Potential Distribution and Response to Climate Change in Puccinellia tenuiflora in China Projected Using Optimized MaxEnt Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
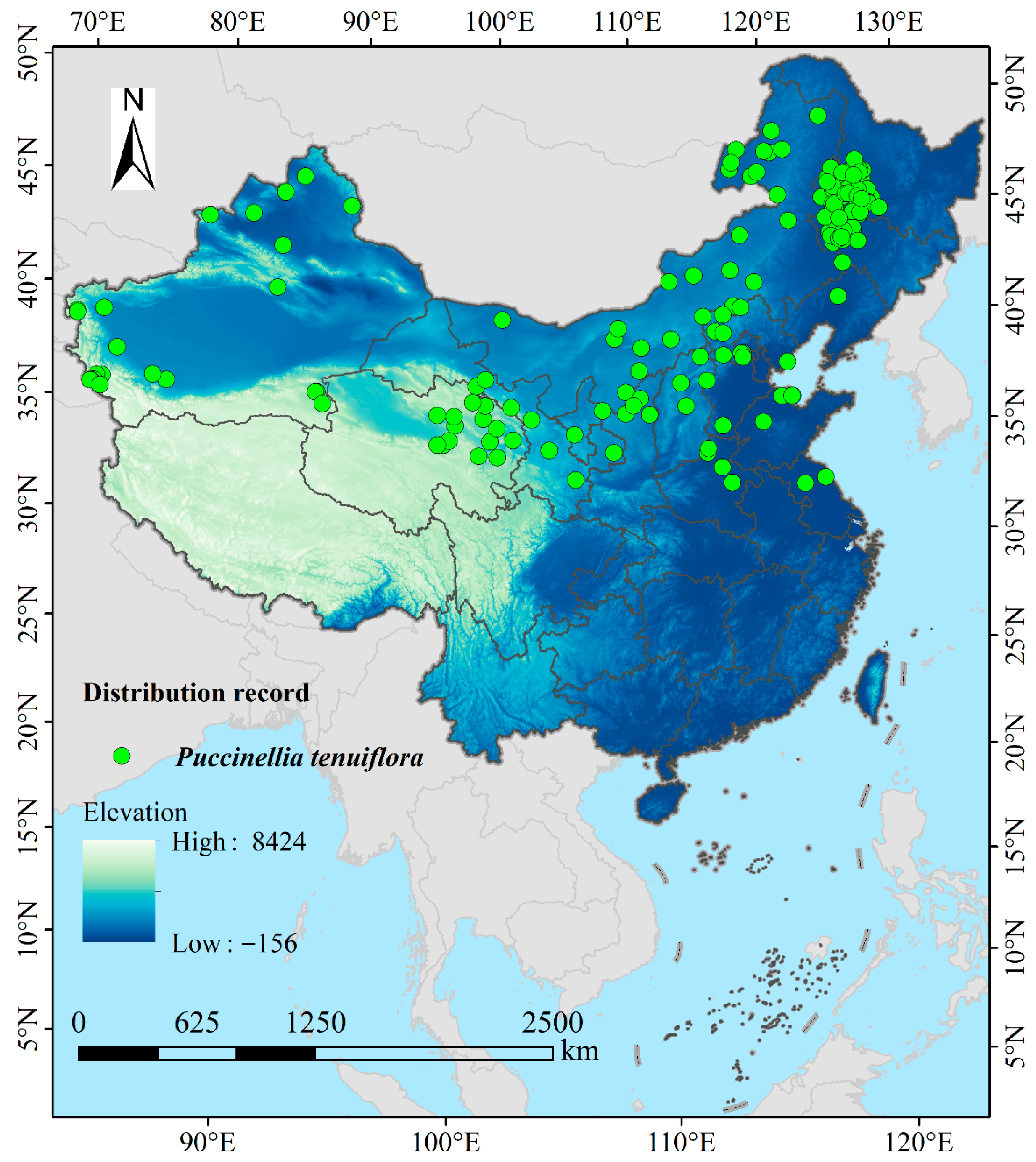
2.1. Occurrence Data Acquisition and Processing
2.2. Acquisition and Processing of Environmental Variables
2.3. Optimization and Prediction Using MaxEnt Model
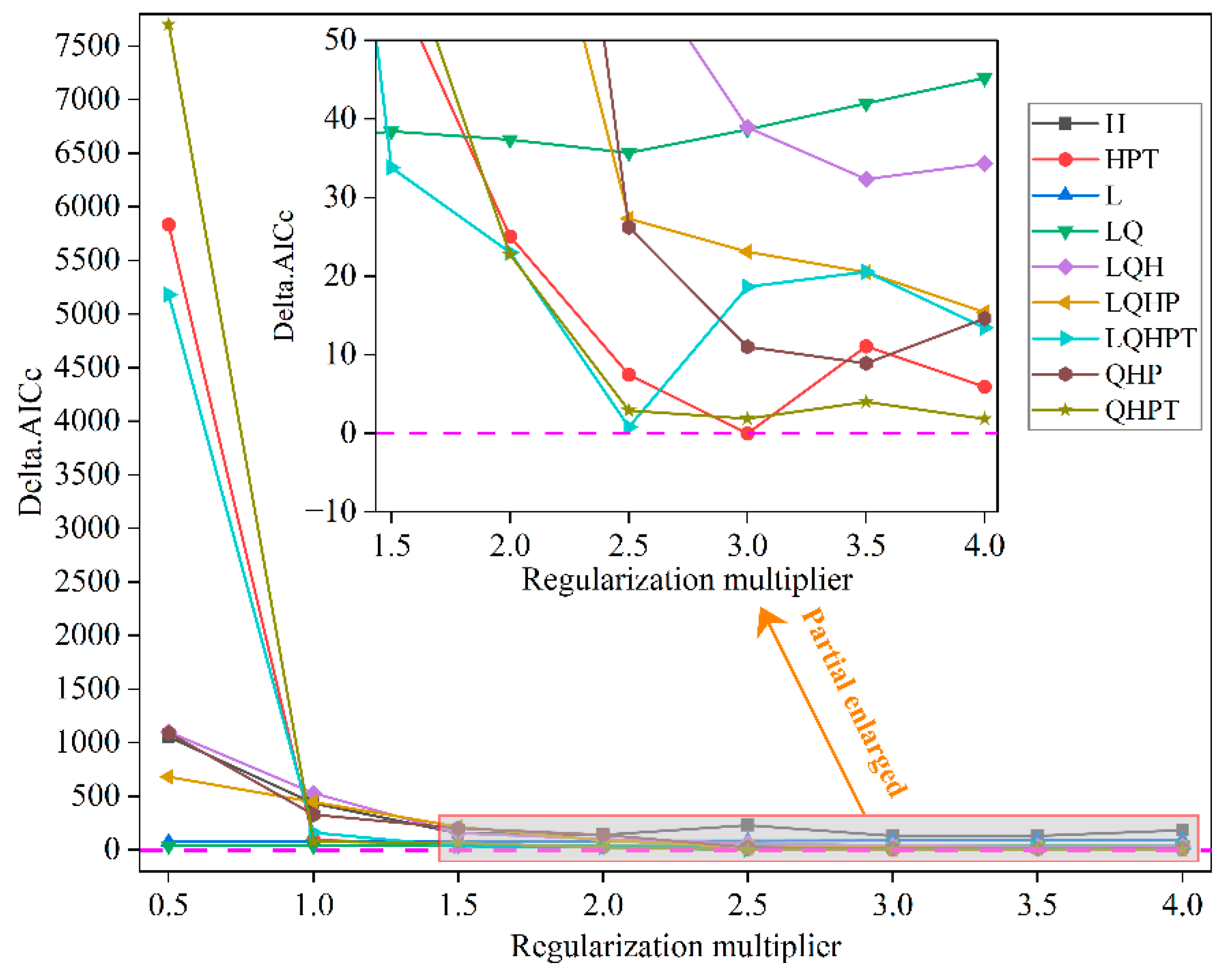
2.3.1. Parameter Optimization of MaxEnt Model
2.3.2. Parameter Configuration of the MaxEnt Model
2.3.3. Evaluation of Optimized Model Prediction Accuracy
2.4. Delineation of Potential Suitable Habitats
2.5. Spatial Pattern Changes in Potential Suitable Habitats
2.6. Centroid Shift in Potential Suitable Habitats
3. Results
3.1. Calibration and Accuracy Evaluation of the Maxent Model
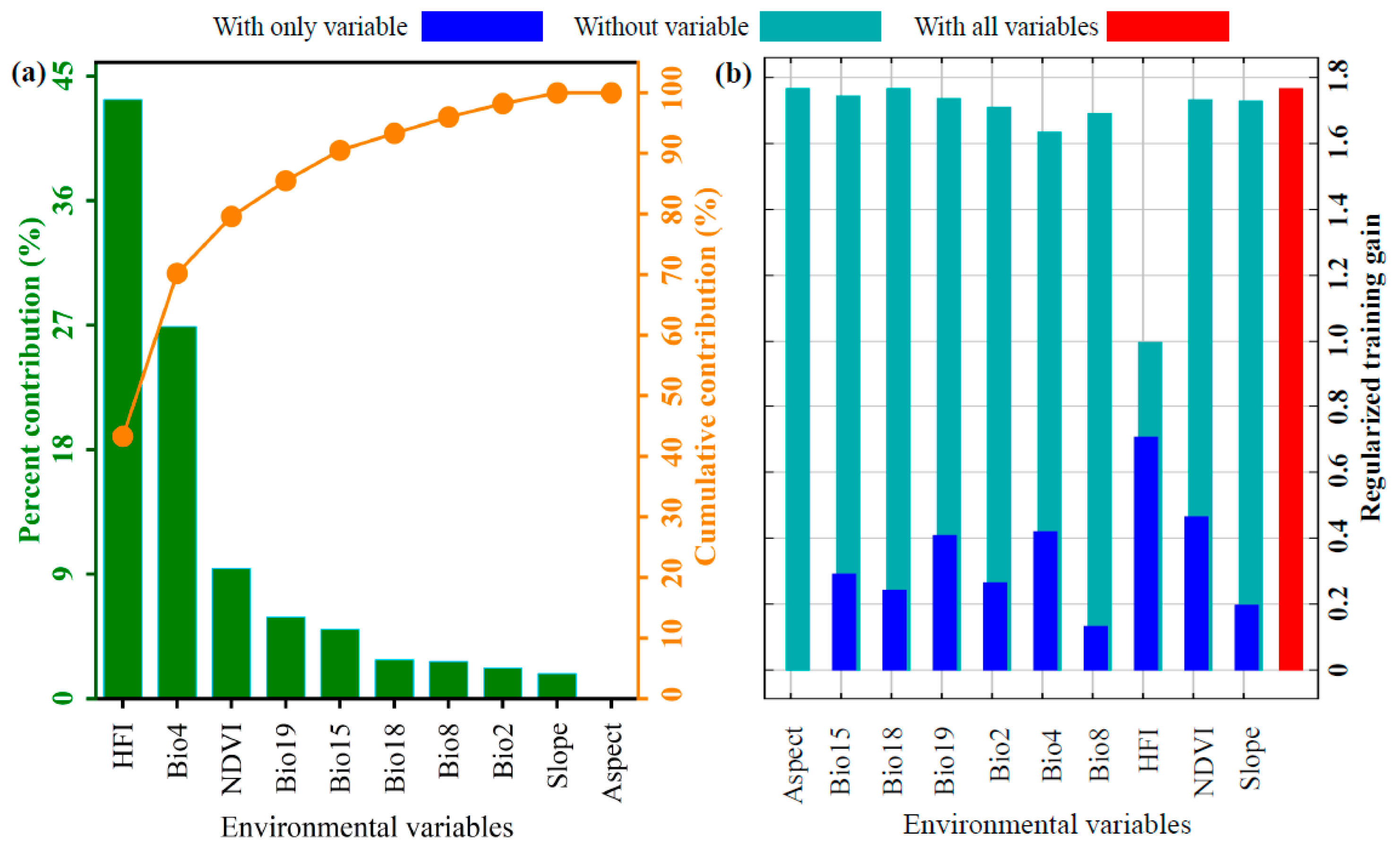
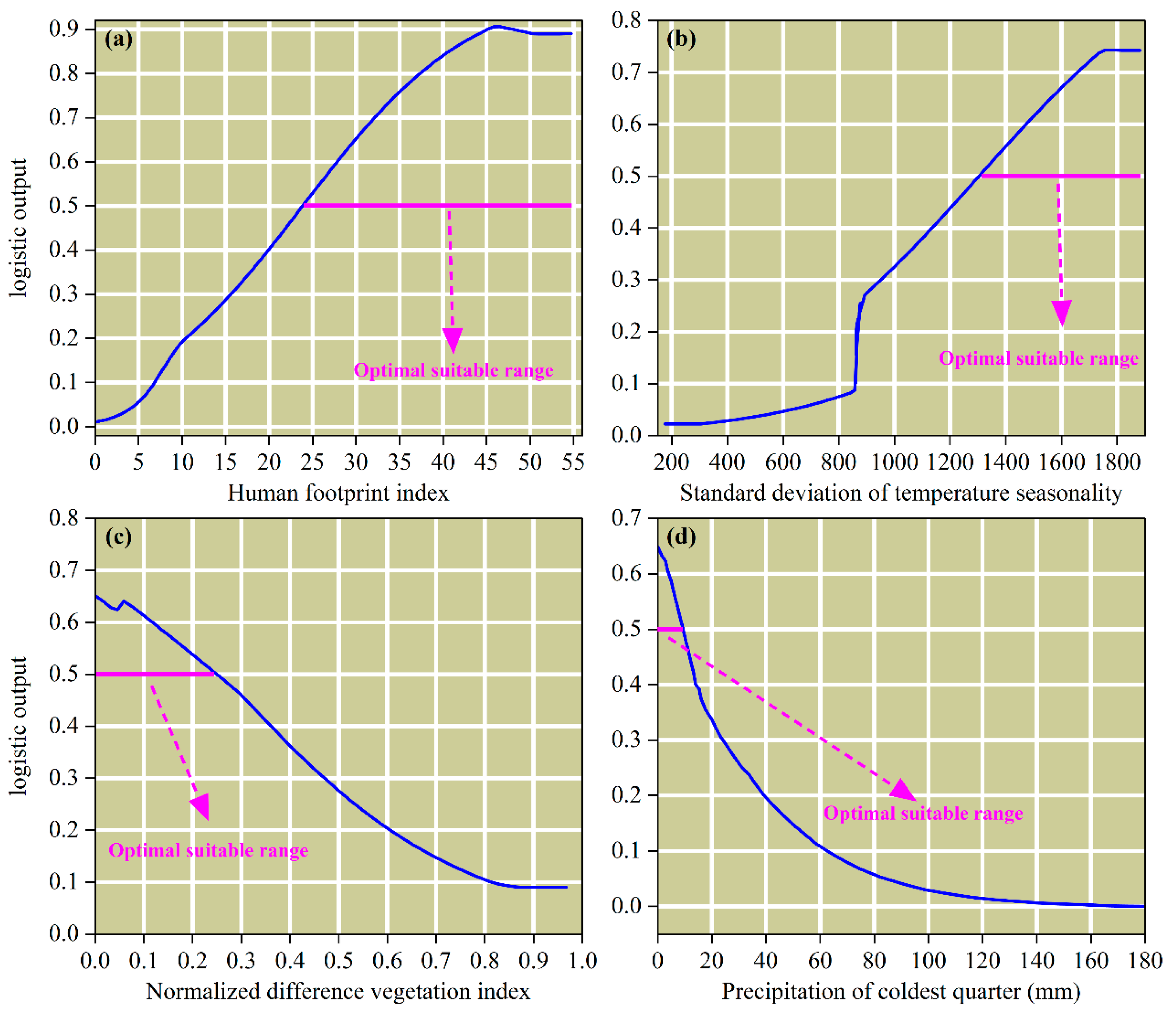
3.2. Key Environmental Variables Influencing the Distribution of P. tenuiflora
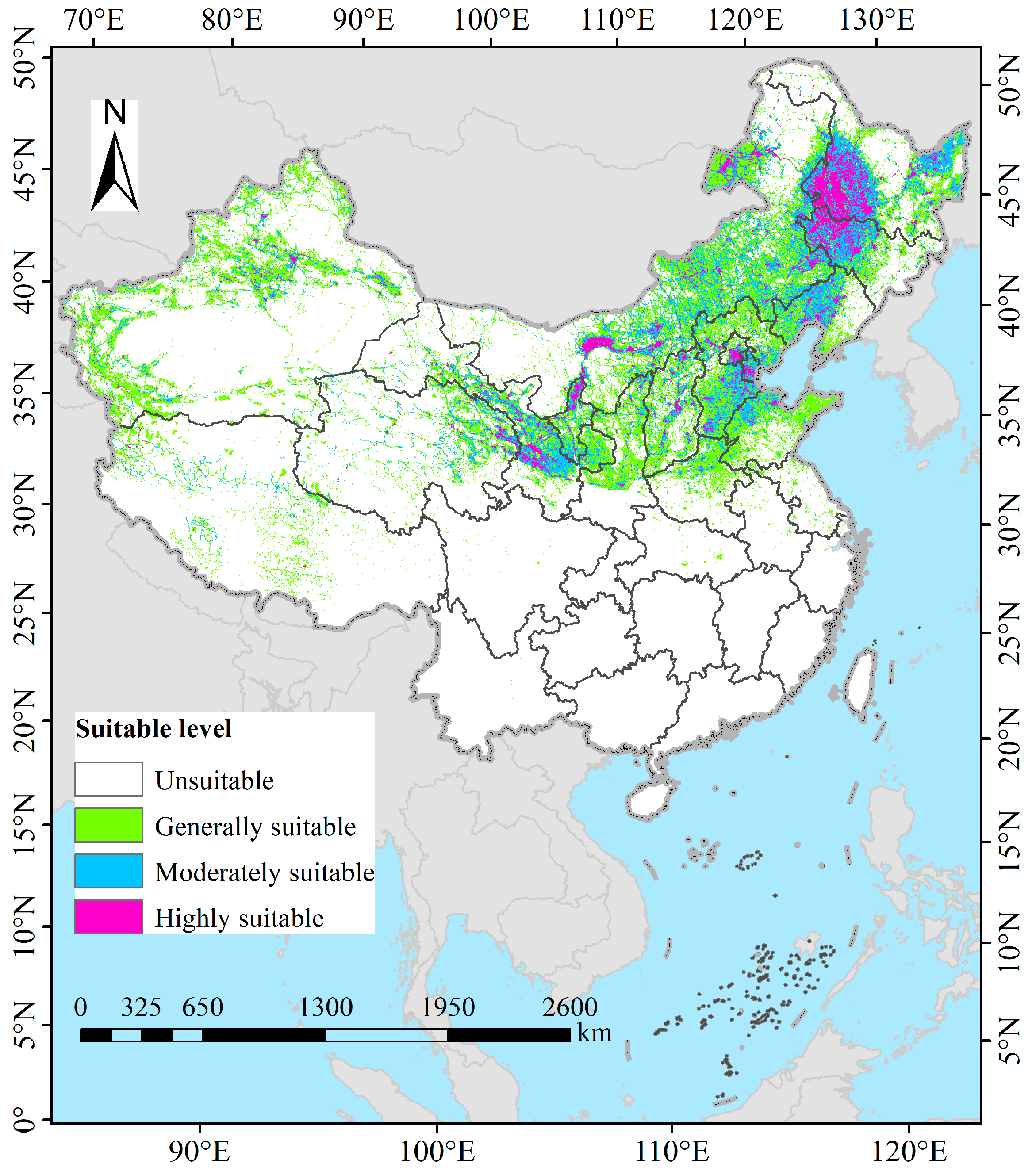
3.3. Potential Suitable Habitats of P. tenuiflora in China Under Current Climatic Conditions
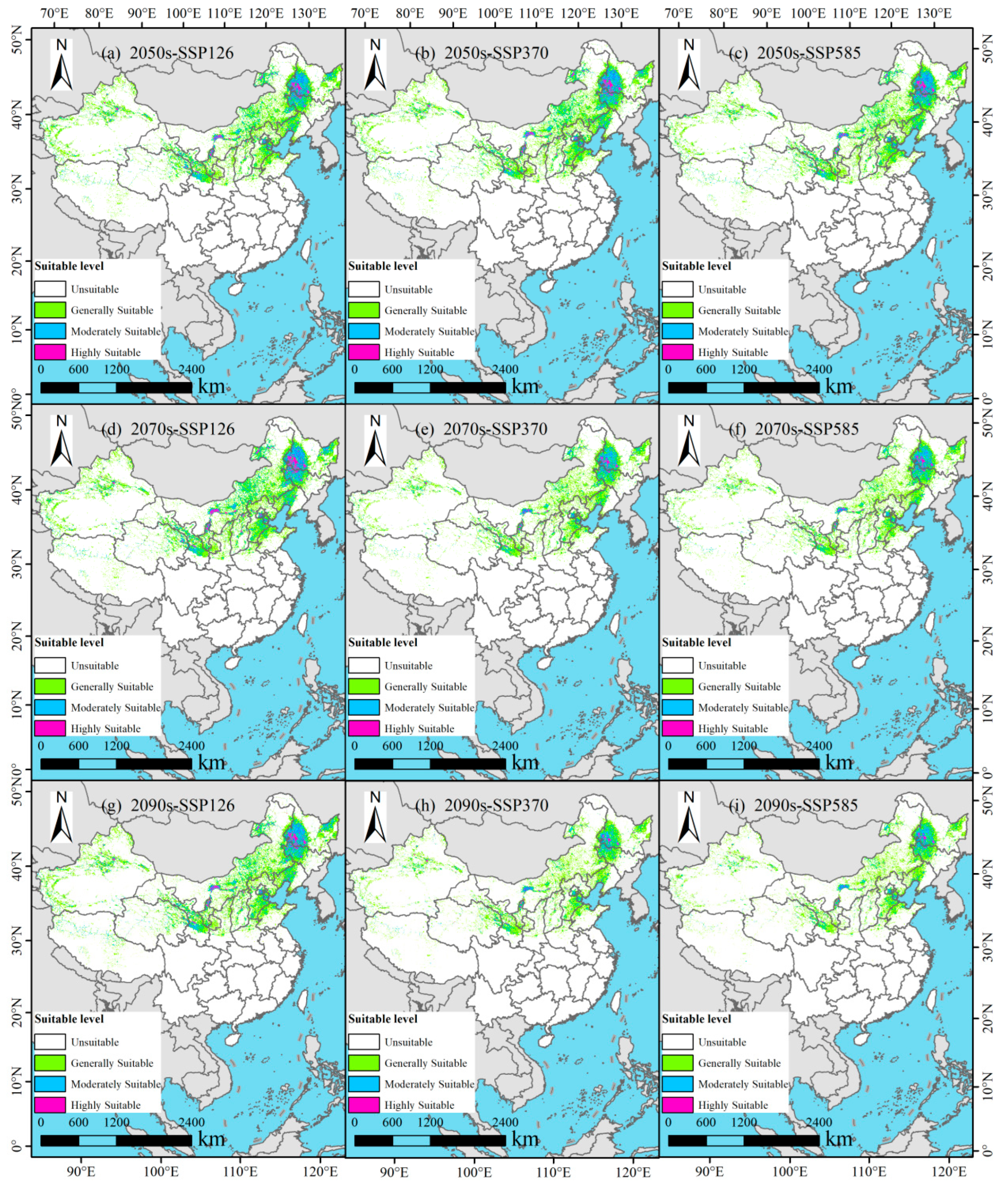
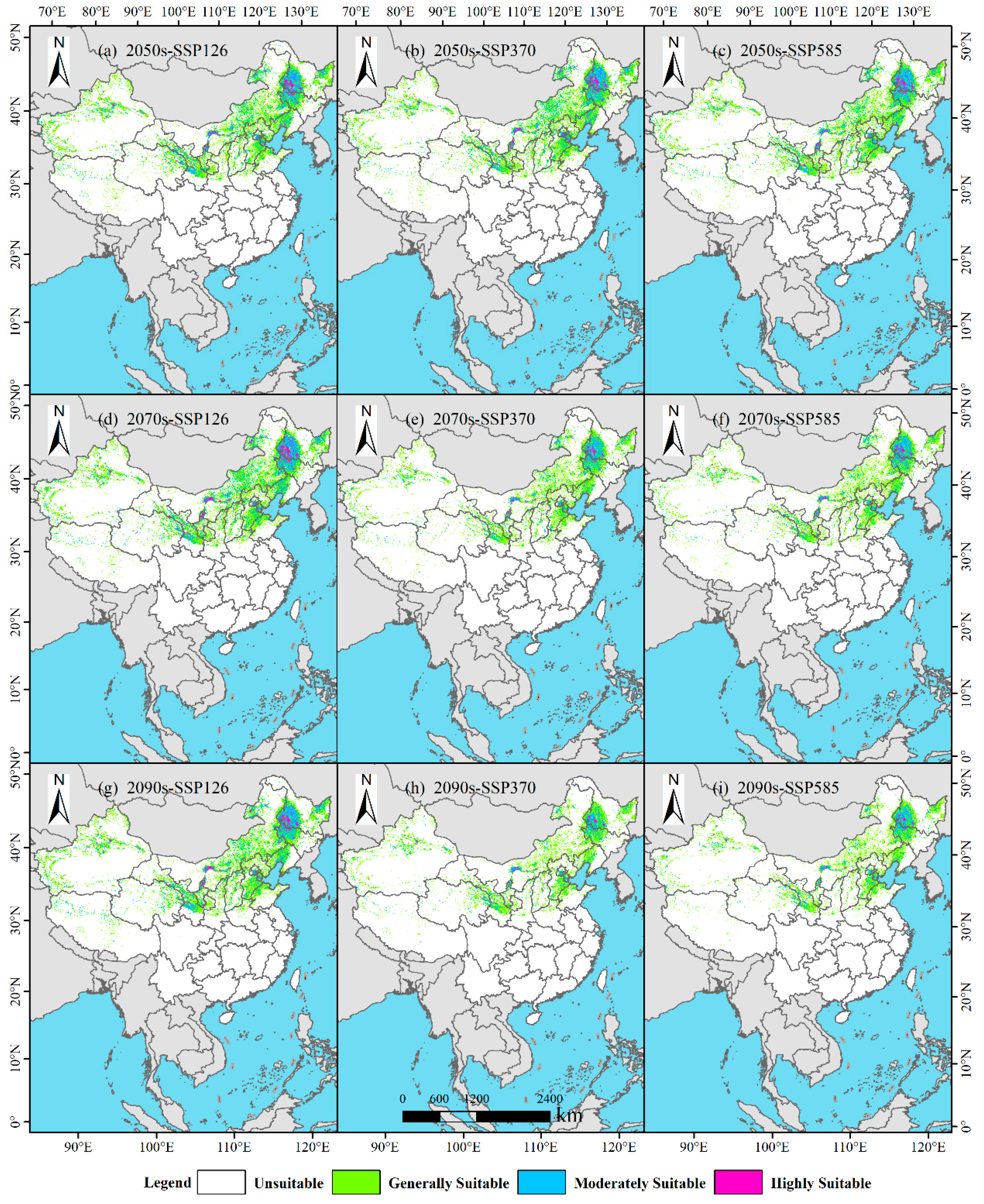
3.4. Potential Suitable Habitats of P. tenuiflora in China Under Future Climate Scenarios
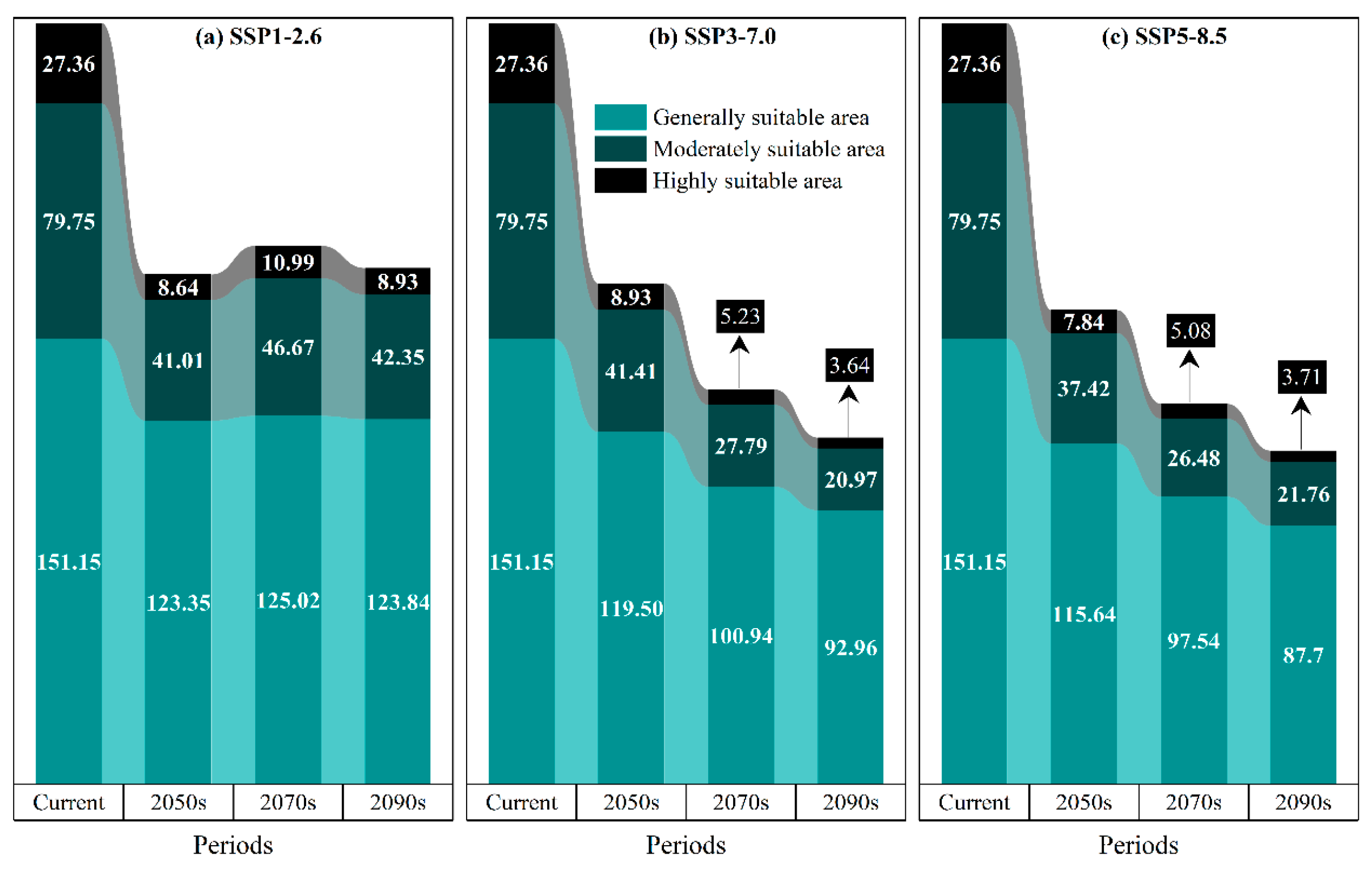
3.5. Change Patterns of Suitable Habitats for P. tenuiflora Across Different Periods
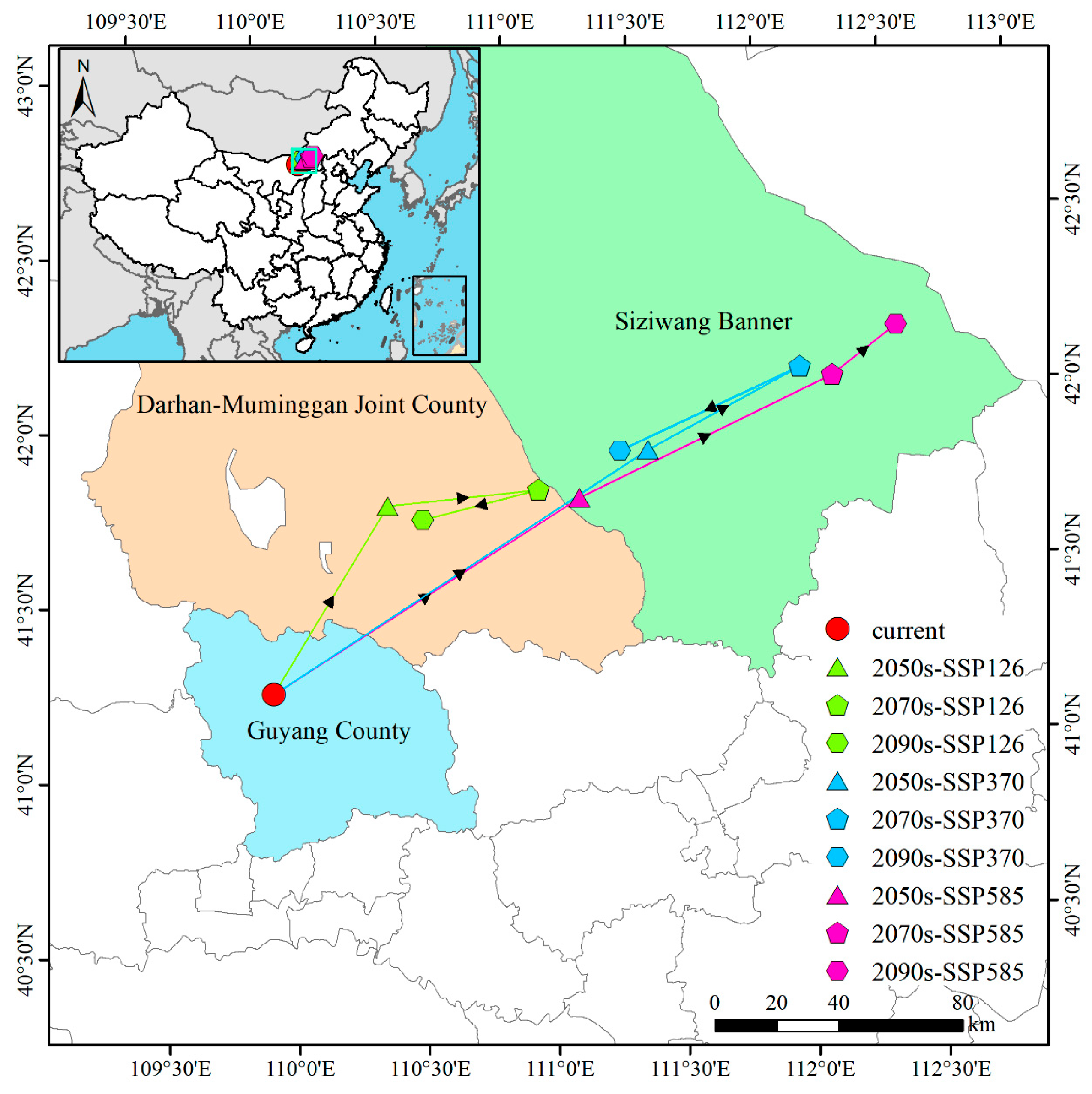
3.6. Centroid Shifts in Suitable Habitats for P. tenuiflora Under Different Climate Scenarios
4. Discussion
4.1. Optimization and Validation of the MaxEnt Model for Predicting the Distribution of P. tenuiflora
4.2. Key Environmental Factors Influencing the Distribution of Suitable Habitats for P. tenuiflora
4.3. Change Trends in Suitable Habitats of P. tenuiflora Under Climate Change Scenarios
4.4. Limitations of This Study and Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Howarth, C.; Viner, D. Integrating adaptation practice in assessments of climate change science: The case of IPCC Working Group II reports. Environ. Sci. Policy 2022, 135, 1–5. [Google Scholar] [CrossRef]
- Petrie, M.D.; Peters, D.P.C.; Yao, J.; Blair, J.M.; Burruss, N.D.; Collins, S.L.; Derner, J.D.; Gherardi, L.A.; Hendrickson, J.R.; Sala, O.E.; et al. Regional grassland productivity responses to precipitation during multiyear above- and below-average rainfall periods. Glob. Change Biol. 2018, 24, 1935–1951. [Google Scholar] [CrossRef]
- González-Orenga, S.; Boscaiu, M.; Vicente, O. Halophytes and Climate Change: Elucidation of Salt-Tolerance Mechanisms and Biodiversity Conservation. In Progress in Botany; Lüttge, U., Cánovas, F.M., Almeida, M.C.R., Leuschner, C., Pretzsch, H., Eds.; Springer Nature: Cham, Switzerland, 2024; Volume 85, pp. 223–263. [Google Scholar]
- Becerra-López, J.L.; Ramírez-Bautista, A.; Romero-Méndez, U.; Pavón, N.P.; Sánchez-Rojas, G. Effect of climate change on halophytic grasslands loss and its impact in the viability of Gopherus flavomarginatus. Nat. Conserv. 2017, 21, 39–55. [Google Scholar] [CrossRef]
- Bernatchez, L.; Ferchaud, A.-L.; Berger, C.S.; Venney, C.J.; Xuereb, A. Genomics for monitoring and understanding species responses to global climate change. Nat. Rev. Genet. 2024, 25, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Perret, D.L.; Evans, M.E.K.; Sax, D.F. A species’ response to spatial climatic variation does not predict its response to climate change. Proc. Natl. Acad. Sci. USA 2024, 121, e2304404120. [Google Scholar] [CrossRef]
- Malanoski, C.M.; Farnsworth, A.; Lunt, D.J.; Valdes, P.J.; Saupe, E.E. Climate change is an important predictor of extinction risk on macroevolutionary timescales. Science 2024, 383, 1130–1134. [Google Scholar] [CrossRef]
- Frans, V.F.; Liu, J. Gaps and opportunities in modelling human influence on species distributions in the Anthropocene. Nat. Ecol. Evol. 2024, 8, 1365–1377. [Google Scholar] [CrossRef]
- Wessely, J.; Essl, F.; Fiedler, K.; Gattringer, A.; Hülber, B.; Ignateva, O.; Moser, D.; Rammer, W.; Dullinger, S.; Seidl, R. A climate-induced tree species bottleneck for forest management in Europe. Nat. Ecol. Evol. 2024, 8, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, B.; Bacher, S.; Barbosa, A.M.; Gallien, L.; González-Moreno, P.; Martínez-Bolea, V.; Sorte, C.; Vimercati, G.; Vilà, M. Risks posed by invasive species to the provision of ecosystem services in Europe. Nat. Commun. 2024, 15, 2631. [Google Scholar] [CrossRef]
- Sorbe, F.; Gränzig, T.; Förster, M. Evaluating sampling bias correction methods for invasive species distribution modeling in Maxent. Ecol. Inform. 2023, 76, 102124. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, S.; Chen, S. Predicting the potential habitat suitability of Saussurea species in China under future climate scenarios using the optimized Maximum Entropy (MaxEnt) model. J. Clean. Prod. 2024, 474, 143552. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Guillera-Arroita, G.; Lahoz-Monfort, J.J. A review of evidence about use and performance of species distribution modelling ensembles like BIOMOD. Divers. Distrib. 2019, 25, 839–852. [Google Scholar] [CrossRef]
- Han, Q.Q.; Wang, Y.P.; Li, J.; Li, J.; Yin, X.C.; Jiang, X.Y.; Yu, M.; Wang, S.M.; Shabala, S.; Zhang, J.L. The mechanistic basis of sodium exclusion in Puccinellia tenuiflora under conditions of salinity and potassium deprivation. Plant J. 2022, 112, 322–338. [Google Scholar] [CrossRef]
- Han, L.; Gao, Z.; Li, L.; Li, C.; Yan, H.; Xiao, B.; Ma, Y.; Wang, H.; Yang, C.; Xun, H. Adaptive Strategy of the Perennial Halophyte Grass Puccinellia tenuiflora to Long-Term Salinity Stress. Plants 2024, 13, 3445. [Google Scholar] [CrossRef]
- Xu, T.; Zhang, M.; Ding, S.; Liu, B.; Chang, Q.; Zhao, X.; Wang, Y.; Wang, J.; Wang, L. Grassland degradation with saline-alkaline reduces more soil inorganic carbon than soil organic carbon storage. Ecol. Indic. 2021, 131, 108194. [Google Scholar] [CrossRef]
- Wang, J.; Xu, T.; Feng, X.; Zhu, W.; Zhang, L.; Pan, D.; Akram, N.A.; Ma, Q.; Zhong, Z.; Mahroof, S.; et al. Simulated grazing and nitrogen addition facilitate spatial expansion of Leymus chinensis clones into saline-alkali soil patches: Implications for Songnen grassland restoration in northeast China. Land Degrad. Dev. 2022, 33, 710–722. [Google Scholar] [CrossRef]
- Jin, L.; Li, X.; Sun, H.; Wang, J.; Zhang, J.; Zhang, Y. Effects of restoration years on vegetation and soil characteristics under different artificial measures in Alpine Mining Areas, West China. Sustainability 2022, 14, 10889. [Google Scholar] [CrossRef]
- Li, S.; Liu, W.; Shi, Z.; Liu, K.; Wang, W.; Liu, L.; Duan, H. Production performance in cultivated mixed-sown grasslands combining Poa pratensis L. and various Poaceae forage grasses. PLoS ONE 2025, 20, e0324084. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, C.; Han, L.; Li, C.; Xiao, B.; Wang, H.; Yang, C. Extensive secretion of phenolic acids and fatty acids facilitates rhizosphere pH regulation in halophyte Puccinellia tenuiflora under alkali stress. Physiol. Plant. 2022, 174, e13678. [Google Scholar] [CrossRef]
- Chao, Y.; Wang, Y.; Li, S.; Wang, X. Introduction test of Floret alkali grass onsaline-alkali land in Qaidam basin. Chin. Qinghai J. Anim. Vet. Sci. 2018, 48, 9–11, (In Chinese with English abstract). [Google Scholar]
- Zhang, Y.; Qin, C.; Liu, S.; Xu, Y.; Li, Y.; Zhang, Y.; Song, Y.; Sun, M.; Fu, C.; Qin, Z.; et al. Establishment of an efficient Agrobacterium-mediated genetic transformation system in halophyte Puccinellia tenuiflora. Mol. Breed. 2021, 41, 55. [Google Scholar] [CrossRef]
- Yang, N.; Song, X.; Lu, X.; Chen, Q.; Liu, J.; Liu, Y.; Wang, H.; Zhang, Z.; Tang, Z. Comparative study on metabolites and elements of two dominant plant communities in saline-alkali grassland. Environ. Exp. Bot. 2021, 190, 104587. [Google Scholar] [CrossRef]
- Sun, W.; Zhou, B.; Zhou, H.; Zhao, T.; Shi, M.; Xiao, R. Climatic suitability zoning of natural pasture cultivation in Qinghai Province. Pratacultural Sci. 2023, 40, 885–894. [Google Scholar]
- Chen, J.; Liu, J.; Zhu, R.; Li, J.; Di, G.; Zhang, Q.; Mao, D.; Kong, X. Analysis of suitable bioclimatic characteristics of Leymus chinensis Using MaxEnt Model. Acta Agrestia Sin. 2019, 27, 35–42. [Google Scholar]
- Wu, Z.; Hou, X.; Ren, W.; Wang, Z.; Chang, C.; Yang, Y.; Yang, Y. Prediction of the potential geographic distribution of Levmus chinensis based on MaxEnt and colection and protection of germplasm. Acta Prataculturae Sin. 2018, 27, 125–135. [Google Scholar]
- Zhang, Y.; Liu, G.; Lu, Q.; Xiong, D.; Li, G.; Du, S. Understanding the limiting climatic factors on the suitable habitat of Chinese Alfalfa. Forests 2022, 13, 482. [Google Scholar] [CrossRef]
- Lv, X.; Zhou, G. Climatic Suitability of the Geographic Distribution of Stipa breviflora in Chinese Temperate Grassland under Climate Change. Sustainability 2018, 10, 3767. [Google Scholar] [CrossRef]
- Rehan, M.; Hassan, A.; Zeb, S.; Ullah, S.; Ahmad, F.; Bohnett, E.; Bosso, L.; Fida, T.; Kabir, M. Application of species distribution models to estimate and manage the Asiatic black bear (Ursus thibetanus) habitat in the Hindu Kush Mountains, Pakistan. Eur. J. Wildl. Res. 2024, 70, 62. [Google Scholar] [CrossRef]
- Mushagalusa, C.A.; Fandohan, A.B.; Glèlè Kakaï, R. Predicting species abundance using machine learning approach: A comparative assessment of random forest spatial variants and performance metrics. Model. Earth Syst. Environ. 2024, 10, 5145–5171. [Google Scholar] [CrossRef]
- Mu, H.; Li, X.; Wen, Y.; Huang, J.; Du, P.; Su, W.; Miao, S.; Geng, M. A global record of annual terrestrial Human Footprint dataset from 2000 to 2018. Sci. Data 2022, 9, 176. [Google Scholar] [CrossRef]
- Didan, K. MODIS/Terra Vegetation Indices Monthly L3 Global 1km SIN Grid V006; NASA Land Processes Distributed Active Archive Center: Sioux Falls, SD, USA, 2015. [CrossRef]
- Wu, T.; Lu, Y.; Fang, Y.; Xin, X.; Li, L.; Li, W.; Jie, W.; Zhang, J.; Liu, Y.; Zhang, L.; et al. The Beijing Climate Center Climate System Model (BCC-CSM): The main progress from CMIP5 to CMIP6. Geosci. Model Dev. 2019, 12, 1573–1600. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Naimi, B.; Hamm, N.A.S.; Groen, T.A.; Skidmore, A.K.; Toxopeus, A.G. Where is positional uncertainty a problem for species distribution modelling? Ecography 2014, 37, 191–203. [Google Scholar] [CrossRef]
- Zong, M.; Han, G.; Li, Y.; Wang, G.; Wang, A.; Yang, X. Predicting the potential distribution of dominant species of the coastal wetland in the Yellow River Delta, China using MaxEnt model. Chin. J. Appl. Ecol. 2017, 28, 1833–1842. [Google Scholar]
- Yang, S.; Wang, H.; Tong, J.; Bai, Y.; Alatalo, J.M.; Liu, G.; Fang, Z.; Zhang, F. Impacts of environment and human activity on grid-scale land cropping suitability and optimization of planting structure, measured based on the MaxEnt model. Sci. Total Environ. 2022, 836, 155356. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L.; Xue, J.; Shi, P.; Liang, S. Habitat Suitability Shifts of Eucommia ulmoides in Southwest China Under Climate Change Projections. Biology 2025, 14, 451. [Google Scholar] [CrossRef]
- Mendes, P.; Velazco, S.J.E.; Andrade, A.F.A.d.; De Marco, P. Dealing with overprediction in species distribution models: How adding distance constraints can improve model accuracy. Ecol. Model. 2020, 431, 109180. [Google Scholar] [CrossRef]
- Kass, J.M.; Muscarella, R.; Galante, P.J.; Bohl, C.L.; Pinilla-Buitrago, G.E.; Boria, R.A.; Soley-Guardia, M.; Anderson, R.P. ENMeval 2.0: Redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods Ecol. Evol. 2021, 12, 1602–1608. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–175. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, X.; Jiang, X.; Fan, T.; Liang, X.; Yan, W. MaxEnt modeling for predicting suitable habitat for endangered tree Keteleeria davidiana (Pinaceae) in China. Forests 2023, 14, 394. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef]
- Phillips, S.J.; Anderson, R.P.; Dudík, M.; Schapire, R.E.; Blair, M.E. Opening the black box: An open-source release of Maxent. Ecography 2017, 40, 887–893. [Google Scholar] [CrossRef]
- Anderson, R.P.; Gonzalez, I. Species-specific tuning increases robustness to sampling bias in models of species distributions: An implementation with Maxent. Ecol. Model. 2011, 222, 2796–2811. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Shi, X.; Wang, J.; Zhang, L.; Chen, S.; Zhao, A.; Ning, X.; Fan, G.; Wu, N.; Zhang, L.; Wang, Z. Prediction of the potentially suitable areas of Litsea cubeba in China based on future climate change using the optimized MaxEnt model. Ecol. Indic. 2023, 148, 110093. [Google Scholar] [CrossRef]
- Wu, Z.; Gao, T.; Luo, Y.; Shi, J. Prediction of the global potential geographical distribution of Hylurgus ligniperda using a maximum entropy model. For. Ecosyst. 2022, 9, 100042. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Zhu, Z.; Tao, Y.; Xiang, J. Predicting the potential suitable distribution area of Emeia pseudosauteri in Zhejiang Province based on the MaxEnt model. Sci. Rep. 2023, 13, 1806. [Google Scholar] [CrossRef]
- Wang, J.; Xu, M.; Ngawang, N.; Chen, Y.; Bonjor, N.; Jia, X.; Zeng, Z.; Qiong, L. The potential habitat of Phlomoides rotata in Tibet was based on an optimized MaxEnt model. Front. Plant Sci. 2025, 16, 1560603. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.K.M.; Stevens, M.H.H. Temperature, topography, soil characteristics, and NDVI drive habitat preferences of a shade-tolerant invasive grass. Ecol. Evol. 2020, 10, 10785–10797. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Z.; Gao, C.; Dong, Y.; Jing, Z.; Du, L.; Hou, X. MaxEnt-Based predictions of suitable potential distribution of Leymus secalinus under current and future climate change. Plants 2025, 14, 293. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Su, M.; Liu, M.; Yang, J.; Wu, Q. Spatial Distribution Patterns of the Key Afforestation Species Cupressus funebris: Insights from an Ensemble Model under Climate Change Scenarios. Forests 2024, 15, 1280. [Google Scholar] [CrossRef]
- Fialas, P.C.; Santini, L.; Russo, D.; Amorim, F.; Rebelo, H.; Novella-Fernandez, R.; Marques, F.; Domer, A.; Vella, A.; Martinoli, A.; et al. Changes in community composition and functional diversity of European bats under climate change. Conserv. Biol. 2025, 39, e70025. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.L.; Bennett, J.R.; French, C.M. SDMtoolbox 2.0: The next generation Python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. PeerJ 2017, 5, e4095. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zhang, L.; Wang, Y.; Liu, Y.; Ma, Y. The optimized Maxent model reveals the pattern of distribution and changes in the suitable cultivation areas for Reaumuria songarica being driven by climate change. Ecol. Evol. 2024, 14, e70015. [Google Scholar] [CrossRef]
- Li, X.; Wu, K.; Hao, S.; Yue, Z.; Ran, Z.; Ma, J. Mapping cropland suitability in China using optimized MaxEnt model. Field Crops Res. 2023, 302, 109064. [Google Scholar] [CrossRef]
- Warren, D.L.; Seifert, S.N. Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, J.; Mao, Y.; Liu, Z.; Zhao, G.; Zhang, F. Prediction of the potentially suitable areas of Elymus dahuricus Turcz in China under climate change based on maxent. Sci. Rep. 2025, 15, 17959. [Google Scholar] [CrossRef]
- Xiang, Y.; Li, S.; Yang, Q.; Ren, J.; Liu, Y.; Luo, Y.; Zhao, L.; Luo, X.; Yao, B.; Guo, X. Forecasting northward range expansion of Switchgrass in China via multi-Scenario MaxEnt simulations. Biology 2025, 14, 1061. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, R.; Gao, L.; Huang, D.; Fan, Y.; Liu, C.; Chen, J. Predicting the current and future distributions of Pennisetum alopecuroides (L.) in China under climate change based on the MaxEnt model. PLoS ONE 2023, 18, e0281254. [Google Scholar] [CrossRef]
- Xing, Y.; Shi, J.; De, K.; Wang, X.; Wang, W.; Ma, Y.; Zhang, H.; He, M.; Liu, Q. The Current Distribution of Carex alatauensis in the Qinghai–Tibet Plateau Estimated by MaxEnt. Agronomy 2023, 13, 564. [Google Scholar] [CrossRef]
- Radosavljevic, A.; Anderson, R.P. Making better Maxent models of species distributions: Complexity, overfitting and evaluation. J. Biogeogr. 2014, 41, 629–643. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Wang, P.; Liu, G.; Li, X.; Su, Y.; Li, X.; Wei, L.; Zhang, J.; Zeng, Y.; Zhou, J. Potential distribution of Elymus nutans in China under future climate scenarios. Chin. J. Ecol. 2025, 44, 590–599. [Google Scholar]
- Tuo, H.; Ghanizadeh, H.; Ji, X.; Yang, M.; Wang, Z.; Huang, J.; Wang, Y.; Tian, H.; Ye, F.; Li, W. Moderate grazing enhances ecosystem multifunctionality through leaf traits and taxonomic diversity in long-term fenced grasslands. Sci. Total Environ. 2024, 957, 177781. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Ren, W.; Chi, Y.; Zheng, H.; Fry, E.; Yuan, F.; Liu, Y. Heavy grazing reduces the potential for grassland restoration: A global meta-analysis. Environ. Res. Lett. 2024, 19, 103001. [Google Scholar] [CrossRef]
- Ma, B.; Sun, J. Predicting the distribution of Stipa purpurea across the Tibetan Plateau via the MaxEnt model. BMC Ecol. 2018, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Zhang, J.L.; Liu, X.S.; Li, Z.; Wu, G.Q.; Cai, J.Y.; Flowers, T.J.; Wang, S.M. Puccinellia tenuiflora maintains a low Na+ level under salinity by limiting unidirectional Na+ influx resulting in a high selectivity for K+ over Na+. Plant Cell Environ. 2009, 32, 486–496. [Google Scholar] [CrossRef]
- Wang, W.; Guo, J. The contribution of root respiration to soil CO2 efflux in Puccinellia tenuiflora dominated community in a semi-arid meadow steppe. Chin. Sci. Bull. 2006, 51, 697–703. [Google Scholar] [CrossRef]
- Yan, S.; Shen, Y. Effects of ecological factors on salt-tolerance of Puccinellia tenuiflora seeds during germination. Acta Phytoecol. Sin. 1996, 20, 414–422. [Google Scholar]
- Deng, L.; Shangguan, Z.; Bell, S.M.; Soromotin, A.V.; Peng, C.; An, S.; Wu, X.; Xu, X.; Wang, K.; Li, J.; et al. Carbon in Chinese grasslands: Meta-analysis and theory of grazing effects. Carbon Res. 2023, 2, 19. [Google Scholar] [CrossRef]
- Gao, J.; Carmel, Y. Can the intermediate disturbance hypothesis explain grazing–diversity relations at a global scale? Oikos 2020, 129, 493–502. [Google Scholar] [CrossRef]
- Liu, H.; Mi, Z.; Lin, L.; Wang, Y.; Zhang, Z.; Zhang, F.; Wang, H.; Liu, L.; Zhu, B.; Cao, G.; et al. Shifting plant species composition in response to climate change stabilizes grassland primary production. Proc. Natl. Acad. Sci. USA 2018, 115, 4051–4056. [Google Scholar] [CrossRef] [PubMed]
- Silvertown, J.; Dodd, M.E.; Gowing, D.J.G.; Mountford, J.O. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature 1999, 400, 61–63. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Environ. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Williams, S.E.; Shoo, L.P.; Isaac, J.L.; Hoffmann, A.A.; Langham, G. Towards an integrated framework for assessing the vulnerability of species to climate change. PLOS Biol. 2008, 6, e325. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Wu, K.; Peng, J.; Mao, Y.; Zhao, G.; Zhang, F. Predicting suitable habitats and conservation areas for Suaeda salsa using MaxEnt and Marxan models. iScience 2025, 28, 112933. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.P.; Hermoso, V.; Lopes-Lima, M.; Miranda, R.; Filipe, A.F.; Sousa, R. The role of connectivity in conservation planning for species with obligatory interactions: Prospects for future climate scenarios. Glob. Change Biol. 2024, 30, e17169. [Google Scholar] [CrossRef]
- Muthukrishnan, R.; Smiley, T.M.; Title, P.O.; Fudickar, A.M.; Jahn, A.E.; Lau, J.A. Chasing the niche: Escaping climate change threats in place, time, and space. Glob. Change Biol. 2025, 31, e70167. [Google Scholar] [CrossRef]
| Category | Abbreviation | Environmental Variables | Units | VIF |
|---|---|---|---|---|
| Bioclimatic | Bio2 | Mean diurnal range (Mean of monthly) | °C | 2.63 |
| Bio4 | Standard deviation of temperature seasonality | 7.04 | ||
| Bio8 | Mean temperature of wettest quarter | °C | 7.13 | |
| Bio15 | Variation in precipitation seasonality | 2.93 | ||
| Bio18 | Precipitation of warmest quarter | mm | 3.71 | |
| Bio19 | Precipitation of coldest quarter | mm | 2.20 | |
| Topographic | Aspect | Aspect | ° | 3.31 |
| Slope | Slope | ° | 1.26 | |
| Human | HFI | Human footprint index | 1.50 | |
| Vegetation | NDVI | Normalized difference vegetation index | 1.57 |
| Period | Area (104 km2) | Rate of Change (%) | ||||
|---|---|---|---|---|---|---|
| Stability | Contraction | Expansion | Stability | Contraction | Expansion | |
| 2050s-SSP126 | 208.70 | 106.35 | 2.34 | 65.76 | 33.51 | 0.74 |
| 2070s-SSP126 | 220.94 | 94.11 | 1.91 | 69.71 | 29.69 | 0.60 |
| 2090s-SSP126 | 211.86 | 103.20 | 1.77 | 66.87 | 32.57 | 0.56 |
| 2050s-SSP370 | 206.24 | 108.81 | 0.94 | 65.27 | 34.44 | 0.30 |
| 2070s-SSP370 | 163.10 | 151.95 | 0.31 | 51.72 | 48.18 | 0.10 |
| 2090s-SSP370 | 142.65 | 172.41 | 0.77 | 45.17 | 54.59 | 0.24 |
| 2050s-SSP585 | 195.19 | 119.86 | 1.09 | 61.74 | 37.91 | 0.35 |
| 2070s-SSP585 | 157.05 | 158.01 | 0.44 | 49.78 | 50.08 | 0.14 |
| 2090s-SSP585 | 137.71 | 177.34 | 0.33 | 43.66 | 56.23 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wei, X.; Zhang, M.; Zhang, J. Potential Distribution and Response to Climate Change in Puccinellia tenuiflora in China Projected Using Optimized MaxEnt Model. Biology 2025, 14, 1426. https://doi.org/10.3390/biology14101426
Yang H, Wei X, Zhang M, Zhang J. Potential Distribution and Response to Climate Change in Puccinellia tenuiflora in China Projected Using Optimized MaxEnt Model. Biology. 2025; 14(10):1426. https://doi.org/10.3390/biology14101426
Chicago/Turabian StyleYang, Hao, Xiaoting Wei, Manyin Zhang, and Jinxin Zhang. 2025. "Potential Distribution and Response to Climate Change in Puccinellia tenuiflora in China Projected Using Optimized MaxEnt Model" Biology 14, no. 10: 1426. https://doi.org/10.3390/biology14101426
APA StyleYang, H., Wei, X., Zhang, M., & Zhang, J. (2025). Potential Distribution and Response to Climate Change in Puccinellia tenuiflora in China Projected Using Optimized MaxEnt Model. Biology, 14(10), 1426. https://doi.org/10.3390/biology14101426







