Wolbachia Surface Protein (wsp) Gene Sequencing of Strains A and B in Native Aedes albopictus of Mérida, Yucatán
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mosquito Sample Collection and Identification
2.2. DNA Extraction and Wolbachia PCR Amplification
2.3. Wolbachia wsp Gene DNA Sequencing Analyses
3. Results and Discussion
3.1. Detection of Wolbachia Strains A and B in Field-Collected Aedes albopictus from Yucatán
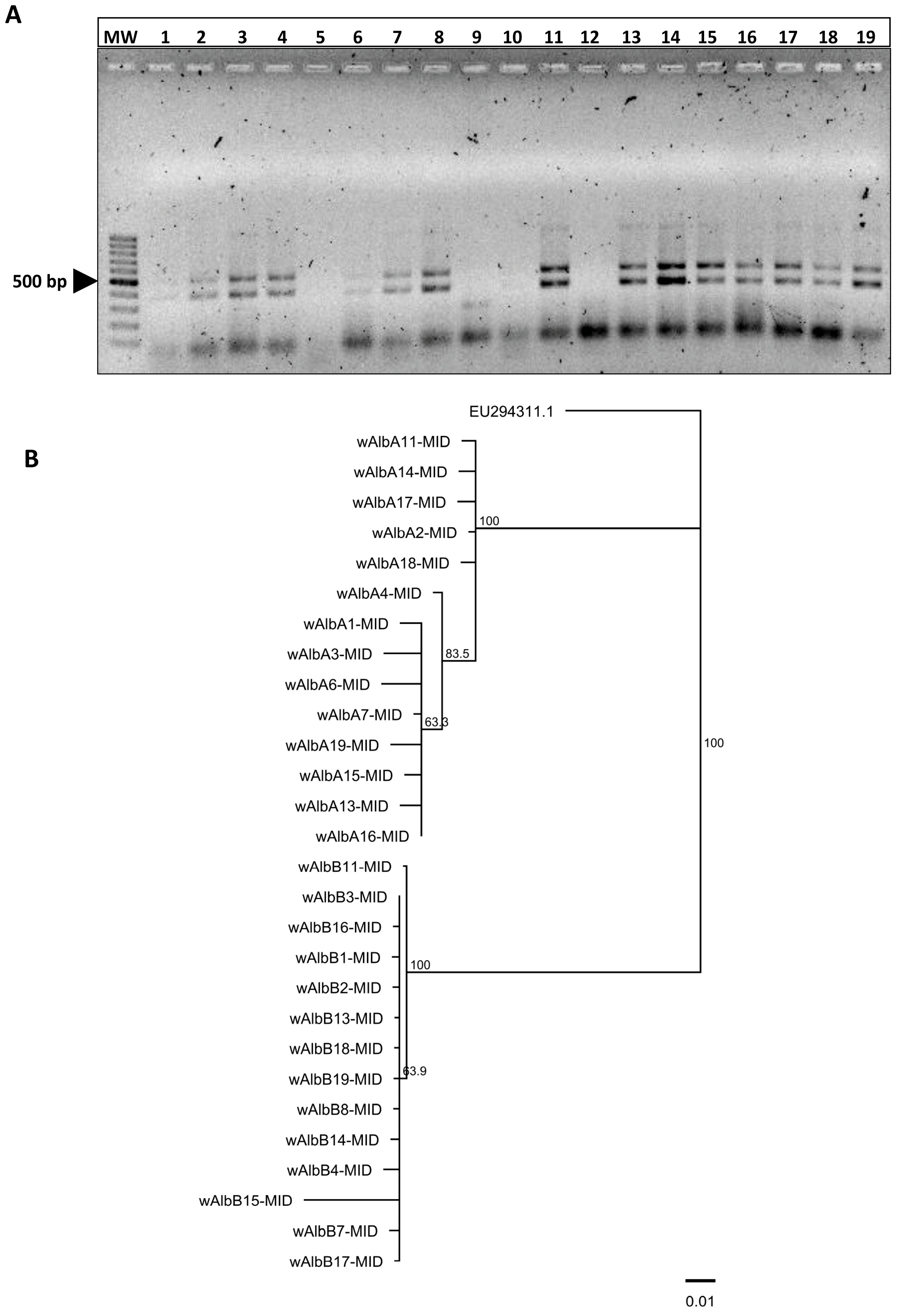
3.2. Nucleotide Comparison Analyses with Wolbachia Strains in Aedes albopictus
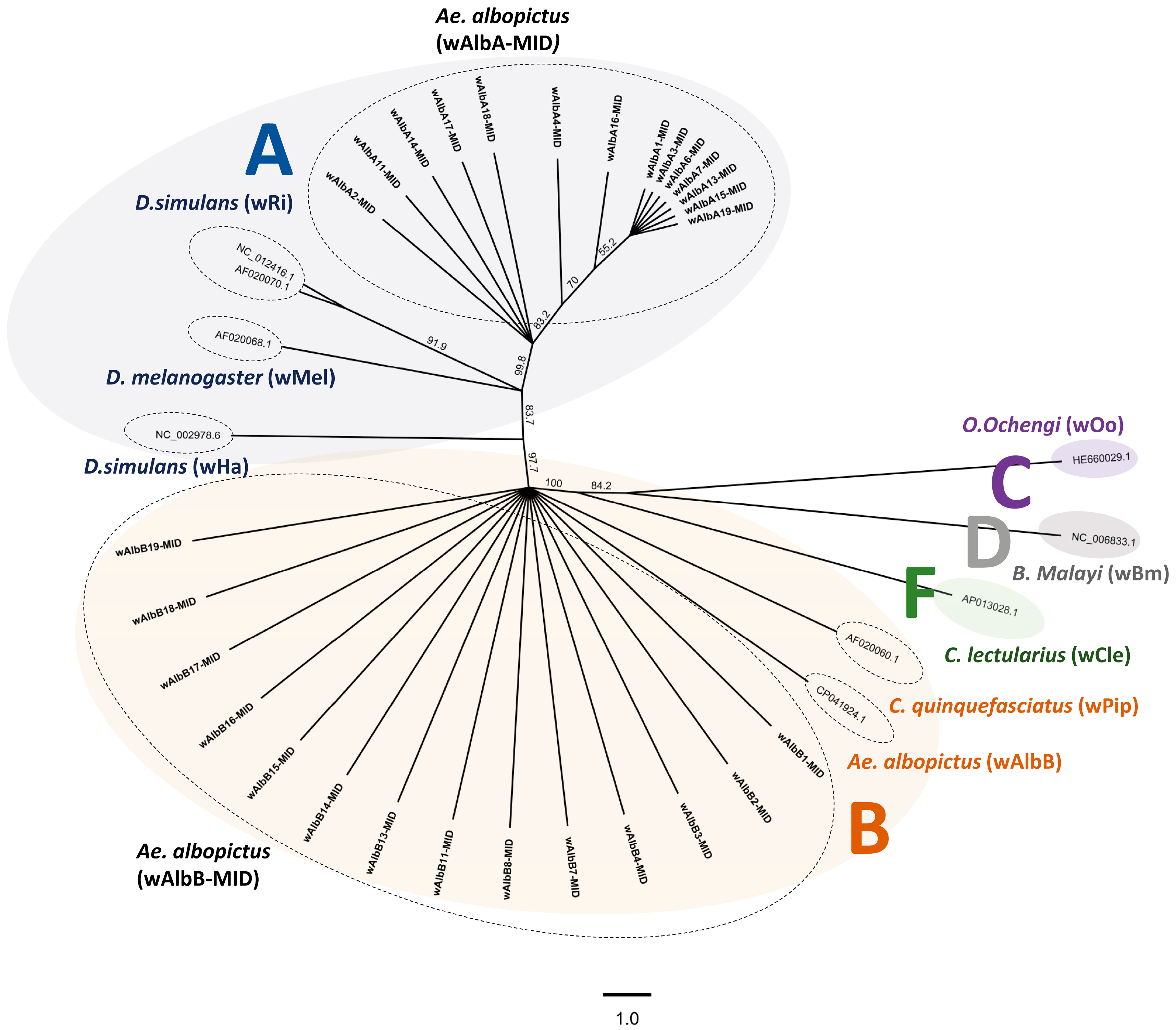

4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| wAlbA | Wolbachia strain A of Ae. albopictus. |
| wAlbB | Wolbachia strain B of Ae. albopictus. |
| wsp | Wolbachia surface protein |
| MID | Mérida |
| ABVs | Aedes-borne human viruses |
| DENV | Dengue virus |
| ZIKV | Zika virus |
| CHIKV | Chikungunya virus |
| ABD | Aedes-borne human diseases |
References
- Hilgenboecker, K.; Hammerstein, P.; Schlattmann, P.; Telschow, A.; Werren, J.H. How many species are infected with Wolbachia?—A statistical analysis of current data. FEMS Microbiol. Lett. 2008, 281, 215–220. [Google Scholar] [CrossRef]
- Werren, J.H.; Baldo, L.; Clark, M.E. Wolbachia: Master manipulators of invertebrate biology. Nat. Rev. Microbiol. 2008, 6, 741–751. [Google Scholar] [CrossRef]
- Zhou, W.; Rousset, F.; O’Neil, S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proc. Biol. Sci. 1998, 265, 509–515. [Google Scholar] [CrossRef]
- Baldo, L.; Dunning Hotopp, J.C.; Jolley, K.A.; Bordenstein, S.R.; Biber, S.A.; Choudhury, R.R.; Hayashi, C.; Maiden, M.C.J.; Tettelin, H.; Werren, J.H. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl. Environ. Microbiol. 2006, 72, 7098–7110. [Google Scholar] [CrossRef] [PubMed]
- Lefoulon, E.; Bain, O.; Makepeace, B.L.; d’Haese, C.; Uni, S.; Martin, C.; Gavotte, L. Breakdown of coevolution between symbiotic bacteria Wolbachia and their filarial hosts. PeerJ 2016, 4, e1840. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, S.L.; Giordano, R.; Colbert, A.M.; Karr, T.L.; Robertson, H.M. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proc. Natl. Acad. Sci. USA 1992, 89, 2699–2702. [Google Scholar] [CrossRef]
- Sinkins, S.P. Wolbachia and cytoplasmic incompatibility in mosquitoes. Insect. Biochem. Mol. Biol. 2004, 34, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Khoo, C.C.; Dobson, S.L. Wolbachia establishment and invasion in an Aedes aegypti laboratory population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef]
- Bourtzis, K. Wolbachia- Based Technologies for Insect Pest Population Control. In Transgenesis and the Management of Vector-Borne Disease Advances in Experimental Medicine and Biology; Aksoy, S., Ed.; Springer: New York, NY, USA, 2008; Volume 627, pp. 104–113. [Google Scholar]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 2011, 476, 450–453. [Google Scholar] [CrossRef]
- Pereira, T.N.; Rocha, M.N.; Sucupira, P.H.F.; Carvalho, F.D.; Moreira, L.A. Wolbachia significantly impacts the vector competence of Aedes aegypti for Mayaro virus. Sci. Rep. 2018, 8, 6889. [Google Scholar] [CrossRef]
- Liang, X.; Tan, C.H.; Sun, Q.; Zhang, M.; Wong, P.S.J.; Li, M.I.; Mak, K.W.; Martín-Park, A.; Contreras-Perera, Y.; Puerta-Guardo, H.; et al. Wolbachia wAlbB remains stable in Aedes aegypti over 15 years but exhibits genetic background-dependent variation in virus blocking. PNAS Nexus 2022, 1, pgac203. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Gasperi, G.; Chen, X.; James, A.A. The invasive mosquito species Aedes albopictus: Current knowledge and future perspectives. Trends Parasitol. 2013, 29, 460–468. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, B.A.; Wilson, A.E.; Zohdy, S. Aedes albopictus is a competent vector of Zika virus: A meta-analysis. PLoS ONE 2019, 14, e0216794. [Google Scholar] [CrossRef]
- Kamal, M.; Kenawy, M.A.; Rady, M.H.; Khaled, A.S.; Samy, A.M. Mapping the global potential distributions of two arboviral vectors Aedes aegypti and Ae. albopictus under changing climate. PLoS ONE 2019, 13, e0210122. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and future spread of the arbovirus vectors Aedes aegypti and Aedes albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of the tiger: Global risk of invasion by the mosquito Aedes albopictus. Vector Borne Zoonotic Dis. 2007, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xi, Z.; Liu, X.; Wang, J.; Guo, Y.; Ren, D.; Wu, H.; Wang, X.; Chen, B.; Liu, Q. Identification and molecular characterization of Wolbachia strains in natural populations of Aedes albopictus in China. Parasites Vectors 2020, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- Noor Afizah, A.; Roziah, A.; Nazni, W.A.; Lee, H.L. Detection of Wolbachia from field collected Aedes albopictus Skuse in Malaysia. Indian J. Med. Res. 2015, 142, 205–210. [Google Scholar]
- Sinkins, S.P.; Braig, H.R.; O’Neill, S.L. Wolbachia superinfections and the expression of cytoplasmic incompatibility. Proc. Biol. Sci. 1995, 261, 325–330. [Google Scholar]
- Dobson, S.L.; Marsland, E.J.; Rattanadechakul, W. Wolbachia-induced cytoplasmic incompatibility in single- and superinfected Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2001, 38, 382–387. [Google Scholar] [CrossRef]
- Bourtzis, K.; Dobson, S.L.; Xi, Z.; Rasgon, J.L.; Calvitti, M.; Moreira, L.A.; Bossin, H.C.; Moretti, R.; Baton, L.A.; Hughes, G.L.; et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 2014, 132, S150–S163. [Google Scholar] [CrossRef]
- Martín-Park, A.; Che-Mendoza, A.; Contreras-Perera, Y.; Pérez-Carrillo, S.; Puerta-Guardo, H.; Villegas-Chim, J.; Guillermo-May, G.; Medina-Barreiro, A.; Delfín-González, H.; Méndez-Vales, R.; et al. Pilot trial using mass field-releases of sterile males produced with the incompatible and sterile insect techniques as part of integrated Aedes aegypti control in Mexico. PLoS Negl. Trop. Dis. 2022, 16, e0010324. [Google Scholar] [CrossRef]
- Paz-Bailey, G.; Jernigan, D.B.; Laserson, K.; Zielinski-Gutierrez, E.; Petersen, L. New solutions against the dengue global threat: Opportunities for Wolbachia interventions. Int. J. Infect. Dis. 2025, 157, 107923. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Yu, W.; Jiang, J.; Sanchez, C.; Karna, A.K.; Martinez, K.J.L.; Hanley, K.A.; Buenemann, M.; Hansen, I.A.; Xue, R.-D.; et al. Wolbachia pipientis occurs in Aedes aegypti populations in New Mexico and Florida, USA. Ecol. Evol. 2019, 9, 6148–6156. [Google Scholar] [CrossRef] [PubMed]
- Dutra, H.L.; Rocha, M.N.; Dias, F.B.; Mansur, S.B.; Caragata, E.P.; Moreira, L.A. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe 2016, 19, 771–774. [Google Scholar] [CrossRef]
- de Albuquerque, A.L.; Magalhães, T.; Ayres, C.F. High prevalence and lack of diversity of Wolbachia pipientis in Aedes albopictus populations from Northeast Brazil. Mem. Inst. Oswaldo Cruz 2011, 106, 773–776. [Google Scholar] [CrossRef]
- Torres-Monzón, J.A.; Casas-Martínez, M.; López-Ordóñez, T. Infection of Aedes mosquitoes by native Wolbachia in urban cemeteries of Southern Mexico. Salud Publica Mex. 2020, 62, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Contreras-Perera, Y.; Perez-Carrillo, S.; Che-Mendoza, A.; Ayora-Talavera, G.; Vazquez-Prokopec, G.; Martin-Park, A.; Zhang, D.; Manrique-Saide, P.; UCBE-LCB Team. Wolbachia in Native Populations of Aedes albopictus (Diptera: Culicidae) From Yucatan Peninsula, Mexico. J. Insect Sci. 2020, 20, 16. [Google Scholar] [CrossRef]
- Shoemaker, D.D.; Machado, C.A.; Molbo, D.; Werren, J.H.; Windsor, D.M.; Herre, E.A. The distribution of Wolbachia in fig wasps: Correlations with host phylogeny, ecology and population structure. Proc. Biol. Sci. 2002, 269, 2257–2267. [Google Scholar] [CrossRef]
- Contreras-Perera, Y.J.; Briceño-Mendez, M.; Flores-Suárez, A.E.; Manrique-Saide, P.; Palacio-Vargas, J.A.; Huerta-Jimenez, H.; Martin-Park, A. New Record of Aedes albopictus In A Suburban Area Of Merida, Yucatan, Mexico. J. Am. Mosq. Control Assoc. 2019, 35, 210–213. [Google Scholar] [CrossRef]
- Rueda, L. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with Dengue Virus Transmission. Zootaxa 2004, 589, 60. [Google Scholar] [CrossRef]
- Ahmad, N.A.; Vythilingam, I.; Lim, Y.A.L.; Zabari, N.; Lee, H.L. Detection of Wolbachia in Aedes albopictus and Their Effects on Chikungunya Virus. Am. J. Trop. Med. Hyg. 2017, 96, 148–156. [Google Scholar] [CrossRef]
- Braig, H.R.; Zhou, W.; Dobson, S.L.; O’Neill, S.L. Cloning and characterization of a gene encoding the major surface protein of the bacterial endosymbiont Wolbachia pipientis. J. Bacteriol. 1998, 180, 2373–2378. [Google Scholar] [CrossRef]
- Gabriel, E.F.; Jeff, U.I. Jukes-Cantor Correction for Phylogenetic Tree Reconstruction. bioRxiv 2024. [Google Scholar] [CrossRef]
- Nugapola, N.W.N.P.; De Silva, W.A.P.P.; Karunaratne, S.H.P.P. Distribution and phylogeny of Wolbachia strains in wild mosquito populations in Sri Lanka. Parasites Vectors 2017, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Ellegaard, K.M.; Klasson, L.; Näslund, K.; Bourtzis, K.; Andersson, S.G.E. Comparative Genomics of Wolbachia and the Bacterial Species Concept. PLoS Genet. 2013, 9, e1003381. [Google Scholar] [CrossRef] [PubMed]
- Klasson, L.; Westberg, J.; Sapountzis, P.; Näslund, K.; Lutnaes, Y.; Darby, A.C.; Veneti, Z.; Chen, L.; Braig, H.R.; Garrett, R.; et al. The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans. Proc. Natl. Acad. Sci. USA 2009, 106, 5725–5730. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 2011, 476, 454–457. [Google Scholar] [CrossRef]
- McMeniman, C.J.; Lane, R.V.; Cass, B.N.; Fong, A.W.; Sidhu, M.; Wang, Y.F.; O’Neill, S.L. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 2009, 323, 141–144. [Google Scholar] [CrossRef]
- Mavingui, P.; Valiente Moro, C.; Tran-Van, V.; Wisniewski-Dyé, F.; Raquin, V.; Minard, G.; Tran, F.-H.; Voronin, D.; Rouy, Z.; Bustos, P.; et al. Whole-genome sequence of Wolbachia strain wAlbB, an endosymbiont of tiger mosquito vector Aedes albopictus. J. Bacteriol. 2012, 194, 1840. [Google Scholar] [CrossRef]
- Glaser, R.L.; Meola, M.A. The Native Wolbachia Endosymbionts of Drosophila melanogaster and Culex quinquefasciatus Increase Host Resistance to West Nile Virus Infection. PLoS ONE 2010, 5, e11977. [Google Scholar] [CrossRef]
- Nikoh, N.; Hosokawa, T.; Moriyama, M.; Oshima, K.; Hattori, M.; Fukatsu, T. Evolutionary origin of insect-Wolbachia nutritional mutualism. Proc. Natl. Acad. Sci. USA 2014, 111, 10257–10262. [Google Scholar] [CrossRef]
- Foster, J.; Ganatra, M.; Kamal, I.; Ware, J.; Makarova, K.; Ivanova, N.; Bhattacharyya, A.; Kapatral, V.; Kumar, S.; Posfai, J.; et al. The Wolbachia genome of Brugia malayi: Endosymbiont evolution within a human pathogenic nematode. PLoS Biol. 2005, 3, e121. [Google Scholar] [CrossRef]
- Kitrayapong, P.; Baimai, V.; O’Neill, S.L. Field prevalence of Wolbachia in the mosquito vector Aedes albopictus. Am. J. Trop. Med. Hyg. 2002, 66, 108–111. [Google Scholar] [CrossRef]
- Baião, G.C.; Schneider, D.I.; Miller, W.J.; Klasson, L. The effect of Wolbachia on gene expression in Drosophila paulistorum and its implications for symbiont-induced host speciation. BMC Genom. 2019, 20, 465. [Google Scholar] [CrossRef]
- Wu, M.; Sun, L.V.; Vamathevan, J.; Riegler, M.; Deboy, R.; Brownlie, J.C.; McGraw, E.A.; Martin, W.; Esser, C.; Ahmadinejad, N.; et al. Phylogenomics of the Reproductive Parasite Wolbachia pipientis wMel: A Streamlined Genome Overrun by Mobile Genetic Elements. PLoS Biol. 2004, 2, e69. [Google Scholar] [CrossRef]
- Darby, A.C.; Armstrong, S.D.; Bah, G.S.; Kaur, G.; Hughes, M.A.; Kay, S.M.; Koldkjær, P.; Rainbow, L.; Radford, A.D.; Blaxter, M.L.; et al. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res. 2012, 22, 2467–2477. [Google Scholar] [CrossRef]
- Zhang, D.; Zheng, X.; Xi, Z.; Bourtzis, K.; Gilles, J.R.L. Combining the Sterile Insect Technique with the Incompatible Insect Technique: I-Impact of Wolbachia Infection on the Fitness of Triple- and Double-Infected Strains of Aedes albopictus. PLoS ONE 2015, 10, e0121126. [Google Scholar] [CrossRef]
- Dean, J.L.; Dobson, S.L. Characterization of Wolbachia infections and interspecific crosses of Aedes (Stegomyia) polynesiensis and Ae. (Stegomyia) riversi (Diptera: Culicidae). J. Med. Entomol. 2004, 41, 894–900. [Google Scholar] [CrossRef]
- Baton, L.A.; Pacidônio, E.C.; Gonçalves, D.S.; Moreira, L.A. wFlu: Characterization and evaluation of a native Wolbachia from the mosquito Aedes fluviatilis as a potential vector control agent. PLoS ONE 2013, 8, e59619. [Google Scholar] [CrossRef]
- Jeffries, C.L.; Tantely, L.M.; Raharimalala, F.N.; Hurn, E.; Boyer, S.; Walker, T. Diverse novel resident Wolbachia strains in Culicine mosquitoes from Madagascar. Sci. Rep. 2018, 8, 17456. [Google Scholar] [CrossRef]
- Lozano-Sardaneta, Y.N.; Viveros-Santos, V.; Mosso-González, C.; Ulloa-García, A.; Torres-Monzón, J.A. Natural coinfection with two Wolbachia supergroups in wild mosquitoes of Aedes albopictus. Salud Pública México 2025, 67, 269–275. [Google Scholar]
- Ant, T.H.; Sinkins, S.P. A Wolbachia triple-strain infection generates self-incompatibility in Aedes albopictus and transmission instability in Aedes aegypti. Parasit Vectors 2018, 11, 295. [Google Scholar] [CrossRef]
- Fu, Y.; Gavotte, L.; Mercer, D.R.; Dobson, S.L. Artificial triple Wolbachia infection in Aedes albopictus yields a new pattern of unidirectional cytoplasmic incompatibility. Appl. Environ. Microbiol. 2010, 76, 5887–5891. [Google Scholar] [CrossRef]
- Garcia-Rejon, J.E.; Navarro, J.C.; Cigarroa-Toledo, N.; Baak-Baak, C.M. An Updated Review of the Invasive Aedes albopictus in the Americas; Geographical Distribution, Host Feeding Patterns, Arbovirus Infection, and the Potential for Vertical Transmission of Dengue Virus. Insects 2021, 12, 967. [Google Scholar] [CrossRef]
- Torres, R.; Hernandez, E.; Flores, V.; Ramirez, J.L.; Joyce, A.L. Wolbachia in mosquitoes from the Central Valley of California, USA. Parasites Vectors 2020, 13, 558. [Google Scholar] [CrossRef] [PubMed]
- Baldo, L.; Lo, N.; Werren, J.H. Mosaic nature of the Wolbachia surface protein. J. Bacteriol. 2005, 187, 5406–5418. [Google Scholar] [CrossRef] [PubMed]
- Baldo, L.; Werren, J.H. Revisiting Wolbachia supergroup typing based on WSP: Spurious lineages and discordance with MLST. Curr. Microbiol. 2007, 55, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Schulenburg, J.H.; Hurst, G.D.; Huigens, T.M.; van Meer, M.M.; Jiggins, F.M.; Majerus, M.E. Molecular evolution and phylogenetic utility of Wolbachia ftsZ and wsp gene sequences with special reference to the origin of male-killing. Mol. Biol. Evol. 2000, 17, 584–600. [Google Scholar] [CrossRef]
| Accession Number | Max Score | Total Score | Percentage Identity | Wolbachia Strain (wsp Gene) |
|---|---|---|---|---|
| MK684349.1 | 494 | 494 | 100 | A |
| KY817484.1 | 494 | 494 | 100 | A |
| KX573028.1 | 494 | 494 | 100 | A |
| KY523670.1 | 494 | 494 | 100 | A |
| KJ140127.1 | 494 | 494 | 100 | A |
| KF725078.1 | 494 | 494 | 100 | A |
| JX476002.1 | 494 | 494 | 100 | A |
| KC668278.1 | 494 | 494 | 100 | A |
| HM007832.1 | 494 | 494 | 100 | A |
| MK684351.1 | 492 | 492 | 99 | A |
| MF805776.1 | 492 | 492 | 100 | A |
| KX118690.1 | 492 | 492 | 100 | A |
| KF725079.1 | 492 | 492 | 100 | A |
| JX475999.1 | 492 | 492 | 100 | A |
| AF020058.1 | 490 | 490 | 100 | A |
| KC668284.1 | 488 | 488 | 99.63 | A |
| EU651894.1 | 481 | 481 | 100 | A |
| MK684350.1 | 475 | 475 | 98.88 | A |
| KU738337.1 | 472 | 472 | 100 | A |
| KJ140133.1 | 466 | 466 | 98.13 | A |
| GQ469985.1 | 466 | 466 | 98.5 | A |
| MH418437.1 | 457 | 457 | 100 | A |
| MN307069.1 | 756 | 756 | 100 | B |
| MK695179.1 | 756 | 756 | 100 | B |
| MK695177.1 | 756 | 756 | 100 | B |
| MK695176.1 | 756 | 756 | 100 | B |
| MK695175.1 | 756 | 756 | 100 | B |
| CP041924.1 | 756 | 756 | 100 | B |
| CP041923.1 | 756 | 756 | 100 | B |
| MH418465.1 | 756 | 756 | 100 | B |
| MH418464.1 | 756 | 756 | 100 | B |
| MH418463.1 | 756 | 756 | 100 | B |
| Host Organism | Name of Strain | Supergroup | Accession Number | Reference |
|---|---|---|---|---|
| D. simulans | wRi (Riverside) | A | AF020070.1 NC_012416.1 | Braig et al., 1998 [35]; Baião et al., 2019 [47] |
| D. simulans | wHa (Hawaii) | A | AF020068.1 | Braig et al., 1998 [35] |
| D. melanogaster | wMel | A | NC_002978.6 | Wu et al., 2004 [48] |
| C. quinquefasciatus | wPip | B | AF020060.1 | Zhou et al., 1998 [3] |
| Ae. albopictus | wAlbB | B | CP041924.1 | Kulkarni et al., 2019 [26] |
| Onchocerca ochengi | wOo | C | HE660029.1 | Darby et al., 2012 [49] |
| Brugia malayi | wBm | D | NC_006833.1 | Foster et al., 2005 [45] |
| Cimex lectularius | wCle | F | AP013028.1 | Nikoh et al., 2014 [44] |
| Accession Number | Country |
|---|---|
| JX476004/7.1 | India (Orissa) |
| AF020058.1 MG765532.1 | USA |
| MH418426/32/34/37.1 KX573017/18/19/22/23/38 KF782059/60/68/72/81/83/88/90 KF782100/02/05/08.1 KC004024.1 | Malaysia |
| MH503767.1 | Pakistan |
| AY462864.1 | Taiwan |
| MK684349/50/51 KX118690/91/92.1 | Mexico |
| EU727139.1 | Italy |
| KU738324/25.1 | China |
| MH777434.1 | Sri Lanka |
| Accession Number | Country |
|---|---|
| JX629464/67 | India (Orissa) |
| JX129186.1 KX5731/32/37.1 MH418463/65.1 KF781999/06/12/28/33/41/42/45/47.1 | USA |
| KX650069.1 | Pakistan (Punjab) |
| MG765533.1 AF020059.1 | USA |
| EU727140.1 | Italy |
| AY462863.1 | Taiwan |
| KU738369/76/82/83/84/85.1 | China |
| MT645169.1 | Singapore |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puerta-Guardo, H.; Contreras-Perera, Y.; Perez-Carrillo, S.; Che-Mendoza, A.; Ciau-Carrillo, K.J.; Parra-Cardeña, M.; Rodriguez-Sanchez, I.; Gomez-Govea, M.A.; Medina-Barreiro, A.; Ayora-Talavera, G.; et al. Wolbachia Surface Protein (wsp) Gene Sequencing of Strains A and B in Native Aedes albopictus of Mérida, Yucatán. Biology 2025, 14, 1399. https://doi.org/10.3390/biology14101399
Puerta-Guardo H, Contreras-Perera Y, Perez-Carrillo S, Che-Mendoza A, Ciau-Carrillo KJ, Parra-Cardeña M, Rodriguez-Sanchez I, Gomez-Govea MA, Medina-Barreiro A, Ayora-Talavera G, et al. Wolbachia Surface Protein (wsp) Gene Sequencing of Strains A and B in Native Aedes albopictus of Mérida, Yucatán. Biology. 2025; 14(10):1399. https://doi.org/10.3390/biology14101399
Chicago/Turabian StylePuerta-Guardo, Henry, Yamili Contreras-Perera, Silvia Perez-Carrillo, Azael Che-Mendoza, Karina Jacqueline Ciau-Carrillo, Manuel Parra-Cardeña, Iram Rodriguez-Sanchez, Mayra A. Gomez-Govea, Anuar Medina-Barreiro, Guadalupe Ayora-Talavera, and et al. 2025. "Wolbachia Surface Protein (wsp) Gene Sequencing of Strains A and B in Native Aedes albopictus of Mérida, Yucatán" Biology 14, no. 10: 1399. https://doi.org/10.3390/biology14101399
APA StylePuerta-Guardo, H., Contreras-Perera, Y., Perez-Carrillo, S., Che-Mendoza, A., Ciau-Carrillo, K. J., Parra-Cardeña, M., Rodriguez-Sanchez, I., Gomez-Govea, M. A., Medina-Barreiro, A., Ayora-Talavera, G., Pavia-Ruz, N., Martin-Park, A., & Manrique-Saide, P. (2025). Wolbachia Surface Protein (wsp) Gene Sequencing of Strains A and B in Native Aedes albopictus of Mérida, Yucatán. Biology, 14(10), 1399. https://doi.org/10.3390/biology14101399






