Long-Term Potentiation and Neurotransmitter Expression Change in Dysautonomia Linked to Binge Eating Disorder: Protective Role of Exercise
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Animals
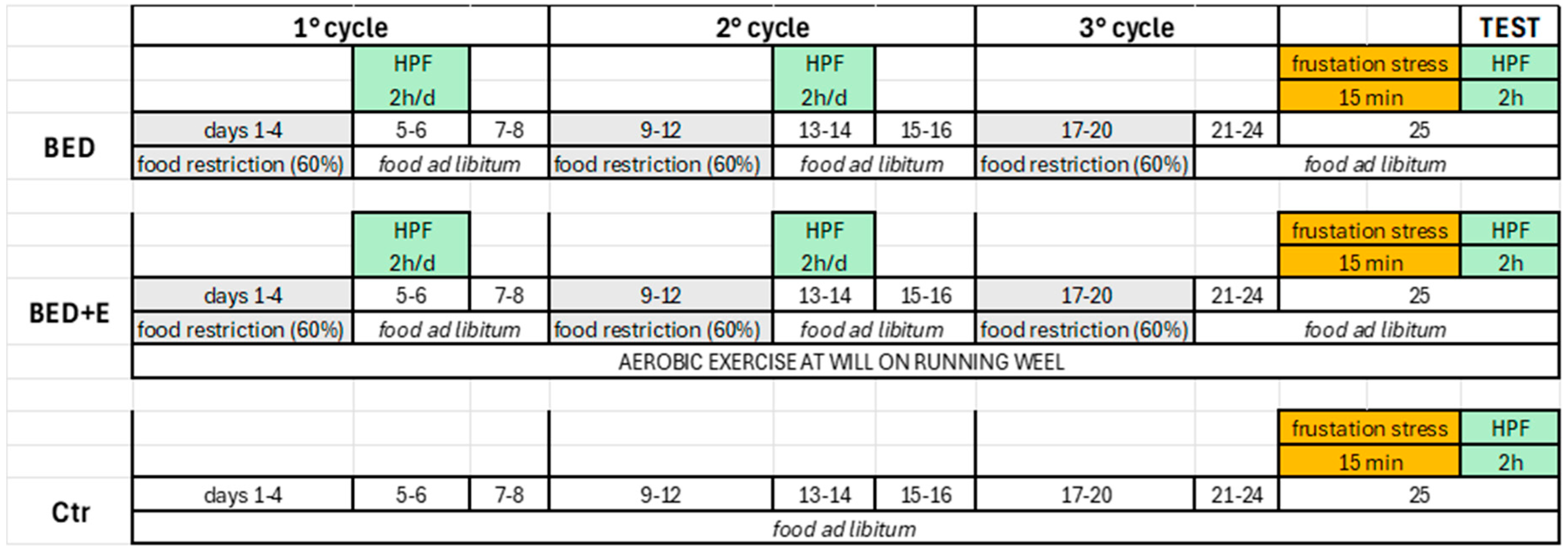
2.2. Induction of the Experimental Model
2.3. Electrophysiological Procedure
2.4. Immunohistochemistry
2.5. Image Acquisition and Analysis
2.6. Statistics
3. Results
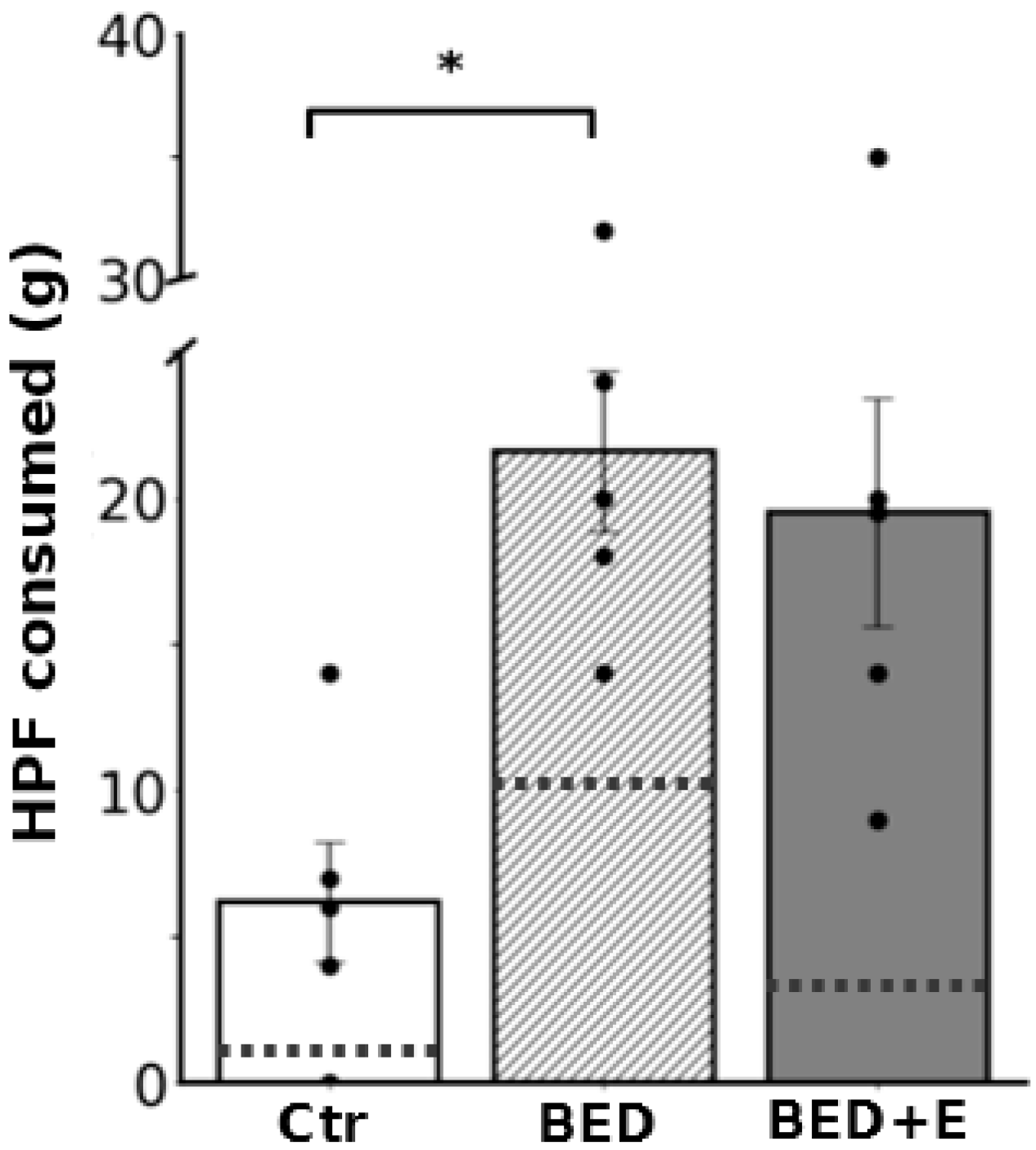
3.1. Protocol of Cycles of Restricted Feeding and Access to HCF Induced the Experimental Model of Binge-like Eating—Exercise Prevented Installation of the Model
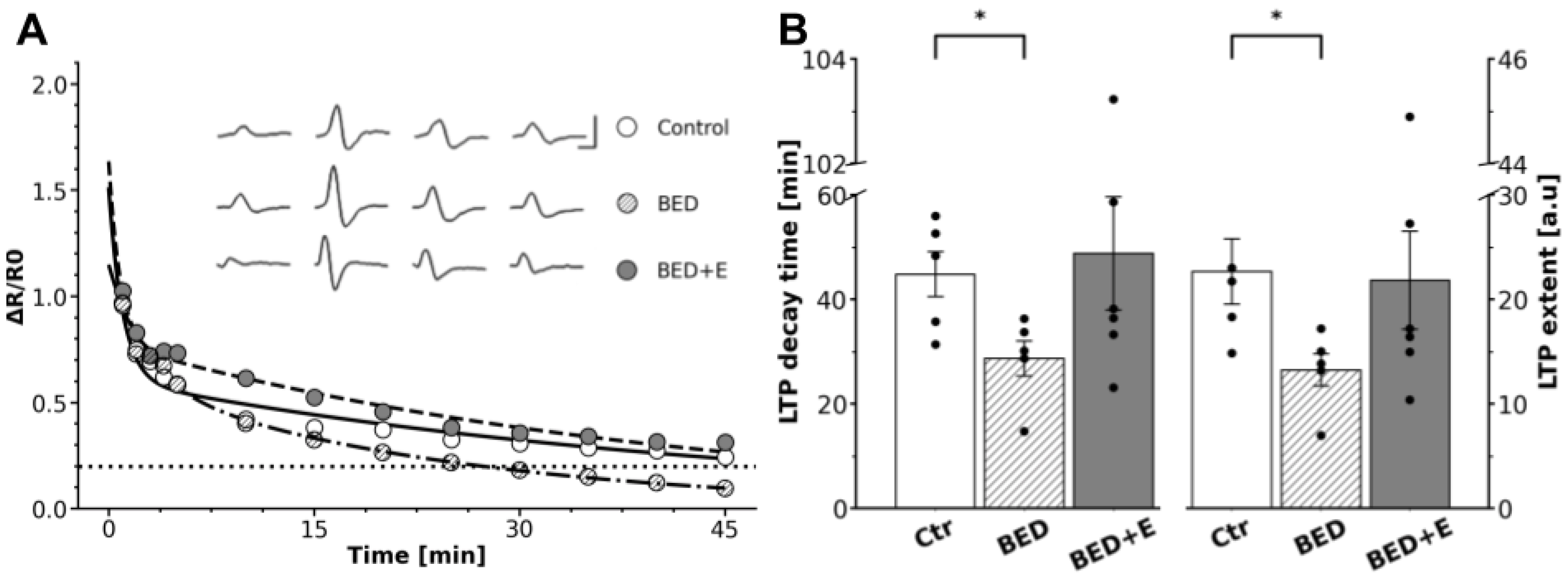
3.2. Ganglia from Binge-Eating Rats Failed to Express gLTP
3.3. Exercise Prevents LTP Loss
3.4. BED-Associated Dysautonomia Enlarged GABA Presence and Did Not Change ACh and Segregation
3.5. Exercise Prevents GABA Upregulation and Reduces ACh-GABA Segregation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cardinali, D.P. Autonomic Nervous System: Basic and Clinical Aspects; Springer International Publishing: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Brown, T.H.; McAfee, D. Long-term synaptic potentiation in the superior cervical ganglion. Science 1982, 215, 1411–1413. [Google Scholar] [CrossRef]
- Martínez, L.A.; Cifuentes, F.; Morales, M.A. Ganglionic Long-Term Potentiation in Prehypertensive and Hypertensive Stages of Spontaneously Hypertensive Rats Depends on GABA Modulation. Neural Plast. 2019, 13, 7437894. [Google Scholar] [CrossRef]
- Elinos, D.; Rodríguez, R.; Martínez, L.A.; Zetina, M.E.; Cifuentes, F.; Morales, M.A. Segregation of Acetylcholine and GABA in the Rat Superior Cervical Ganglia: Functional Correlation. Front. Cell. Neurosci. 2016, 10, 91. [Google Scholar] [CrossRef]
- Vega, A.; Cancino-Rodezno, A.; Valle-Leija, P.; Sánchez-Tafolla, B.M.; Elinos, D.; Cifuentes, F.; Morales, M.A. Neurotrophin-dependent plasticity of neurotransmitter segregation in the rat superior cervical ganglion in vivo. Dev. Neurobiol. 2016, 76, 832–846. [Google Scholar] [CrossRef]
- Merino-Jiménez, C.; Miguel, F.; Feria Pliego, J.A.; Zetina Rosales, M.E.; Cifuentes, F.; Morales, M.A. Sympathetic Hyperactivity and Age Affect Segregation and Expression of Neurotransmitters. Front. Cell. Neurosci. 2018, 12, 411. [Google Scholar] [CrossRef]
- Hernández, A.; González-Sierra, C.; Zetina, M.A.; Cifuentes, F.; Morales, M.A. Age Dependent Changes in the Occurrence and Segregation of GABA and Acetylcholine in the Rat Superior Cervical Ganglia. Int. J. Mol. Sci. 2024, 25, 2588. [Google Scholar] [CrossRef]
- Akselrod, S.; Gordon, D.; Ubel, F.A.; Shannon, D.C.; Berger, A.C.; Cohen, R.J. Power spectrum analysis of heart rate fluctuation: A quantitative probe of beat-to-beat cardiovascular control. Science 1981, 213, 220–222. [Google Scholar] [CrossRef]
- Grassi, G.; Biffi, A.; Dell’Oro, R.; Trevano, F.Q.; Seravalle, G.; Corrao, G.; Perseghin, G.; Mancia, G. Sympathetic neural abnormalities in type 1 and type 2 diabetes: A systematic review and meta-analysis. J. Hypertens. 2020, 38, 1436–1442. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, K.; Senador, D.D.F.; Mostarda, C.; Irigoyen, M.C.; Morris, M. Sympathetic overactivity precedes metabolic dysfunction in a fructose model of glucose intolerance in mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R950–R957. [Google Scholar] [CrossRef] [PubMed]
- Carnagarin, R.; Lambert, G.W.; Kiuchi, M.G.; Nolde, J.M.; Matthews, V.B.; Eikelis, N.; Lambert, E.A.; Schlaich, M.P. Effects of sympathetic modulation in metabolic disease. Ann. N. Y. Acad. Sci. 2019, 1454, 80–89. [Google Scholar] [CrossRef]
- Friederich, H.C.; Schild, S.; Schellberg, D.; Quenter, A.; Bode, C.; Herzog, W.; Zipfel, S. Cardiac parasympathetic regulation in obese women with binge eating disorder. Int. J. Obes. 2006, 30, 534–542. [Google Scholar] [CrossRef]
- Vögele, C.; Florin, I. Psychophysiological responses to food exposure: An experimental study in binge eaters. Int. J. Eat. Disord. 1997, 21, 147–157. [Google Scholar] [CrossRef]
- Guerdjikova, A.I.; Mori, N.; Casuto, L.S.; McElroy, S.L. Binge Eating Disorder. Psychiatr. Clin. N. Am. 2017, 40, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Cuesto, G.; Everaerts, C.; León, L.G.; Acebes, A. Molecular bases of anorexia nervosa, bulimia nervosa and binge eating disorder: Shedding light on the darkness. J. Neurogenet. 2017, 31, 266–287. [Google Scholar] [CrossRef] [PubMed]
- Scherma, M.; Collu, R.; Satta, V.; Giunti, E.; Fadda, P. Animal Models of Eating Disorders. In Psychiatric Disorders: Methods in Molecular Biology; Kobeissy, F., Ed.; Humana: New York, NY, USA, 2019; Volume 2011. [Google Scholar] [CrossRef]
- Corwin, R.L.; Avena, N.M.; Boggiano, M.M. Feeding and reward: Perspectives from three rat models of binge eating. Physiol. Behav. 2011, 104, 87–97. [Google Scholar] [CrossRef]
- Gao, L.; Wang, W.; Liu, D.; Zucker, I.H. Exercise Training Normalizes Sympathetic Outflow by Central Antioxidant Mechanisms in Rabbits with Pacing-Induced Chronic Heart Failure. Circulation 2007, 115, 3095–3102. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Levine, B.D. Exercise and the autonomic nervous system. Handb. Clin. Neurol. 2013, 117, 147–160. [Google Scholar] [CrossRef]
- Albertz, J.; Boersma, G.J.; Tamashiro, K.L.; Moran, T.H. The effects of scheduled running wheel access on binge-like eating behavior and its consequences. Appetite 2018, 126, 176–184. [Google Scholar] [CrossRef]
- Yang, T.Y.; Gao, Z.; Liang, N.-C. Sex-dependent wheel running effects on high fat diet preference, metabolic outcomes, and performance on the barnes maze in rats. Nutrients 2020, 12, 2721. [Google Scholar] [CrossRef]
- Burmester, V.; Graham, E.; Nicholls, D. Physiological, emotional and neural responses to visual stimuli in eating disorders: A review. J. Eat. Disord. 2021, 9, 23. [Google Scholar] [CrossRef]
- Ávila, A.; SanMiguel, N.; Serrano, M.A. Core Symptoms of Eating Disorders and Heart Rate Variability: A Systematic Review. Sci 2025, 7, 89. [Google Scholar] [CrossRef]
- Romano, A.; Di Bonaventura, M.V.M.; Gallelli, C.A.; Koczwara, J.B.; Smeets, D.; Giusepponi, M.E.; De Ceglia, M.; Friuli, M.; Di Bonaventura, E.M.; Scuderi, C.; et al. Oleoylethanolamide decreases frustration stress-induced binge-like eating in female rats: A novel potential treatment for binge eating disorder. Neuropsychopharmacology 2020, 45, 1931–1941. [Google Scholar] [CrossRef]
- Alkadhi, K.A.; Otoom, S.A.; Tanner, F.L.; Sockwell, D.; Hogan, Y.H. Inhibition of ganglionic long-term potentiation decreases blood pressure in spontaneously hypertensive rats. Exp. Biol. Med. 2001, 226, 1024–1030. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Aleisa, A.M.; Alkadhi, K.A. Expression of gLTP in sympathetic ganglia of obese Zucker rats in vivo: Molecular evidence. J. Mol. Neurosci. 2008, 35, 297–306. [Google Scholar] [CrossRef]
- Alzoubi, K.H.; Khabour, O.F.; Alhaidar, I.A.; Aleisa, A.M.; Alkadhi, K.A. Diabetes impairs synaptic plasticity in the superior cervical ganglion: Possible role for BDNF and oxidative stress. J. Mol. Neurosci. 2013, 51, 763–770. [Google Scholar] [CrossRef]
- Veladiz-Gracia, F.; González-Sierra, C.; Cifuentes, F.; Morales, M.A. Alterations in the plasticity of sympathetic synapses are part of the dysautonomias associated with metabolic syndrome and binge eating disorder. Medicina 2024, 84 (Suppl. V), 93. [Google Scholar]
- Arias, E.R.; Valle-Leija, P.; Morales, M.A.; Cifuentes, F. Differential contribution of BDNF and NGF to long-term potentiation in the superior cervical ganglion of the rat. Neuropharmacology 2014, 81, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Barnard, R.J.; Ying, Z.; Roberts, C.K.; Gómez-Pinilla, F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002, 112, 803–814. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Meisel, R.L.; Mullins, A.J.; Davidson, T.L. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav. Brain Res. 2007, 182, 57–66. [Google Scholar] [CrossRef]
- Landsberg, L. Feast or famine: The sympathetic nervous system response to nutrient intake. Cell. Mol. Neurobiol. 2006, 26, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Silveira, R.O.; Zanatto, V.; Appolinario, J.C.; Kapczinski, F. An open trial of reboxetine in obese patients with binge eating disorder. Eat. Weight. Disord. Stud. Anorex. Bulim. Obes. 2005, 10, e93–e96. [Google Scholar] [CrossRef] [PubMed]
- Bello, N.T.; Yeh, C.-Y.; James, M.H. Reduced Sensory-Evoked Locus Coeruleus-Norepinephrine Neural Activity in Female Rats with a History of Dietary-Induced Binge Eating. Front. Psychol. 2019, 10, 1966. [Google Scholar] [CrossRef]
- Berner, L.A.; Bocarsly, M.E.; Hoebel, B.G.; Avena, N.M. Baclofen suppresses binge eating of pure fat but not a sugar-rich or sweet-fat diet. Behav. Pharmacol. 2009, 20, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Ebenezer, I.S. The effects of chronic intraperitoneal administration of the GABA B receptor agonist baclofen on food intake in rats. Eur. J. Pharmacol. 2008, 593, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Moraska, A.; Deak, T.; Spencer, R.L.; Roth, D.; Fleshner, M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1321–R1329. [Google Scholar] [CrossRef] [PubMed]
- Scarpace, P.J.; Matheny, M.; Zhang, Y. Wheel running eliminates high-fat preference and enhances leptin signaling in the ventral tegmental area. Physiol. Behav. 2010, 100, 173–179. [Google Scholar] [CrossRef]
- Molteni, R.; Wu, A.; Vaynman, S.; Ying, Z.; Barnard, R.J.; Gómez-Pinilla, F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience 2004, 123, 429–440. [Google Scholar] [CrossRef]
- Liang, N.C.; Bello, N.T.; Moran, T.H. Wheel running reduces high-fat diet intake, preference and mu-opioid agonist stimulated intake. Behav. Brain Res. 2015, 284, 1–10. [Google Scholar] [CrossRef]
- Raisi, A.; Zerbini, V.; Piva, T.; Belvederi Murri, M.; Menegatti, E.; Caruso, L.; Masotti, S.; Grazzi, G.; Mazzoni, G.; Mandini, S. Treating Binge Eating Disorder With Physical Exercise: A Systematic Review and Meta-analysis. J. Nutr. Educ. Behav. 2023, 55, 523–530. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veladiz-Gracia, F.; Elinos, D.; González-Sierra, C.; Rubio-Galicia, A.; Cifuentes, F.; Morales, M.A. Long-Term Potentiation and Neurotransmitter Expression Change in Dysautonomia Linked to Binge Eating Disorder: Protective Role of Exercise. Biology 2025, 14, 1410. https://doi.org/10.3390/biology14101410
Veladiz-Gracia F, Elinos D, González-Sierra C, Rubio-Galicia A, Cifuentes F, Morales MA. Long-Term Potentiation and Neurotransmitter Expression Change in Dysautonomia Linked to Binge Eating Disorder: Protective Role of Exercise. Biology. 2025; 14(10):1410. https://doi.org/10.3390/biology14101410
Chicago/Turabian StyleVeladiz-Gracia, Fernanda, Diana Elinos, Constanza González-Sierra, Angel Rubio-Galicia, Fredy Cifuentes, and Miguel Angel Morales. 2025. "Long-Term Potentiation and Neurotransmitter Expression Change in Dysautonomia Linked to Binge Eating Disorder: Protective Role of Exercise" Biology 14, no. 10: 1410. https://doi.org/10.3390/biology14101410
APA StyleVeladiz-Gracia, F., Elinos, D., González-Sierra, C., Rubio-Galicia, A., Cifuentes, F., & Morales, M. A. (2025). Long-Term Potentiation and Neurotransmitter Expression Change in Dysautonomia Linked to Binge Eating Disorder: Protective Role of Exercise. Biology, 14(10), 1410. https://doi.org/10.3390/biology14101410






