PIWIs Regulate Spermatogonia Self-Renewal and Differentiation by Wnt/β-Catenin Signaling Pathway in Eriocheir sinensis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. RNA Interference
2.3. RNA Extraction and Real-Time PCR
2.4. Western Blotting
2.5. EdU Incorporation Assay
2.6. Cell Culture and Expression of PIWIs
2.7. Immunofluorescence
2.8. Cell Cycle Analysis
2.9. TCF Reporter Assay
2.10. Statistical Analysis
3. Results
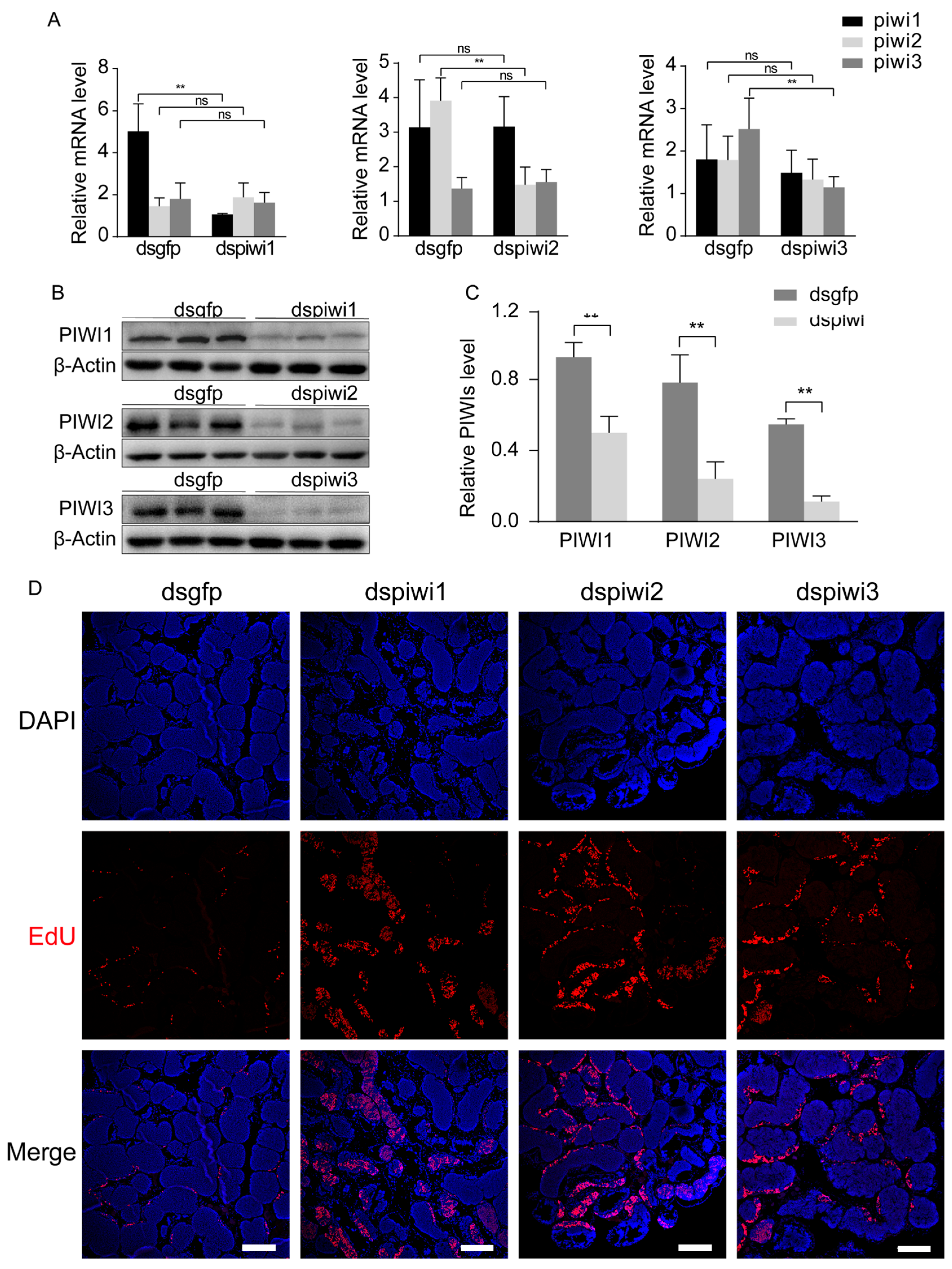
3.1. Effects of PIWI Deficiency on Spermatogonia Proliferation and Differentiation in E. sinensis
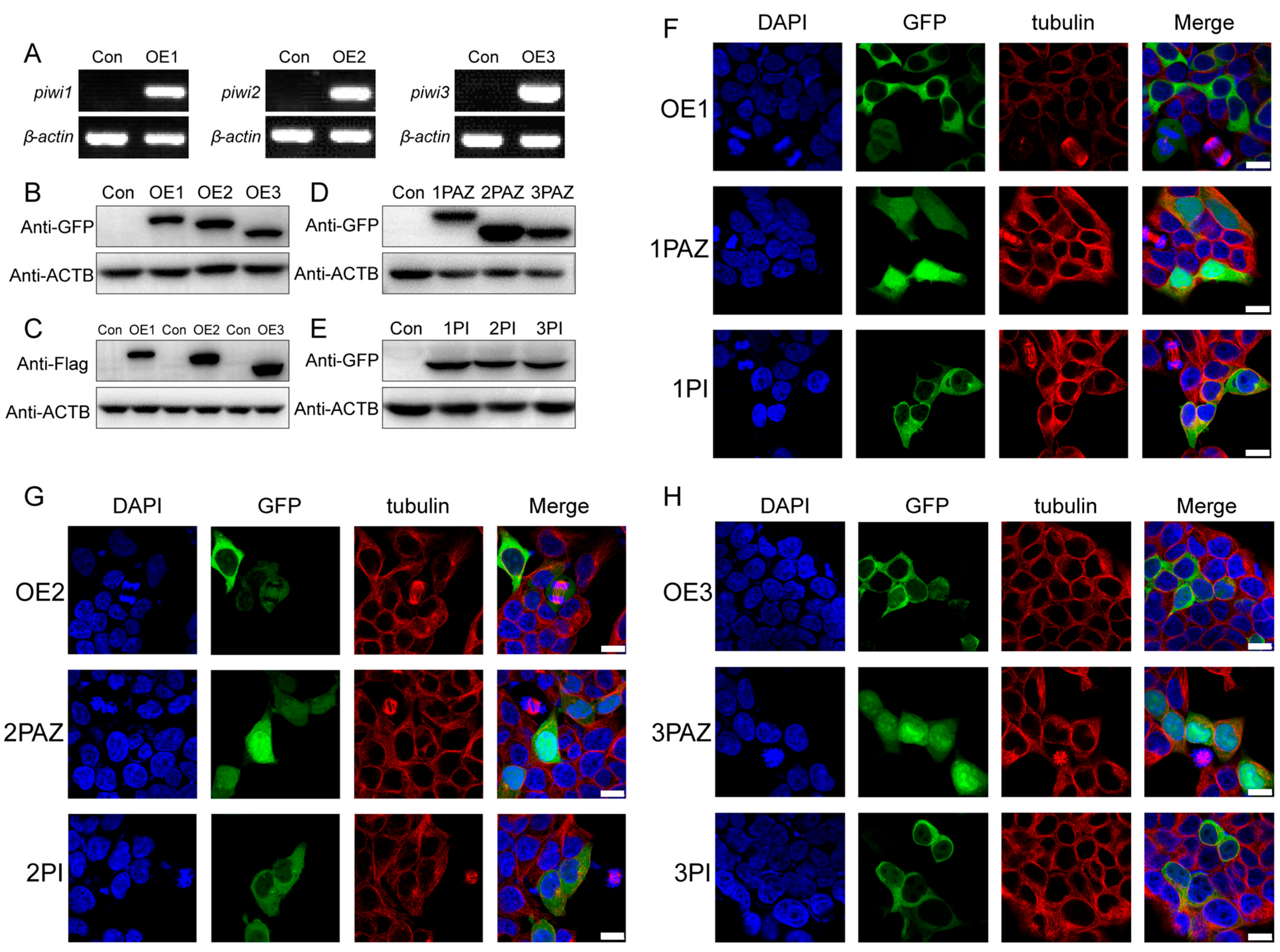
3.2. Localization of PIWIs in HEK 293T Cells
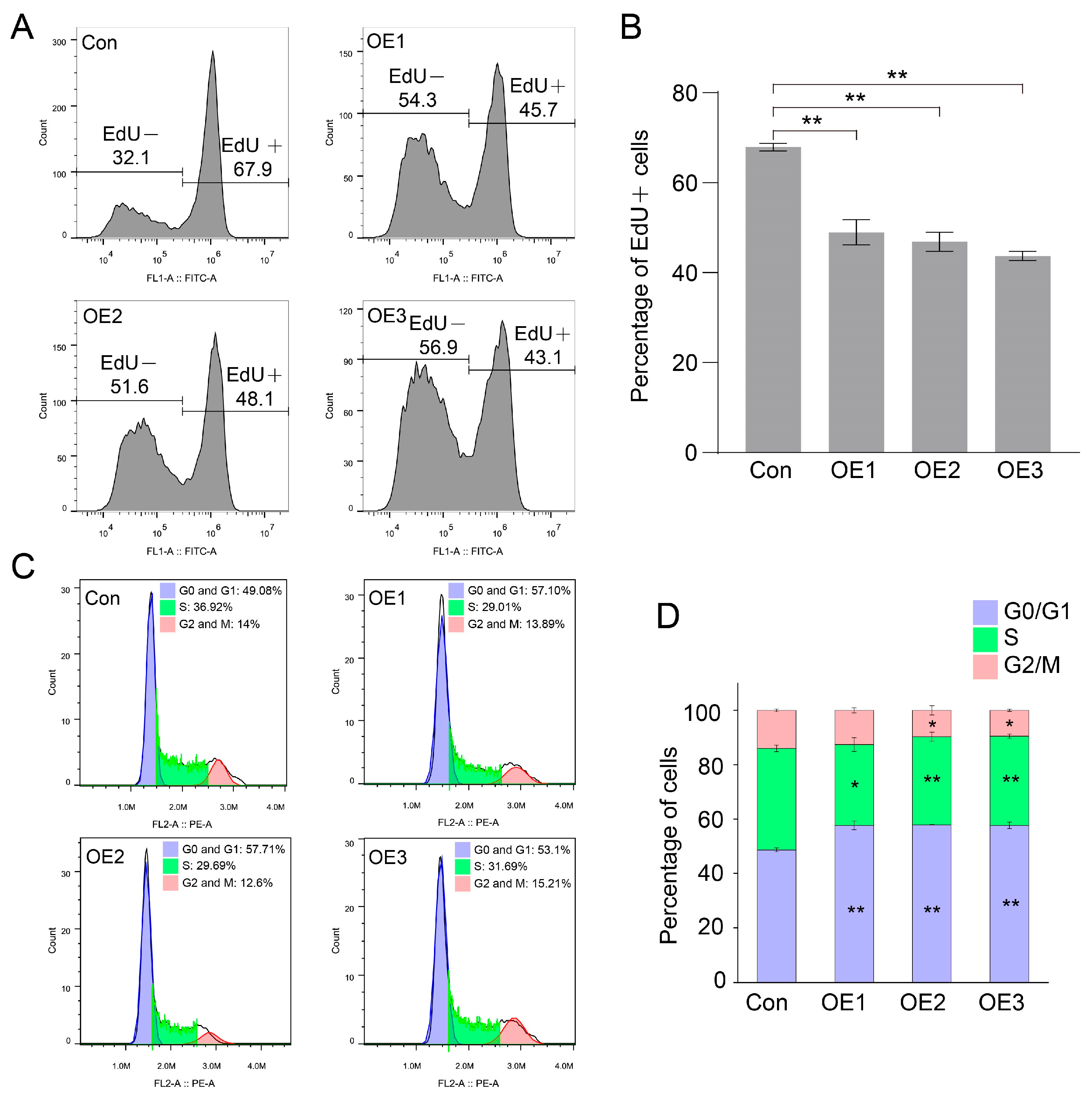
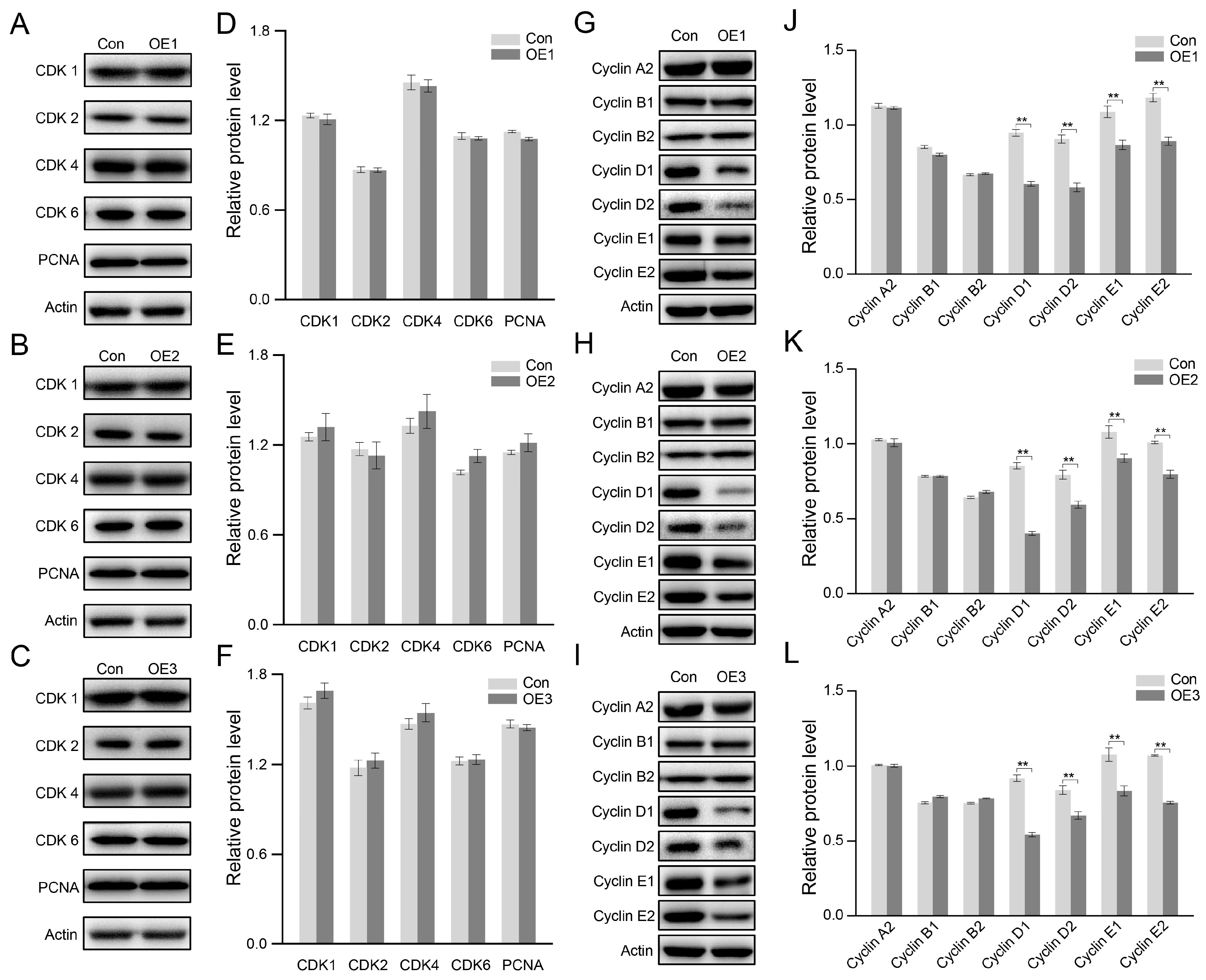
3.3. Cell Proliferation Inhibited by Down-Regulating Cyclin After PIWI Expression
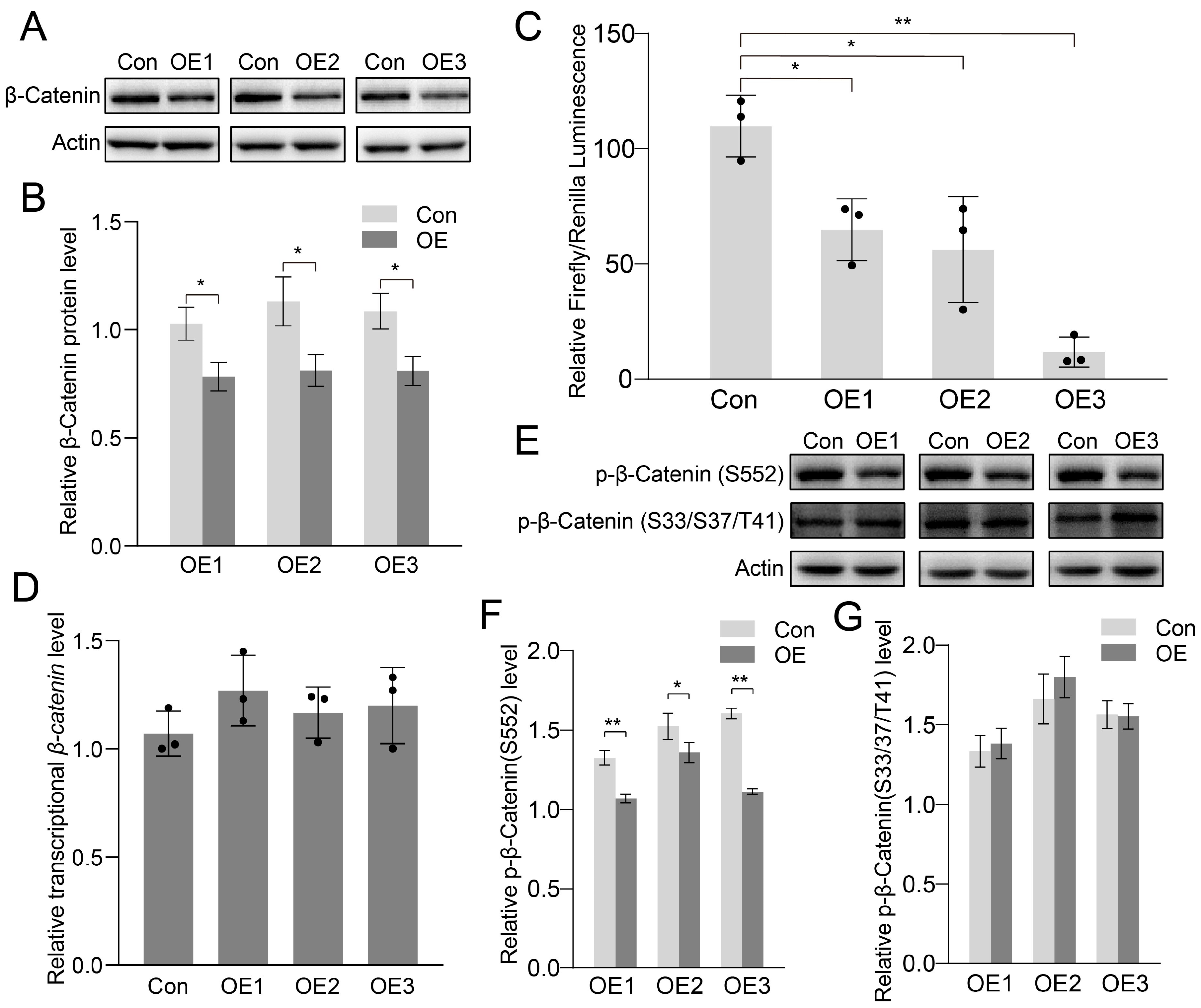
3.4. Canonical Wnt-Signaling Pathway Inhibited After PIWI Expression
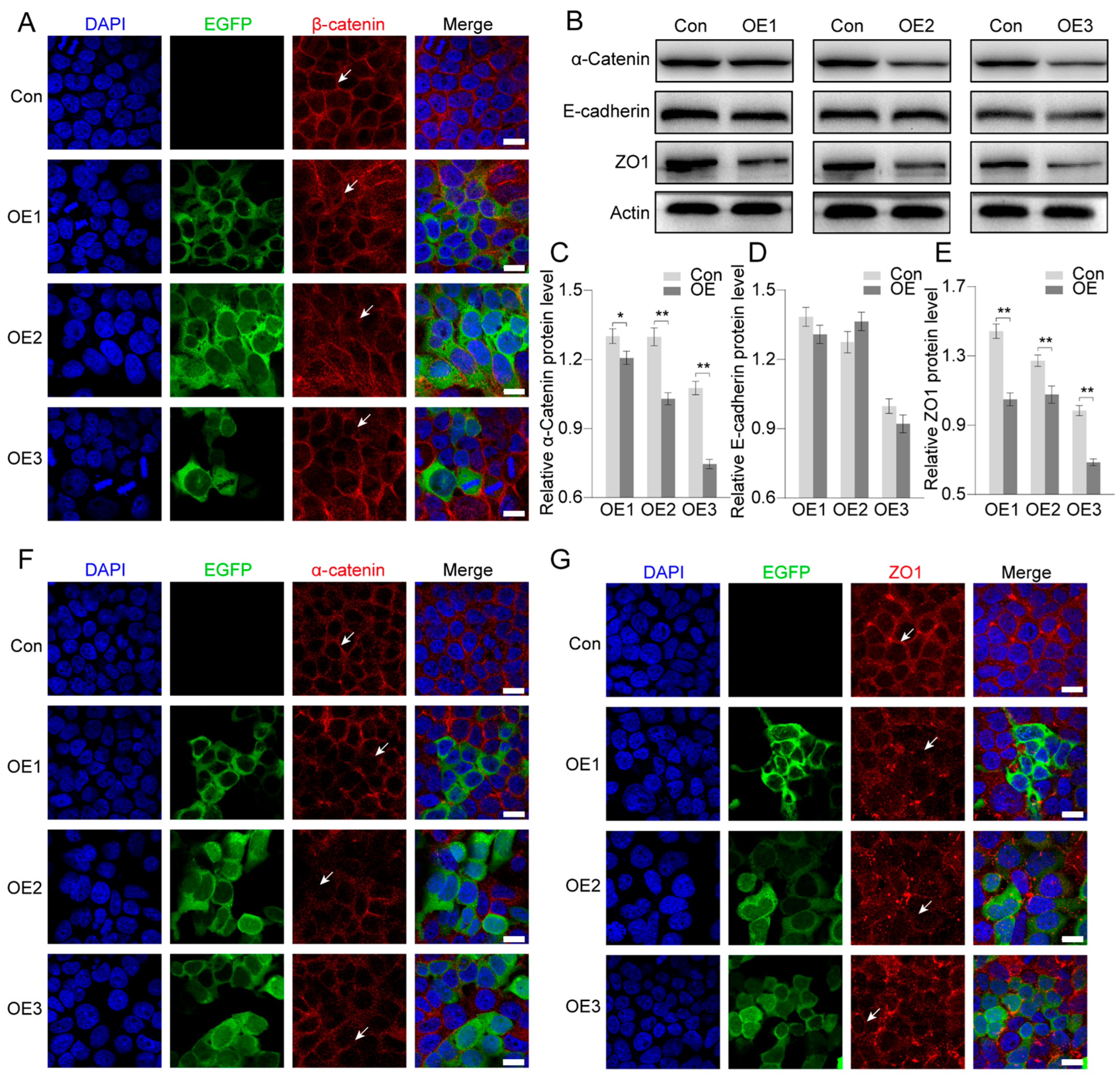
3.5. Effects on Regulation of Cell Junction Proteins After PIWI Expression
4. Discussion and Perspectives
4.1. Novel Functions of PIWIs in Regulating Spermatogonia Self-Renewal and Differentiation During E. sinensis Spermatogenesis
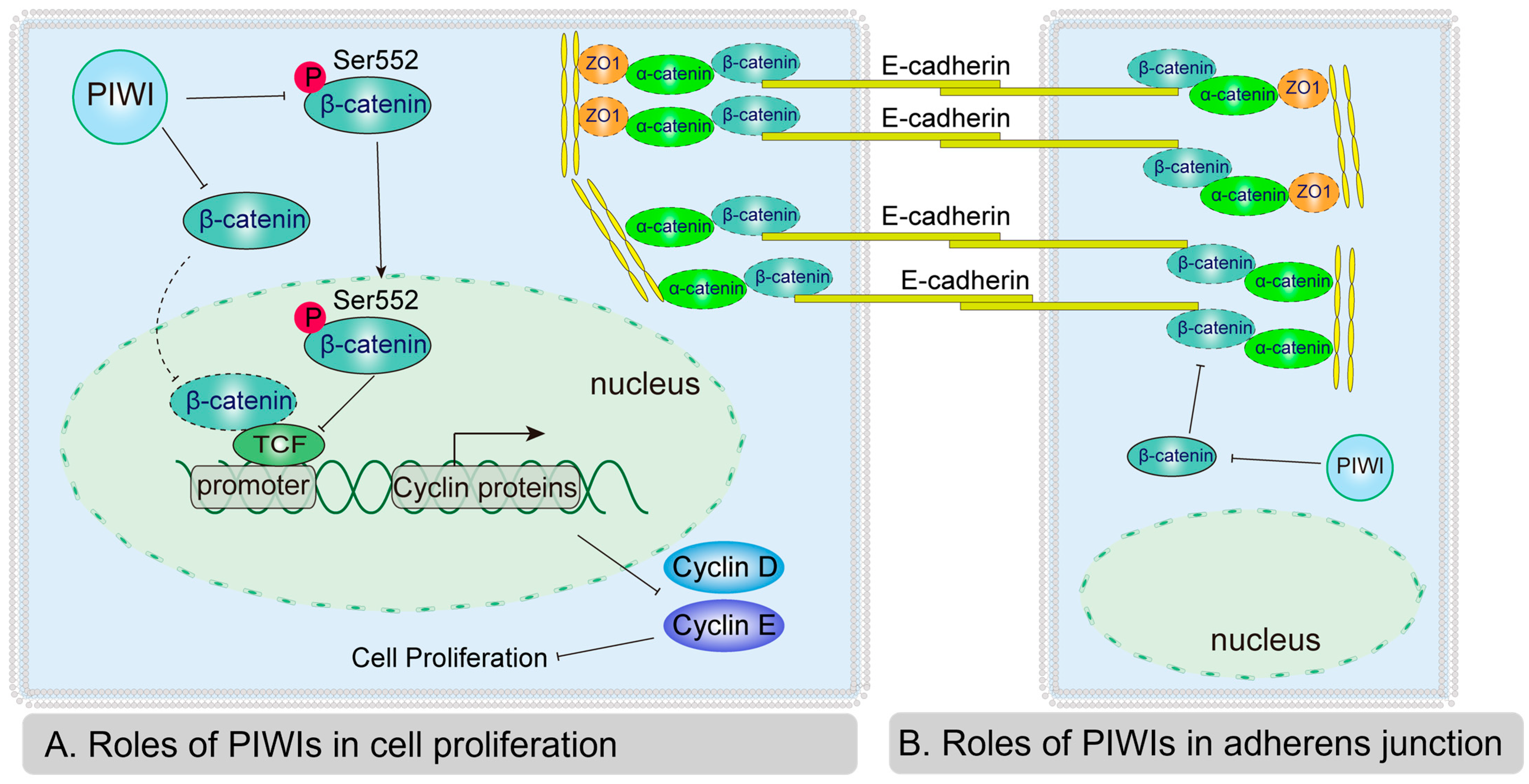
4.2. PIWIs Suppressed Spermatogonia Proliferation and Differentiation Through Wnt Signaling Pathway
4.3. PIWIs Affect Adherens Junctions by Regulating α-Catenin and ZO1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stukenborg, J.B.; Kjartansdóttir, K.R.; Reda, A.; Colon, E.; Albersmeier, J.P.; Söder, O. Male germ cell development in humans. Horm. Res. Paediatr. 2014, 81, 2–12. [Google Scholar] [CrossRef]
- Matson, C.K.; Murphy, M.W.; Griswold, M.D.; Yoshida, S.; Bardwell, V.J.; Zarkower, D. The Mammalian Doublesex Homolog DMRT1 Is a Transcriptional Gatekeeper that Controls the Mitosis versus Meiosis Decision in Male Germ Cells. Dev. Cell 2010, 19, 612–624. [Google Scholar] [CrossRef]
- Mei, X.X.; Wang, J.; Wu, J. Extrinsic and intrinsic factors controlling spermatogonial stem cell self-renewal and differentiation. Asian J. Androl. 2015, 17, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Liao, H.F.; Yu, C.Y.; Mo, C.F.; Lin, S.P. Epigenetic factors in the regulation of prospermatogonia and spermatogonial stem cells. Reproduction 2015, 150, R77–R91. [Google Scholar] [CrossRef] [PubMed]
- Juliano, C.; Wang, J.; Lin, H. Uniting germline and stem cells: The function of Piwi proteins and the piRNA pathway in diverse organisms. Annu. Rev. Genet. 2011, 45, 447–469. [Google Scholar] [CrossRef] [PubMed]
- Ramat, A.; Simonelig, M. Functions of PIWI Proteins in Gene Regulation: New Arrows Added to the piRNA Quiver. Trends Genet. 2021, 37, 188–200. [Google Scholar] [CrossRef]
- Lin, H.; Spradling, A.C. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 1997, 124, 2463–2476. [Google Scholar] [CrossRef]
- Meister, G. Argonaute proteins: Functional insights and emerging roles. Nat. Rev. Genet. 2013, 14, 447–459. [Google Scholar] [CrossRef]
- Cox, D.N.; Chao, A.; Baker, J.; Chang, L.; Qiao, D.; Lin, H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998, 12, 3715–3727. [Google Scholar] [CrossRef]
- Thomson, T.; Lin, H. The biogenesis and function of PIWI proteins and piRNAs: Progress and prospect. Annu. Rev. Cell Dev. Biol. 2009, 25, 355–376. [Google Scholar] [CrossRef]
- Megosh, H.B.; Cox, D.N.; Campbell, C.; Lin, H. The role of PIWI and the miRNA machinery in Drosophila germline determination. Curr. Biol. 2006, 16, 1884–1894. [Google Scholar] [CrossRef]
- Li, M.; Hong, N.; Gui, J.; Hong, Y. Medaka piwi is essential for primordial germ cell migration. Curr. Mol. Med. 2012, 12, 1040–1049. [Google Scholar] [CrossRef]
- Kuramochi-Miyagawa, S.; Kimura, T.; Ijiri, T.W.; Isobe, T.; Asada, N.; Fujita, Y.; Ikawa, M.; Iwai, N.; Okabe, M.; Deng, W.; et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 2004, 131, 839–849. [Google Scholar] [CrossRef]
- Unhavaithaya, Y.; Hao, Y.; Beyret, E.; Yin, H.; Kuramochi-Miyagawa, S.; Nakano, T.; Lin, H. MILI, a PIWI-interacting RNA-binding protein, is required for germ line stem cell self-renewal and appears to positively regulate translation. J. Biol. Chem. 2009, 284, 6507–6519. [Google Scholar] [CrossRef]
- Deng, W.; Lin, H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell 2002, 2, 819–830. [Google Scholar] [CrossRef]
- Carmell, M.A.; Girard, A.; van de Kant, H.J.; Bourc’his, D.; Bestor, T.H.; de Rooij, D.G.; Hannon, G.J. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell 2007, 12, 503–514. [Google Scholar] [CrossRef]
- Houwing, S.; Kamminga, L.M.; Berezikov, E.; Cronembold, D.; Girard, A.; van den Elst, H.; Filippov, D.V.; Blaser, H.; Raz, E.; Moens, C.B.; et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell 2007, 129, 69–82. [Google Scholar] [CrossRef]
- Houwing, S.; Berezikov, E.; Ketting, R.F. Zili is required for germ cell differentiation and meiosis in zebrafish. EMBO J. 2008, 27, 2702–2711. [Google Scholar] [CrossRef] [PubMed]
- Meistrich, M.L.; Mohapatra, B.; Shirley, C.R.; Zhao, M. Roles of transition nuclear proteins in spermiogenesis. Chromosoma 2003, 111, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Gou, L.T.; Kang, J.Y.; Dai, P.; Wang, X.; Li, F.; Zhao, S.; Zhang, M.; Hua, M.M.; Lu, Y.; Zhu, Y.; et al. Ubiquitination-deficient Mutations in human Piwi cause male infertility by impairing histone-to-protamine exchange during spermiogenesis. Cell 2017, 169, 1090–1104.e13. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Wang, X.; Liu, M.F. A dual role of the PIWI/piRNA machinery in regulating mRNAs during mouse spermiogenesis. Sci. China Life Sci. 2020, 63, 447–449. [Google Scholar] [CrossRef]
- Gou, L.T.; Dai, P.; Yang, J.H.; Xue, Y.; Hu, Y.P.; Zhou, Y.; Kang, J.Y.; Wang, X.; Li, H.; Hua, M.M.; et al. Pachytene piRNAs instruct massive mRNA elimination during late spermiogenesis. Cell Res. 2014, 24, 680–700. [Google Scholar] [CrossRef]
- Vourekas, A.; Zheng, Q.; Alexiou, P.; Maragkakis, M.; Kirino, Y.; Gregory, B.D.; Mourelatos, Z. Mili and Miwi target RNA repertoire reveals piRNA biogenesis and function of Miwi in spermiogenesis. Nat. Struct. Mol. Biol. 2012, 19, 773–781. [Google Scholar] [CrossRef]
- Dai, P.; Wang, X.; Gou, L.T.; Li, Z.T.; Wen, Z.; Chen, Z.G.; Hua, M.M.; Zhong, A.; Wang, L.; Su, H.; et al. A Translation-activating function of MIWI/piRNA during mouse spermiogenesis. Cell 2019, 179, 1566–1581.e16. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Sun, W.J.; He, L.; Li, Q.; Wang, Q. Morphological alterations of all stages of spermatogenesis and acrosome reaction in Chinese mitten crab Eriocheir sinensis. Cell Tissue Res. 2015, 360, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, K.; Martínez-Soler, F.; Ausió, J.; Chiva, M. Histones and nucleosomes in cancer sperm (Decapod: Crustacea) previously described as lacking basic DNA-associated proteins: A new model of sperm chromatin. J. Cell. Biochem. 2008, 105, 574–584. [Google Scholar] [CrossRef]
- Wei, B.H.; Ni, J.H.; Yang, T.; Hao, S.L.; Yang, W.X. PIWIs maintain testis apoptosis to remove abnormal germ cells in Eriocheir sinensis. Reproduction 2021, 162, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Szakmary, A.; Cox, D.N.; Wang, Z.; Lin, H. Regulatory relationship among piwi, pumilio, and bag-of-marbles in Drosophila germline stem cell self-renewal and differentiation. Curr. Biol. 2005, 15, 171–178. [Google Scholar] [CrossRef]
- Tetsu, O.; McCormick, F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398, 422–426. [Google Scholar] [CrossRef]
- Shtutman, M.; Zhurinsky, J.; Simcha, I.; Albanese, C.; D’Amico, M.; Pestell, R.; Ben-Ze’ev, A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA 1999, 96, 5522–5527. [Google Scholar] [CrossRef]
- Logan, C.Y.; Nusse, R. The Wnt signaling pathway in development and disease. Cell Dev. Biol. 2004, 20, 781–810. [Google Scholar] [CrossRef]
- Seifert, J.R.; Mlodzik, M. Frizzled/PCP signalling: A conserved mechanism regulating cell polarity and directed motility. Nat. Rev. Genet. 2007, 8, 126–138. [Google Scholar] [CrossRef]
- Semenov, M.V.; Habas, R.; Macdonald, B.T.; He, X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell 2007, 131, 1378. [Google Scholar] [CrossRef]
- Ku, H.Y.; Lin, H. PIWI proteins and their interactors in piRNA biogenesis, germline development and gene expression. Natl. Sci. Rev. 2014, 1, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Yost, C.; Torres, M.; Miller, J.R.; Huang, E.; Kimelman, D.; Moon, R.T. The axis-inducing activity, stability, and subcellular distribution of beta-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev. 1996, 10, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Morin, P.J.; Sparks, A.B.; Korinek, V.; Barker, N.; Clevers, H.; Vogelstein, B.; Kinzler, K.W. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275, 1787–1790. [Google Scholar] [CrossRef] [PubMed]
- Taurin, S.; Sandbo, N.; Qin, Y.; Browning, D.; Dulin, N.O. Phosphorylation of beta-catenin by cyclic AMP-dependent protein kinase. J. Biol. Chem. 2006, 281, 9971–9976. [Google Scholar] [CrossRef]
- Fang, D.; Hawke, D.; Zheng, Y.; Xia, Y.; Meisenhelder, J.; Nika, H.; Mills, G.B.; Kobayashi, R.; Hunter, T.; Lu, Z. Phosphorylation of beta-catenin by AKT promotes beta-catenin transcriptional activity. J. Biol. Chem. 2007, 282, 11221–11229. [Google Scholar] [CrossRef]
- Wheelock, M.J.; Johnson, K.R. Cadherins as modulators of cellular phenotype. Annu. Rev. Cell Dev. Biol. 2003, 19, 207–235. [Google Scholar] [CrossRef] [PubMed]
- Özata, D.M.; Yu, T.; Mou, H.; Gainetdinov, I.; Colpan, C.; Cecchini, K.; Kaymaz, Y.; Wu, P.H.; Fan, K.; Kucukural, A.; et al. Evolutionarily conserved pachytene piRNA loci are highly divergent among modern humans. Nat. Ecol. Evol. 2020, 4, 156–168. [Google Scholar] [CrossRef]
- Cox, D.N.; Chao, A.; Lin, H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 2000, 127, 503–514. [Google Scholar] [CrossRef]
- Dowling, M.; Homolka, D.; Raad, N.; Gos, P.; Pandey, R.R.; Pillai, R.S. In Vivo PIWI slicing in mouse testes deviates from rules established in vitro. RNA Soc. 2023, 29, 308–316. [Google Scholar] [CrossRef]
- Reuter, M.; Berninger, P.; Chuma, S.; Shah, H.; Hosokawa, M.; Funaya, C.; Antony, C.; Sachidanandam, R.; Pillai, R.S. Miwi catalysis is required for piRNA amplification-independent LINE1 transposon silencing. Nature 2011, 480, 264–267. [Google Scholar] [CrossRef]
- Lv, X.; Xiao, W.; Lai, Y.; Zhang, Z.; Zhang, H.; Qiu, C.; Hou, L.; Chen, Q.; Wang, D.; Gao, Y.; et al. The non-redundant functions of PIWI family proteins in gametogenesis in golden hamsters. Nat. Commun. 2023, 14, 5267. [Google Scholar] [CrossRef]
- Sukthaworn, S.; Panyim, S.; Udomkit, A. Homologues of Piwi control transposable elements and development of male germline in Penaeus monodon. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 250, 110807. [Google Scholar] [CrossRef]
- Wang, T.; Lu, L.; Liu, F.; Liu, A.; Ye, H. Transcriptome analysis of testis in response to silencing of piwi2 sheds light on spermatogenesis in the mud crab scylla paramamosain. Aquac. Rep. 2025, 40, 102550. [Google Scholar] [CrossRef]
- Kwon, C.; Tak, H.; Rho, M.; Chang, H.R.; Kim, Y.H.; Kim, K.T.; Balch, C.; Lee, E.K.; Nam, S. Detection of PIWI and piRNAs in the mitochondria of mammalian cancer cells. Biochem. Biophys. Res. Commun. 2014, 446, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Wan, H.; Zhao, S.; Xu, H.; Tang, T.; Oware, K.A.; Qu, S. Role of PIWI-interacting RNAs on cell survival: Proliferation, apoptosis, and cycle. IUBMB Life 2020, 72, 1870–1878. [Google Scholar] [CrossRef]
- Mai, D.; Ding, P.; Tan, L.; Zhang, J.; Pan, Z.; Bai, R.; Li, C.; Li, M.; Zhou, Y.; Tan, W.; et al. PIWI-interacting RNA-54265 is oncogenic and a potential therapeutic target in colorectal adenocarcinoma. Theranostics 2018, 8, 5213–5230. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Luo, Y.; Gao, Y.; Yang, Y.; Wang, Y.; Xu, Y.; Tan, S.; Zhang, Y.; Duan, J.; Yang, Y. piR-651 promotes tumor formation in non-small cell lung carcinoma through the upregulation of cyclin D1 and CDK4. Int. J. Mol. Med. 2016, 38, 927–936. [Google Scholar] [CrossRef]
- Peng, L.; Song, L.; Liu, C.; Lv, X.; Li, X.; Jie, J.; Zhao, D.; Li, D. piR-55490 inhibits the growth of lung carcinoma by suppressing mTOR signaling. Tumour Biol. 2016, 37, 2749–2756. [Google Scholar] [CrossRef]
- Das, B.; Roy, J.; Jain, N.; Mallick, B. Tumor suppressive activity of PIWI-interacting RNA in human fibrosarcoma mediated through repression of RRM2. Mol. Carcinog. 2019, 58, 344–357. [Google Scholar] [CrossRef]
- Clevers, H.; Loh, K.M.; Nusse, R. Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef]
- Yeh, J.R.; Zhang, X.; Nagano, M.C. Wnt5a is a cell-extrinsic factor that supports self-renewal of mouse spermatogonial stem cells. J. Cell Sci. 2011, 124 Pt 14, 2357–2366. [Google Scholar] [CrossRef] [PubMed]
- Yeh, J.R.; Zhang, X.; Nagano, M.C. Indirect effects of Wnt3a/β-catenin signalling support mouse spermatogonial stem cells in vitro. PLoS ONE 2012, 7, e40002. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.M.; Nusse, R. Paracrine Wnt/β-catenin signaling mediates proliferation of undifferentiated spermatogonia in the adult mouse testis. Proc. Natl. Acad. Sci. USA 2016, 113, E1489–E1497. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Zeng, J.; Zhao, X.; Huang, L.; Ma, T.; Zhu, Y.; Liu, M.; Tao, D.; Liu, Y.; Lu, Y.; et al. PIWIL2 stabilizes β-catenin to promote cell cycle and proliferation in tumor cells. Biochem. Biophys. Res. Commun. 2019, 516, 819–824. [Google Scholar] [CrossRef]
- Lin, K.Y.; Wang, W.D.; Lin, C.H.; Rastegari, E.; Su, Y.H.; Chang, Y.T.; Liao, Y.F.; Chang, Y.C.; Pi, H.; Yu, B.Y.; et al. Piwi reduction in the aged niche eliminates germline stem cells via Toll-GSK3 signaling. Nat. Commun. 2020, 11, 3147. [Google Scholar] [CrossRef]
- Robine, N.; Lau, N.C.; Balla, S.; Jin, Z.; Okamura, K.; Kuramochi-Miyagawa, S.; Blower, M.D.; Lai, E.C. A broadly conserved pathway generates 3′UTR-directed primary piRNAs. Curr. Biol. 2009, 19, 2066–2076. [Google Scholar] [CrossRef]
- Rojas-Ríos, P.; Simonelig, M. piRNAs and PIWI proteins: Regulators of gene expression in development and stem cells. Development 2018, 145, dev161786. [Google Scholar] [CrossRef]
- Stamos, J.L.; Weis, W.I. The β-catenin destruction complex. Cold Spring Harb. Perspect. Biol. 2013, 5, a007898. [Google Scholar] [CrossRef]
- Grivna, S.T.; Pyhtila, B.; Lin, H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 13415–13420. [Google Scholar] [CrossRef] [PubMed]
- Ramat, A.; Garcia-Silva, M.R.; Jahan, C.; Naït-Saïdi, R.; Dufourt, J.; Garret, C.; Chartier, A.; Cremaschi, J.; Patel, V.; Decourcelle, M.; et al. The PIWI protein Aubergine recruits eIF3 to activate translation in the germ plasm. Cell Res. 2020, 30, 421–435. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Regulation of cadherin-mediated adhesion in morphogenesis. Nat. Rev. Mol. Cell Biol. 2005, 6, 622–634. [Google Scholar] [CrossRef]
- Maker, A.; Gumbiner, B.M. Reconstitution of the full transmembrane cadherin-catenin complex. Protein Expr. Purif. 2022, 193, 106056. [Google Scholar] [CrossRef]
- Harrison, O.J.; Jin, X.; Hong, S.; Bahna, F.; Ahlsen, G.; Brasch, J.; Wu, Y.; Vendome, J.; Felsovalyi, K.; Hampton, C.M.; et al. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure 2011, 19, 244–256. [Google Scholar] [CrossRef] [PubMed]
- Pokutta, S.; Weis, W.I. Structure of the dimerization and beta-catenin-binding region of alpha-catenin. Mol. Cell 2000, 5, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.H.; Weis, W.I. The structure of the beta-catenin/E-cadherin complex and the molecular basis of diverse ligand recognition by beta-catenin. Cell 2001, 105, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Nagafuchi, A.; Moroi, S.; Tsukita, S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to alpha catenin and actin filaments. J. Cell Biol. 1997, 138, 181–192. [Google Scholar] [CrossRef]
| Gene | Primer Sequence (5′–3′) | Purpose | |
|---|---|---|---|
| Piwi1 | F | ACACCCCAAAATCCGAGACC | qPCR |
| R | CTTGTCCATGCTCCCCTTGT | ||
| Piwi2 | F | TTGATGCGCCGATGTATGGA | qPCR |
| R | GGATGGTGCTGGTGTATCCC | ||
| Piwi3 | F | AAGGCATACCAAGTAGCCGTG | qPCR |
| R | TAGCAATGACTCCATGGTCGG | ||
| esβ-actin | F | CGAGGCTACACCTTCACGAC | qPCR |
| R | ACGCGGCAGTGGTCATTT | ||
| β-catenin | F | CATCTACACAGTTTGATGCTGCT | qPCR |
| R | GCAGTTTTGTCAGTTCAGGGA | ||
| hβ-actin | F | CTCCATCCTGGCCTCGCTGT | qPCR |
| R | GCTGTCACCTTCACCGTTCC | ||
| 1PAZ | F | TCGAGCTCAAGCTTCGAATTCCAGCACTTGCCTCAATGTAATGG | expression |
| R | TTATCTAGATCCGGTGGATCCAGTGGGTGTGACACGGG | ||
| 1PI | F | TCGAGCTCAAGCTTCGAATTCGTTGGCAGTCATAATCTTTCCAAC | expression |
| R | TTATCTAGATCCGGTGGATCCCTTCACATTCTGACCAATGAGATA | ||
| 2PAZ | F | TCGAGCTCAAGCTTCGAATTCGGAGACAGCCTACACCGTCG | expression |
| R | TTATCTAGATCCGGTGGATCCGTCTGGGGCGGTGCG | ||
| 2PI | F | TCGAGCTCAAGCTTCGAATTCGCTGGTGCTGGTGGTGCTC | expression |
| R | TTATCTAGATCCGGTGGATCCTGCCTTGTTGACGATCTGGC | ||
| 3PAZ | F | TCGAGCTCAAGCTTCGAATTCCGACACTGTGTACAACCTCCTTCG | expression |
| R | TTATCTAGATCCGGTGGATCCGTCTGGGCTGAGGCGTGT | ||
| 3PI | F | TCGAGCTCAAGCTTCGAATTCCCTGGTGGTGTGCGTCATCCC | expression |
| R | TTATCTAGATCCGGTGGATCCATGGATTGCTTGGCCTGTGATGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, B.-H.; Zhao, Z.; Qi, H.-Y.; Li, Z.-F.; Yang, W.-X.; Hao, S.-L. PIWIs Regulate Spermatogonia Self-Renewal and Differentiation by Wnt/β-Catenin Signaling Pathway in Eriocheir sinensis. Biology 2025, 14, 1440. https://doi.org/10.3390/biology14101440
Wei B-H, Zhao Z, Qi H-Y, Li Z-F, Yang W-X, Hao S-L. PIWIs Regulate Spermatogonia Self-Renewal and Differentiation by Wnt/β-Catenin Signaling Pathway in Eriocheir sinensis. Biology. 2025; 14(10):1440. https://doi.org/10.3390/biology14101440
Chicago/Turabian StyleWei, Bang-Hong, Zhan Zhao, Hong-Yu Qi, Zhen-Fang Li, Wan-Xi Yang, and Shuang-Li Hao. 2025. "PIWIs Regulate Spermatogonia Self-Renewal and Differentiation by Wnt/β-Catenin Signaling Pathway in Eriocheir sinensis" Biology 14, no. 10: 1440. https://doi.org/10.3390/biology14101440
APA StyleWei, B.-H., Zhao, Z., Qi, H.-Y., Li, Z.-F., Yang, W.-X., & Hao, S.-L. (2025). PIWIs Regulate Spermatogonia Self-Renewal and Differentiation by Wnt/β-Catenin Signaling Pathway in Eriocheir sinensis. Biology, 14(10), 1440. https://doi.org/10.3390/biology14101440







