Ectoplana limuli, a Parasite of the Horseshoe Crab (Tachypleus tridentatus): A New Record in China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Management
2.2. Prevalence Analysis
2.3. Parasite Morphological Observation
2.4. Histological Observation
2.5. Molecular Biological Identification
2.6. Phylogenetic Analysis
3. Results
3.1. Prevalence
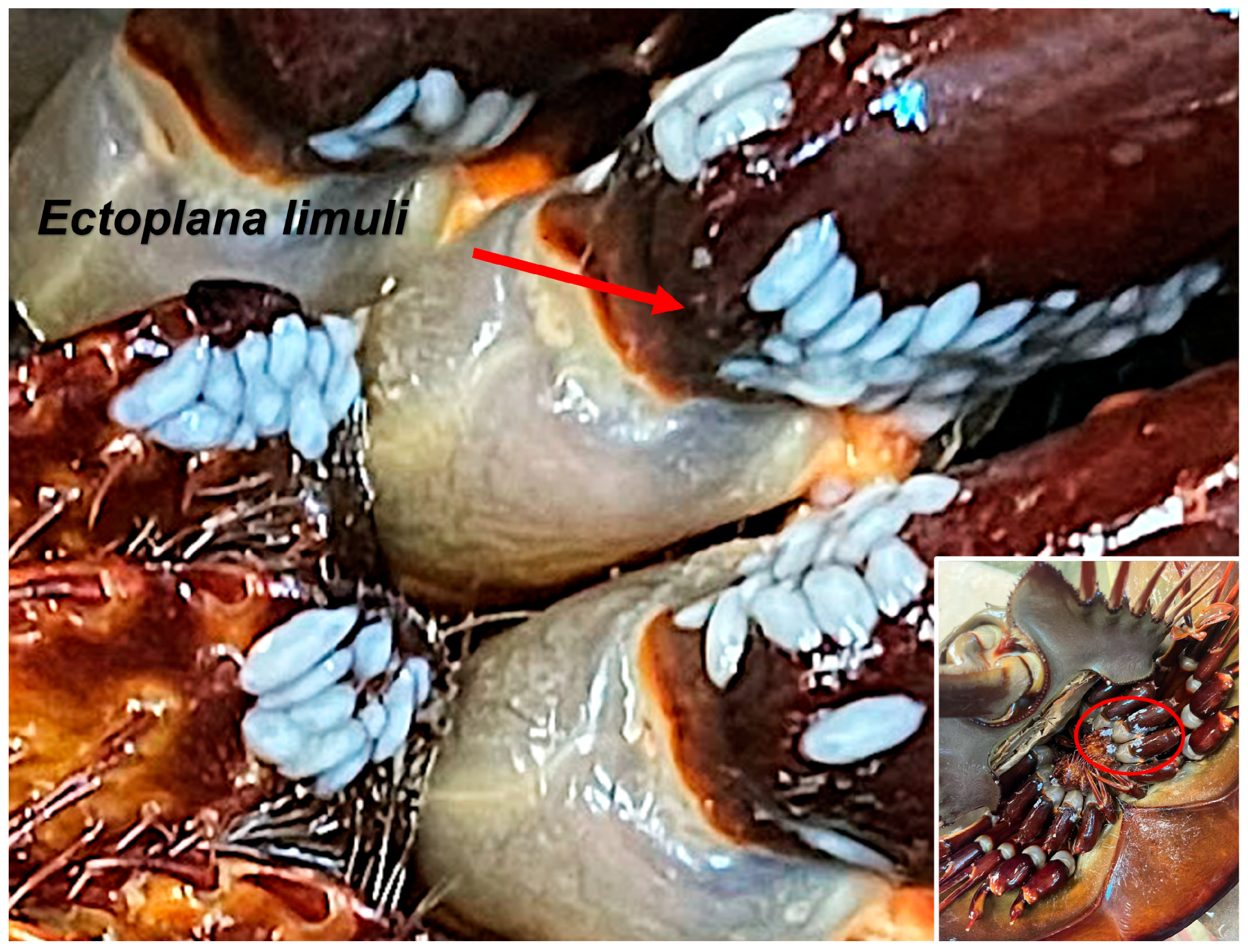
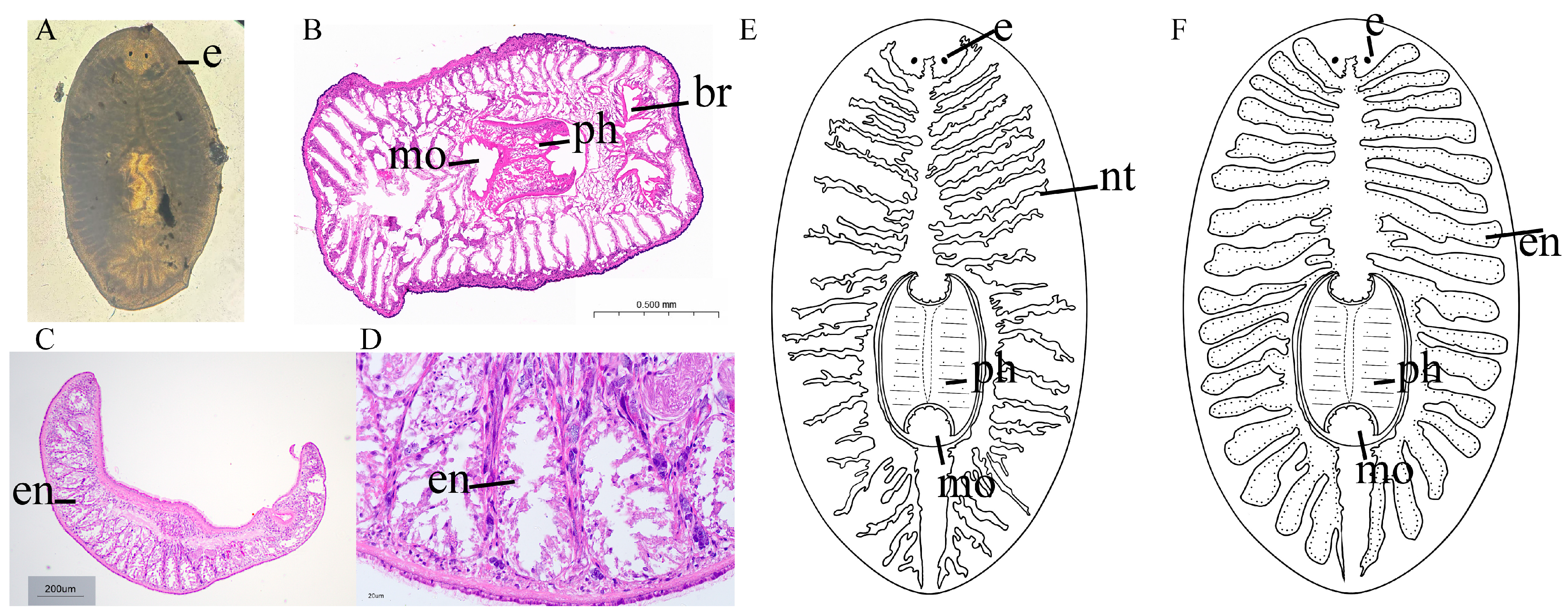
3.2. Morphological Observation
3.3. Molecular Identification
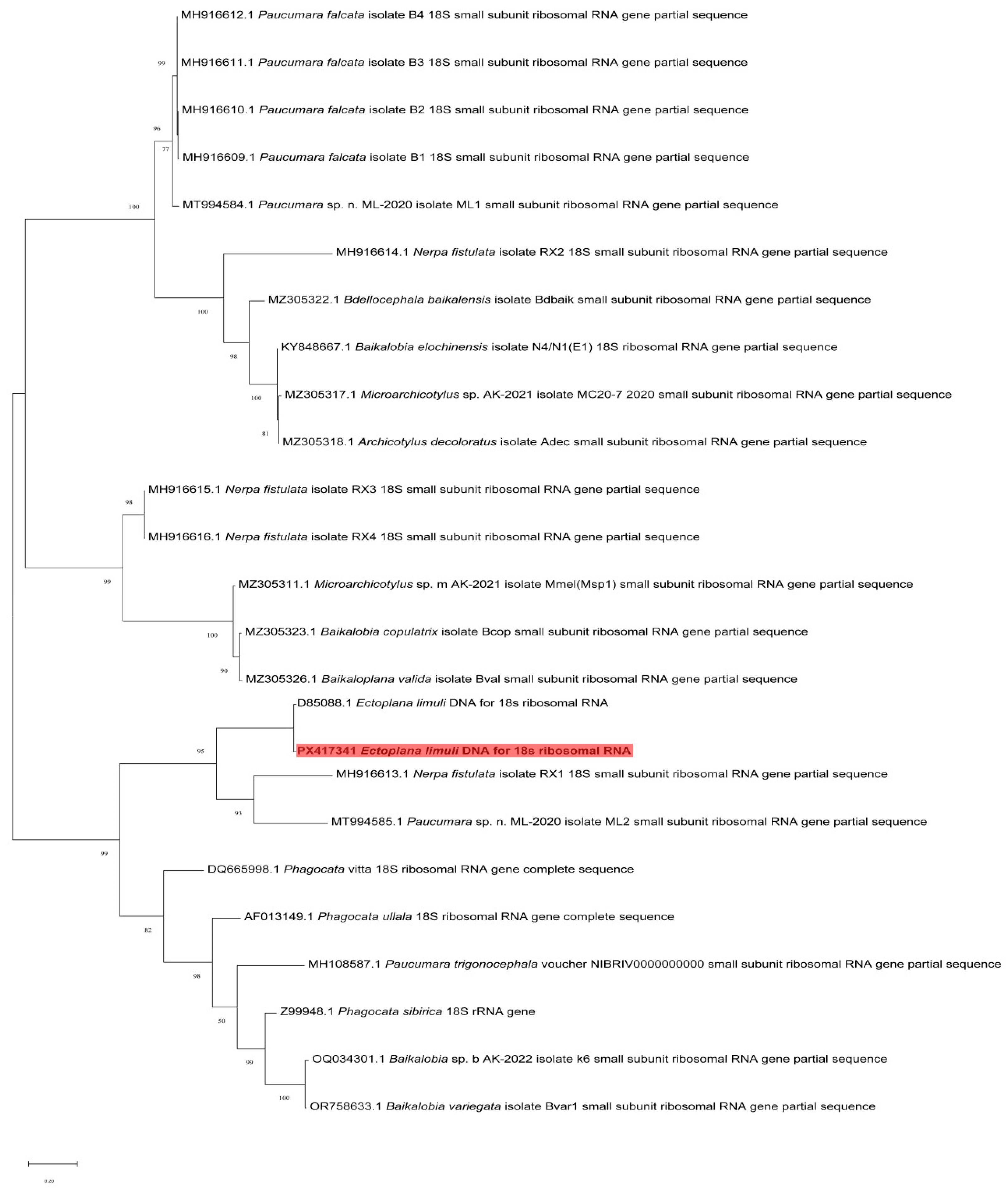
3.4. Phylogenetic Tree
4. Discussion
4.1. Morphological Characteristics of Ectoplana limuli
4.2. Phylogenetic Analysis of Ectoplana limuli
4.3. Prevalence of Ectoplana limuli in T. tridentatus
4.4. Potential Impact of Parasitic Infections on Juvenile T. tridentatus Mortality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ijima, I.; Kaburaki, T. Preliminary Descriptions of Some Japanese Triclads. Annot. Zool. Jpn. 1916, 9, 153–171. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; Mcveigh, R.; O’Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: A comprehensive update on curation, resources and tools. Database 2020, 2020, baaa062. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.Y.; Sun, Z.Y.; Li, W.X.; Sluys, R.; Li, S.F.; Wang, A.T. Addition to the known diversity of Chinese freshwater planarians: Integrative description of a new species of Dugesia Girard, 1850 (Platyhelminthes, Tricladida, Dugesiidae). Zoosyst. Evol. 2022, 98, 233–243. [Google Scholar] [CrossRef]
- Kawakatsu, M.; Oki, I.; Tamura, S. Taxonomy and geographical distribution of Dugesia japonica and D. ryukyuensis in the Far East. Hydrobiologia 1995, 305, 55–61. [Google Scholar] [CrossRef]
- Momčilović, S.; Cantacessi, C.; Arsić-Arsenijević, V.; Otranto, D.; Tasić-Otašević, S. Rapid diagnosis of parasitic diseases: Current scenario and future needs. Clin. Microbiol. Infect. 2019, 25, 290–309. [Google Scholar] [CrossRef]
- Huang, G.; Peng, X. Genus Bithynia: Morphological classification to molecular identification. Parasites Vectors 2024, 17, 496. [Google Scholar] [CrossRef]
- Chen, X.; Gu, Y.G.; Ying, Z.; Luo, Z.; Zhang, W.; Xie, X. Impact assessment of human activities on resources of juvenile horseshoe crabs in Hainan coastal areas, China. Mar. Pollut. Bull. 2023, 188, 114726. [Google Scholar] [CrossRef]
- Itaya, S.; Koyama, A.; Shuuno, M.; Onikura, N.; Tai, A.; Yano, S. Spawning habitat suitability maps for the conservation of the tri-spine horseshoe crab Tachypleus tridentatus in Tsuyazaki Cove, Fukuoka, Japan. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 884–896. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, Y.; Yan, Q.; Zhang, L.; Chen, D.; Ruan, L.; Kong, Y.; Shi, H.; Chen, M.; Chen, J. The draft genome of horseshoe crab Tachypleus tridentatus reveals its evolutionary scenario and well-developed innate immunity. BMC Genom. 2020, 21, 137. [Google Scholar] [CrossRef]
- Rudkin, D.M.; Young, G.A. Horseshoe crabs—An ancient ancestry revealed. In Biology and Conservation of Horseshoe Crabs; Tanacredi, J.T., Botton, M.L., Smith, D., Eds.; Springer: Boston, MA, USA, 2009; pp. 25–44. [Google Scholar] [CrossRef]
- Vestbo, S.; Obst, M.; Quevedo Fernandez, F.J.; Intanai, I.; Funch, P. Present and potential future distributions of Asian horseshoe crabs determine areas for conservation. Front. Mar. Sci. 2018, 5, 164. [Google Scholar] [CrossRef]
- Xu, P.; Bai, H.; Xie, X.; Wang, C.-C.; Huang, X.; Wang, X.; Zhang, M.; Ye, Z.; Zhu, J.; Zhen, W.; et al. Tri-spine horseshoe crab aquaculture, ranching and stock enhancement: Perspectives and challenges. Front. Mar. Sci. 2021, 8, 608155. [Google Scholar] [CrossRef]
- Ying, Z.; Bao, Y.; Li, Y.; Ye, G.; Zhang, S.; Xu, P.; Zhu, J.; Xie, X. Impact of Different Diets on Adult Tri-Spine Horseshoe Crab, Tachypleus tridentatus. J. Ocean Univ. China 2022, 21, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Bang, F. A bacterial disease of Limulus polyphemus. Bull Johns Hopkins Hosp. 1956, 98, 325–351. [Google Scholar]
- Nolan, M.W.; Smith, S.A. Clinical evaluation, common diseases, and veterinary care of the horseshoe crab, Limulus polyphemus. In Biology and Conservation of Horseshoe Crabs; Tanacredi, J.T., Botton, M.L., Smith, D., Eds.; Springer: Boston, MA, USA, 2009; pp. 479–499. [Google Scholar] [CrossRef]
- Irshath, A.A.; Rajan, A.P.; Vimal, S.; Prabhakaran, V.-S.; Ganesan, R. Bacterial Pathogenesis in Various Fish Diseases: Recent Advances and Specific Challenges in Vaccine Development. Vaccines 2023, 11, 470. [Google Scholar] [CrossRef]
- Leibovitz, L.; Lewbart, G.A. The American Horseshoe Crab; Harvard University Press: Cambridge, MA, USA, 2003. [Google Scholar]
- Bush, A.O.; Lafferty, K.D.; Lotz, J.M.; Shostak, A.W. Parasitology meets ecology on its own terms: Margolis et al. revisited. J. Parasitol. 1997, 83, 575–583. [Google Scholar] [CrossRef]
- Liu, S.; Song, L.; Huang, S.; Liu, Z.; Xu, Y.; Wang, Z.; Qiu, H.; Wang, J.; Chen, Z.; Xiao, Y.; et al. Hydroxyapatite microspheres encapsulated within hybrid hydrogel promote skin regeneration through the activation of Calcium Signaling and Motor Protein pathway. Bioact. Mater. 2025, 50, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- Wang, H.; Dumack, K.; Rissi, D.V.; Finn, D.R.; Bonkowski, M.; Tebbe, C.C. Profiling the eukaryotic soil microbiome with differential primers and an antifungal peptide nucleic acid probe (PNA): Implications for diversity assessment. Appl. Soil Ecol. 2024, 200, 105464. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Rai, S.; Singh, L.S.; Shaanker, R.U.; Jeyaram, K.; Parija, T.; Sahoo, D. Endophytic fungi of Panax sokpayensis produce bioactive ginsenoside Compound K in flask fermentation. Sci. Rep. 2024, 14, 9318. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Y.; Wu, Y.; Hu, S.; Sun, B. Molecular epidemiology and recombination of enterovirus D68 in China. Infect. Genet. Evol. 2023, 115, 105512. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Zuckerkandl, E.; Pauling, L. Evolutionary Divergence and Convergence in Proteins. In Evolving Genes and Proteins; Academic Press: Oxford, UK, 1965; pp. 97–166. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Deochand, N.; Costello, M.S.; Deochand, M.E. Behavioral Research with Planaria. Perspect. Behav. Sci. 2018, 41, 447–464. [Google Scholar] [CrossRef]
- Wang, C.; Yue, F.; Kuang, S. Muscle Histology Characterization Using H&E Staining and Muscle Fiber Type Classification Using Immunofluorescence Staining. BIO-Protocol 2017, 7, e2279. [Google Scholar] [CrossRef]
- Rompolas, P.; Patel-King, R.S.; King, S.M. Chapter 4—Schmidtea mediterranea: A model system for analysis of motile cilia. In Methods in Cell Biology; King, S.M., Pazour, G.J., Eds.; Academic Press: Oxford, UK, 2009; pp. 81–98. [Google Scholar] [CrossRef]
- Cabej, N.R. Chapter 4—Cambrian explosion: Sudden burst of animal bauplaene and morphological diversification. In Epigenetic Mechanisms of the Cambrian Explosion; Cabej, N.R., Ed.; Academic Press: Oxford, UK, 2020; pp. 137–211. [Google Scholar] [CrossRef]
- Cui, Y.Y.; Ye, L.T.; Wu, L.; Wang, J.Y. Seasonal occurrence of Perkinsus spp. and tissue distribution of P. olseni in clam (Soletellina acuta) from coastal waters of Wuchuan County, southern China. Aquaculture 2018, 492, 300–305. [Google Scholar] [CrossRef]
- Bininda-Emonds, O.R.P. 18S rRNA variability maps reveal three highly divergent, conserved motifs within Rotifera. BMC Ecol. Evol. 2021, 21, 118. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.; Zhang, Y.; Li, M.; Wang, T.; Su, Y. Geographic isolation and environmental heterogeneity contribute to genetic differentiation in Cephalotaxus oliveri. Ecol. Evol. 2023, 13, e9869. [Google Scholar] [CrossRef]
- Fišer, C.; Robinson, C.T.; Malard, F. Cryptic species as a window into the paradigm shift of the species concept. Mol. Ecol. 2018, 27, 613–635. [Google Scholar] [CrossRef]
- Struck, T.H.; Feder, J.L.; Bendiksby, M.; Birkeland, S.; Cerca, J.; Gusarov, V.I.; Kistenich, S.; Larsson, K.H.; Liow, L.H.; Nowak, M.D.; et al. Finding evolutionary processes hidden in cryptic species. Trends Ecol. Evol. 2018, 33, 153–163. [Google Scholar] [CrossRef]
- Huggins, L.G.; Waite, J.H. Eggshell formation in Bdelloura candida, an ectoparasitic turbellarian of the horseshoe crab Limulus polyphemus. J. Exp. Zool. 1993, 265, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Riesgo, A.; Burke, E.A.; Laumer, C.; Giribet, G. Genetic variation and geographic differentiation in the marine triclad Bdelloura candida (Platyhelminthes, Tricladida, Maricola), ectocommensal on the American horseshoe crab Limulus polyphemus. Mar. Biol. 2017, 164, 111. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Li, W.X.; Sluys, R.; Wu, M.Q.; Wang, L.; Li, S.F.; Wang, A.T. Two new species of marine flatworm from southern China facilitate determination of the phylogenetic position of the genus Nerpa Marcus, 1948 and the histochemical structure of the nervous system in the genus Paucumara Sluys, 1989 (Platyhelminthes, Tricladida, Maricola). Zootaxa 2019, 4568, 149–167. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Nishioka, M.; Yamamoto, M. Phylogenetic Relationships Among Turbellarian Orders Inferred from 18S rDNA Sequences. Zool. Sci. 1996, 13, 747–756. [Google Scholar] [CrossRef]
- Li, M.Y.; Ma, X.Y.; Li, W.X.; Yang, Y.; Sluys, R.; Chen, J.J.; Li, S.F.; Wang, A.T. A new species of Pacifides from the Western Pacific Coast and the first fully freshwater species of the maricolan planarian genus Paucumara (Platyhelminthes, Tricladida, Maricola). Syst. Biodivers. 2021, 19, 488–506. [Google Scholar] [CrossRef]
- Goupil, L.S.; Ivry, S.L.; Hsieh, I.; Suzuki, B.M.; Craik, C.S.; O’Donoghue, A.J.; McKerrow, J.H. Cysteine and aspartyl proteases contribute to protein digestion in the gut of freshwater planaria. PLoS Neglected Trop. Dis. 2016, 10, e0004893. [Google Scholar] [CrossRef]
- Kawakatsu, M.; Sekiguchi, K. Rediscriptions of Ectoplana limuli (Ijima et Kaburaki, 1916) and Ectoplana undata Slys 1983 (Turbellaria, Tricladidia, Maricola), collected from the three extant species of Asian horseshoe crabs. Jôbu J. Manag. Inf. Sci. 1988, 5, 57–94. [Google Scholar]
- Tavakol, S.; Luus-Powell, W.J.; Smit, W.J.; Baker, C.; Hoffman, A.; Halajian, A. First introduction of two Australian temnocephalan species into Africa with an alien host: Double trouble. J. Parasitol. 2016, 102, 653–658. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Fu, Y.; Zhong, M.; Kuang, Y.; Bai, H.; Zhang, C.; Zhen, W.; Xu, P.; Wang, C.C.; Zhu, J. Spatiotemporal Distribution of Asian Horseshoe Crab Eggs Are Highly Intermingled with Anthropogenic Structures in Northern Beibu Gulf, China. J. Ocean Univ. China 2022, 21, 531–540. [Google Scholar] [CrossRef]
- Ismail, S.; Farner, J.; Couper, L.; Mordecai, E.; Lyberger, K. Temperature and intraspecific variation affect host–parasite interactions. Oecologia 2024, 204, 389–399. [Google Scholar] [CrossRef]
- Mignatti, A.; Boag, B.; Cattadori, I.M. Host immunity shapes the impact of climate changes on the dynamics of parasite infections. Proc. Natl. Acad. Sci. USA 2016, 113, 2970–2975. [Google Scholar] [CrossRef]
- Chen, X.; Gu, Y.G.; Xie, X.; Ying, Z.; Luo, Z.; Zhang, W.; Xie, M.; Fan, J. A new strategy for optimizing marine protection networks by considering functional connectivity: An example of Tachypleus tridentatus. Sci. Total Environ. 2024, 911, 168763. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Bao, Y.; Xie, X.; Lin, W.; Chen, X.; Zhao, X.; Xiao, X. An effective way to monitor the population of juvenile horseshoe crabs in the Beibu Gulf of China. Reg. Stud. Mar. Sci. 2024, 79, 103831. [Google Scholar] [CrossRef]
- Marcogliese, D.J. The impact of climate change on the parasites and infectious diseases of aquatic animals. Rev. Sci. Et Tech. (Int. Off. Epizoot.) 2008, 27, 467–484. [Google Scholar] [CrossRef]
- Marcogliese, D.J. Climate Change and Parasitism in Aquatic Ecosystems. In Aquatic Parasitology: Ecological and Environmental Concepts and Implications of Marine and Freshwater Parasites; Smit, N.J., Sures, B., Eds.; Springer Nature Switzerland: Cham, Switzerland, 2025; pp. 547–593. [Google Scholar] [CrossRef]
- Byers, J.E. Effects of climate change on parasites and disease in estuarine and nearshore environments. PLoS Biol. 2020, 18, e3000743. [Google Scholar] [CrossRef]
| Sequence ID | Number of bp | Percentage |
|---|---|---|
| PX417341 | 183/184 | 99% |
| PX417342 | 172/173 | 99% |
| PX417343 | 178/178 | 100% |
| PX417344 | 183/184 | 99% |
| PX417345 | 185/186 | 99% |
| PX417346 | 188/194 | 97% |
| PX417347 | 183/184 | 99% |
| PX417348 | 183/184 | 99% |
| PX417349 | 174/175 | 99% |
| PX417350 | 183/184 | 99% |
| PX417351 | 183/184 | 99% |
| PX417352 | 183/184 | 99% |
| PX417353 | 183/184 | 99% |
| PX417354 | 178/178 | 100% |
| PX417355 | 183/184 | 99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Ye, L.; Ying, Z.; Deng, C.; Xie, X.; Chen, X.; Li, T. Ectoplana limuli, a Parasite of the Horseshoe Crab (Tachypleus tridentatus): A New Record in China. Biology 2025, 14, 1412. https://doi.org/10.3390/biology14101412
Luo Z, Ye L, Ying Z, Deng C, Xie X, Chen X, Li T. Ectoplana limuli, a Parasite of the Horseshoe Crab (Tachypleus tridentatus): A New Record in China. Biology. 2025; 14(10):1412. https://doi.org/10.3390/biology14101412
Chicago/Turabian StyleLuo, Zimeng, Lingtong Ye, Ziwei Ying, Chenxiang Deng, Xiaoyong Xie, Xiaohai Chen, and Ting Li. 2025. "Ectoplana limuli, a Parasite of the Horseshoe Crab (Tachypleus tridentatus): A New Record in China" Biology 14, no. 10: 1412. https://doi.org/10.3390/biology14101412
APA StyleLuo, Z., Ye, L., Ying, Z., Deng, C., Xie, X., Chen, X., & Li, T. (2025). Ectoplana limuli, a Parasite of the Horseshoe Crab (Tachypleus tridentatus): A New Record in China. Biology, 14(10), 1412. https://doi.org/10.3390/biology14101412






