Cancer-Associated Fibroblasts Arising from Endothelial-to-Mesenchymal Transition: Induction Factors, Functional Roles, and Transcriptomic Evidence
Simple Summary
Abstract
1. Introduction
1.1. Cancer-Associated Fibroblasts (CAFs)
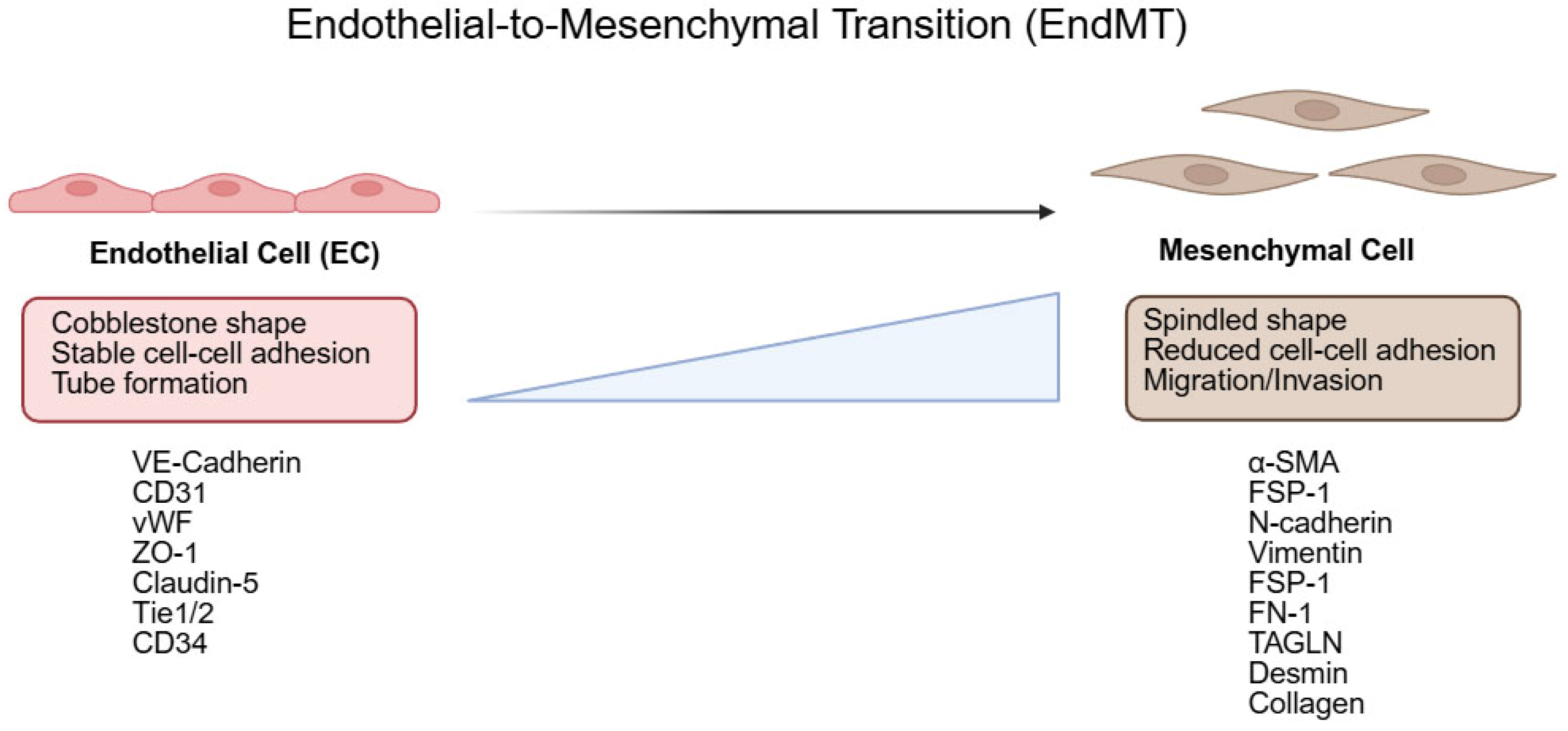
1.2. Endothelial-to-Mesenchymal Transition (EndMT)
2. EndMT Induction in Cancer: Models and Factors
2.1. Induction of EndMT Using Mouse Tumor Models
Zeisberg et al. (2007) [7]
2.2. Induction of EndMT Using Cancer-Conditioned Medium (CM)
2.2.1. Wojciech M. Ciszewski et al. (2017) [30]
2.2.2. Krizbai et al. (2015) [14]
2.2.3. Valentin Platel et al. (2022) [31]
2.2.4. Clara Bourreau et al. (2025) [32]
2.3. Induction of EndMT via Co-Culture
2.4. Induction of EndMT via 3D Modeling
2.4.1. Se-Hyuk Kim et al. (2019) [35]
2.4.2. Ju Hun Yeon et al. (2018) [36]
2.5. EndMT Induced by Tumor Microenvironment (TME) Constituents
2.5.1. Wen-Fei Wei et al. (2023) [29]
2.5.2. Tze-Sing Huang Group (Chi-Shuan Fan et al., 2018 [33]; Fan et al., 2019 [38])
2.6. Induction of EndMT via Targeted Gene Perturbation
2.6.1. Roselyne Tournaire Group (Julie Garcia et al., 2012 [39]; Marjorie Adjuto-Saccone et al., 2021 [40])
2.6.2. Seo-Hyun Choi Group (Seo-Hyun Choi et al., 2016 [41]; 2018 [28])
2.7. Virus-Induced EndMT
Paola Gasperini et al. (2012) [43]
3. EndMT-CAFs Revealed by Bioinformatics Tools
3.1. Han Luo et al. (2022) [44]
3.2. Quanzhong Liu et al. (2025) [21]
3.3. Minghui Hou et al. (2025) [45]
3.4. Li Ji et al. (2025) [46]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 3D | Three-dimensional |
| ACM | Activated conditioned medium |
| BEAM | Branch Expression Analysis Modeling |
| CAF | Cancer-associated fibroblast |
| CAFEndMT | EndMT-like cancer-associated fibroblast subtype |
| CAFmyo | Cancer-associated myofibroblast subtype |
| CGGA | Chinese Glioma Genome Atlas |
| CM | Conditioned medium |
| CRC | Colorectal cancer |
| CSCC | Cervical squamous cell carcinoma |
| DEG | Differentially expressed gene |
| DMVEC | Dermal microvascular endothelial cell |
| DSP | Differentially secreted protein |
| EAC | Esophageal adenocarcinoma |
| EGA | European Genome-phenome Archive |
| EC | Endothelial cell |
| ECM | Extracellular matrix |
| EdMTS | EndMT signature (score) |
| EMT | Epithelial-to-mesenchymal transition |
| EndMT | Endothelial-to-mesenchymal transition |
| GBC | Gallbladder cancer |
| GBM | Glioblastoma |
| GC | Gastric cancer |
| GO | Gene Ontology |
| GSEA | Gene Set Enrichment Analysis |
| HCC | Hepatocellular carcinoma |
| HCMEC | Human cerebral microvascular endothelial cell |
| HDLEC | Human dermal lymphatic endothelial cell |
| HEMEC | Human esophageal microvascular endothelial cell |
| HMEC-1 | Human microvascular endothelial cell line |
| HMVEC | Human microvascular endothelial cell |
| HPAEC | Human pulmonary arterial endothelial cell |
| HPMEC | Human pulmonary microvascular endothelial cell |
| HUVEC | Human umbilical vein endothelial cell |
| IBD | Inflammatory bowel disease |
| IFF | Interstitial fluid flow |
| IF | Immunofluorescence |
| KD | Knockdown |
| KO | Knockout |
| KP | KrasG12D;Trp53flox/flox (lung adenocarcinoma mouse model) |
| KS | Kaposi’s sarcoma |
| KSHV | Kaposi’s sarcoma-associated herpesvirus |
| LFQ | Label-free quantitation |
| lncRNA | Long non-coding RNA |
| LSL | LoxP-STOP-LoxP |
| MLEC | Mouse lung endothelial cell |
| MSC | Mesenchymal stem cell |
| mRNA | Messenger RNA |
| NSCLC | Non-small-cell lung cancer |
| PCA | Principal component analysis |
| PDAC | Pancreatic ductal adenocarcinoma |
| R26R-LacZ | Rosa26 reporter–LacZ |
| RBEC | Rat brain endothelial cell |
| rGBM | Recurrent glioblastoma |
| RIP1-Tag2 | RIP1-Tag2 pancreatic islet tumor mouse model |
| RNA-seq | RNA sequencing |
| ROS | Reactive oxygen species |
| SAEndo2 | Scar-associated endothelial subset |
| scRNA-seq | Single-cell RNA sequencing |
| shRNA | Short hairpin RNA |
| siRNA | Small interfering RNA |
| STAD | Stomach adenocarcinoma (TCGA cohort) |
| ST-seq | Spatial transcriptomics sequencing |
| TAM | Tumor-associated macrophage |
| TCGA | The Cancer Genome Atlas |
| tdTomato | Tandem dimer Tomato |
| TEER | Transendothelial electrical resistance |
| TEC | Tumor endothelial cell |
| TME | Tumor microenvironment |
| TMZ | Temozolomide |
| TMZ-R | Temozolomide-resistant |
| TMZ-S | Temozolomide-sensitive |
References
- Olumi, A.F.; Grossfeld, G.D.; Hayward, S.W.; Carroll, P.R.; Tlsty, T.D.; Cunha, G.R. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999, 59, 5002–5011. [Google Scholar] [CrossRef]
- Gaggioli, C.; Hooper, S.; Hidalgo-Carcedo, C.; Grosse, R.; Marshall, J.F.; Harrington, K.; Sahai, E. Fibroblast-led collective invasion of carcinoma cells with differing roles for RhoGTPases in leading and following cells. Nat. Cell Biol. 2007, 9, 1392–1400. [Google Scholar] [CrossRef]
- Tsujino, T.; Seshimo, I.; Yamamoto, H.; Ngan, C.Y.; Ezumi, K.; Takemasa, I.; Ikeda, M.; Sekimoto, M.; Matsuura, N.; Monden, M. Stromal myofibroblasts predict disease recurrence for colorectal cancer. Clin. Cancer Res. 2007, 13, 2082–2090. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Yao, H.H.; Zhu, Z.Q.; Ning, Z.L.; Huang, Q. Stromal Myofibroblasts Are Associated with Poor Prognosis in Solid Cancers: A Meta-Analysis of Published Studies. PLoS ONE 2016, 11, e0159947. [Google Scholar] [CrossRef]
- Ozdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef]
- Rhim, A.D.; Oberstein, P.E.; Thomas, D.H.; Mirek, E.T.; Palermo, C.F.; Sastra, S.A.; Dekleva, E.N.; Saunders, T.; Becerra, C.P.; Tattersall, I.W.; et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007, 67, 10123–10128. [Google Scholar] [CrossRef]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; Diprete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [CrossRef]
- Bochet, L.; Lehuede, C.; Dauvillier, S.; Wang, Y.Y.; Dirat, B.; Laurent, V.; Dray, C.; Guiet, R.; Maridonneau-Parini, I.; Le Gonidec, S.; et al. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013, 73, 5657–5668. [Google Scholar] [CrossRef]
- Ohlund, D.; Handly-Santana, A.; Biffi, G.; Elyada, E.; Almeida, A.S.; Ponz-Sarvise, M.; Corbo, V.; Oni, T.E.; Hearn, S.A.; Lee, E.J.; et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 2017, 214, 579–596. [Google Scholar] [CrossRef]
- Markwald, R.R.; Fitzharris, T.P.; Smith, W.N. Sturctural analysis of endocardial cytodifferentiation. Dev. Biol. 1975, 42, 160–180. [Google Scholar] [CrossRef]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef]
- Kokudo, T.; Suzuki, Y.; Yoshimatsu, Y.; Yamazaki, T.; Watabe, T.; Miyazono, K. Snail is required for TGFbeta-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J. Cell Sci. 2008, 121, 3317–3324. [Google Scholar] [CrossRef] [PubMed]
- Krizbai, I.A.; Gasparics, A.; Nagyoszi, P.; Fazakas, C.; Molnar, J.; Wilhelm, I.; Bencs, R.; Rosivall, L.; Sebe, A. Endothelial-mesenchymal transition of brain endothelial cells: Possible role during metastatic extravasation. PLoS ONE 2015, 10, e0123845. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, S.; Fontana, F.; Li, Y.; Xiao, W.; Gao, Z.; Krudewig, A.; Affolter, M.; Belting, H.G.; Abdelilah-Seyfried, S.; et al. The tight junction protein Claudin-5 limits endothelial cell motility. J. Cell Sci. 2021, 134, jcs248237. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Stahl, P.L.; Salmen, F.; Vickovic, S.; Lundmark, A.; Navarro, J.F.; Magnusson, J.; Giacomello, S.; Asp, M.; Westholm, J.O.; Huss, M.; et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016, 353, 78–82. [Google Scholar] [CrossRef]
- Marx, V. Method of the Year: Spatially resolved transcriptomics. Nat. Methods 2021, 18, 9–14. [Google Scholar] [CrossRef]
- Tirosh, I.; Izar, B.; Prakadan, S.M.; Wadsworth, M.H., 2nd; Treacy, D.; Trombetta, J.J.; Rotem, A.; Rodman, C.; Lian, C.; Murphy, G.; et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016, 352, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Villani, A.C.; Satija, R.; Reynolds, G.; Sarkizova, S.; Shekhar, K.; Fletcher, J.; Griesbeck, M.; Butler, A.; Zheng, S.; Lazo, S.; et al. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017, 356, eaah4573. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, M.; Lin, Z.; Wu, L.; Xia, P.; Zhu, M.; Huang, B.; Wu, W.; Zhang, R.; Li, K.; et al. COL1A1-positive endothelial cells promote gastric cancer progression via the ANGPTL4-SDC4 axis driven by endothelial-to-mesenchymal transition. Cancer Lett. 2025, 623, 217731. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Tarnavski, O.; Zeisberg, M.; Dorfman, A.L.; McMullen, J.R.; Gustafsson, E.; Chandraker, A.; Yuan, X.; Pu, W.T.; Roberts, A.B.; et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat. Med. 2007, 13, 952–961. [Google Scholar] [CrossRef] [PubMed]
- Zeisberg, E.M.; Potenta, S.E.; Sugimoto, H.; Zeisberg, M.; Kalluri, R. Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J. Am. Soc. Nephrol. 2008, 19, 2282–2287. [Google Scholar] [CrossRef]
- Evrard, S.M.; Lecce, L.; Michelis, K.C.; Nomura-Kitabayashi, A.; Pandey, G.; Purushothaman, K.R.; d’Escamard, V.; Li, J.R.; Hadri, L.; Fujitani, K.; et al. Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 2016, 7, 11853. [Google Scholar] [CrossRef] [PubMed]
- Ranchoux, B.; Antigny, F.; Rucker-Martin, C.; Hautefort, A.; Pechoux, C.; Bogaard, H.J.; Dorfmuller, P.; Remy, S.; Lecerf, F.; Plante, S.; et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 2015, 131, 1006–1018. [Google Scholar] [CrossRef]
- Maddaluno, L.; Rudini, N.; Cuttano, R.; Bravi, L.; Giampietro, C.; Corada, M.; Ferrarini, L.; Orsenigo, F.; Papa, E.; Boulday, G.; et al. EndMT contributes to the onset and progression of cerebral cavernous malformations. Nature 2013, 498, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Kessler, S.P.; West, G.A.; Bhilocha, S.; de la Motte, C.; Sadler, T.M.; Gopalan, B.; Stylianou, E.; Fiocchi, C. Inflammation-induced endothelial-to-mesenchymal transition: A novel mechanism of intestinal fibrosis. Am. J. Pathol. 2011, 179, 2660–2673. [Google Scholar] [CrossRef]
- Choi, S.H.; Kim, A.R.; Nam, J.K.; Kim, J.M.; Kim, J.Y.; Seo, H.R.; Lee, H.J.; Cho, J.; Lee, Y.J. Tumour-vasculature development via endothelial-to-mesenchymal transition after radiotherapy controls CD44v6(+) cancer cell and macrophage polarization. Nat. Commun. 2018, 9, 5108. [Google Scholar] [CrossRef]
- Wei, W.F.; Zhou, H.L.; Chen, P.Y.; Huang, X.L.; Huang, L.; Liang, L.J.; Guo, C.H.; Zhou, C.F.; Yu, L.; Fan, L.S.; et al. Cancer-associated fibroblast-derived PAI-1 promotes lymphatic metastasis via the induction of EndoMT in lymphatic endothelial cells. J. Exp. Clin. Cancer Res. 2023, 42, 160. [Google Scholar] [CrossRef]
- Ciszewski, W.M.; Sobierajska, K.; Wawro, M.E.; Klopocka, W.; Chefczynska, N.; Muzyczuk, A.; Siekacz, K.; Wujkowska, A.; Niewiarowska, J. The ILK-MMP9-MRTF axis is crucial for EndMT differentiation of endothelial cells in a tumor microenvironment. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2283–2296. [Google Scholar] [CrossRef]
- Platel, V.; Lechevalier, D.; Bourreau, C.; Renault, S.; Soborova, I.; Jeanniere, C.; Martin, L.; Herault, O.; Corre, I.; Clere, N. NOX1 and NOX2: Two enzymes that promote endothelial-to-mesenchymal transition induced by melanoma conditioned media. Pharmacol. Res. 2022, 177, 106097. [Google Scholar] [CrossRef]
- Bourreau, C.; Navarro, E.; Cotinat, M.; Krejbich, M.; Guillonneau, F.; Guette, C.; Boissard, A.; Henry, C.; Corre, I.; Treps, L.; et al. Secretomes From Non-Small Cell Lung Cancer Cells Induce Endothelial Plasticity Through a Partial Endothelial-to-Mesenchymal Transition. Cancer Med. 2025, 14, e70707. [Google Scholar] [CrossRef]
- Fan, C.S.; Chen, W.S.; Chen, L.L.; Chen, C.C.; Hsu, Y.T.; Chua, K.V.; Wang, H.D.; Huang, T.S. Osteopontin-integrin engagement induces HIF-1alpha-TCF12-mediated endothelial-mesenchymal transition to exacerbate colorectal cancer. Oncotarget 2018, 9, 4998–5015. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Lyros, O.; Medda, R.; Jovanovic, N.; Schmidt, J.L.; Otterson, M.F.; Johnson, C.P.; Behmaram, B.; Shaker, R.; Rafiee, P. Endothelial-mesenchymal transition in normal human esophageal endothelial cells cocultured with esophageal adenocarcinoma cells: Role of IL-1beta and TGF-beta2. Am. J. Physiol. Cell Physiol. 2014, 307, C859–C877. [Google Scholar] [CrossRef]
- Kim, S.H.; Song, Y.; Seo, H.R. GSK-3beta regulates the endothelial-to-mesenchymal transition via reciprocal crosstalk between NSCLC cells and HUVECs in multicellular tumor spheroid models. J. Exp. Clin. Cancer Res. 2019, 38, 46. [Google Scholar] [CrossRef]
- Yeon, J.H.; Jeong, H.E.; Seo, H.; Cho, S.; Kim, K.; Na, D.; Chung, S.; Park, J.; Choi, N.; Kang, J.Y. Cancer-derived exosomes trigger endothelial to mesenchymal transition followed by the induction of cancer-associated fibroblasts. Acta Biomater. 2018, 76, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Xu, C.; Zhang, Y.; Xue, C.; Yang, C.; Bi, H.; Qian, X.; Wu, M.; Ji, K.; Zhao, Y.; et al. Umbilical Cord-Derived Mesenchymal Stem Cell-Derived Exosomal MicroRNAs Suppress Myofibroblast Differentiation by Inhibiting the Transforming Growth Factor-beta/SMAD2 Pathway During Wound Healing. Stem Cells Transl. Med. 2016, 5, 1425–1439. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.S.; Chen, L.L.; Hsu, T.A.; Chen, C.C.; Chua, K.V.; Li, C.P.; Huang, T.S. Endothelial-mesenchymal transition harnesses HSP90alpha-secreting M2-macrophages to exacerbate pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2019, 12, 138. [Google Scholar] [CrossRef]
- Garcia, J.; Sandi, M.J.; Cordelier, P.; Binetruy, B.; Pouyssegur, J.; Iovanna, J.L.; Tournaire, R. Tie1 deficiency induces endothelial-mesenchymal transition. EMBO Rep. 2012, 13, 431–439. [Google Scholar] [CrossRef]
- Adjuto-Saccone, M.; Soubeyran, P.; Garcia, J.; Audebert, S.; Camoin, L.; Rubis, M.; Roques, J.; Binetruy, B.; Iovanna, J.L.; Tournaire, R. TNF-alpha induces endothelial-mesenchymal transition promoting stromal development of pancreatic adenocarcinoma. Cell Death Dis. 2021, 12, 649. [Google Scholar] [CrossRef]
- Choi, S.H.; Nam, J.K.; Kim, B.Y.; Jang, J.; Jin, Y.B.; Lee, H.J.; Park, S.; Ji, Y.H.; Cho, J.; Lee, Y.J. HSPB1 Inhibits the Endothelial-to-Mesenchymal Transition to Suppress Pulmonary Fibrosis and Lung Tumorigenesis. Cancer Res. 2016, 76, 1019–1030. [Google Scholar] [CrossRef]
- Kim, M.; Choi, S.H.; Jin, Y.B.; Lee, H.J.; Ji, Y.H.; Kim, J.; Lee, Y.S.; Lee, Y.J. The effect of oxidized low-density lipoprotein (ox-LDL) on radiation-induced endothelial-to-mesenchymal transition. Int. J. Radiat. Biol. 2013, 89, 356–363. [Google Scholar] [CrossRef]
- Gasperini, P.; Espigol-Frigole, G.; McCormick, P.J.; Salvucci, O.; Maric, D.; Uldrick, T.S.; Polizzotto, M.N.; Yarchoan, R.; Tosato, G. Kaposi sarcoma herpesvirus promotes endothelial-to-mesenchymal transition through Notch-dependent signaling. Cancer Res. 2012, 72, 1157–1169. [Google Scholar] [CrossRef]
- Luo, H.; Xia, X.; Huang, L.B.; An, H.; Cao, M.; Kim, G.D.; Chen, H.N.; Zhang, W.H.; Shu, Y.; Kong, X.; et al. Pan-cancer single-cell analysis reveals the heterogeneity and plasticity of cancer-associated fibroblasts in the tumor microenvironment. Nat. Commun. 2022, 13, 6619. [Google Scholar] [CrossRef]
- Hou, M.; Chen, J.; Jiang, Y.; Liu, C.; Wang, X.; Liu, E.; Zong, Y.; Gu, M.; Meng, Z.; Wang, S.; et al. Single-cell analysis reveals CD34(+)CD90(+) endothelial cells promote tumor metastasis in gallbladder cancer. NPJ Precis. Oncol. 2025, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Xia, D.; Zhou, Y.; Hu, Y.; Yang, Z.; Yin, Y.; Wang, J.; Zhang, B.; Gong, L.; Li, K.; et al. Spatial transcriptomics and multi-omics reveal relapse and resistance mechanisms of EndMT-derived CAFs mediated by TNC and FLNC in glioblastoma. J. Transl. Med. 2025, 23, 702. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Xia, D.; Xu, D.; Yin, Y.; Xu, F.; Zhang, B.; Li, K.; Yang, Z.; Zou, J. Neovascularization directed by CAVIN1/CCBE1/VEGFC confers TMZ-resistance in glioblastoma. Cancer Lett. 2024, 582, 216593. [Google Scholar] [CrossRef] [PubMed]


| Reference | Model of Induction | Induction Protocol | EndMT Key Phenotype |
|---|---|---|---|
| Zeisberg et al., 2007 [7] | Tumor implantation, spontaneous tumor induction | Subcutaneous implantation of B16F10 in C57BL/6; spontaneous tumors in Rip1-Tag2 transgenic mice | β-gal+/FSP1+ and β-gal+/α-SMA+ traced cells, CD31+/FSP1+ and CD31+/α-SMA+ cells (partial EndMT), FSP1+/TGF-β1+ cells |
| Ciszewski et al., 2017 [30] | Cancer CM | CM from colon cancer cells after 72 h culture mixed with EC medium at 1:2 and applied to HMEC-1 for 216 h | Spindle-like morphology; stress fibers increased; endothelial markers decreased (claudin-5, ZO-1); mesenchymal markers increased (α-SMA, FSP-1, N-cadherin) |
| Krizbai et al., 2015 [14] | Cancer CM | ACM (latent TGF-β activated by heating 80 °C, 10 min) treated on ECs for 48 h | Endothelial markers decreased (VE-cadherin, claudin-5); mesenchymal markers increased (fibronectin, β1-integrin, calponin, α-SMA); TEER decreased |
| Platel et al., 2022 [31] | Cancer CM | CM (72 h culture) mixed 1:1 with EC medium and treated for 24, 48, 72 h | Tube formation decreased; migration increased; actin stress fibers increased; vWF+/α-SMA+ double-positive cells increased (partial EndMT) |
| Bourreau et al., 2025 [32] | Cancer CM | CM (72 h) mixed 1:1 with EC medium; treated for 48–72 h | Spindle-like morphology; stress fibers increased; loss of endothelial marker vWF; gain of mesenchymal markers α-SMA and CD44 (increased vWF−/α-SMA+ and CD31−/CD44+ cells); secretome showed enrichment of EndMT, angiogenesis, and ROS-related pathways |
| Nie et al., 2014 [34] | Cancer co-culture, cancer CM | Transwell co-culture with OE33 for 3, 6, 10 days; CM (24 h cancer culture in EC medium) applied to HEMEC for 6 days | Endothelial markers decreased (CD31, VE-cadherin, vWF); mesenchymal markers increased (FSP1, α-SMA, vimentin, desmin, COL1A2, Snail); migration increased; tube formation decreased; collagen gel contraction increased; CD31+/FSP1+ cells in EAC tissue; VEGF secretion increased with VEGFR2 decreased |
| Se-Hyuk Kim et al., 2019 [35] | 3D spheroid co-culture, 3D-culture cancer CM | CM from 3D spheroids (3 days) applied to HUVECs for 24–48 h; ultra-low-attachment 3D co-culture for 3 days | Endothelial markers decreased (CD31, VE-cadherin); mesenchymal marker α-SMA increased; spheroid compactness increased; in vivo with CHIR99021: fibrosis and CD31 signal decreased; drug resistance mitigated |
| Ju Hun Yeon et al., 2018 [36] | 3D microfluidic device with IFF, cancer exosomes | IFF generated by reservoir height difference; exosomes (1, 10, 50 μg/mL) for 1–7 days (from B16BL6-bearing C57BL/6J mice) | Morphological change with active filopodia; mesenchymal markers increased (vimentin, FSP-1); endothelial marker decreased (VE-cadherin) |
| Wen-Fei Wei et al., 2023 [29] | CAF CM | CM from patient-derived CAFs cultured 48 h in EC medium, applied to HDLEC for 24 h | Endothelial marker decreased (VE-cadherin); mesenchymal markers increased (α-SMA, vimentin); α-SMA+/LYVE-1+ lymphatic vessels increased; transendothelial migration increased |
| Chi-Shuan Fan et al., 2018 [33] | OPN treatment | Recombinant OPN 0.3 μg/mL for 15–24 h | Endothelial markers decreased (VE-cadherin, Tie1, Tie2, CD31); mesenchymal markers increased (α-SMA, fibronectin); migration and invasion increased; gap-junction activity decreased; cancer growth and metastasis increased; CD44+/CD326+ stemness population increased |
| Chi-Shuan Fan et al., 2019 [38] | OPN treatment | OPN 0.3 μg/mL for 24 h | Endothelial markers decreased (VE-cadherin, Tie1, Tie2, CD31); mesenchymal markers increased (α-SMA, fibronectin); lncRNA (LOC340340, LOC101927256, MNX1-AS1) decreased; tumor growth increased; M2 macrophage infiltration increased |
| Julie Garcia et al., 2012 [39] | Tie1 expression modulation | Tie1 siRNA knockdown for 48–72 h | Spindle-like morphology; migration increased; endothelial markers decreased (CD31, VE-cadherin, CD34, FVIII); mesenchymal markers increased (α-SMA, S100A4, COL1A1, SM22α, N-cadherin) |
| Marjorie Adjuto-Saccone et al., 2021 [40] | Recombinant TNF-α | 20, 50, 100 ng/mL TNF-α (mainly 100 ng/mL) for 24–168 h (CD31 decreased from 24 h; α-SMA increased from 48 h) | Spindle-like morphology; migration increased; angiogenesis decreased; endothelial markers decreased (CD31, VE-cadherin, CD34); mesenchymal markers increased (α-SMA, S100A4, COL1A1, SM22α, N-cadherin) |
| Choi et al., 2016 [41] | HSPB1 si/shRNA | siRNA 1–3 days; nasal delivery of Hspb1 shRNA after 2 weeks of tumor induction, analyzed at 14 weeks | Endothelial markers decreased (VE-cadherin, CD31); mesenchymal marker α-SMA increased; VEGF-driven tube formation decreased; in vivo α-SMA+/CD31+ cells and collagen deposition increased; TGF-β1 and FSP1 increased; tumor fibrosis and progression increased |
| Choi et al., 2018 [28] | Irradiation | Single 20 Gy dose of radiation; assessed 1–23 days post-irradiation | α-SMA+/CD31+ partial EndMT increased; collagen deposition increased; tumor growth and metastasis increased; CD44v6+ cancer stem-like cells increased; α-SMA+/NG2+ pericytes increased; lineage-traced EndMT in tdTomato Ecs |
| Paola Gasperini et al., 2012 [43] | KSHV infection | Infection with rKSHV.219 (2.5 mL supernatant of virus-producing VERO cells), 12-day culture with puromycin selection | Actin reorganization; migration increased; endothelial markers decreased (CD31, VE-cadherin, Tie2, CD34); mesenchymal markers increased (CD146, NG-2, vimentin, PDGFRβ, α-SMA); in KS lesions, LANA+ cells showed low CD31 and high mesenchymal markers |
| Reference | Sequencing Type | EndMT Distinguishing Markers | EndMT Key Phenotype |
|---|---|---|---|
| Han Luo et al., 2022 [44] | scRNA-seq | CAF subtype co-expressing endothelial markers (e.g., vWF, PLVAP) and mesenchymal markers (e.g., ACTA2, RGS5) designated as CAFEndMT; Endothelial Cell-Specific Molecule 1 (ESM1) proposed as a CAFEndMT-specific marker. | TEC, CAFEndMT, and CAFmyo continuity suggested by trajectory analysis; CAFEndMT shows a high angiogenesis hallmark signature; in some cancers, patients with high CAFEndMT gene-set signatures exhibit poorer survival; strong predicted interaction with SPP1+ TAMs. |
| Liu Q. et al., 2025 [21] | scRNA-seq, ST-seq, bulk RNA-seq | PECAM1+ endothelial cluster with high expression of mesenchymal markers (ACTA2, COL1A1, RGS5) and enrichment of matrix-related pathway gene sets designated as COL1A1+ EC; differentially expressed genes with significant increases used to define an EndMT signature (EdMTS). | EdMTS is higher in GC than in adjacent normal tissue, higher in advanced GC, and highest in liver metastasis; strongly positively correlated with cancer-related processes (hypoxia, invasion, migration, TGF-β signaling); contributes to immune suppression/evasion; associated with poorer survival. |
| Minghui Hou et al., 2025 [45] | scRNA-seq, bulk RNA-seq | After primary classification by endothelial markers (CD34, PECAM1, VWF), a secondary CD90-positive mesenchymal pattern was used to designate the cluster SAEndo2; ESM1 selectively expressed. | In SAEndo2, mesenchymal/ECM-related pathways (ECM remodeling, EMT) are upregulated; CD34+CD90+ ECs display high expression of mesenchymal markers (FN1, α-SMA, COL4A2, MMP-9, PDGF) and EndMT-related transcription factors (TWIST, SLUG, SNAI); linked to poor survival and adverse prognosis; promotes tumor-cell migration and invasion. |
| Li Ji et al., 2025 [46] | ST-seq, bulk RNA-seq, LFQ proteomics | No explicit EndMT cluster defined; within fibroblast subsets, an rGBM-enriched cluster with high ECM Gene Ontology enrichment and high CAF markers (COL3A1, COL1A1, COL1A2, FN1) designated as a CAF-enriched cluster. | In HCMECs, endothelial markers (VE-cadherin, CD31) decrease, mesenchymal markers (α-SMA, COL1A1, FN1) increase, TGF-β is upregulated, and drug resistance is implicated. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Kim, E.-G.; Kim, B.Y.; Soung, N.-K. Cancer-Associated Fibroblasts Arising from Endothelial-to-Mesenchymal Transition: Induction Factors, Functional Roles, and Transcriptomic Evidence. Biology 2025, 14, 1403. https://doi.org/10.3390/biology14101403
Han J, Kim E-G, Kim BY, Soung N-K. Cancer-Associated Fibroblasts Arising from Endothelial-to-Mesenchymal Transition: Induction Factors, Functional Roles, and Transcriptomic Evidence. Biology. 2025; 14(10):1403. https://doi.org/10.3390/biology14101403
Chicago/Turabian StyleHan, Junyeol, Eung-Gook Kim, Bo Yeon Kim, and Nak-Kyun Soung. 2025. "Cancer-Associated Fibroblasts Arising from Endothelial-to-Mesenchymal Transition: Induction Factors, Functional Roles, and Transcriptomic Evidence" Biology 14, no. 10: 1403. https://doi.org/10.3390/biology14101403
APA StyleHan, J., Kim, E.-G., Kim, B. Y., & Soung, N.-K. (2025). Cancer-Associated Fibroblasts Arising from Endothelial-to-Mesenchymal Transition: Induction Factors, Functional Roles, and Transcriptomic Evidence. Biology, 14(10), 1403. https://doi.org/10.3390/biology14101403








