Dissecting the Functional Interplay Between Heme Oxygenase LjHO1 and Leghemoglobins in Lotus japonicus Nodules
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material, Bacterial Strains and Growth Conditions
2.2. Hairy Root Transformation in L. japonicus
2.3. GUS Staining
2.4. Subcellular Localizations
2.5. CRISPR-Cas9-Mediated Genome Editing in L. japonicus
2.6. Acetylene Reduction Assay
2.7. Heme Extraction and UPLC-MS/MS Analysis
2.8. Statistical Analysis
3. Results
3.1. LjHO1 Expression Dynamics Under Lb Deficiency
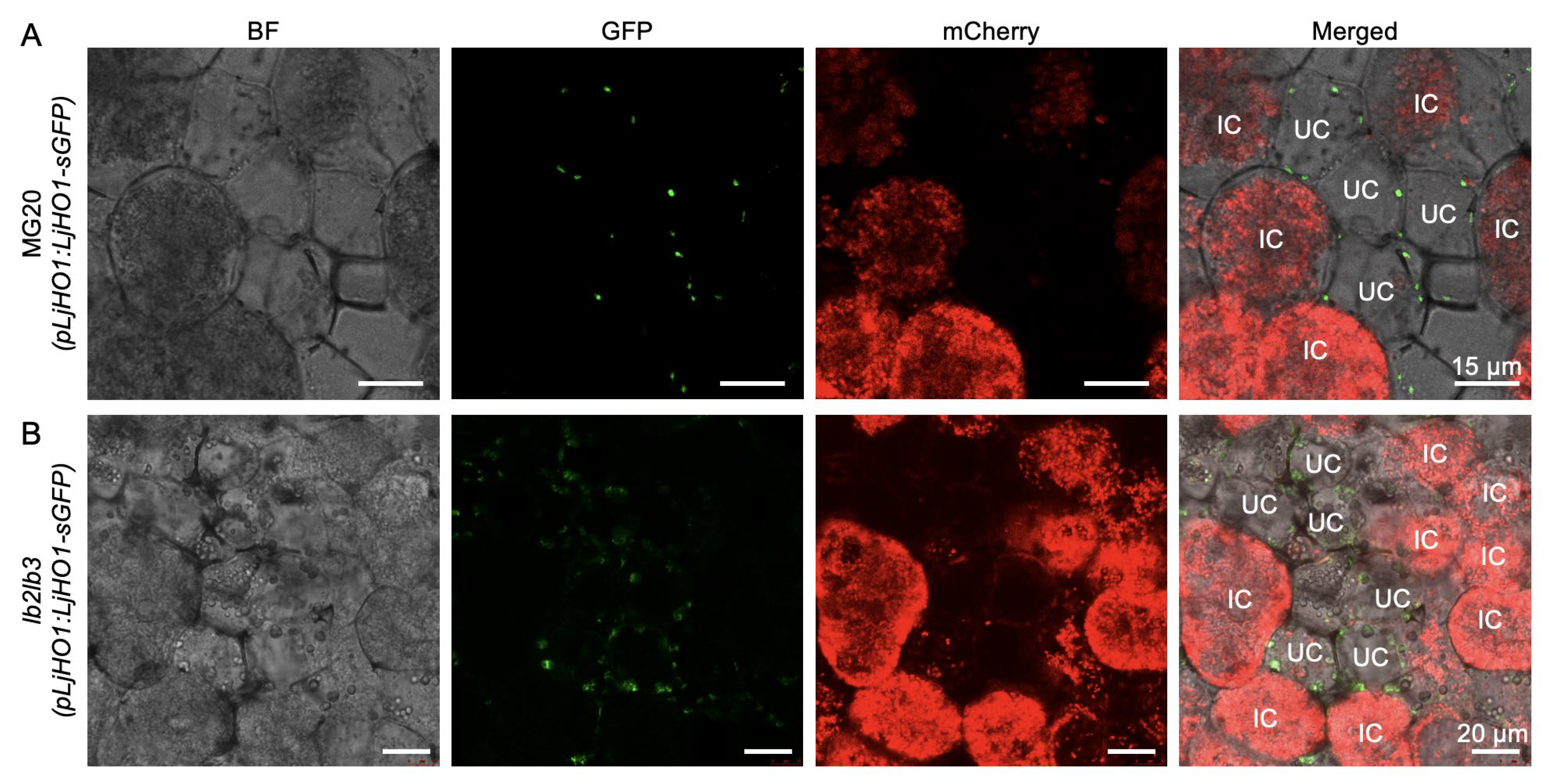
3.2. Lbs Deficiency Does Not Alter LjHO1 Localization in Uninfected Cells
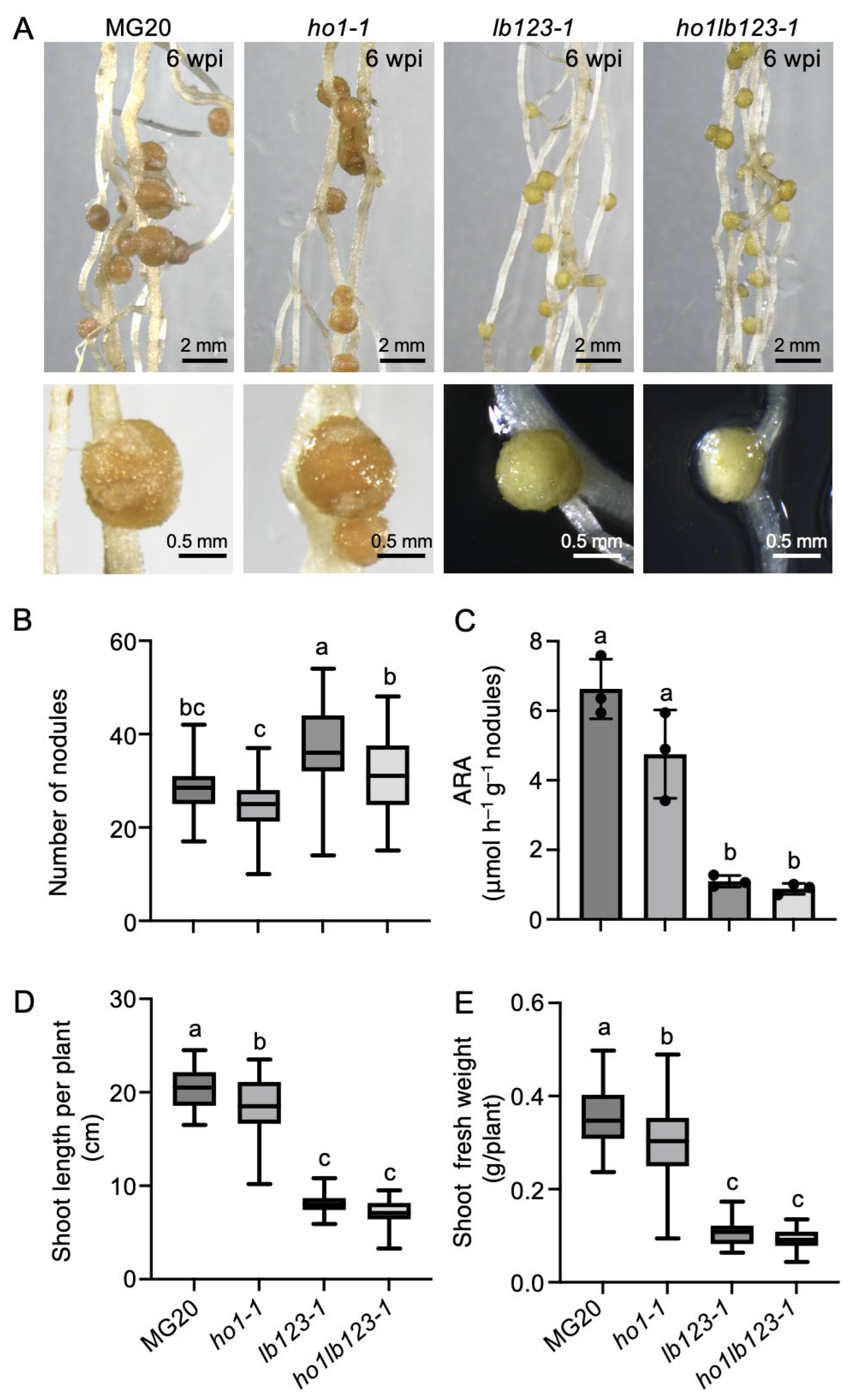
3.3. Phenotypic Analysis of the ho1lb123-1 Quadruple Mutant
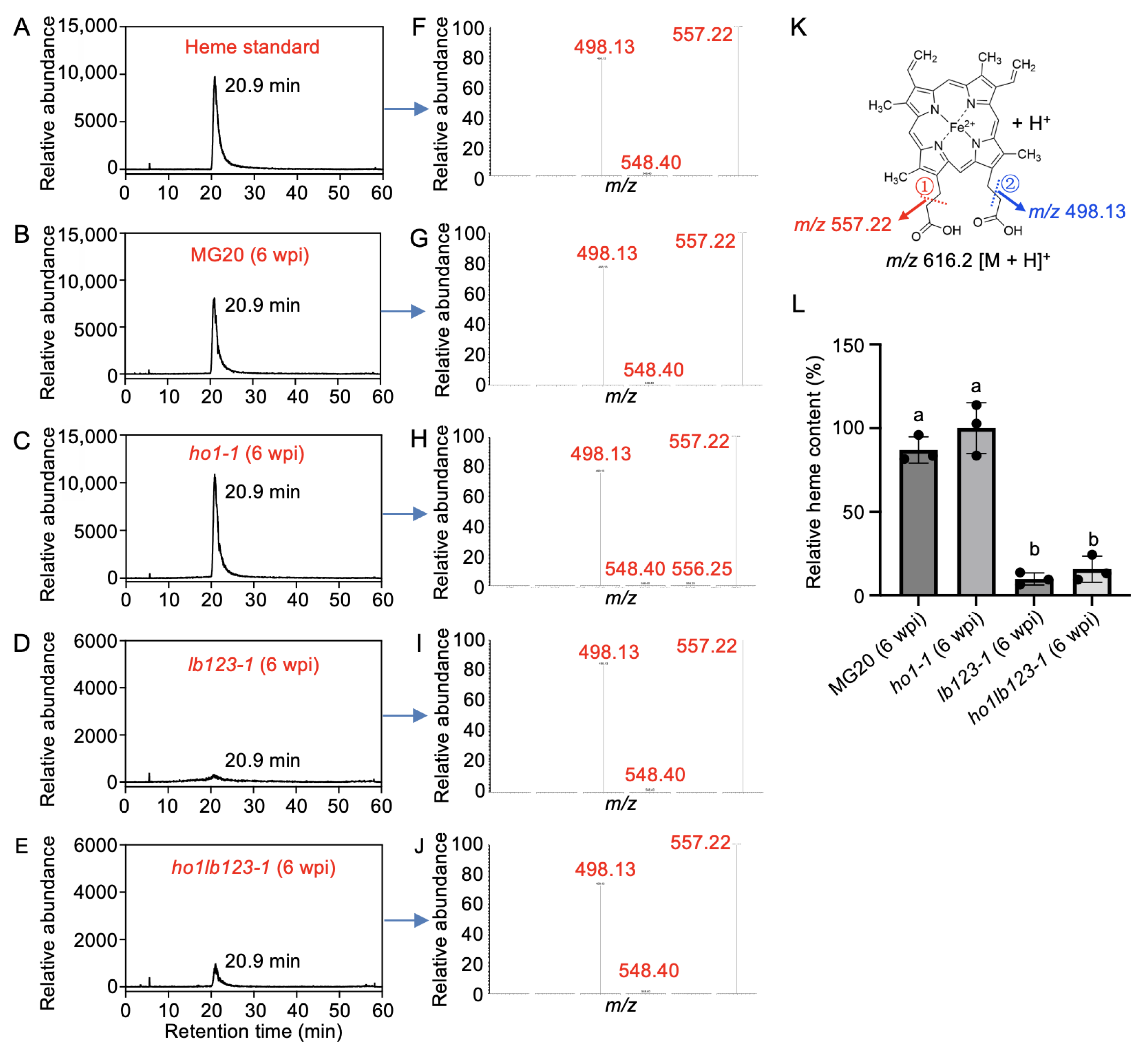
3.4. Loss of LjHO1 Slightly Elevates Heme Levels in ho1lb123 Mutant Nodules
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARA | Acetylene reduction activity |
| BF | Bright-field |
| BV | Biliverdin |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| GluTR | Glutamyl-tRNA reductase |
| GUS | β-Glucuronidase |
| HO | Heme oxygenase |
| Lb | Leghemoglobin |
| ROS | Reactive oxygen species |
| SNF | Symbiotic nitrogen fixation |
| UPLC-MS/MS | Ultra-performance liquid chromatography-tandem mass spectrometry |
| WT | Wild type |
References
- Roy, S.; Liu, W.; Nandety, R.S.; Crook, A.; Mysore, K.S.; Pislariu, C.I.; Frugoli, J.; Dickstein, R.; Udvardi, M.K. Celebrating 20 Years of Genetic Discoveries in Legume Nodulation and Symbiotic Nitrogen Fixation. Plant Cell 2020, 32, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Udvardi, M.; Poole, P.S. Transport and metabolism in legume-rhizobia symbioses. Annu. Rev. Plant Biol. 2013, 64, 781–805. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Ott, T.; van Dongen, J.T.; Gunther, C.; Krusell, L.; Desbrosses, G.; Vigeolas, H.; Bock, V.; Czechowski, T.; Geigenberger, P.; Udvardi, M.K. Symbiotic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr. Biol. 2005, 15, 531–535. [Google Scholar] [CrossRef]
- Larrainzar, E.; Villar, I.; Rubio, M.C.; Perez-Rontome, C.; Huertas, R.; Sato, S.; Mun, J.H.; Becana, M. Hemoglobins in the legume-Rhizobium symbiosis. New Phytol. 2020, 228, 472–484. [Google Scholar] [CrossRef]
- Baulcombe, D.; Verma, D.P. Preparation of a complementary DNA for leghaemoglobin and direct demonstration that leghaemoglobin is encoded by the soybean genome. Nucleic Acids Res. 1978, 5, 4141–4154. [Google Scholar] [CrossRef]
- Wang, L.; Rubio, M.C.; Xin, X.; Zhang, B.; Fan, Q.; Wang, Q.; Ning, G.; Becana, M.; Duanmu, D. CRISPR/Cas9 knockout of leghemoglobin genes in Lotus japonicus uncovers their synergistic roles in symbiotic nitrogen fixation. New Phytol. 2019, 224, 818–832. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Jardinaud, M.F.; Gao, J.; Pecrix, Y.; Wen, J.; Mysore, K.; Xu, P.; Sanchez-Canizares, C.; Ruan, Y.; Li, Q.; et al. NIN-like protein transcription factors regulate leghemoglobin genes in legume nodules. Science 2021, 374, 625–628. [Google Scholar] [CrossRef]
- Minguillon, S.; Roman, A.; Perez-Rontome, C.; Wang, L.; Xu, P.; Murray, J.D.; Duanmu, D.; Rubio, M.C.; Becana, M. Dynamics of hemoglobins during nodule development, nitrate response, and dark stress in Lotus japonicus. J. Exp. Bot. 2024, 75, 1547–1564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, L.; Rubio, M.C.; Perez-Rontome, C.; Zhou, Y.; Qi, Y.; Tian, T.; Zhang, W.; Fan, Q.; Becana, M.; et al. Heme catabolism mediated by heme oxygenase in uninfected interstitial cells enables efficient symbiotic nitrogen fixation in Lotus japonicus nodules. New Phytol. 2023, 239, 1989–2006. [Google Scholar] [CrossRef]
- Wang, L.; Tian, T.; Deng, Y.; Ji, J.; Liang, J.; Guan, Y.; Li, R.; Huang, X.; Wang, Y.; Ning, G.; et al. Plant glutamyl-tRNA reductases coordinate plant and rhizobial heme biosynthesis in nitrogen-fixing nodules. Plant Cell 2025, 37, koaf095. [Google Scholar] [CrossRef]
- Sangwan, I.; O’Brian, M.R. Expression of a soybean gene encoding the tetrapyrrole-synthesis enzyme glutamyl-tRNA reductase in symbiotic root nodules. Plant Physiol. 1999, 119, 593–598. [Google Scholar] [CrossRef]
- Sangwan, I.; O’Brian, M.R. Expression of the soybean (Glycine max) glutamate 1-semialdehyde aminotransferase gene in symbiotic root nodules. Plant Physiol. 1993, 102, 829–834. [Google Scholar] [CrossRef]
- Madsen, O.; Sandal, L.; Sandal, N.N.; Marcker, K.A. A soybean coproporphyrinogen oxidase gene is highly expressed in root nodules. Plant Mol. Biol. 1993, 23, 35–43. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhou, Y.; Li, R.H.; Liang, J.J.; Tian, T.; Ji, J.; Chen, R.Z.; Zhou, Y.M.; Fan, Q.L.; Ning, G.G.; et al. Single cell-type transcriptome profiling reveals genes that promote nitrogen fixation in the infected and uninfected cells of legume nodules. Plant Biotechnol. J. 2022, 20, 616. [Google Scholar] [CrossRef]
- Pfeiffer, N.E.; Torres, C.M.; Wagner, F.W. Proteolytic Activity in Soybean Root Nodules: Activity in Host Cell Cytosol and Bacteroids throughout Physiological Development and Senescence. Plant Physiol. 1983, 71, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Puppo, A.; Groten, K.; Bastian, F.; Carzaniga, R.; Soussi, M.; Lucas, M.M.; de Felipe, M.R.; Harrison, J.; Vanacker, H.; Foyer, C.H. Legume nodule senescence: Roles for redox and hormone signalling in the orchestration of the natural aging process. New Phytol. 2005, 165, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Herrada, G.; Puppo, A.; Moreau, S.; Day, D.A.; Rigaud, J. How is leghemoglobin involved in peribacteroid membrane degradation during nodule senescence? FEBS Lett. 1993, 326, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Nishimura, K.; Tokizawa, M.; Yamamoto, Y.Y.; Oka, Y.; Matsushita, T.; Hanada, K.; Shirai, K.; Mano, S.; Shimizu, T.; et al. Alternative localization of HEME OXYGENASE 1 in plant cells regulates cytosolic heme catabolism. Plant Physiol. 2024, 195, 2937–2951. [Google Scholar] [CrossRef]
- Gisk, B.; Yasui, Y.; Kohchi, T.; Frankenberg-Dinkel, N. Characterization of the haem oxygenase protein family in Arabidopsis thaliana reveals a diversity of functions. Biochem. J. 2010, 425, 425–434. [Google Scholar] [CrossRef]
- Muramoto, T.; Yokota, T.K.A.; Hwang, I.; Goodman, H.M. The Arabidopsis Photomorphogenic Mutant hy1 Is Deficient in Phytochrome Chromophore Biosynthesis as a Result of a Mutation in a Plastid Heme Oxygenase. Plant Cell 1999, 11, 335–347. [Google Scholar] [CrossRef]
- Shekhawat, G.S.; Verma, K. Haem oxygenase (HO): An overlooked enzyme of plant metabolism and defence. J. Exp. Bot. 2010, 61, 2255–2270. [Google Scholar] [CrossRef]
- Mullebner, A.; Moldzio, R.; Redl, H.; Kozlov, A.V.; Duvigneau, J.C. Heme Degradation by Heme Oxygenase Protects Mitochondria but Induces ER Stress via Formed Bilirubin. Biomolecules 2015, 5, 679–701. [Google Scholar] [CrossRef]
- Emborg, T.J.; Walker, J.M.; Noh, B.; Vierstra, R.D. Multiple heme oxygenase family members contribute to the biosynthesis of the phytochrome chromophore in Arabidopsis. Plant Physiol. 2006, 140, 856–868. [Google Scholar] [CrossRef]
- Zilli, C.G.; Balestrasse, K.B.; Yannarelli, G.G.; Polizio, A.H.; Santa-Cruz, D.M.; Tomaro, M.L. Heme oxygenase up-regulation under salt stress protects nitrogen metabolism in nodules of soybean plants. Environ. Exp. Bot. 2008, 64, 83–89. [Google Scholar] [CrossRef]
- Coale, T.H.; Loconte, V.; Turk-Kubo, K.A.; Vanslembrouck, B.; Mak, W.K.E.; Cheung, S.; Ekman, A.; Chen, J.H.; Hagino, K.; Takano, Y.; et al. Nitrogen-fixing organelle in a marine alga. Science 2024, 384, 217–222. [Google Scholar] [CrossRef]
- Baudouin, E.; Frendo, P.; Le Gleuher, M.; Puppo, A. A Medicago sativa haem oxygenase gene is preferentially expressed in root nodules. J. Exp. Bot. 2004, 55, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Bjork, P.K.; Kolte, M.V.; Poulsen, E.; Dedic, E.; Drace, T.; Andersen, S.U.; Nadzieja, M.; Liu, H.; Castillo-Michel, H.; et al. Zinc mediates control of nitrogen fixation via transcription factor filamentation. Nature 2024, 631, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Broughton, W.; Dilworth, M. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971, 125, 1075–1080. [Google Scholar] [CrossRef]
- Saeki, K.; Kouchi, H. The Lotus symbiont, Mesorhizobium loti: Molecular genetic techniques and application. J. Plant Res. 2000, 113, 457–465. [Google Scholar] [CrossRef]
- Kaneko, T.; Nakamura, Y.; Sato, S.; Asamizu, E.; Kato, T.; Sasamoto, S.; Watanabe, A.; Idesawa, K.; Ishikawa, A.; Kawashima, K. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 2000, 7, 331–338. [Google Scholar] [CrossRef]
- Díaz, C.L.; Grønlund, M.; Schlaman, H.R.; Spaink, H.P. Induction of hairy roots for symbiotic gene expression studies. In Lotus japonicus Handbook; Springer: Dordrecht, The Netherlands, 2005; pp. 261–277. [Google Scholar]
- Wang, L.X.; Wang, L.L.; Tan, Q.; Fang, Q.L.; Zhu, H.; Hong, Z.L.; Zhang, Z.M.; Duanmu, D.Q. Efficient Inactivation of Symbiotic Nitrogen Fixation Related Genes in Lotus japonicus Using CRISPR-Cas9. Front. Plant Sci. 2016, 7, 1333. [Google Scholar] [CrossRef]
- Lombari, P.; Ercolano, E.; Alaoui, H.; Chiurazzi, M. Agrobacterium-mediated in vitro transformation. In Lotus japonicus Handbook; Springer: Dordrecht, The Netherlands, 2005; pp. 251–259. [Google Scholar]
- Bisseling, T.; van Straten, J.; Houwaard, F. Turnover of nitrogenase and leghemoglobin in root nodules of Pisum sativum. Biochim. Biophys. Acta 1980, 610, 360–370. [Google Scholar] [CrossRef]
- Liu, Z.; Kong, X.; Long, Y.; Liu, S.; Zhang, H.; Jia, J.; Cui, W.; Zhang, Z.; Song, X.; Qiu, L.; et al. Integrated single-nucleus and spatial transcriptomics captures transitional states in soybean nodule maturation. Nat. Plants 2023, 9, 515–524. [Google Scholar] [CrossRef]
- Nagababu, E.; Rifkind, J.M. Heme degradation by reactive oxygen species. Antioxid. Redox Signal. 2004, 6, 967–978. [Google Scholar]
- Gao, J.P.; Su, Y.; Jiang, S.; Liang, W.; Lou, Z.; Frugier, F.; Xu, P.; Murray, J.D. Applying conventional and cell-type-specific CRISPR/Cas9 genome editing in legume plants. aBIOTECH 2025, 6, 346–360. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Tian, T.; Ji, J.; Tan, L.; Peng, K.; Liu, Z.; Zhao, W.; Wang, C.; Liu, F.; Zhang, X. Dissecting the Functional Interplay Between Heme Oxygenase LjHO1 and Leghemoglobins in Lotus japonicus Nodules. Biology 2025, 14, 1401. https://doi.org/10.3390/biology14101401
Zhou Y, Tian T, Ji J, Tan L, Peng K, Liu Z, Zhao W, Wang C, Liu F, Zhang X. Dissecting the Functional Interplay Between Heme Oxygenase LjHO1 and Leghemoglobins in Lotus japonicus Nodules. Biology. 2025; 14(10):1401. https://doi.org/10.3390/biology14101401
Chicago/Turabian StyleZhou, Yu, Tao Tian, Jie Ji, Liting Tan, Kexin Peng, Zhuocheng Liu, Wenlong Zhao, Chuanzhi Wang, Fawang Liu, and Xingtao Zhang. 2025. "Dissecting the Functional Interplay Between Heme Oxygenase LjHO1 and Leghemoglobins in Lotus japonicus Nodules" Biology 14, no. 10: 1401. https://doi.org/10.3390/biology14101401
APA StyleZhou, Y., Tian, T., Ji, J., Tan, L., Peng, K., Liu, Z., Zhao, W., Wang, C., Liu, F., & Zhang, X. (2025). Dissecting the Functional Interplay Between Heme Oxygenase LjHO1 and Leghemoglobins in Lotus japonicus Nodules. Biology, 14(10), 1401. https://doi.org/10.3390/biology14101401





