Unraveling Melasma: From Epidermal Pigmentation to Microenvironmental Dysregulation
Abstract
Simple Summary
Abstract
1. Introduction
2. The Epidermal Landscape of Melasma: Beyond Melanocyte Hyperfunction
2.1. Melanocyte Activity and Melanin Production
2.2. Epidermal Barrier Dysfunction and Melanin Retention
3. Dermal Remodeling and the Photoaging Connection
3.1. Solar Elastosis and Extracellular Matrix (ECM) Abnormalities
3.2. Basement Membrane (BM) Disruption
3.3. Increased Vascularity (Angiogenesis)
3.4. Senescent Dermal Fibroblasts
3.5. Sebaceous Glands
4. Immune Dysregulation and Chronic Inflammation
4.1. Mast Cell Involvement
4.2. T Cells and Macrophages
4.3. Neurogenic Inflammation and the Skin Microenvironment
5. Key Modulating Factors in Melasma Pathogenesis
5.1. Ultraviolet (UV) Radiation: The Primary Environmental Trigger
5.2. Hormonal Fluctuations: Endogenous Modulators
5.3. Genetic Predisposition: The Underlying Susceptibility
5.4. Recently Recognized Factors
6. Therapeutic Implications and Future Directions
6.1. Photoprotection as a Cornerstone
6.2. Advanced Tyrosinase Inhibition Strategies
6.3. Targeting Vascularity and Inflammation
6.4. Modulating Oxidative Stress and Photoaging
6.5. Addressing Immune Dysregulation
6.6. Tranexamic Acid: A Multifaceted Agent
6.7. Holistic and Personalized Approaches
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | adenylate cyclase |
| AMPK | AMP-activated protein kinase |
| bFGF | basic fibroblast growth factor |
| BM | basement membrane |
| cAMP | cyclic adenosine phosphate |
| Cer | ceramide |
| CGRP | calcitonin gene-related peptide |
| CLC7 | chloride channel 7 |
| COC | combined oral contraceptive |
| COX-2 | cyclooxygenase-2 |
| CREB | cAMP-response element binding protein |
| DDX5 | DEAD-Box Helicase 5 |
| ECM | extracellular matrix |
| EDN1 | endothelin 1 |
| EDNRB | endothelin receptor B |
| ERK | extracellular signal-regulated kinase |
| ESR | estrogen receptor |
| ET | endothelin |
| H2 | histamine type 2 |
| HEVL | high-energy visible light |
| HGF | hepatocyte growth factor |
| hIUD | hormonal intrauterine device |
| IGF | insulin-like growth factor |
| IL | interleukin |
| KGF | keratinocyte growth factor |
| LED | light-emitting diode |
| MAPK | mitogen-activated protein kinase |
| MC1R | melanocortin 1 receptor |
| MITF | microphthalm-associated transcription factor |
| MMP | matrix metalloproteinase |
| NEP | neural endopeptidase |
| NGF | nerve growth factor |
| NGFR | nerve growth factor receptor |
| OCA2 | oculocutaneous albinism 2 |
| OXT | oxytocin |
| PDL | pulsed-dye laser |
| PKA | protein kinase A |
| PKC | protein kinase C |
| POC | progestin-only contraceptive |
| ROS | reactive oxygen species |
| SASP | senescence-associated secretory phenotype |
| SCF | stem cell factor |
| SG | sebaceous gland |
| SLC | solute carrier |
| SNP | single-nucleotide polymorphism |
| TPC2 | two-pore channel 2 |
| TRPV | transient receptor potential vanilloid |
| TXA | tranexamic acid |
| TYR | tyrosinase |
| TYRP | tyrosinase-related protein |
| ULC | ultra-long chain |
| UV | ultraviolet |
| UVB | ultraviolet B |
| UVR | ultraviolet radiation |
| V-ATPase | vacuolar-type ATPase |
| VDR | vitamin D receptor |
| VEGF | vascular endothelial growth factor |
| VL | visible light |
| VLC | very long chain |
| αMSH | α-melanocyte stimulating hormone |
References
- Desai, S.R.; Alexis, A.F.; Elbuluk, N.; Grimes, P.E.; Weiss, J.; Hamzavi, I.H.; Taylor, S.C. Best Practices in the Treatment of Melasma with a Focus on Patients with Skin of Color. J. Am. Acad. Dermatol. 2024, 90, 269–279. [Google Scholar] [CrossRef]
- Gan, C.; Rodrigues, M. An Update on New and Existing Treatments for the Management of Melasma. Am. J. Clin. Dermatol. 2024, 25, 717–733. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.N.; Kincaid, C.M.; Mesinkovska, N.A. The Burden of Melasma: Race, Ethnicity, and Comorbidities. J. Drugs Dermatol. JDD 2024, 23, 691–693. [Google Scholar] [CrossRef]
- Udomwech, L.; Eden, C.; Tawanwongsri, W. Relationship between Facial Melasma and Ocular Photoaging Diseases. Med. Sci. 2025, 13, 61. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, Q.; Xia, Y. New Mechanistic Insights of Melasma. Clin. Cosmet. Investig. Dermatol. 2023, 16, 429–442. [Google Scholar] [CrossRef]
- Mpofana, N.; Paulse, M.; Gqaleni, N.; Makgobole, M.U.; Pillay, P.; Hussein, A.; Dlova, N.C. The Effect of Melasma on the Quality of Life in People with Darker Skin Types Living in Durban, South Africa. Int. J. Environ. Res. Public Health 2023, 20, 7068. [Google Scholar] [CrossRef]
- Karadogan, S.K.; Irer, B. Sexual Dysfunction in Women with Facial Melasma: A Case Control Study. Arch. Dermatol. Res. 2025, 317, 779. [Google Scholar] [CrossRef] [PubMed]
- Artzi, O.; Horovitz, T.; Bar-Ilan, E.; Shehadeh, W.; Koren, A.; Zusmanovitch, L.; Mehrabi, J.N.; Salameh, F.; Isman Nelkenbaum, G.; Zur, E.; et al. The Pathogenesis of Melasma and Implications for Treatment. J. Cosmet. Dermatol. 2021, 20, 3432–3445. [Google Scholar] [CrossRef]
- Yao, H.; Shen, S.; Gao, X.; Feng, J.; Song, X.; Xiang, W. Definition of Refractory Melasma and Its Treatment: A Review. Lasers Med. Sci. 2024, 39, 118. [Google Scholar] [CrossRef]
- Hafeez, F.; Mata, D.A.; Lian, C.G.; Poulos, E.G. Prominent Transepidermal Melanin Deposition Is a Distinguishing Histopathological Feature of Melasma: A Clinicopathologic Study. Dermatology 2021, 237, 145–147. [Google Scholar] [CrossRef]
- Guarneri, F. Etiopathogenesis of Melasma. J. Pigment. Disord. 2014, S1, 003. [Google Scholar] [CrossRef]
- Espósito, A.C.C.; Cassiano, D.P.; Da Silva, C.N.; Lima, P.B.; Dias, J.A.F.; Hassun, K.; Bagatin, E.; Miot, L.D.B.; Miot, H.A. Update on Melasma—Part I: Pathogenesis. Dermatol. Ther. 2022, 12, 1967–1988. [Google Scholar] [CrossRef] [PubMed]
- Suryaningsih, B.E.; Sadewa, A.H.; Wirohadidjojo, Y.W.; Soebono, H. Association between Heterozygote Val92Met MC1R Gene Polymorphisms with Incidence of Melasma: A Study of Javanese Women Population in Yogyakarta. Clin. Cosmet. Investig. Dermatol. 2019, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Morgado-Carrasco, D.; Piquero-Casals, J.; Granger, C.; Trullàs, C.; Passeron, T. Melasma: The Need for Tailored Photoprotection to Improve Clinical Outcomes. Photodermatol. Photoimmunol. Photomed. 2022, 38, 515–521. [Google Scholar] [CrossRef]
- Sarkar, R.; Jagadeesan, S.; Basavapura Madegowda, S.; Verma, S.; Hassan, I.; Bhat, Y.; Minni, K.; Jha, A.; Das, A.; Jain, G.; et al. Clinical and Epidemiologic Features of Melasma: A Multicentric Cross-Sectional Study from India. Int. J. Dermatol. 2019, 58, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Guo, S.; Tan, C. Diet and Living Environment as Novel Etiological Factors for Melasma: The Results form a Retrospective Case-Control Study of 150 Chinese Patients. J. Cosmet. Dermatol. 2025, 24, e70038. [Google Scholar] [CrossRef]
- He, X.; Chen, L.; Jin, S.; Ding, Y.; Xu, Z.; Xiang, L.; Zhang, C. Correlation of Biorhythmic Disorders with Melasma: A Cross-Sectional Survey. J. Am. Acad. Dermatol. 2025, 92, 185–187. [Google Scholar] [CrossRef]
- Getachew, E.D.; Kamal, K.; Young, K.; Xiang, D.H.; Semenov, Y.; Mostaghimi, A.; Theodosakis, N. A Case-Control Study of Racial Differences in Melasma Risk Factors and Incidence in a Diverse National United States Population. J. Am. Acad. Dermatol. 2024, 91, 539–541. [Google Scholar] [CrossRef]
- Ning, X.; Yang, J.; Ouyang, H.; Jiang, L.; Yu, R.; Li, C.; Sheng, Y.; Song, X.; Xu, J.; Zuo, X.; et al. In Vivo Evaluation of Melasma Pathologic Features and Treatment Response by 2-Photon Microscopy. JAMA Dermatol. 2025, e252790, Epub ahead of print. [Google Scholar] [CrossRef]
- Gu, D.; Pan, R.; Meng, X.; Liu, T.; Zhong, H.; Chen, N.; Xu, Y. What Lies behind Melasma: A Review of the Related Skin Microenvironment. Int. J. Dermatol. 2025, 64, 256–265. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Park, K.-C. Clues to the Pathogenesis of Melasma from Its Histologic Findings. J. Pigment. Disord. 2014, 1, 141. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Hwang, Y.-J.; Lee, S.-K.; Park, K.-C. Heterogeneous Pathology of Melasma and Its Clinical Implications. Int. J. Mol. Sci. 2016, 17, 824. [Google Scholar] [CrossRef]
- Ali, L.; Al Niaimi, F. Pathogenesis of Melasma Explained. Int. J. Dermatol. 2025, 64, 1201–1212. [Google Scholar] [CrossRef]
- Chang, C.-C.; Wang, Y.-J.; Huang, L.; Chen, I.-L.; Shih, Y.-C.; Shen, J.-W.; Lu, M.-E.; Chiang, H.-M.; Lin, B.-S.; Wu, Y.-H. Photoaging Features of Melasma: An in Vivo Layered and Quantitative Analysis Using Computer-Aided Detection of Cellular Resolution Full-Field Optical Coherence Tomography. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e870–e873. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Jin, S.; Xuan, Y.; Yang, Y.; Lu, X.; Wang, C.; Chen, L.; Xiang, L.; Zhang, C. 590 Nm LED Irradiation Improved Erythema through Inhibiting Angiogenesis of Human Microvascular Endothelial Cells and Ameliorated Pigmentation in Melasma. Cells 2022, 11, 3949. [Google Scholar] [CrossRef]
- Bastonini, E.; Kovacs, D.; Maresca, V.; Ottaviani, M.; Di Nardo, A.; Flori, E.; Cardinali, G.; Briganti, S. Lipidome Complexity in Physiological and Pathological Skin Pigmentation. Int. J. Mol. Sci. 2025, 26, 6785. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Lu, H.; Luo, H.; Hu, Y.; Chen, Y.; Xie, B.; Du, X.; Hua, Y.; Song, X. Tape Stripping and Lipidomics Reveal Skin Surface Lipid Abnormity in Female Melasma. Pigment. Cell Melanoma Res. 2021, 34, 1105–1111. [Google Scholar] [CrossRef]
- Liu, H.-M.; Cheng, M.-Y.; Xun, M.-H.; Zhao, Z.-W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible Mechanisms of Oxidative Stress-Induced Skin Cellular Senescence, Inflammation, and Cancer and the Therapeutic Potential of Plant Polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef] [PubMed]
- Mpofana, N.; Peter, C.; Lukman, H.Y.; Makgobole, M.U.; Dlova, N.C.; Gqaleni, N.; Hussein, A.; Sabiu, S. Mechanisms of Selected Cassipourea Metabolites for Melasma Treatment: Network Pharmacology and Molecular Dynamics Study. F1000Research 2024, 13, 952. [Google Scholar] [CrossRef]
- Phansuk, K.; Vachiramon, V.; Jurairattanaporn, N.; Chanprapaph, K.; Rattananukrom, T. Dermal Pathology in Melasma: An Update Review. Clin. Cosmet. Investig. Dermatol. 2022, 15, 11–19. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Na, J.-I.; Choi, J.-Y.; Park, K.-C. Melasma: Updates and Perspectives. Exp. Dermatol. 2019, 28, 704–708. [Google Scholar] [CrossRef]
- Serre, C.; Busuttil, V.; Botto, J.-M. Intrinsic and Extrinsic Regulation of Human Skin Melanogenesis and Pigmentation. Int. J. Cosmet. Sci. 2018, 40, 328–347. [Google Scholar] [CrossRef]
- Dall’Olmo, L.; Papa, N.; Surdo, N.C.; Marigo, I.; Mocellin, S. Alpha-Melanocyte Stimulating Hormone (α-MSH): Biology, Clinical Relevance and Implication in Melanoma. J. Transl. Med. 2023, 21, 562. [Google Scholar] [CrossRef] [PubMed]
- da Silva, C.N.; Miot, H.A.; Grassi, T.F.; Dias-Melício, L.A.; Santos, L.; Espósito, A.C.C. Expression of Endothelin-1, Endothelin Receptor-a, and Endothelin Receptor-B in Facial Melasma Compared to Adjacent Skin. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2847–2853. [Google Scholar] [CrossRef]
- Regazzetti, C.; De Donatis, G.M.; Ghorbel, H.H.; Cardot-Leccia, N.; Ambrosetti, D.; Bahadoran, P.; Chignon-Sicard, B.; Lacour, J.-P.; Ballotti, R.; Mahns, A.; et al. Endothelial Cells Promote Pigmentation through Endothelin Receptor B Activation. J. Investig. Dermatol. 2015, 135, 3096–3104. [Google Scholar] [CrossRef]
- Wang, J.; Jarrold, B.; Zhao, W.; Deng, G.; Moulton, L.; Laughlin, T.; Hakozaki, T. The Combination of Sucrose Dilaurate and Sucrose Laurate Suppresses HMGB1: An Enhancer of Melanocyte Dendricity and Melanosome Transfer to Keratinocytes. J. Eur. Acad. Dermatol. Venereol. 2022, 36 (Suppl. S3), 3–11. [Google Scholar] [CrossRef]
- Bao, M.; Gempeler, M.; Campiche, R. Melanosome Transport and Processing in Skin Pigmentation: Mechanisms and Targets for Pigmentation Modulation. Int. J. Mol. Sci. 2025, 26, 8630. [Google Scholar] [CrossRef]
- Feng, C.; Chen, X.; Yin, X.; Jiang, Y.; Zhao, C. Matrix Metalloproteinases on Skin Photoaging. J. Cosmet. Dermatol. 2024, 23, 3847–3862. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.J.; Navarro, C.; Durán, P.; Galan-Freyle, N.J.; Parra Hernández, L.A.; Pacheco-Londoño, L.C.; Castelanich, D.; Bermúdez, V.; Chacin, M. Antioxidants in Photoaging: From Molecular Insights to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 2403. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 682414. [Google Scholar] [CrossRef] [PubMed]
- Hajialiasgary Najafabadi, A.; Soheilifar, M.H.; Masoudi-Khoram, N. Exosomes in Skin Photoaging: Biological Functions and Therapeutic Opportunity. Cell Commun. Signal. 2024, 22, 32. [Google Scholar] [CrossRef]
- Hara, Y.; Shibata, T. Characteristics of Dermal Vascularity in Melasma and Solar Lentigo. Photodermatol. Photoimmunol. Photomed. 2024, 40, e12953. [Google Scholar] [CrossRef]
- Kumar, D.; Sood, R.; Tiwari, P. Melasma Management: Unveiling Recent Breakthroughs through Literature Analysis. Health Sci. Rev. 2025, 14, 100213. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, H.; Yang, Y.; Zhang, S.; Wang, J.; Zhang, D.; Yu, H. Metformin Attenuates UVA-Induced Skin Photoaging by Suppressing Mitophagy and the PI3K/AKT/mTOR Pathway. Int. J. Mol. Sci. 2022, 23, 6960. [Google Scholar] [CrossRef]
- Liu, L.-X.; Liao, Z.-K.; Dong, B.-Q.; Jiang, S.; Lei, T.-C. Tranexamic Acid Ameliorates Skin Hyperpigmentation by Downregulating Endothelin-1 Expression in Dermal Microvascular Endothelial Cells. Ann. Dermatol. 2024, 36, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yu, Z.; Wu, W.; Du, Y.; Du, Q.; Huang, H.; Li, Y.; Xuan, T.; Liang, Y.-C.; Liu, Y.; et al. Fibroblast Bioelectric Signaling Drives Hair Growth. Cell 2025, 188, 5175–5193. [Google Scholar] [CrossRef]
- Chen, L.; Hu, Y.; Zhang, M.; Liu, L.; Ma, J.; Xu, Z.; Zhang, J.; Gu, H.; Chen, K. METTL14 Affects UVB-induced Human Dermal Fibroblasts Photoaging via miR-100-3p Biogenesis in an m6A-dependent Manner. Aging Cell 2024, 23, e14123. [Google Scholar] [CrossRef]
- Chen, Y.; Lian, N.; Chen, S.; Xiao, T.; Ke, Y.; Zhang, Y.; Song, C.; Yang, Y.; Xu, S.; Gu, H.; et al. GSDME Deficiency Leads to the Aggravation of UVB-Induced Skin Inflammation through Enhancing Recruitment and Activation of Neutrophils. Cell Death Dis. 2022, 13, 841. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Photoaging: UV Radiation-Induced Inflammation and Immunosuppression Accelerate the Aging Process in the Skin. Inflamm. Res. 2022, 71, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, R.; Dhatwalia, S.K.; Kumar, R.; Rani, S.; Parsad, D. Emerging Role of Dermal Compartment in Skin Pigmentation: Comprehensive Review. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Liao, Z.; Dong, B.; Jiang, S.; Lei, T. Targeting Senescent Dermal Fibroblasts Responsible for Hyperactive Melanocytes in Melasma. Chin. Med. J. 2023, 136, 1563–1565. [Google Scholar] [CrossRef]
- Espósito, A.C.C.; Brianezi, G.; Miot, L.D.B.; Miot, H.A. Fibroblast Morphology, Growth Rate and Gene Expression in Facial Melasma. An. Bras. Dermatol. 2022, 97, 575–582. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, H.; Wu, X.; Xu, Y.; Tan, Q. Resveratrol Activates Autophagy and Protects from UVA-Induced Photoaging in Human Skin Fibroblasts and the Skin of Male Mice by Regulating the AMPK Pathway. Biogerontology 2024, 25, 649–664. [Google Scholar] [CrossRef]
- Mosca, S.; Ottaviani, M.; Briganti, S.; Di Nardo, A.; Flori, E. The Sebaceous Gland: A Key Player in the Balance between Homeostasis and Inflammatory Skin Diseases. Cells 2025, 14, 747. [Google Scholar] [CrossRef]
- Veniaminova, N.A.; Jia, Y.Y.; Hartigan, A.M.; Huyge, T.J.; Tsai, S.-Y.; Grachtchouk, M.; Nakagawa, S.; Dlugosz, A.A.; Atwood, S.X.; Wong, S.Y. Distinct Mechanisms for Sebaceous Gland Self-Renewal and Regeneration Provide Durability in Response to Injury. Cell Rep. 2023, 42, 113121. [Google Scholar] [CrossRef]
- Abdel-Naser, M.B.; Seltmann, H.; Zouboulis, C.C. SZ95 Sebocytes Induce Epidermal Melanocyte Dendricity and Proliferation in Vitro. Exp. Dermatol. 2012, 21, 393–395. [Google Scholar] [CrossRef]
- Abdel-Naser, M.B.; Nikolakis, G.; Zouboulis, C.C. Preservation of Epidermal Melanocyte Integrity in an Ex Vivo Co-culture Skin Model with Sebocytes. Exp. Dermatol. 2023, 32, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Flori, E.; Mastrofrancesco, A.; Mosca, S.; Ottaviani, M.; Briganti, S.; Cardinali, G.; Filoni, A.; Cameli, N.; Zaccarini, M.; Zouboulis, C.C.; et al. Sebocytes Contribute to Melasma Onset. iScience 2022, 25, 103871. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Kim, S.L.; Lee, J.S.; Kwon, K.-Y.; Lee, S.-J.; Kim, D.W.; Lee, W.J. Possible Existence of Melanocytes or Melanoblasts in Human Sebaceous Glands. Ann. Dermatol. 2014, 26, 469–473. [Google Scholar] [CrossRef]
- Dileepan, K.N.; Raveendran, V.V.; Sharma, R.; Abraham, H.; Barua, R.; Singh, V.; Sharma, R.; Sharma, M. Mast Cell-Mediated Immune Regulation in Health and Disease. Front. Med. 2023, 10, 1213320. [Google Scholar] [CrossRef]
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front. Cell. Neurosci. 2019, 13, 422. [Google Scholar] [CrossRef]
- Marek-Jozefowicz, L.; Nedoszytko, B.; Grochocka, M.; Żmijewski, M.A.; Czajkowski, R.; Cubała, W.J.; Slominski, A.T. Molecular Mechanisms of Neurogenic Inflammation of the Skin. Int. J. Mol. Sci. 2023, 24, 5001. [Google Scholar] [CrossRef]
- Sabat, R.; Wolk, K.; Loyal, L.; Döcke, W.-D.; Ghoreschi, K. T Cell Pathology in Skin Inflammation. Semin. Immunopathol. 2019, 41, 359–377. [Google Scholar] [CrossRef]
- Ni, X.; Xu, Y.; Wang, W.; Kong, B.; Ouyang, J.; Chen, J.; Yan, M.; Wu, Y.; Chen, Q.; Wang, X.; et al. IL-17D-Induced Inhibition of DDX5 Expression in Keratinocytes Amplifies IL-36R-Mediated Skin Inflammation. Nat. Immunol. 2022, 23, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Moradzad, M.; Ghalamkari, S.; Fadaei, M.; Cowin, A.J.; Hassanshahi, M. Macrophage-Mediated Inflammation in Skin Wound Healing. Cells 2022, 11, 2953. [Google Scholar] [CrossRef]
- Shutova, M.S.; Boehncke, W.-H. Mechanotransduction in Skin Inflammation. Cells 2022, 11, 2026. [Google Scholar] [CrossRef]
- Byun, J.W.; Park, I.S.; Choi, G.S.; Shin, J. Role of Fibroblast-Derived Factors in the Pathogenesis of Melasma. Clin. Exp. Dermatol. 2016, 41, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Bak, H.; Lee, H.J.; Chang, S.-E.; Choi, J.-H.; Kim, M.N.; Kim, B.J. Increased Expression of Nerve Growth Factor Receptor and Neural Endopeptidase in the Lesional Skin of Melasma. Dermatol. Surg. 2009, 35, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Serhan, N.; Basso, L.; Sibilano, R.; Petitfils, C.; Meixiong, J.; Bonnart, C.; Reber, L.L.; Marichal, T.; Starkl, P.; Cenac, N.; et al. House Dust Mites Activate Nociceptor–Mast Cell Clusters to Drive Type 2 Skin Inflammation. Nat. Immunol. 2019, 20, 1435–1443. [Google Scholar] [CrossRef]
- Chaijaras, S.; Boonpethkaew, S.; Chirasuthat, S.; Sakpuwadol, N.; Yongpisarn, T.; Anansiripun, P.; Vachiramon, V. Efficacy of Botulinum Toxin a for the Management of Melasma: A Split-Face, Randomized Control Study. J. Cosmet. Dermatol. 2025, 24, e70376. [Google Scholar] [CrossRef]
- Rahimi, H.; Mirnezami, M.; Yazdabadi, A. Bilirubin as a New Antioxidant in Melasma. J. Cosmet. Dermatol. 2022, 21, 5800–5803. [Google Scholar] [CrossRef]
- Cui, B.; Wang, Y.; Jin, J.; Yang, Z.; Guo, R.; Li, X.; Yang, L.; Li, Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid. Med. Cell. Longev. 2022, 2022, 6037303. [Google Scholar] [CrossRef]
- Liu, L.; Xu, Z.; Dai, X.; Zhou, X.; Chen, L.; Luan, C.; Huang, D.; Chen, H.; Zhang, J.; Hu, Y.; et al. Mechanistic Insights Into 5′-tiRNA-His-GTG Mediated Activation of the JNK Pathway in Skin Photoaging. Aging Cell 2025, 24, e70049. [Google Scholar] [CrossRef]
- Cario, M. How Hormones May Modulate Human Skin Pigmentation in Melasma: An in Vitro Perspective. Exp. Dermatol. 2019, 28, 709–718. [Google Scholar] [CrossRef]
- Gauthier, Y.; Cario, M.; Pain, C.; Lepreux, S.; Benzekri, L.; Taieb, A. Oestrogen Associated with Ultraviolet B Irradiation Recapitulates the Specific Melanosome Distribution Observed in Caucasoid Melasma. Br. J. Dermatol. 2019, 180, 951–953. [Google Scholar] [CrossRef]
- Goandal, N.F.; Rungby, J.; Karmisholt, K.E. The Role of Sex Hormones in the Pathogenesis of Melasma. Ugeskr. Laeger 2022, 184, V10210769. [Google Scholar] [PubMed]
- Filoni, A.; Mariano, M.; Cameli, N. Melasma: How Hormones Can Modulate Skin Pigmentation. J. Cosmet. Dermatol. 2019, 18, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.; Lipner, S.R. Variations in Melasma Risk Associated with Different Synthetic Progestins: A Population-Based Analysis of TriNetX. J. Am. Acad. Dermatol. 2025, 93, 837–839. [Google Scholar] [CrossRef]
- Cheng, D.; Gaurav, A.; Xiang, D.; Ji, H.; Hirsh, D.A.; Chen, S.T.; Mostaghimi, A.; Ma, K.S.-K.; Theodosakis, N. Hormonal Intrauterine Devices Are Associated with Lower Long-Term Melasma Risk Compared with Combined and Progestin-Only Oral Contraceptives: A Population-Based Cohort Study. J. Am. Acad. Dermatol. 2025, 93, 473–475. [Google Scholar] [CrossRef] [PubMed]
- Locci-Molina, N.; Wang, A.; Kroumpouzos, G. Melasma Improving Spontaneously upon Switching from a Combined Oral Contraceptive to a Hormone-Releasing Intrauterine Device: A Report of Four Cases. Acta Derm. Venereol. 2015, 95, 624–625. [Google Scholar] [CrossRef]
- Alanazi, M.M.; Alsanea, S.; Kumar, A.; Alehaideb, Z.; Matou-Nasri, S.; AlGhamdi, K.M. Modulatory Effects of Oxytocin on Normal Human Cultured Melanocyte Proliferation, Migration, and Melanogenesis. Tissue Cell 2024, 91, 102579. [Google Scholar] [CrossRef]
- Seleit, I.; Bakry, O.A.; Masoud, E.; Nabil, S. Identification of Genotypes and Allelic Frequencies of Vitamin D Receptor Gene Polymorphism (TaqI) in Egyptian Melasma Patients. Indian Dermatol. Online J. 2017, 8, 443–448. [Google Scholar] [CrossRef]
- Mpofana, N.; Mlambo, Z.P.; Makgobole, M.U.; Dlova, N.C.; Naicker, T. Association of Genetic Polymorphisms in SLC45A2, TYR, HERC2, and SLC24A in African Women with Melasma: A Pilot Study. Int. J. Mol. Sci. 2025, 26, 1158. [Google Scholar] [CrossRef]
- Mun, S.J.; Lee, V.; Gupta, M. Sunscreens in Pigmentary Disorders: Time to Revise the Message. Photochem. Photobiol. Sci. 2025, 24, 215–225. [Google Scholar] [CrossRef]
- He, M.; Chen, X.; Jin, S.; Zhang, C. Visible Light Protection: An Updated Review of Tinted Sunscreens. Photodermatol. Photoimmunol. Photomed. 2025, 41, e70033. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, S.; Xie, H.; Meng, X.; Cui, B.; Xiao, Z. Melasma Secondary to Drugs: A Real-World Pharmacovigilance Study of the FDA Adverse Event Reporting System (FAERS). BMC Pharmacol. Toxicol. 2025, 26, 73. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, J.W.; Kim, Y.; Park, T.J.; Lee, J.Y.; Kang, H.Y. Alitretinoin Induces Skin Pigmentation: Implications for Melasma. Eur. J. Dermatol. 2022, 32, 140–142. [Google Scholar] [CrossRef]
- Grimes, P.E.; Paturi, J.; Chen, Y.; Wangari-Olivero, J.; Dumbuya, H.; Yan, X.; Lynch, S.; Zheng, Q. Photoprotection Efficacy of Sun Protection Factor and Iron Oxide Formulations in Diverse Skin with Melasma and Photodamage. J. Drugs Dermatol. 2025, 24, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Karkoszka, M.; Rok, J.; Wrześniok, D. Melanin Biopolymers in Pharmacology and Medicine—Skin Pigmentation Disorders, Implications for Drug Action, Adverse Effects and Therapy. Pharmaceuticals 2024, 17, 521. [Google Scholar] [CrossRef] [PubMed]
- Yardman-Frank, J.M.; Fisher, D.E. Skin Pigmentation and Its Control: From Ultraviolet Radiation to Stem Cells. Exp. Dermatol. 2021, 30, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting Tyrosinase in Hyperpigmentation: Current Status, Limitations and Future Promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef] [PubMed]
- Koirala, M.; Shashikala, H.B.M.; Jeffries, J.; Wu, B.; Loftus, S.K.; Zippin, J.H.; Alexov, E. Computational Investigation of the pH Dependence of Stability of Melanosome Proteins: Implication for Melanosome Formation and Disease. Int. J. Mol. Sci. 2021, 22, 8273. [Google Scholar] [CrossRef]
- Bellono, N.W.; Escobar, I.E.; Oancea, E. A Melanosomal Two-Pore Sodium Channel Regulates Pigmentation. Sci. Rep. 2016, 6, 26570. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Sharma, A.; Motiani, R.K. A Novel Gain of Function Mutation in TPC2 Reiterates pH-Pigmentation Interplay: Emerging Role of Ionic Homeostasis as a Master Pigmentation Regulator. Cell Calcium 2023, 111, 102705. [Google Scholar] [CrossRef]
- Miao, F.; Su, M.-Y.; Jiang, S.; Luo, L.-F.; Shi, Y.; Lei, T.-C. Intramelanocytic Acidification Plays a Role in the Antimelanogenic and Antioxidative Properties of Vitamin C and Its Derivatives. Oxid. Med. Cell. Longev. 2019, 2019, 2084805. [Google Scholar] [CrossRef]
- Cho, E.; Hyung, K.E.; Choi, Y.-H.; Chun, H.; Kim, D.; Jun, S.-H.; Kang, N.-G. Modulating OCA2 Expression as a Promising Approach to Enhance Skin Brightness and Reduce Dark Spots. Biomolecules 2024, 14, 1284. [Google Scholar] [CrossRef]
- Liu, Y.; Chi, W.; Tao, L.; Wang, G.; Deepak, R.N.V.K.; Sheng, L.; Chen, T.; Feng, Y.; Cao, X.; Cheng, L.; et al. Ablation of Proton/Glucose Exporter SLC45A2 Enhances Melanosomal Glycolysis to Inhibit Melanin Biosynthesis and Promote Melanoma Metastasis. J. Investig. Dermatol. 2022, 142, 2744–2755.e9. [Google Scholar] [CrossRef]
- Scales, J.L.; Koroma, D.C.; Oancea, E. Chapter Thirteen—Single Organelle Measurements of Melanosome pH Using the Novel Ratiometric Indicator RpHiMEL. In Methods in Enzymology; Ion Channels: Channel Chemical Biology, Engineering, and Physiological Function; Minor, D.L., Colecraft, H.M., Eds.; Academic Press: Cambridge, MA, USA, 2021; Volume 654, pp. 315–344. [Google Scholar]
- Collins, M.P.; Forgac, M. Regulation and Function of V-ATPases in Physiology and Disease. Biochim. Biophys. Acta (BBA)-Biomembr. 2020, 1862, 183341. [Google Scholar] [CrossRef]
- Wiriyasermkul, P.; Moriyama, S.; Nagamori, S. Membrane Transport Proteins in Melanosomes: Regulation of Ions for Pigmentation. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183318. [Google Scholar] [CrossRef]
- Yousaf, S.; Sethna, S.; Chaudhary, M.A.; Shaikh, R.S.; Riazuddin, S.; Ahmed, Z.M. Molecular Characterization of SLC24A5 Variants and Evaluation of Nitisinone Treatment Efficacy in a Zebrafish Model of OCA6. Pigm. Cell Melanoma Res. 2020, 33, 556–565. [Google Scholar] [CrossRef]
- Koroma, D.C.; Scales, J.L.; Trotman, J.C.; Wakamatsu, K.; Ito, S.; Oancea, E. The Lysosomal Chloride-Proton Exchanger CLC7 Functions in Melanosomes as a Negative Regulator of Human Pigmentation. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kong, S.H.; Suh, H.S.; Choi, Y.S. Treatment of Melasma with Pulsed-Dye Laser and 1,064-Nm Q-Switched Nd:YAG Laser: A Split-Face Study. Ann. Dermatol. 2018, 30, 1–7. [Google Scholar] [CrossRef]
- Speeckaert, R.; Bulat, V.; Speeckaert, M.M.; van Geel, N. The Impact of Antioxidants on Vitiligo and Melasma: A Scoping Review and Meta-Analysis. Antioxidants 2023, 12, 2082. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, H.; Su, T.; Huang, W.; Wu, X.; Chen, X.; Ye, S.; Zhong, J.; Li, C.; Li, Y. Luteolin Mitigates Photoaging Caused by UVA-Induced Fibroblast Senescence by Modulating Oxidative Stress Pathways. Int. J. Mol. Sci. 2025, 26, 1809. [Google Scholar] [CrossRef] [PubMed]
- Holanda, I.R.M.; de Almeida Corrêa Alfredo, M.; Cassiano, D.P.; Esposito, A.C.C.; Lima, P.B.; Bagatin, E.; Miot, H.A. Efficacy of Oral 5 Mg Melatonin in the Treatment of Facial Melasma in Women: A Double-Blind, Randomized, Placebo-Controlled Clinical Trial. J. Eur. Acad. Dermatol. Venereol. 2024, 38, e607–e609. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Jin, J.; Huang, J.; Guo, Y.; Qian, Q. Combining Large-Spot Low-Fluence 1064-Nm and Fractional 1064-Nm Picosecond Lasers for Promoting Protective Melanosome Autophagy via the PI3K/Akt/mTOR Signalling Pathway for the Treatment of Melasma. Exp. Dermatol. 2024, 33, e15094. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.a.F.; Lima, P.B.; Cassiano, D.P.; Espósito, A.C.C.; Bagatin, E.; Miot, L.D.B.; Miot, H.A. Oral Ketotifen Associated with Famotidine for the Treatment of Facial Melasma: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e123–e125. [Google Scholar] [CrossRef]
- Chin, N.E.; Austin, A.H. Expanding Inclusivity: Tranexamic Acid for the Treatment of Melasma in Males. J. Drugs Dermatol. 2024, 23, e110–e112. [Google Scholar] [CrossRef]
- Bagherani, N. The Efficacy of Tranexamic Acid in the Treatment of Melasma: The Efficacy of Tranexamic Acid in the Treatment of Melasma. Dermatol. Ther. 2015, 28, 265. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, Y.; Zhao, Y.; Geng, Q.; Guan, C.; Xu, J.; Xie, B.; Song, X. Tranexamic Acid May Promote Melanocores Clustering in Keratinocytes through Upregulation of Rab5b. Exp. Dermatol. 2023, 32, 777–786. [Google Scholar] [CrossRef]
- Demirbas, A.; Ulutas Demirbas, G.; Diremsizoglu, E.; Esen, M. Dual Benefits of Oral Tranexamic Acid: Reducing Melasma Severity and Inflammation. J. Cosmet. Dermatol. 2025, 24, e70257. [Google Scholar] [CrossRef]
- Kuceki, G.; Mendez, D.; Garza-Dueñas, G.G.; Kruithoff, C.; Fonseca, L.O.; Lal, K. No Thromboembolic Events Observed in RCTs of Oral Tranexamic Acid: A Systematic Review and Descriptive Synthesis. J. Am. Acad. Dermatol. 2025, 93, 1086–1088. [Google Scholar] [CrossRef]
- Liang, R.; Luo, H.; Pan, W.; Yang, S.; Peng, X.; Kuang, B.; Huang, H.; Liu, C. Comparative Efficacy and Safety of Tranexamic Acid for Melasma by Different Administration Methods: A Systematic Review and Network Meta-Analysis. J. Cosmet. Dermatol. 2024, 23, 1150–1164. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Chan, L.; Handog, E.; Djojoseputro, L.; Lim, J.; Ling, R.; Nguyen, H.; Tam, E.; Tang, J.; Thng, S.; et al. Optimizing Melasma Management with Topical Tranexamic Acid: An Expert Consensus. J. Drugs Dermatol. 2023, 22, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Alfredo, M.-G.; Maribel, P.-M.; Eloy, P.-R.; Susana, G.-E.; Luis, L.G.S.; Carmen, G.M. Depigmenting Topical Therapy Based on a Synergistic Combination of Compounds Targeting the Key Pathways Involved in Melasma Pathophysiology. Exp. Dermatol. 2023, 32, 611–619. [Google Scholar] [CrossRef] [PubMed]
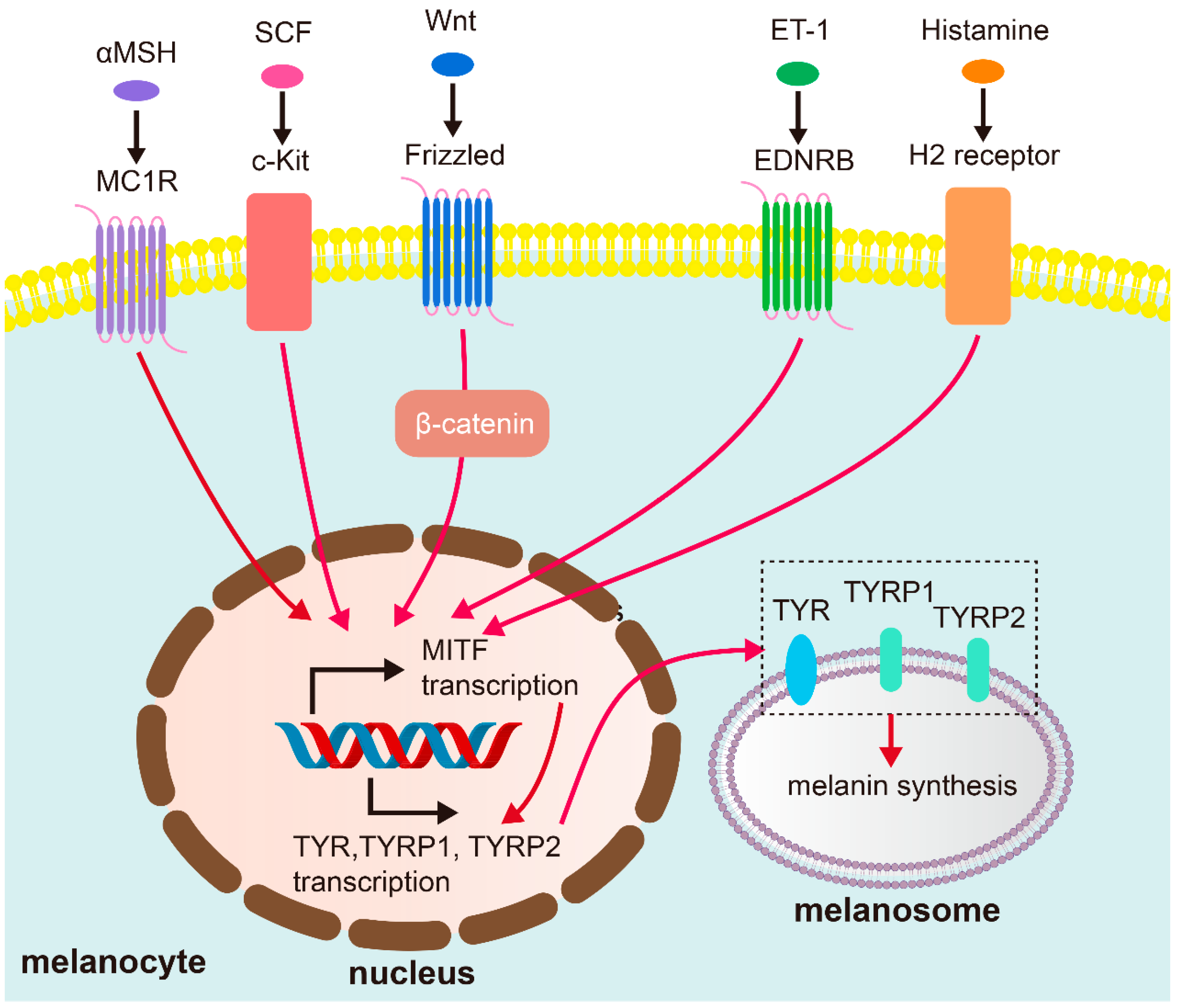
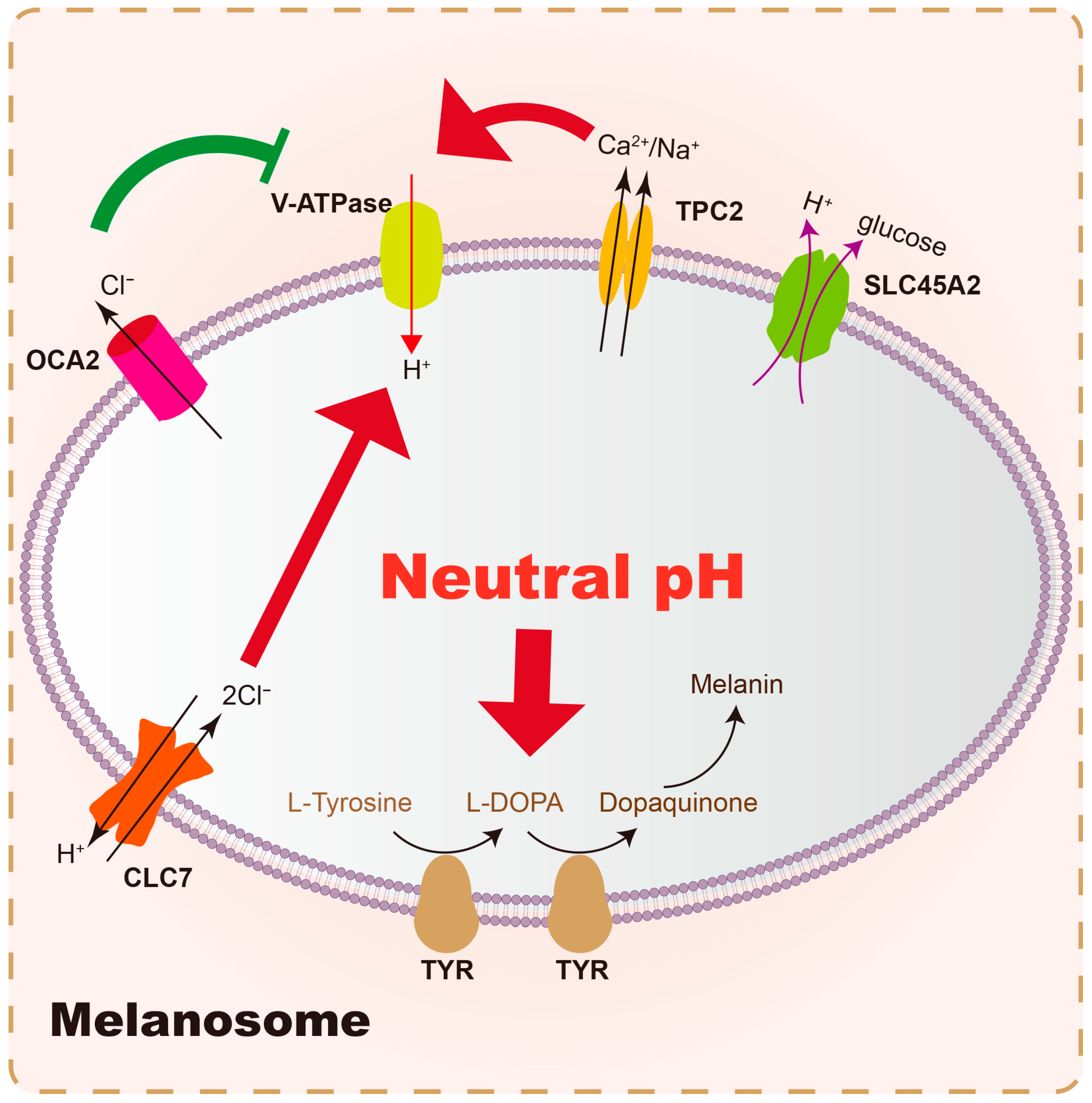
| Gene | Polymorphism/SNP | Population/Study | Association/Risk | Reference (Year) |
|---|---|---|---|---|
| MC1R | Val92Met (rs2228479) | Javanese women | Increased risk of melasma | Suryaningsih et al. (2019) [13] |
| VDR | TaqI (t allele/tt genotype) | Egyptian women | Associated with melasma | Seleit et al. (2017) [83] |
| TYR | rs1042602 (AA genotype) | African women (pilot) | Marked risk increase | Mpofana et al. (2025) [84] |
| HERC2 | rs1129038 | African women (pilot) | Population variation | Mpofana et al. (2025) [84] |
| SLC24A5 | rs1426654 | African women (pilot) | Population variation | Mpofana et al. (2025) [84] |
| Results | Membrane Proteins | Function | Reference (Year) |
|---|---|---|---|
| Raise pH | SLC45A2 | An H+-coupled glucose exporter in melanosomes | Liu et al. (2022) [98] |
| OCA2 | Outward transport of Cl− from the melanosome lumen, which decreases the driving force for inward H+ transport by V-ATPase | Scales et al. (2021) [99] | |
| Lower pH | V-ATPase | Pump the H+ into melanosomes in an ATP-dependent way | Collins et al. (2020) [100] |
| TPC2 | Generates membrane voltage (membrane potential) by the positive conductance, thus controlling the function of V-ATPase for H+ influx | Wiriyasermkul et al. (2020) [101] | |
| SLC24A5 | Transport Ca2+ and K+ into the melanosome in exchange for Na+ | Yousaf et al. (2021) [102] | |
| CLC7 | As a 2Cl−/1H+ antiporter, pumping Cl− into the lumen, which increases the V-ATPase driving force and acidifies the melanosome lumen | Koroma et al. (2021) [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, F.; Wan, J.; Zhou, Y.; Shi, Y. Unraveling Melasma: From Epidermal Pigmentation to Microenvironmental Dysregulation. Biology 2025, 14, 1402. https://doi.org/10.3390/biology14101402
Miao F, Wan J, Zhou Y, Shi Y. Unraveling Melasma: From Epidermal Pigmentation to Microenvironmental Dysregulation. Biology. 2025; 14(10):1402. https://doi.org/10.3390/biology14101402
Chicago/Turabian StyleMiao, Fang, Jing Wan, Youwen Zhou, and Ying Shi. 2025. "Unraveling Melasma: From Epidermal Pigmentation to Microenvironmental Dysregulation" Biology 14, no. 10: 1402. https://doi.org/10.3390/biology14101402
APA StyleMiao, F., Wan, J., Zhou, Y., & Shi, Y. (2025). Unraveling Melasma: From Epidermal Pigmentation to Microenvironmental Dysregulation. Biology, 14(10), 1402. https://doi.org/10.3390/biology14101402






