 185.00 ± 6.56 a | 52.20 ± 1.33 a | 23.42 ± 0.18 c | 28.78 ± 1.15 a | 26.50 ± 0.66 a |
| Treatment | pH | EC (μS·cm−1) | Alkali-Hydrolyzed N (mg·kg−1) | Available P (mg·kg−1) | Available K (mg·kg−1) | SOM (g·kg−1) | CEC (cmol·kg−1) |
|---|---|---|---|---|---|---|---|
| CK | 6.78 ± 0.03 c | 378.67 ± 12.90 d | 32.20 ± 2.10 a | 10.84 ± 0.98 ab | 80.33 ± 2.72 b | 16.83 ± 1.08 b | 12.27 ± 0.06 b |
| SS | 6.73 ± 0.03 d | 1558.67 ± 20.55 b | 30.57 ± 1.46 a | 12.24 ± 0.90 a | 72.59 ± 0.63 c | 16.17 ± 1.12 b | 12.47 ± 0.59 ab |
| SS+P | 6.78 ± 0.03 c | 1499.33 ± 13.20 c | 28.00 ± 2.10 a | 10.65 ± 0.86 ab | 62.07 ± 1.50 e | 16.93 ± 0.45 b | 12.80 ± 0.20 ab |
| SS+B | 7.01 ± 0.01 b | 1575.67 ± 30.24 b | 31.50 ± 3.21 a | 10.46 ± 0.96 b | 89.19 ± 3.33 a | 21.77 ± 0.60 a | 12.47 ± 0.60 ab |
| SS+P+B | 7.12 ± 0.01 a | 1668.00 ± 12.29 a | 28.00 ± 2.42 a | 10.02 ± 0.50 b | 66.64 ± 2.31 d | 22.87 ± 0.40 a | 13.30 ± 0.70 a |
| Treatment | Catalase (mg/g·h) | Urease (mg/g·24 h) | Sucrase (mg/g·24 h) | Neutral Phosphatase (mg/g·h) |
|---|---|---|---|---|
| CK | 18.00 ± 0.70 b | 0.52 ± 0.06 b | 30.67 ± 3.98 a | 0.02 ± 0.00 a |
| SS | 18.23 ± 1.08 b | 0.55 ± 0.03 b | 29.53 ± 7.97 a | 0.02 ± 0.01 a |
| SS+P | 19.43 ± 0.47 ab | 0.43 ± 0.06 b | 40.63 ± 5.14 a | 0.01 ± 0.01 a |
| SS+B | 19.23 ± 2.06 ab | 1.11 ± 0.21 a | 42.20 ± 6.78 a | 0.02 ± 0.01 a |
| SS+P+B | 21.00 ± 0.56 a | 1.43 ± 0.61 a | 39.60 ± 7.70 a | 0.02 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, D.; Liu, F.; Liu, B.; Peng, L.; Sun, M.; Ma, H.; Du, Z. Effects of Biochar and PGPR Application on the Physicochemical Properties and Humus Components of Soil Used for Planting Fruit Mulberry Seedlings Under Salt Stress. Biology 2025, 14, 1441. https://doi.org/10.3390/biology14101441
Jing D, Liu F, Liu B, Peng L, Sun M, Ma H, Du Z. Effects of Biochar and PGPR Application on the Physicochemical Properties and Humus Components of Soil Used for Planting Fruit Mulberry Seedlings Under Salt Stress. Biology. 2025; 14(10):1441. https://doi.org/10.3390/biology14101441
Chicago/Turabian StyleJing, Dawei, Fangchun Liu, Binghua Liu, Lin Peng, Mingjie Sun, Hailin Ma, and Zhenyu Du. 2025. "Effects of Biochar and PGPR Application on the Physicochemical Properties and Humus Components of Soil Used for Planting Fruit Mulberry Seedlings Under Salt Stress" Biology 14, no. 10: 1441. https://doi.org/10.3390/biology14101441
APA StyleJing, D., Liu, F., Liu, B., Peng, L., Sun, M., Ma, H., & Du, Z. (2025). Effects of Biochar and PGPR Application on the Physicochemical Properties and Humus Components of Soil Used for Planting Fruit Mulberry Seedlings Under Salt Stress. Biology, 14(10), 1441. https://doi.org/10.3390/biology14101441









