Molecular Docking of Detoxification Enzymes from Oides leucomelaena with Volatiles of Star Anise
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Collection and Transcriptome Sequencing
2.2. Gene Identification
2.3. Sequence Analysis and Expression Profiling Construction
2.4. Molecular Docking
3. Results
3.1. Identification and Expression Profile of the oleuCYP Sequence
3.2. Identification and Expression Profile of the oleuGST Sequence
3.3. Identification and Expression Profile of the oleuCarE Sequence
3.4. Strong Binding Affinity of Key CYP to Ligands
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patra, J.K.; Das, G.; Bose, S.; Banerjee, S.; Vishnuprasad, C.N.; Del Pilar Rodriguez-Torres, M.; Shin, H.S. Star anise (Illicium verum): Chemical compounds, antiviral properties, and clinical relevance. Phytother. Res. 2020, 34, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Huang, Y.; Zhang, W.; Lu, C.; Yuan, J. A Comprehensive Review of the Pharmacology, Chemistry, Traditional Uses and Quality Control of Star Anise (Illicium verum Hook. F.): An Aromatic Medicinal Plant. Molecules 2023, 28, 7378. [Google Scholar] [CrossRef]
- Sharafan, M.; Jafernik, K.; Ekiert, H.; Kubica, P.; Kocjan, R.; Blicharska, E.; Szopa, A. Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules 2022, 27, 650. [Google Scholar] [CrossRef]
- De, M.; De, A.K.; Sen, P.; Banerjee, A.B. Antimicrobial properties of star anise (Illicium verum Hook f). Phytother. Res. 2002, 16, 94–95. [Google Scholar] [CrossRef]
- Zhang, S.; Kuang, X.; Guo, C.; Jiang, Y.; Zhang, F.; Devahastin, S.; Du, M.; Yi, J. Advanced strategies for the processing and utilization of star Anise (Illicium verum hook. F.): Blanching, drying and bioactive compounds extraction. Food Res. Int. 2025, 217, 116863. [Google Scholar] [CrossRef]
- Yang, M.; Shen, J.; Ding, C.; Yang, X. A Review of Chinese Species of the Genus Oides Weber, 1801 (Coleoptera: Chrysomelidae: Galerucinae). Insects 2024, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Ji, M.; Ning, D.; Chen, H.; Han, M.; Feng, Z. The Occurrence and Control of Oides leucomelaena Weise. Anhui Agric. Sci. Bull. 2007, 13, 2. [Google Scholar]
- Wu, X. Integrated Pest Management Methods for Oides leucomelaena Weise. Guangxi For. Sci. 2001, 1, 34. [Google Scholar]
- Volonté, M.; Traverso, L.; Estivalis, J.M.L.; Almeida, F.C.; Ons, S. Comparative analysis of detoxification-related gene superfamilies across five hemipteran species. BMC Genom. 2022, 23, 757. [Google Scholar] [CrossRef]
- Wu, J.; Tang, W.; Li, Z.; Chakraborty, A.; Zhou, C.; Li, F.; He, S. Duplications and Losses of the Detoxification Enzyme Glycosyltransferase 1 Are Related to Insect Adaptations to Plant Feeding. Int. J. Mol. Sci. 2024, 25, 6080. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Song, Y.; Zeng, R. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Schama, R.; Pedrini, N.; Juárez, M.P.; Nelson, D.R.; Torres, A.Q.; Valle, D.; Mesquita, R.D. Rhodnius prolixus supergene families of enzymes potentially associated with insecticide resistance. Insect Biochem. Mol. Biol. 2016, 69, 91–104. [Google Scholar] [CrossRef]
- Jin, Y.; Gao, Y.; Zhang, H.; Wang, L.; Yang, K.; Dong, H. Detoxification enzymes associated with butene-fipronil resistance in Epacromius coerulipes. Pest Manag. Sci. 2020, 76, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Amezian, D.; Nauen, R.; Le Goff, G. Transcriptional regulation of xenobiotic detoxification genes in insects—An overview. Pestic. Biochem. Physiol. 2021, 174, 104822. [Google Scholar] [CrossRef] [PubMed]
- Amezian, D.; Nauen, R.; Le Goff, G. Comparative analysis of the detoxification gene inventory of four major Spodoptera pest species in response to xenobiotics. Insect Biochem. Mol. Biol. 2021, 138, 103646. [Google Scholar] [CrossRef]
- Jin, M.; Liao, C.; Fu, X.; Holdbrook, R.; Wu, K.; Xiao, Y. Adaptive regulation of detoxification enzymes in Helicoverpa armigera to different host plants. Insect Mol. Biol. 2019, 28, 628–636. [Google Scholar] [CrossRef]
- Halon, E.; Eakteiman, G.; Moshitzky, P.; Elbaz, M.; Alon, M.; Pavlidi, N.; Vontas, J.; Morin, S. Only a minority of broad-range detoxification genes respond to a variety of phytotoxins in generalist Bemisia tabaci species. Sci. Rep. 2015, 5, 17975. [Google Scholar] [CrossRef]
- Kim, Y.H.; Soumaila Issa, M.; Cooper, A.M.; Zhu, K.Y. RNA interference: Applications and advances in insect toxicology and insect pest management. Pestic. Biochem. Physiol. 2015, 120, 109–117. [Google Scholar] [CrossRef]
- Wilding, C.S. Regulating resistance: CncC:Maf, antioxidant response elements and the overexpression of detoxification genes in insecticide resistance. Curr. Opin. Insect Sci. 2018, 27, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Wang, H.; Li, Y.; Dong, R.; Pang, B. Comparative Transcriptome Analysis of the Pest Galeruca daurica (Coleoptera: Chrysomelidae) Larvae in Response to Six Main Metabolites from Allium mongolicum (Liliaceae). Insects 2024, 15, 847. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Li, C.; Wang, Z.; Liu, J.; Zeng, X. Identification of detoxification genes in imidacloprid-resistant Asian citrus psyllid (Hemiptera: Lividae) and their expression patterns under stress of eight insecticides. Pest Manag. Sci. 2019, 75, 1400–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Li, K.; Ma, H.; Hu, L.; Yang, Y.; Liu, L. Molecular Characterization of Odorant-Binding Protein Genes Associated with Host-Seeking Behavior in Oides leucomelaena. Int. J. Mol. Sci. 2024, 25, 9436. [Google Scholar] [CrossRef] [PubMed]
- Huifen, M.; Ling, L.; Lianrong, H.; Mei, J. Analysis of Volatile Chemicals in the Leaves, Flowers and Fruits of Funing Illicium verum. J. West China For. Sci. 2024, 53, 52–58. [Google Scholar]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.X.; Cao, Y. CB-Dock2: Improved protein-ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, W159–W164. [Google Scholar] [CrossRef]
- Chakraborty, P.; Biswas, A.; Dey, S.; Bhattacharjee, T.; Chakrabarty, S. Cytochrome P450 Gene Families: Role in Plant Secondary Metabolites Production and Plant Defense. J. Xenobiot. 2023, 13, 402–423. [Google Scholar] [CrossRef]
- Yang, A.J.; Yin, N.N.; Chen, D.L.; Guo, Y.R.; Zhao, Y.J.; Liu, N.Y. Identification and characterization of candidate detoxification genes in Pharsalia antennata Gahan (Coleoptera: Cerambycidae). Front. Physiol. 2022, 13, 1015793. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Wang, Z.Q.; Zhu, J.Y.; Liu, N.Y. Identification and characterization of detoxification genes in two cerambycid beetles, Rhaphuma horsfieldi and Xylotrechus quadripes (Coleoptera: Cerambycidae: Clytini). Comp. Biochem. physiology. Part B Biochem. Mol. Biol. 2020, 243–244, 110431. [Google Scholar] [CrossRef]
- Wu, C.; Ding, C.; Chen, S.; Wu, X.; Zhang, L.; Song, Y.; Li, W.; Zeng, R. Exposure of Helicoverpa armigera Larvae to Plant Volatile Organic Compounds Induces Cytochrome P450 Monooxygenases and Enhances Larval Tolerance to the Insecticide Methomyl. Insects 2021, 12, 238. [Google Scholar] [CrossRef]
- Xiong, T.; Ling, S.Q.; Liu, J.L.; Zeng, X.N. Insecticidal and P450 mediate metabolism of fluralaner against red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae). Pestic. Biochem. Physiol. 2022, 187, 105184. [Google Scholar] [CrossRef]
- Zhu, F.; Moural, T.W.; Shah, K.; Palli, S.R. Integrated analysis of cytochrome P450 gene superfamily in the red flour beetle, Tribolium castaneum. BMC Genom. 2013, 14, 174. [Google Scholar] [CrossRef]
- Xiong, W.; Gao, S.; Mao, J.; Wei, L.; Xie, J.; Liu, J.; Bi, J.; Song, X.; Li, B. CYP4BN6 and CYP6BQ11 mediate insecticide susceptibility and their expression is regulated by Latrophilin in Tribolium castaneum. Pest Manag. Sci. 2019, 75, 2744–2755. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, G.Y.; Li, L.; Song, Q.S.; Stanley, D.; Wei, S.J.; Zhu, J.Y. Genome-wide and expression-profiling analyses of the cytochrome P450 genes in Tenebrionidea. Arch. Insect Biochem. Physiol. 2022, 111, e21954. [Google Scholar] [CrossRef]
- Wan, P.J.; Shi, X.Q.; Kong, Y.; Zhou, L.T.; Guo, W.C.; Ahmat, T.; Li, G.Q. Identification of cytochrome P450 monooxygenase genes and their expression profiles in cyhalothrin-treated Colorado potato beetle, Leptinotarsa decemlineata. Pestic. Biochem. Physiol. 2013, 107, 360–368. [Google Scholar] [CrossRef]
- Dai, L.; Ma, M.; Wang, C.; Shi, Q.; Zhang, R.; Chen, H. Cytochrome P450s from the Chinese white pine beetle, Dendroctonus armandi (Curculionidae: Scolytinae): Expression profiles of different stages and responses to host allelochemicals. Insect Biochem. Mol. Biol. 2015, 65, 35–46. [Google Scholar] [CrossRef]
- Dai, L.; Wang, C.; Zhang, X.; Yu, J.; Zhang, R.; Chen, H. Two CYP4 genes of the Chinese white pine beetle, Dendroctonus armandi (Curculionidae: Scolytinae), and their transcript levels under different development stages and treatments. Insect Mol. Biol. 2014, 23, 598–610. [Google Scholar] [CrossRef]
- Wu, L.; Li, L.; Xu, Y.; Li, Q.; Liu, F.; Zhao, H. Identification and characterization of CYP307A1 as a molecular target for controlling the small hive beetle, Aethina tumida. Pest Manag. Sci. 2023, 79, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Scharf, M.E.; Parimi, S.; Meinke, L.J.; Chandler, L.D.; Siegfried, B.D. Expression and induction of three family 4 cytochrome P450 (CYP4)* genes identified from insecticide-resistant and susceptible western corn rootworms, Diabrotica virgifera virgifera. Insect Mol. Biol. 2001, 10, 139–146. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Gao, S.S.; Xue, S.; An, S.H.; Zhang, K.P. Disruption of the cytochrome P450 CYP6BQ7 gene reduces tolerance to plant toxicants in the red flour beetle, Tribolium castaneum. Int. J. Biol. Macromol. 2021, 172, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Fang, Z.; Wu, Q.; Tan, L.; Weng, Q. CYP4BN4v7 regulates the population density dependent oocyte maturity rate in bean beetles. Sci. Rep. 2024, 14, 28574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yang, J.; Hu, J.; Yang, F.; Liang, J.; Xue, H.; Wei, X.; Fu, B.; Huang, M.; Du, H.; et al. Glutathione S-transferase directly metabolizes imidacloprid in the whitefly, Bemisia tabaci. Pestic. Biochem. Physiol. 2024, 201, 105863. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Liu, Y.; Dai, B.; Han, Y.; Zhang, Y.; Deng, Z.; Wang, L.; Li, X. GSTD1 Mediates the Tolerance to Abamectin and Beta-Cypermethrin in the Fall Armyworm Spodoptera frugiperda. Insects 2025, 16, 299. [Google Scholar] [CrossRef]
- Shi, H.; Pei, L.; Gu, S.; Zhu, S.; Wang, Y.; Zhang, Y.; Li, B. Glutathione S-transferase (GST) genes in the red flour beetle, Tribolium castaneum, and comparative analysis with five additional insects. Genomics 2012, 100, 327–335. [Google Scholar] [CrossRef]
- Hu, F.; Ye, K.; Tu, X.F.; Lu, Y.J.; Thakur, K.; Jiang, L.; Wei, Z.J. Identification and expression profiles of twenty-six glutathione S-transferase genes from rice weevil, Sitophilus oryzae (Coleoptera: Curculionidae). Int. J. Biol. Macromol. 2018, 120, 1063–1071. [Google Scholar] [CrossRef]
- Dai, L.; Ma, J.; Ma, M.; Zhang, H.; Shi, Q.; Zhang, R.; Chen, H. Characterisation of GST genes from the Chinese white pine beetle Dendroctonus armandi (Curculionidae: Scolytinae) and their response to host chemical defence. Pest Manag. Sci. 2016, 72, 816–827. [Google Scholar] [CrossRef]
- Song, X.W.; Zhong, Q.S.; Ji, Y.H.; Zhang, Y.M.; Tang, J.; Feng, F.; Bi, J.X.; Xie, J.; Li, B. Characterization of a sigma class GST (GSTS6) required for cellular detoxification and embryogenesis in Tribolium castaneum. Insect Sci. 2022, 29, 215–229. [Google Scholar] [CrossRef]
- Liu, X.Y.; Sun, H.M.; Luo, Y.H.; Li, M.Y.; Liu, H.B.; Liu, S. Identification of 14 glutathione S-transferase genes from Lasioderma serricorne and characterization of LsGSTe1 involved in lambda-cyhalothrin detoxification. Pestic. Biochem. Physiol. 2023, 193, 105425. [Google Scholar] [CrossRef]
- Yang, Y.L.; Li, X.; Wang, J.; Song, Q.S.; Stanley, D.; Wei, S.J.; Zhu, J.Y. Comparative genomic analysis of carboxylesterase genes in Tenebrio molitor and other four tenebrionids. Arch. Insect Biochem. Physiol. 2022, 111, e21967. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, H.; Ye, J.; Fu, D.; Sun, Y.; Chen, H. Isolation of CarE genes from the Chinese white pine beetle Dendroctonus armandi (Curculionidae: Scolytinae) and their response to host chemical defense. Pest Manag. Sci. 2019, 75, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Gao, H.; Chen, H. Expression Levels of Detoxification Enzyme Genes from Dendroctonus armandi (Coleoptera: Curculionidae) Fed on a Solid Diet Containing Pine Phloem and Terpenoids. Insects 2021, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Gao, S.; Xiong, W.; Liu, J.; Mao, J.; Lu, Y.; Song, X.; Li, B. Latrophilin mediates insecticides susceptibility and fecundity through two carboxylesterases, esterase4 and esterase6, in Tribolium castaneum. Bull. Entomol. Res. 2019, 109, 534–543. [Google Scholar] [CrossRef]
- Tsubota, T.; Minakuchi, C.; Nakakura, T.; Shinoda, T.; Shiotsuki, T. Molecular characterization of a gene encoding juvenile hormone esterase in the red flour beetle, Tribolium castaneum. Insect Mol. Biol. 2010, 19, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Pang, Y.P.; Park, Y.; Gao, X.; Yao, J.; Zhang, X.; Zhu, K.Y. Genome organization, phylogenies, expression patterns, and three-dimensional protein models of two acetylcholinesterase genes from the red flour beetle. PLoS ONE 2012, 7, e32288. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Xing, L.Y.; Ni, Z.W.; Wu, G. Identification and characterization of ace1-type acetylcholinesterase in insecticide-resistant and -susceptible Propylaea japonica (Thunberg). Bull. Entomol. Res. 2018, 108, 253–262. [Google Scholar] [CrossRef] [PubMed]
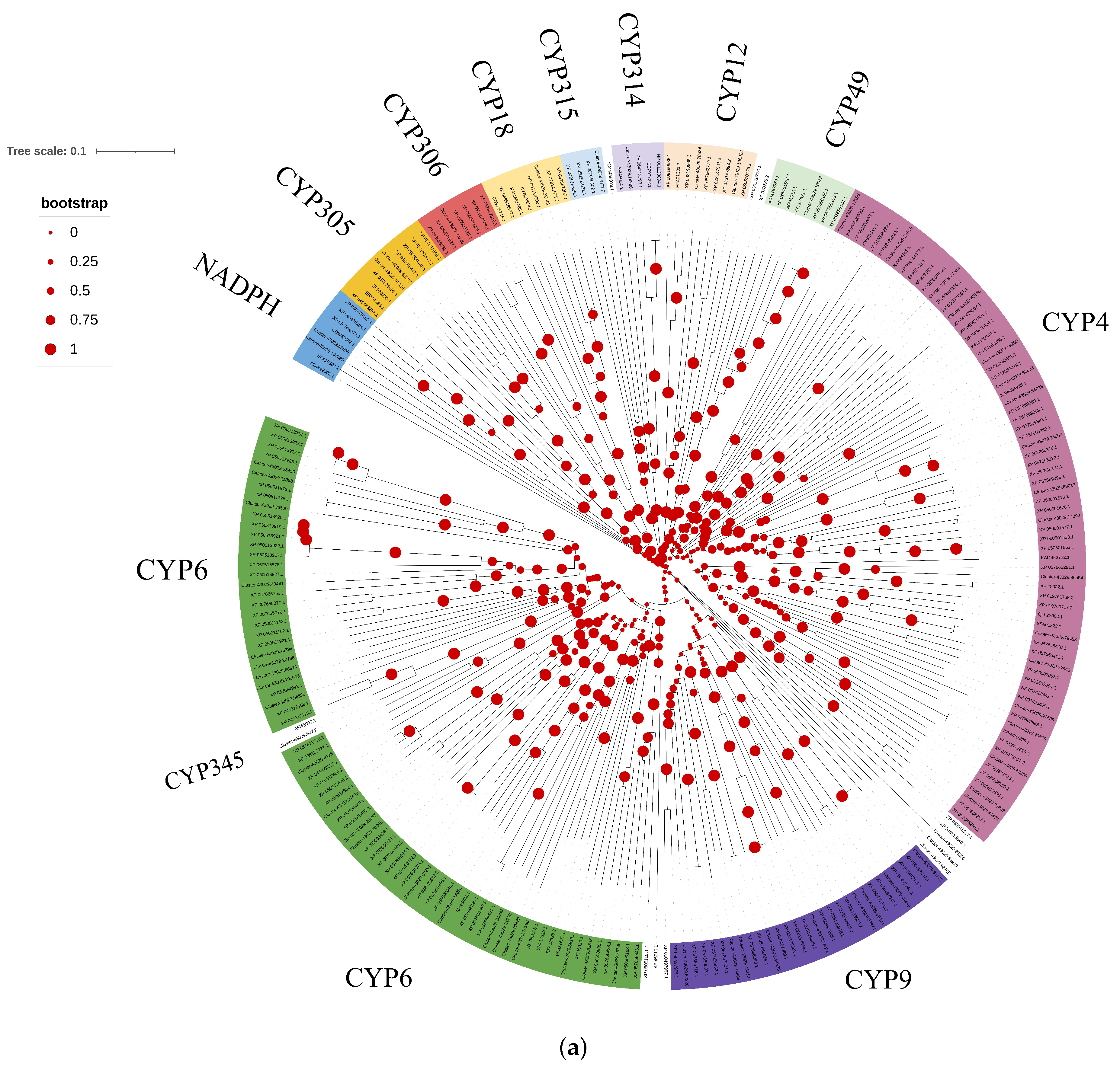
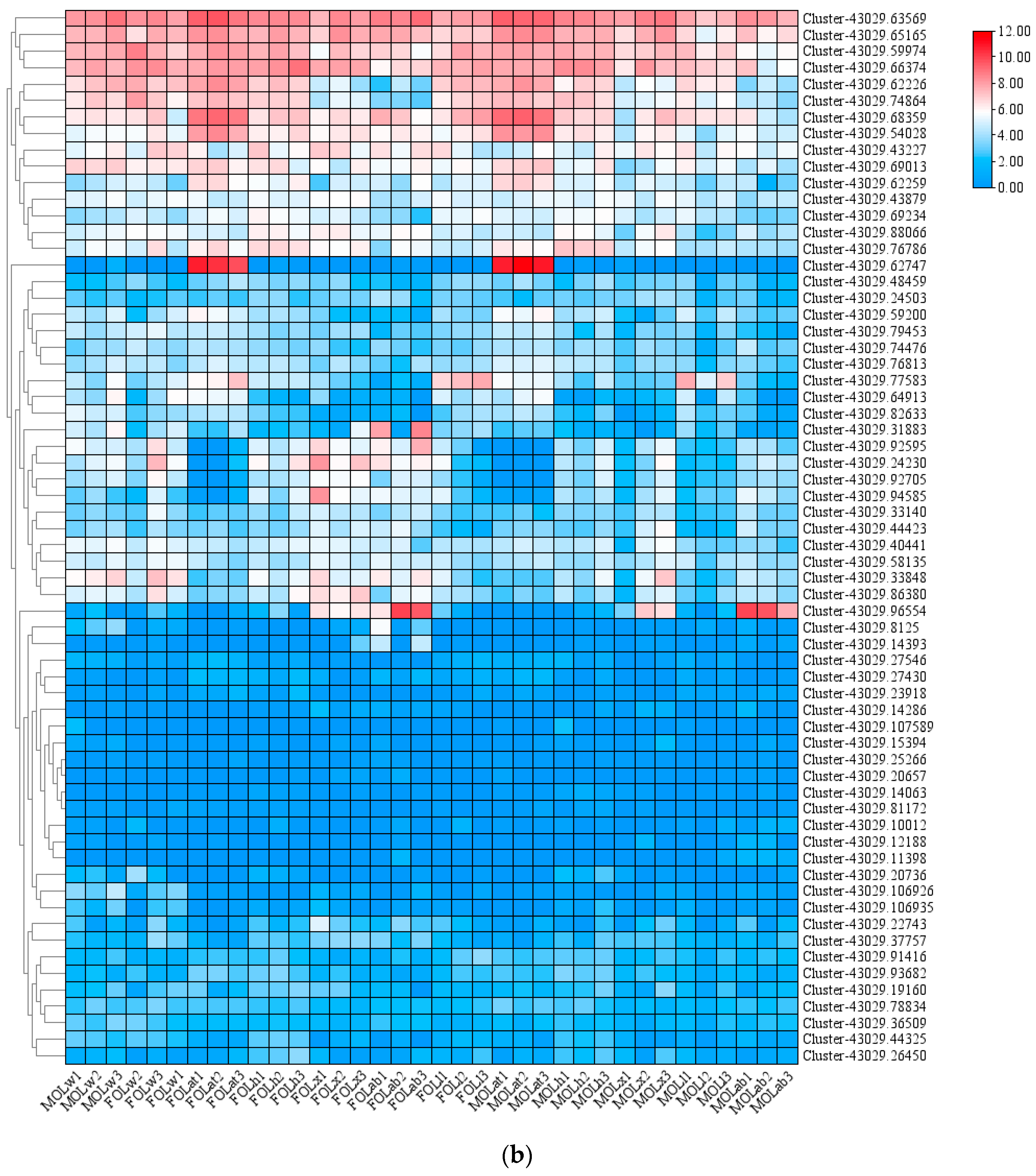
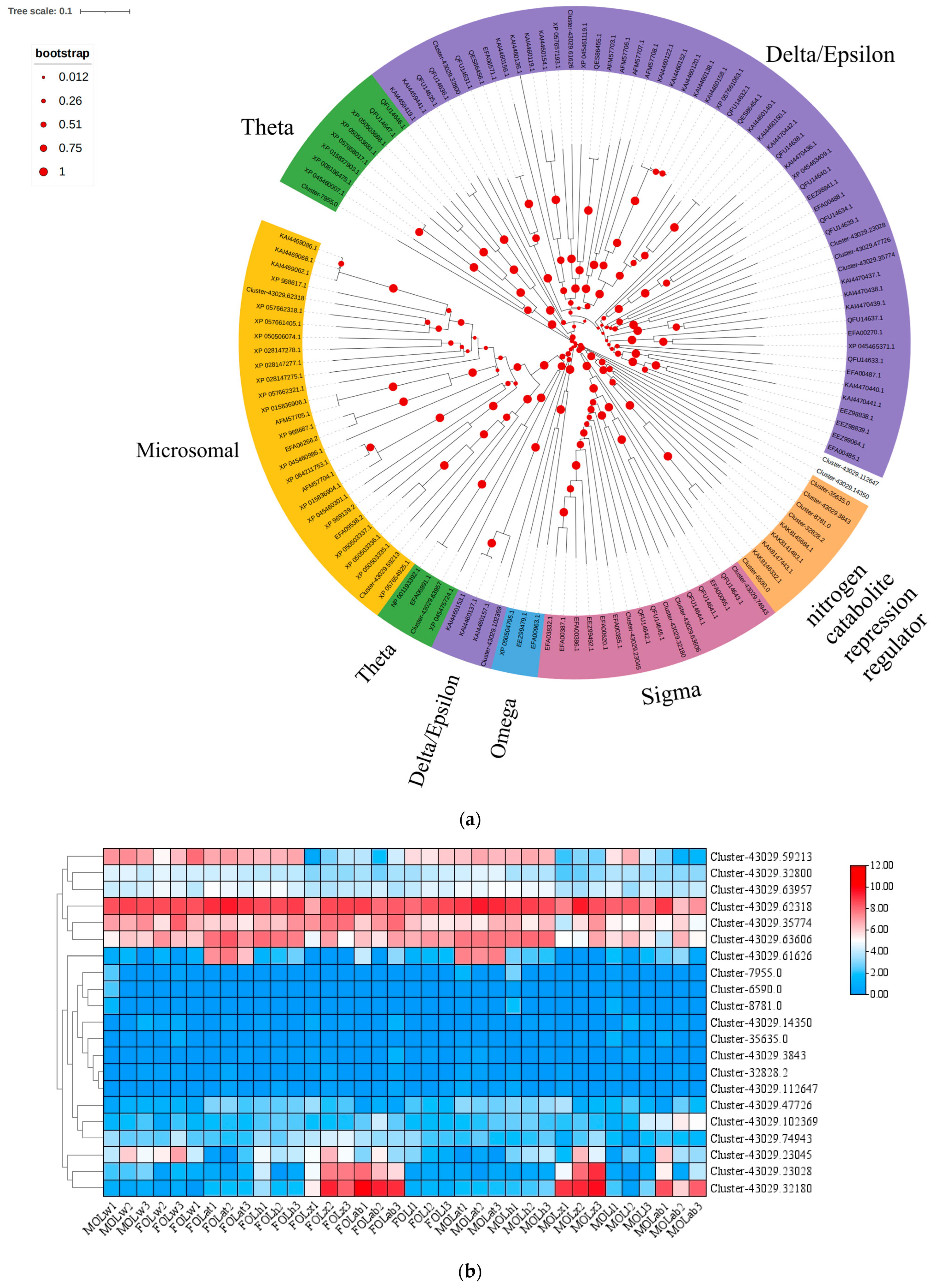



| ID | Name | ORF (aa) | Scientific | E Value | Per Ident | Accession |
|---|---|---|---|---|---|---|
| Cluster-43029.107589 | OleuCYP-NADPH | 595 | Beauveria bassiana | 0 | 99.66% | PMB72824.1 |
| Cluster-43029.63569 | OleuCYP-NADPH | 272 | Diabrotica undecimpunctata | 0 | 90.44% | XP_072381184.1 |
| Cluster-43029.22743 | OleuCYP18a1 | 528 | Diabrotica virgifera virgifera | 0 | 88.45% | XP_028141979.1 |
| Cluster-43029.10012 | OleuCYP49a1 | 544 | Diabrotica undecimpunctata | 0 | 84.71% | XP_072391690.1 |
| Cluster-43029.8125 | OleuCYP6k1 | 499 | Diorhabda carinulata | 0 | 81.26% | XP_057671775.1 |
| Cluster-43029.14063 | OleuCYP6a14 | 515 | Diabrotica undecimpunctata | 0 | 78.29% | XP_072379934.1 |
| Cluster-43029.25266 | OleuCYP302a1 | 291 | Diabrotica virgifera virgifera | 9.00 × 10−140 | 71.13% | XP_028142564.1 |
| Cluster-43029.79453 | OleuCYP4d2 | 505 | Diorhabda carinulata | 0 | 75.30% | XP_057655410.1 |
| Cluster-43029.43227 | OleuCYP305a1 | 448 | Diabrotica virgifera virgifera | 0 | 75.58% | XP_050508447.1 |
| Cluster-43029.96554 | OleuCYP4g15 | 557 | Diabrotica undecimpunctata | 0 | 75.85% | XP_072385487.1 |
| Cluster-43029.33848 | OleuCYP6a8 | 518 | Diabrotica virgifera virgifera | 0 | 75.05% | XP_050508500.1 |
| Cluster-43029.78834 | OleuCYP12a5 | 354 | Diabrotica virgifera virgifera | 2.00 × 10−171 | 65.92% | XP_028147894.2 |
| Cluster-43029.88066 | OleuCYP6a13 | 517 | Diabrotica virgifera virgifera | 0 | 71.48% | XP_050508496.1 |
| Cluster-43029.43879 | OleuCYP4c1 | 494 | Diabrotica undecimpunctata | 0 | 71.25% | XP_072382925.1 |
| Cluster-43029.62226 | OleuCYP9e2 | 525 | Diabrotica virgifera virgifera | 0 | 68.38% | XP_050497957.1 |
| Cluster-43029.33140 | OleuCYP306a1 | 510 | Diabrotica undecimpunctata | 0 | 69.74% | XP_072388304.1 |
| Cluster-43029.59200 | OleuCYP4aa1 | 471 | Diabrotica undecimpunctata | 0 | 70.70% | XP_072396269.1 |
| Cluster-43029.76786 | OleuCYP6a2 | 396 | Diabrotica virgifera virgifera | 0 | 68.10% | XP_050509163.1 |
| Cluster-43029.62259 | OleuCYP6bj70 | 421 | Monolepta hieroglyphica | 0 | 65.95% | XHH54104.1 |
| Cluster-43029.74476 | OleuCYP9e2 | 528 | Diabrotica undecimpunctata | 0 | 65.57% | XP_072396671.1 |
| Cluster-43029.68359 | OleuCYP4c1 | 458 | Diabrotica undecimpunctata | 0 | 66.22% | XP_072390792.1 |
| Cluster-43029.48459 | OleuCYP9e2 | 532 | Diabrotica virgifera virgifera | 0 | 65.69% | XP_050497942.1 |
| Cluster-43029.81172 | OleuCYP9e2 | 513 | Diabrotica virgifera virgifera | 0 | 64.58% | XP_050497945.1 |
| Cluster-43029.59974 | OleuCYP9e2 | 523 | Diabrotica undecimpunctata | 0 | 64.05% | XP_072397933.1 |
| Cluster-43029.91416 | OleuCYP305a1 | 493 | Diorhabda carinulata | 0 | 63.69% | XP_057671993.1 |
| Cluster-43029.69013 | OleuCYP4d14 | 499 | Diabrotica virgifera virgifera | 0 | 61.69% | XP_050501618.1 |
| Cluster-43029.37757 | OleuCYP315a1 | 462 | Diabrotica undecimpunctata | 0 | 66.31% | XP_072382045.1 |
| Cluster-43029.14393 | OleuCYP4c1 | 496 | Diabrotica undecimpunctata | 0 | 63.77% | XP_072402566.1 |
| Cluster-43029.40441 | OleuCYP6k1 | 503 | Diorhabda sublineata | 0 | 60.04% | XP_056644762.1 |
| Cluster-43029.92595 | OleuCYP4c1 | 488 | Diabrotica undecimpunctata | 0 | 66.60% | XP_072382925.1 |
| Cluster-43029.69234 | OleuCYP9e2 | 524 | Diabrotica virgifera virgifera | 0 | 61.83% | XP_028133915.2 |
| Cluster-43029.12188 | OleuCYP4c3 | 481 | Diabrotica undecimpunctata | 0 | 61.20% | XP_072379783.1 |
| Cluster-43029.74864 | OleuCYP9e2 | 502 | Diabrotica virgifera virgifera | 0 | 61.43% | XP_050497948.1 |
| Cluster-43029.54028 | OleuCYP4bn80 | 522 | Monolepta hieroglyphica | 0 | 64.44% | WKR34928.1 |
| Cluster-43029.76813 | OleuCYP9e2 | 502 | Diabrotica virgifera virgifera | 0 | 60.64% | XP_050497948.1 |
| Cluster-43029.24503 | OleuCYP4d | 498 | Diabrotica undecimpunctata | 0 | 53.04% | XP_072402574.1 |
| Cluster-43029.14286 | OleuCYP314a1 | 490 | Colaphellus bowringi | 0 | 72.39% | UYL69089.1 |
| Cluster-43029.20657 | OleuCYP6a23 | 455 | Diabrotica undecimpunctata | 0 | 62.72% | XP_072392648.1 |
| Cluster-43029.44423 | OleuCYP4c1 | 507 | Diorhabda carinulata | 0 | 59.21% | XP_057666257.1 |
| Cluster-43029.64913 | OleuCYP | 531 | Leptinotarsa decemlineata | 0 | 48.77% | AAZ94269.1 |
| Cluster-43029.106926 | OleuCYP12a2 | 337 | Diabrotica virgifera virgifera | 2.00 × 10−138 | 57.96% | XP_050510784.1 |
| Cluster-43029.27430 | OleuCYP6a2 | 520 | Diabrotica virgifera virgifera | 0 | 58.11% | XP_050512634.1 |
| Cluster-43029.86380 | OleuCYP6a20 | 497 | Diorhabda sublineata | 0 | 57.75% | XP_056634040.1 |
| Cluster-43029.44325 | OleuCYP9e2 | 527 | Diabrotica undecimpunctata | 0 | 57.58% | XP_072390190.1 |
| Cluster-43029.31883 | OleuCYP | 506 | Agasicles hygrophila | 0 | 62.08% | AZR39463.1 |
| Cluster-43029.15394 | OleuCYP6k | 494 | Diabrotica virgifera virgifera | 0 | 57.40% | XP_050511921.1 |
| Cluster-43029.77583 | OleuCYP | 571 | Agasicles hygrophila | 0 | 59.27% | AZR39479.1 |
| Cluster-43029.58135 | OleuCYP6a | 501 | Anoplophora glabripennis | 4.00 × 10−178 | 49.60% | XP_023310525.1 |
| Cluster-43029.26450 | OleuCYP6k | 507 | Diabrotica virgifera virgifera | 0 | 56.02% | XP_050513923.1 |
| Cluster-43029.82633 | OleuCYP4v | 482 | Diorhabda sublineata | 5.00 × 10−160 | 46.06% | XP_056640038.1 |
| Cluster-43029.62747 | OleuCYP345h | 499 | Monolepta hieroglyphica | 0 | 63.47% | XHM34208.1 |
| Cluster-43029.65165 | OleuCYP | 489 | Agasicles hygrophila | 2.00 × 10−154 | 46.75% | AZR39465.1 |
| Cluster-43029.92705 | OleuCYP | 490 | Pharsalia antennata | 0 | 50.96% | WCC58103.1 |
| Cluster-43029.11398 | OleuCYP6k | 420 | Diabrotica virgifera virgifera | 2.00 × 10−164 | 53.83% | XP_050513923.1 |
| Cluster-43029.23918 | OleuCYP4c | 506 | Diabrotica virgifera virgifera | 2.00 × 10−127 | 39.60% | XP_028132814.2 |
| Cluster-43029.24230 | OleuCYP6a | 499 | Diorhabda sublineata | 0 | 52.51% | XP_056634040.1 |
| Cluster-43029.66374 | OleuCYP6k | 347 | Diorhabda sublineata | 4.00 × 10−128 | 53.62% | XP_056633285.1 |
| Cluster-43029.27546 | OleuCYP4c | 489 | Diabrotica virgifera virgifera | 4.00 × 10−176 | 50.72% | XP_050502063.1 |
| Cluster-43029.36509 | OleuCYP6 | 496 | Diabrotica undecimpunctata | 2.00 × 10−135 | 39.31% | XP_072393601.1 |
| Cluster-43029.94585 | OleuCYP6k | 500 | Diorhabda sublineata | 0 | 50.72% | XP_056633285.1 |
| Cluster-43029.93682 | OleuCYP6a | 496 | Diorhabda sublineata | 3.00 × 10−180 | 48.90% | XP_056634040.1 |
| Cluster-43029.106935 | OleuCYP6k | 496 | Diorhabda sublineata | 2.00 × 10−175 | 48.58% | XP_056633285.1 |
| Cluster-43029.20736 | OleuCYP6k | 495 | Diabrotica virgifera virgifera | 1.00 × 10−167 | 47.98% | XP_050511921.1 |
| Cluster-43029.19160 | OleuCYP6a | 492 | Diabrotica virgifera virgifera | 2.00 × 10−175 | 47.76% | XP_028130066.2 |
| Cluster-43029.59974 | Cluster-43029.62226 | Cluster-43029.62747 | Cluster-43029.63569 | Cluster-43029.65165 | Cluster-43029.66374 | Cluster-43029.74864 | |
|---|---|---|---|---|---|---|---|
| γ-Gurjunene | −6.6 | −6.4 | −7.2 | −7.1 | −7.7 | −7.2 | −7.5 |
| β-Caryophyllene | −6.7 | −6.9 | −7.2 | −7.1 | −8.0 | −6.7 | −7.5 |
| β-Elemene | −6.3 | −5.9 | −6.4 | −6.8 | −7.1 | −7.9 | −6.5 |
| γ-Elemene | −6.6 | −6 | −6.5 | −6.9 | −7.5 | −7.5 | −6.6 |
| Anisene | −7.3 | −8.7 | −7.7 | −7.5 | −7.1 | −7 | −7.4 |
| Anethole | −6.2 | −6 | −6.2 | −6 | −6 | −5.7 | −6.0 |
| Foeniculin | −7.1 | −7.5 | −7.2 | −6.9 | −7.1 | −6.9 | −7.2 |
| β-Sesquiphellandrene | −6.7 | −6.3 | −6.4 | −7.4 | −7.2 | −7 | −7.6 |
| a-Farnesene | −6.4 | −5.9 | −6.7 | −6.6 | −7 | −6.9 | −7.1 |
| Estragole | −5.8 | −5.6 | −6.3 | −5.9 | −5.7 | −5.6 | −5.9 |
| γ-Gurjunene | −6.6 | −6.4 | −7.2 | −7.1 | −7.7 | −7.2 | −7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhang, Z.; Ma, H.; Hu, L.; Li, K.; Zhao, N.; Liu, L.; Zhou, J. Molecular Docking of Detoxification Enzymes from Oides leucomelaena with Volatiles of Star Anise. Biology 2025, 14, 1411. https://doi.org/10.3390/biology14101411
Yang Y, Zhang Z, Ma H, Hu L, Li K, Zhao N, Liu L, Zhou J. Molecular Docking of Detoxification Enzymes from Oides leucomelaena with Volatiles of Star Anise. Biology. 2025; 14(10):1411. https://doi.org/10.3390/biology14101411
Chicago/Turabian StyleYang, Yingxue, Zhixiao Zhang, Huifen Ma, Lianrong Hu, Kai Li, Ning Zhao, Ling Liu, and Jielong Zhou. 2025. "Molecular Docking of Detoxification Enzymes from Oides leucomelaena with Volatiles of Star Anise" Biology 14, no. 10: 1411. https://doi.org/10.3390/biology14101411
APA StyleYang, Y., Zhang, Z., Ma, H., Hu, L., Li, K., Zhao, N., Liu, L., & Zhou, J. (2025). Molecular Docking of Detoxification Enzymes from Oides leucomelaena with Volatiles of Star Anise. Biology, 14(10), 1411. https://doi.org/10.3390/biology14101411






