The Ubiquitination of Mycobacterium tuberculosis Rv3717 Promotes Proteasomal Degradation of Interleukin Enhancer-Binding Factor
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
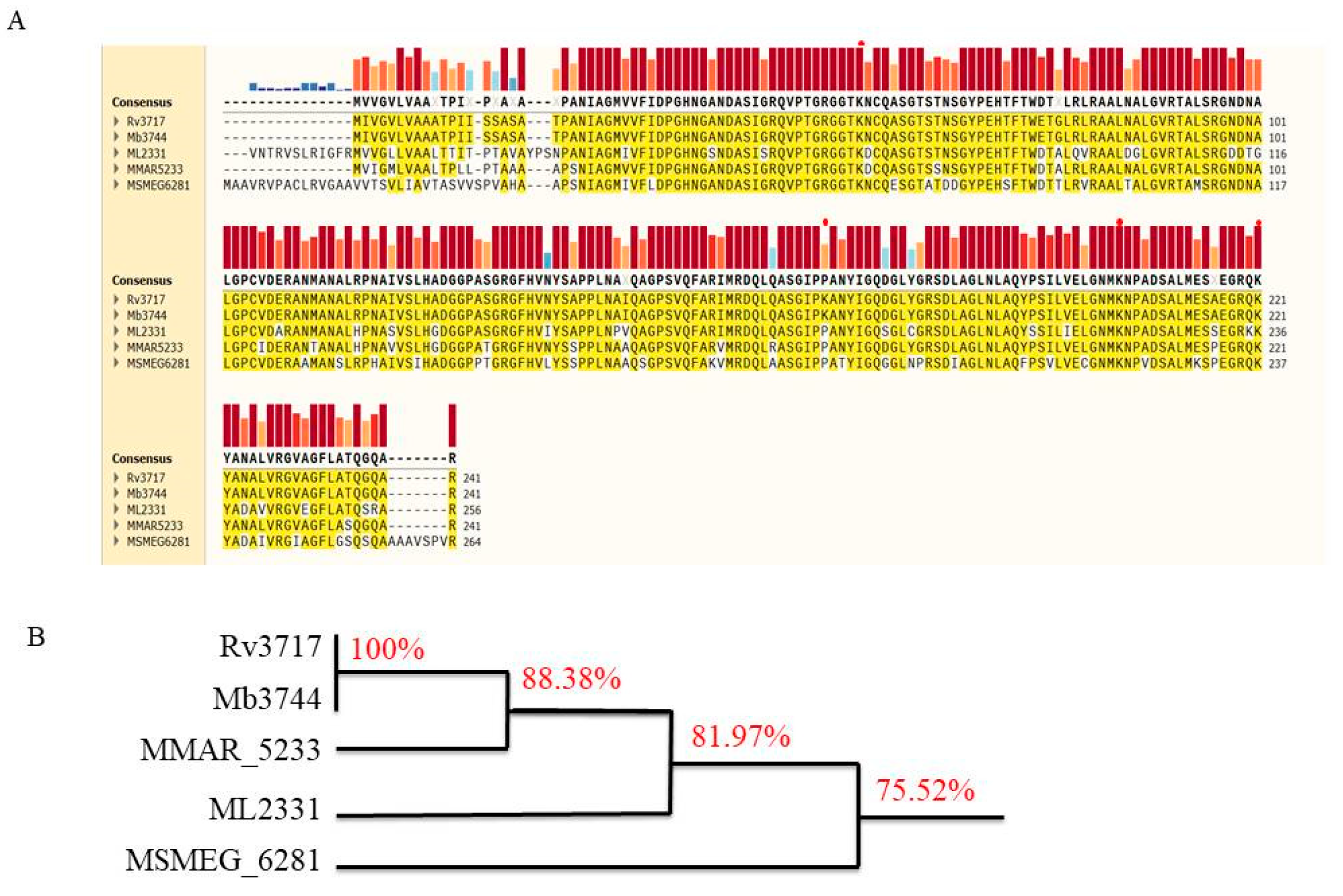
2.1. Cells and Plasmid Construction
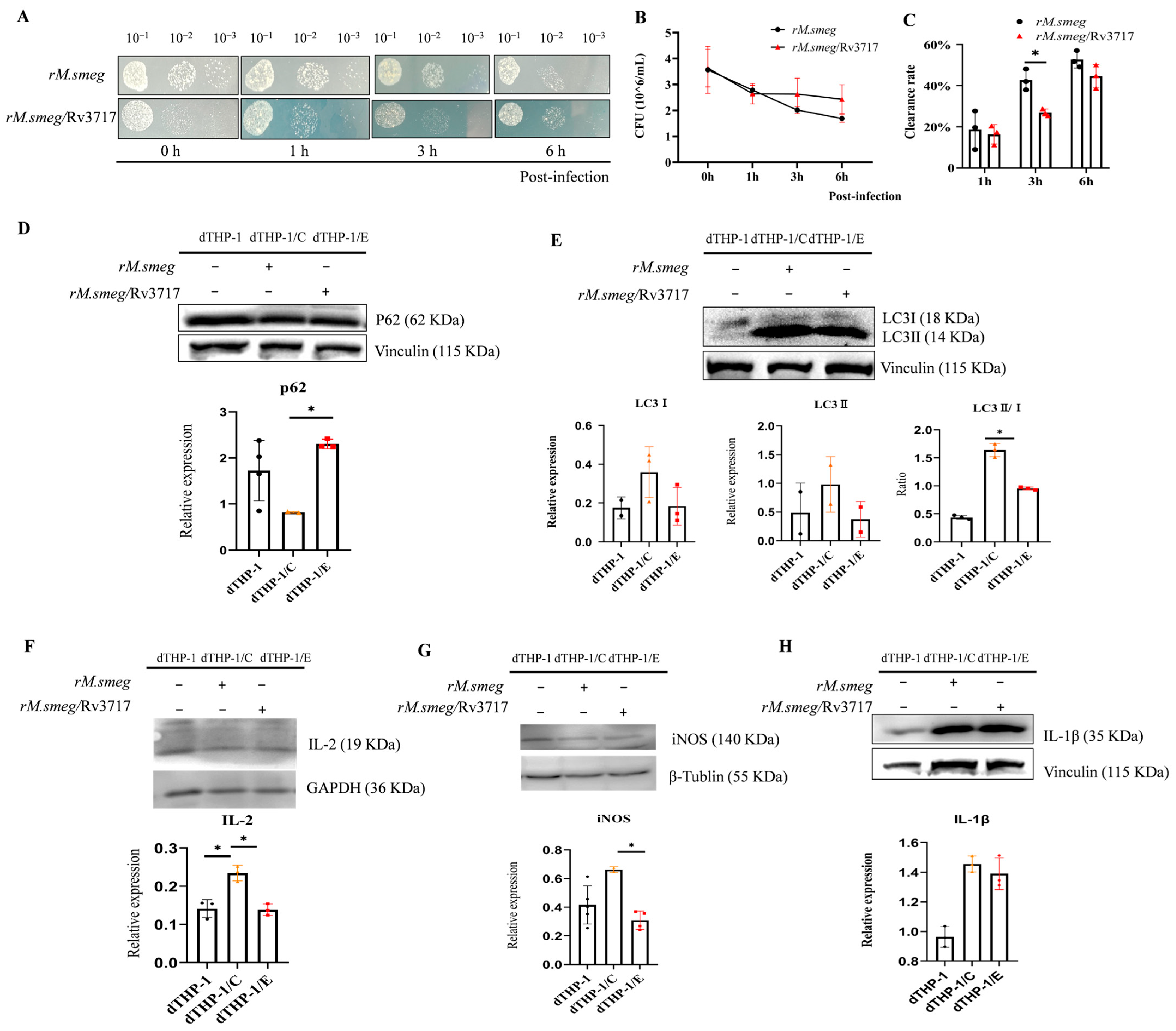
2.2. The Detection of Mycobacterial Clearance Rate in Macrophages
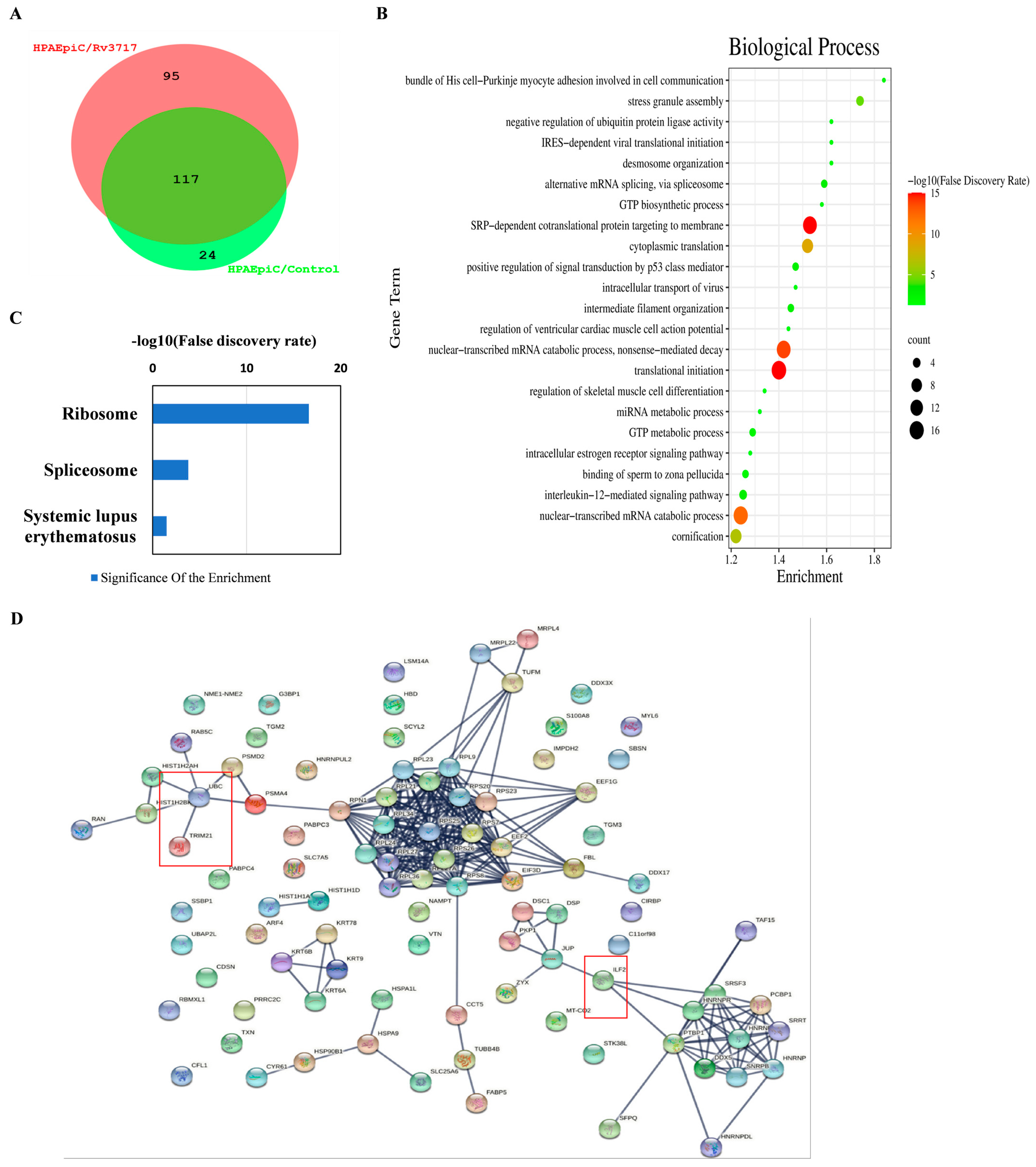
2.3. Co-Immunoprecipitation and LC–MS/MS
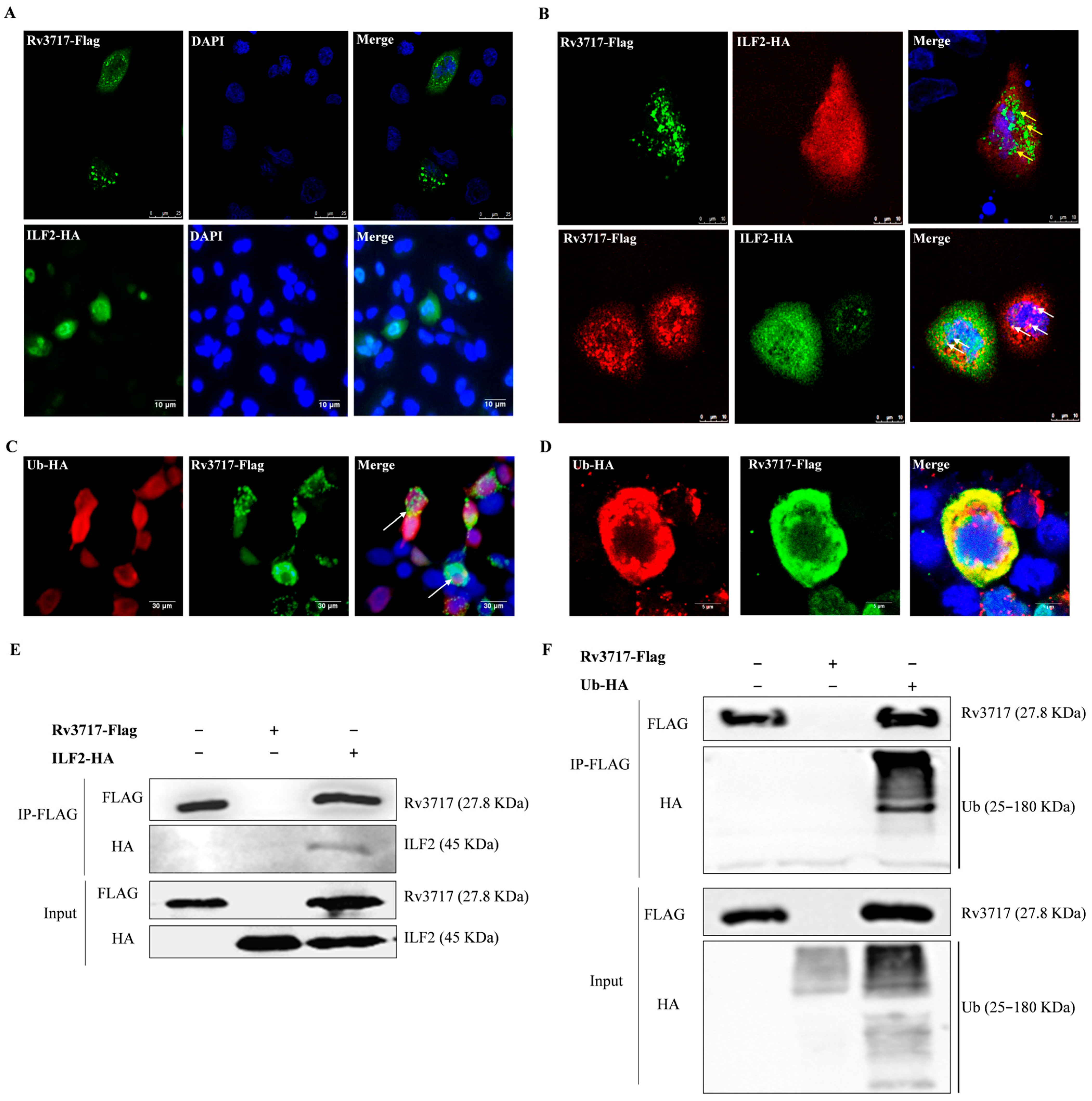
2.4. Immunofluorescence and Western Blotting
2.5. The Assessment of Proteasomal Degradation of Intracellular Proteins
3. Results
3.1. The Impact of Rv3717 on Mycobacterial Clearance and Immune Responses in Host Cells
3.2. Identification of Rv3717-Interacting Proteins
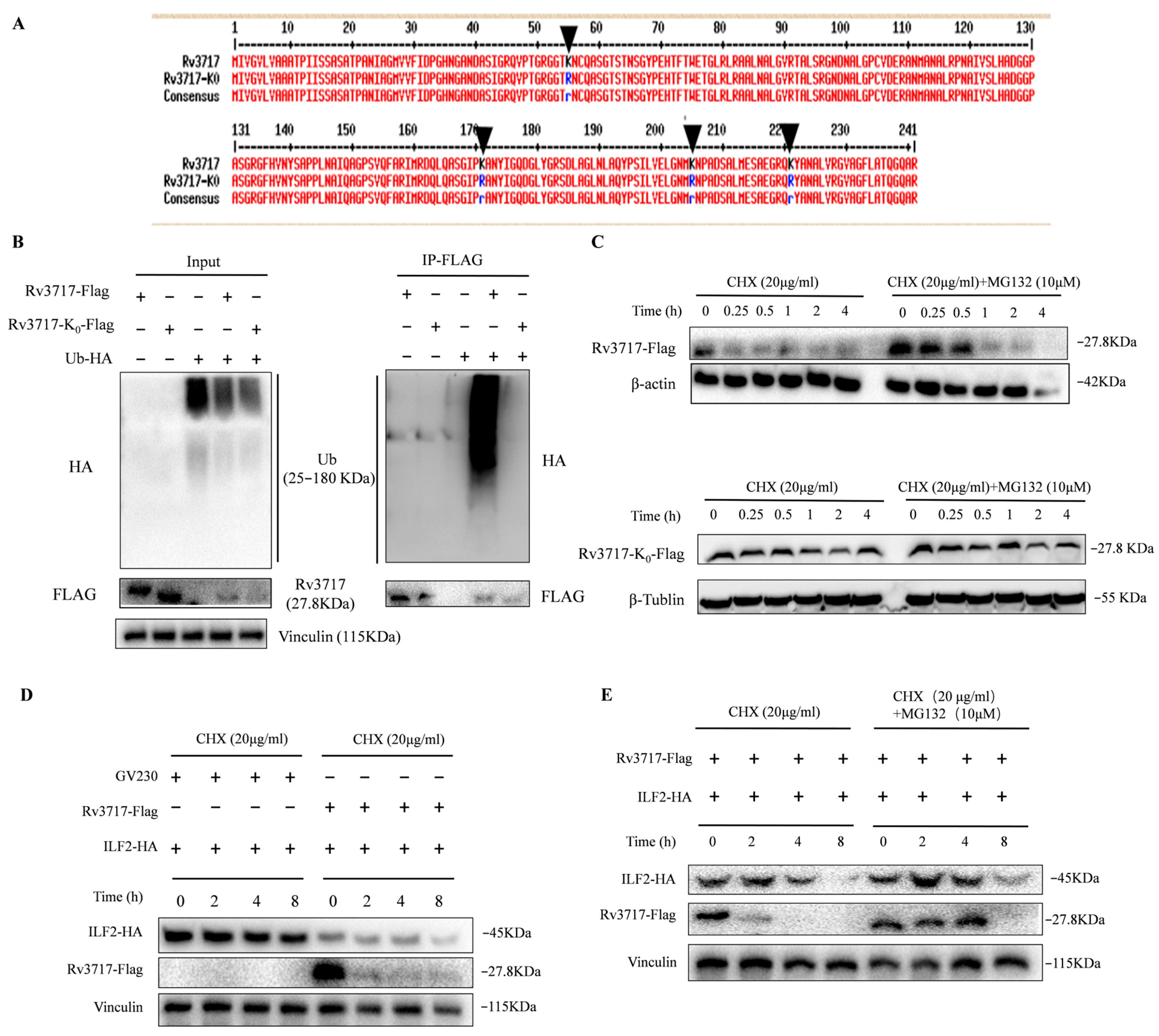
3.3. Rv3717-ILF2 and Rv3717-Ubiquitin Interactions
3.4. The Ubiquitination of Rv3717 Promotes Proteasomal Degradation of ILF2
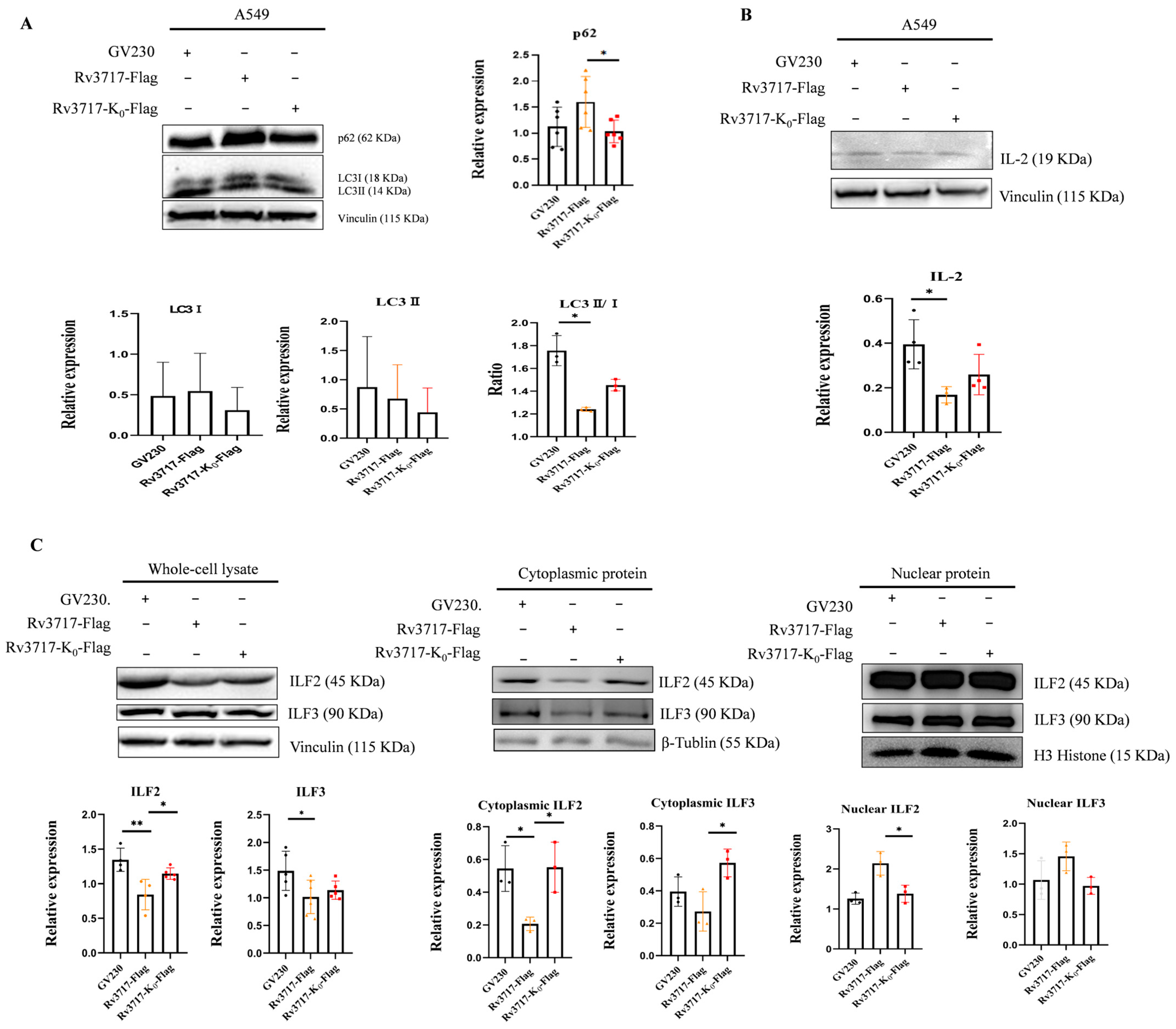
3.5. Effects of Rv3717 Ubiquitination on the Expression of Intracellular Immunity-Related Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2024. [Google Scholar]
- Athman, J.J.; Sande, O.J.; Groft, S.G.; Reba, S.M.; Nagy, N.; Wearsch, P.A.; Richardson, E.T. Mycobacterium tuberculosis Membrane Vesicles Inhibit T Cell Activation. J. Immunol. 2017, 198, 2028–2037. [Google Scholar] [CrossRef]
- Prados-Rosales, R.; Baena, A.; Martinez, L.R.; Luque-Garcia, J.; Kalscheuer, R.; Veeraraghavan, U.; Camara, C.; Nosanchuk, J.D.; Besra, G.S.; Chen, B.; et al. Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J. Clin. Investig. 2011, 121, 1471–1483. [Google Scholar] [CrossRef]
- Palacios, A.; Gupta, S.; Rodriguez, G.M.; Prados-Rosales, R. Extracellular vesicles in the context of Mycobacterium tuberculosis infection. Mol. Immunol. 2021, 133, 175–181. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Kumar, D.; Mishra, A.; Dewangan, R.P.; Shrivastava, P.; Ramachandran, S.; Taneja, B. The structure of Rv3717 reveals a novel amidase from Mycobacterium tuberculosis. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 2543–2554. [Google Scholar] [CrossRef]
- Küssau, T.; Van Wyk, N. Functional Characterization of the N-Acetylmuramyl-l-Alanine Amidase, Ami1, from Mycobacterium abscessus. Cells 2020, 9, 2410. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fu, W.; Zhao, S.; Zhang, C.; Sun, T.; Jiang, T. Lack of MSMEG_6281, a peptidoglycan amidase, affects cell wall integrity and virulence of Mycobacterium smegmatis. Microb. Pathog. 2019, 128, 405–413. [Google Scholar] [CrossRef]
- Li, X.; He, J.; Fu, W.; Cao, P.; Zhang, S.; Jiang, T. Effect of Mycobacterium tuberculosis Rv3717 on cell division and cell adhesion. Microb. Pathog. 2018, 117, 184–190. [Google Scholar] [CrossRef]
- Senzani, S.; Li, D.; Bhaskar, A.; Ealand, C.; Chang, J.; Rimal, B.; Liu, C.; Joon Kim, S.; Dhar, N.; Kana, B. An Amidase_3 domain-containing N-acetylmuramyl-L-alanine amidase is required for mycobacterial cell division. Sci. Rep. 2017, 7, 1140. [Google Scholar] [CrossRef]
- Gong, Z.; Yang, W.; Zhang, H.; Xiang, X.; Zeng, J.; Han, S.; Yang, J.; Xie, J. Mycobacterium tuberculosis Rv3717 enhances the survival of Mycolicibacterium smegmatis by inhibiting host innate immune and caspase-dependent apoptosis. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2020, 84, 104412. [Google Scholar] [CrossRef]
- Healy, C.; Gouzy, A.; Ehrt, S. Peptidoglycan Hydrolases RipA and Ami1 Are Critical for Replication and Persistence of Mycobacterium tuberculosis in the Host. mBio 2020, 11, 10–1128. [Google Scholar] [CrossRef]
- Sassetti, C.M.; Rubin, E.J. Genetic requirements for mycobacterial survival during infection. Proc. Natl. Acad. Sci. USA 2003, 100, 12989–12994. [Google Scholar] [CrossRef]
- Chandra, P.; Grigsby, S.J. Immune evasion and provocation by Mycobacterium tuberculosis. Nat. Rev. Microbiol. 2022, 20, 750–766. [Google Scholar] [CrossRef] [PubMed]
- Witt, K.D. Role of MHC class I pathways in Mycobacterium tuberculosis antigen presentation. Front. Cell. Infect. Microbiol. 2023, 13, 1107884. [Google Scholar] [CrossRef]
- Raunak, R.; Rakshit, R.; Bahl, A.; Sinha, S.; Pandey, S.; Kant, S.; Tripathi, D. Functional Characterization of MIP_07528 of Mycobacterium indicus pranii for Tyrosine Phosphatase Activity Displays Sensitivity to Oxidative Inactivation and Plays a Role in Immunomodulation. Biology 2025, 14, 565. [Google Scholar] [CrossRef]
- Höner zu Bentrup, K.; Russell, D.G. Mycobacterial persistence: Adaptation to a changing environment. Trends Microbiol. 2001, 9, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hanafiah, K.; Arifin, N.; Sanders, P.R.; Othman, N.; Garcia, M.L.; Anderson, D.A. Proteomic Analysis of Antigen 60 Complex of M. bovis Bacillus Calmette-Guérin Reveals Presence of Extracellular Vesicle Proteins and Predicted Functional Interactions. Vaccines 2019, 7, 80. [Google Scholar] [CrossRef]
- Jeong, M.; Jeon, H.; Shin, D. Ubiquitin-regulating effector proteins from Legionella. BMB Rep. 2022, 55, 316–322. [Google Scholar] [CrossRef]
- Kubori, T.; Shinzawa, N.; Kanuka, H.; Nagai, H. Legionella metaeffector exploits host proteasome to temporally regulate cognate effector. PLoS Pathog. 2010, 6, e1001216. [Google Scholar] [CrossRef]
- Shariq, M.; Quadir, N.; Sheikh, J.A.; Singh, A.K.; Bishai, W.R.; Ehtesham, N.Z.; Hasnain, S.E. Post translational modifications in tuberculosis: Ubiquitination paradox. Autophagy 2021, 17, 814–817. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, J.; Li, J.; Yang, H.; Tang, T.; Liang, H.; Zuo, M.; Wang, J.; Liu, H.; Liu, F.; et al. Host-mediated ubiquitination of a mycobacterial protein suppresses immunity. Nature 2020, 577, 682–688. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, Y. A mycobacterial effector promotes ferroptosis-dependent pathogenicity and dissemination. Nat. Commun. 2023, 14, 1430. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhu, C.; Xiao, W.; Huang, K.; Wang, X.; Shi, C.; Lin, D.; Zhang, H.; Liu, X.; Peng, B.; et al. Mycobacterium tuberculosis hijacks host TRIM21- and NCOA4-dependent ferritinophagy to enhance intracellular growth. J. Clin. Investig. 2023, 133, e159941. [Google Scholar] [CrossRef]
- Stricker, R.L.; Behrens, S.E.; Mundt, E. Nuclear factor NF45 interacts with viral proteins of infectious bursal disease virus and inhibits viral replication. J. Virol. 2010, 84, 10592–10605. [Google Scholar] [CrossRef]
- Tsai, H.I.; Zeng, X. NF45/NF90-mediated rDNA transcription provides a novel target for immunosuppressant development. EMBO Mol. Med. 2021, 13, e12834. [Google Scholar] [CrossRef] [PubMed]
- Wolkowicz, U.M.; Cook, A.G. NF45 dimerizes with NF90, Zfr and SPNR via a conserved domain that has a nucleotidyltransferase fold. Nucleic Acids Res. 2012, 40, 9356–9368. [Google Scholar] [CrossRef] [PubMed]
| Accession | Protein Names | Gene Names | MW [kDa] | Protein Score | Sequence Coverage (%) | Unique Peptides | Peptides | # PSMs |
|---|---|---|---|---|---|---|---|---|
| P15924 | Desmoplakin | DSP | 331.57 | 389.59 | 3.45 | 8 | 8 | 8 |
| Q15942 | Zyxin | ZYX | 61.24 | 150.67 | 11.71 | 4 | 4 | 4 |
| P26599 | Polypyrimidine tract-binding protein 1 | PTBP1 | 57.19 | 149.23 | 8.1 | 2 | 2 | 2 |
| P52597 | Heterogeneous nuclear ribonucleoprotein F | HNRNPF | 45.64 | 142.03 | 8.19 | 2 | 2 | 3 |
| P14923 | Junction plakoglobin | JUP | 81.69 | 139.51 | 4.43 | 3 | 3 | 3 |
| P17844 | Probable ATP-dependent RNA helicase DDX5 | DDX5 | 69.1 | 138.77 | 6.03 | 2 | 3 | 3 |
| P23246 | Splicing factor, proline- and glutamine-rich | SFPQ | 76.1 | 135.79 | 3.82 | 2 | 2 | 2 |
| Q08554 | Desmocollin-1 | DSC1 | 99.92 | 111.94 | 2.24 | 1 | 1 | 1 |
| Q9BXP5 | Serrate RNA effector molecule homolog | SRRT | 100.6 | 94.49 | 4.45 | 3 | 3 | 3 |
| P51991 | Heterogeneous nuclear ribonucleoprotein A3 | HNRNPA3 | 39.57 | 79.1 | 6.61 | 2 | 2 | 2 |
| O14979 | Heterogeneous nuclear ribonucleoprotein D-like | HNRNPDL | 46.41 | 76.05 | 3.81 | 1 | 1 | 1 |
| Q92804 | TATA-binding protein-associated factor 2N | TAF15 | 61.79 | 71.83 | 4.05 | 1 | 2 | 2 |
| Q12905 | Interleukin enhancer-binding factor 2 | ILF2 | 43.04 | 70.35 | 3.59 | 1 | 1 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gui, X.-W.; Zhang, T.-F.; Zheng, A.-Q.; Guo, M.-X.; Dong, Q.-W.; Jiang, T. The Ubiquitination of Mycobacterium tuberculosis Rv3717 Promotes Proteasomal Degradation of Interleukin Enhancer-Binding Factor. Biology 2025, 14, 1414. https://doi.org/10.3390/biology14101414
Gui X-W, Zhang T-F, Zheng A-Q, Guo M-X, Dong Q-W, Jiang T. The Ubiquitination of Mycobacterium tuberculosis Rv3717 Promotes Proteasomal Degradation of Interleukin Enhancer-Binding Factor. Biology. 2025; 14(10):1414. https://doi.org/10.3390/biology14101414
Chicago/Turabian StyleGui, Xu-Wen, Teng-Fei Zhang, An-Qi Zheng, Ming-Xin Guo, Qian-Wei Dong, and Tao Jiang. 2025. "The Ubiquitination of Mycobacterium tuberculosis Rv3717 Promotes Proteasomal Degradation of Interleukin Enhancer-Binding Factor" Biology 14, no. 10: 1414. https://doi.org/10.3390/biology14101414
APA StyleGui, X.-W., Zhang, T.-F., Zheng, A.-Q., Guo, M.-X., Dong, Q.-W., & Jiang, T. (2025). The Ubiquitination of Mycobacterium tuberculosis Rv3717 Promotes Proteasomal Degradation of Interleukin Enhancer-Binding Factor. Biology, 14(10), 1414. https://doi.org/10.3390/biology14101414






