Cholinergic Transmission Dysregulation and Neurodegeneration Induced by Thyroid Signaling Disruption Following Butylparaben Single and Repeated Treatment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Culture Conditions
2.3. TRα Activity Determination
2.4. Quantification of Acetylcholine Levels
2.5. Quantification of AChE Activity
2.6. Assessment of Oxidative Stress
2.7. Quantification of Beta-Amyloid and Tau Peptide
2.8. Quantification of Target Proteins
2.9. Gene Expression Measurement
2.10. siRNA Transfection and Gene Silencing Validation
2.11. Cell Viability Determination (Caspases 3/7 and MTT Assays)
2.12. Statistical Analysis
3. Results
3.1. Quantification of D3 Levels and TRα Activity and Levels
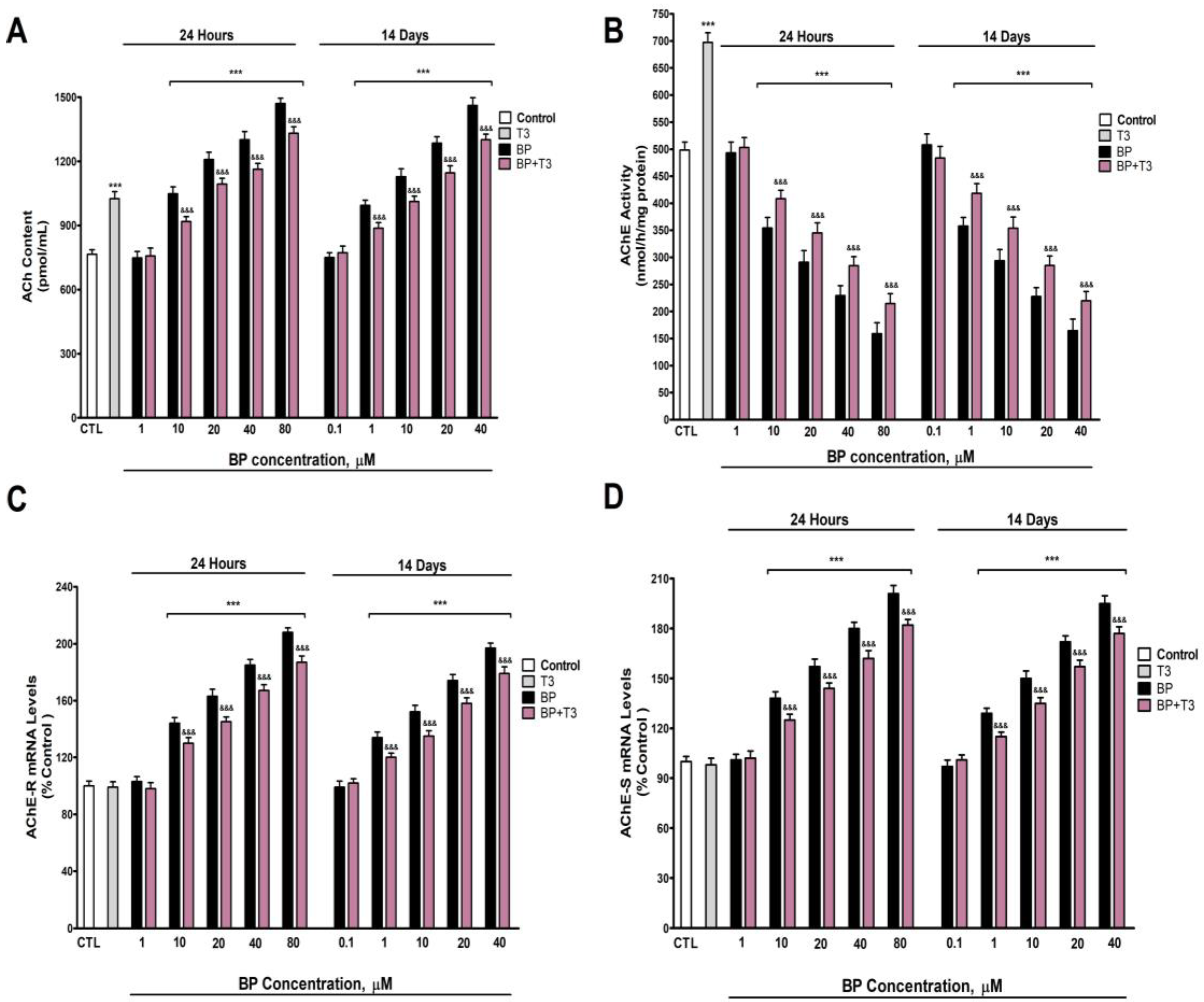
3.2. Quantification of ACh Levels and AChE Activity and Variants Expression
3.3. NRF2 Pathway Assessment (HO1, SOD1, and NRF2 Protein Levels Quantification)
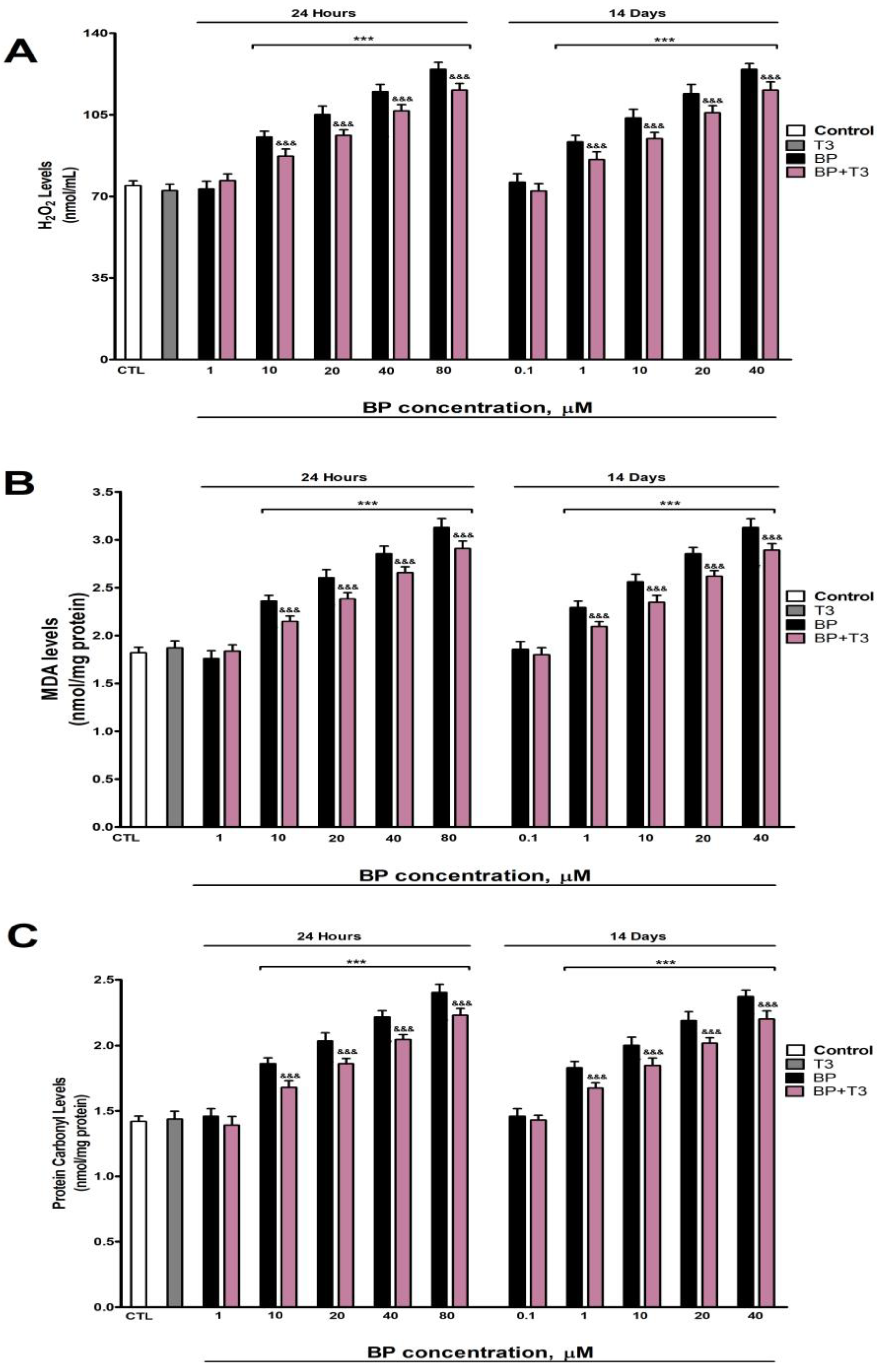
3.4. Oxidative Stress Analysis
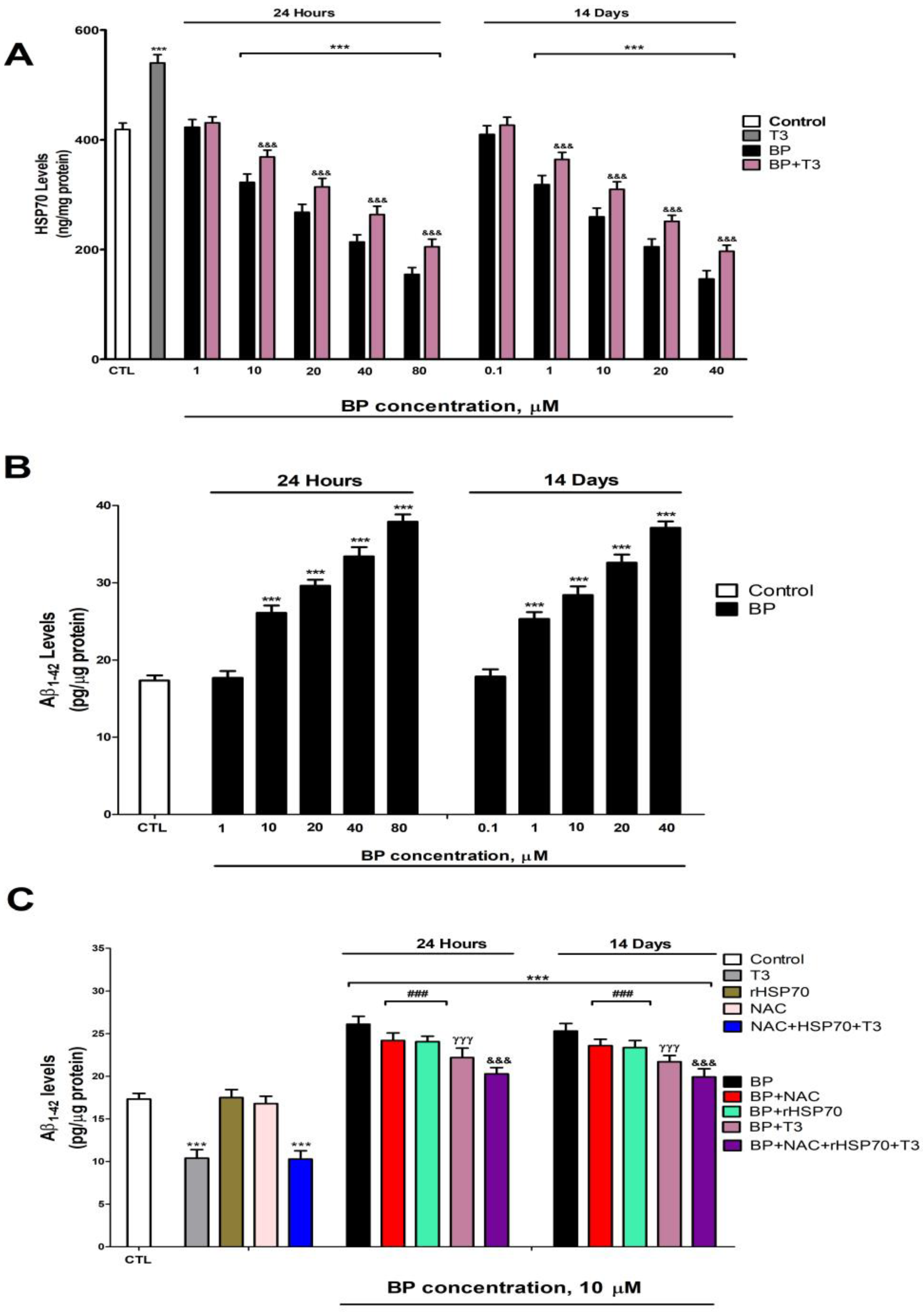
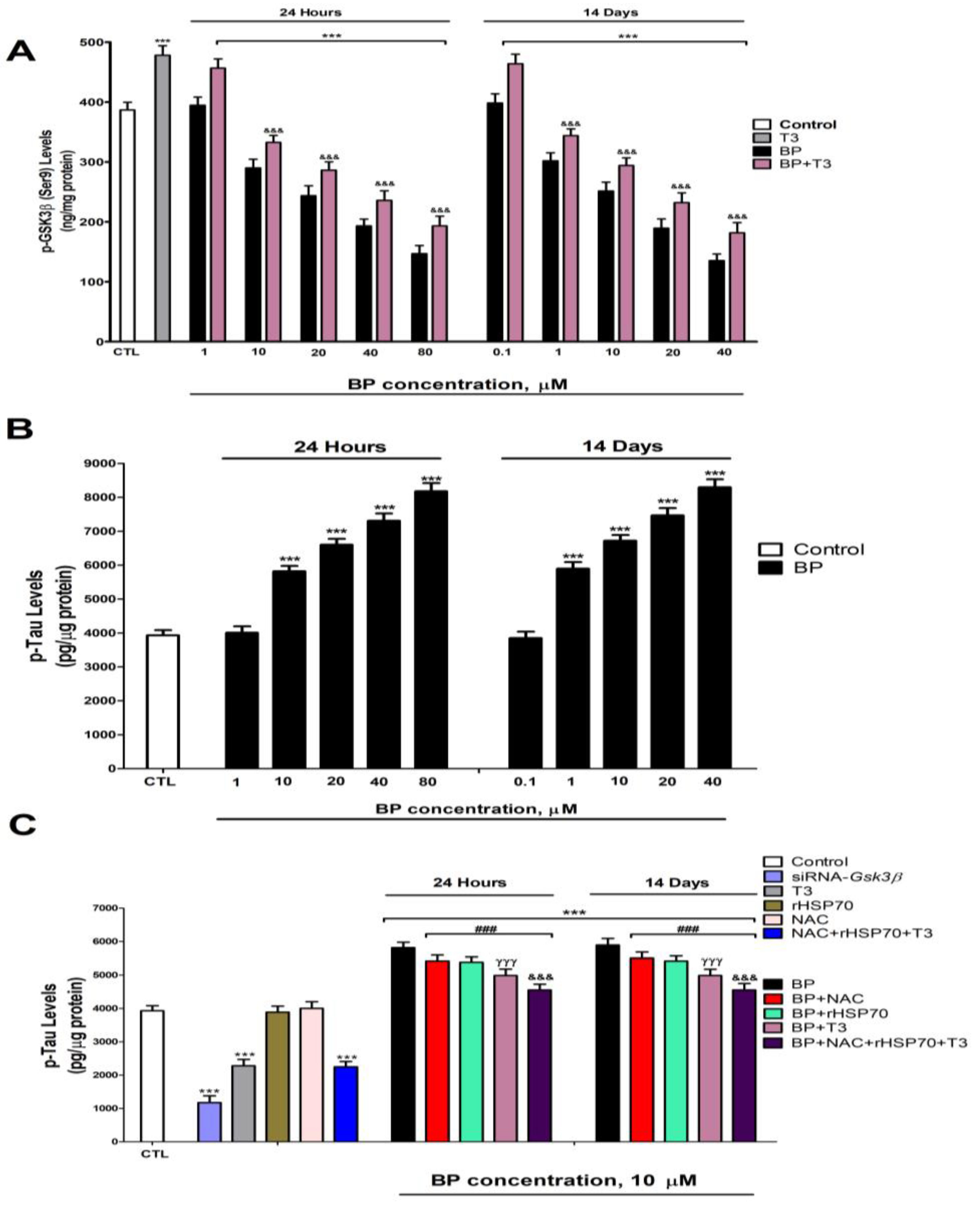
3.5. Quantification of HSP70 and p-GSK3β (Ser9) Levels
3.6. Quantification of Tau and β-Amyloid Peptide Levels
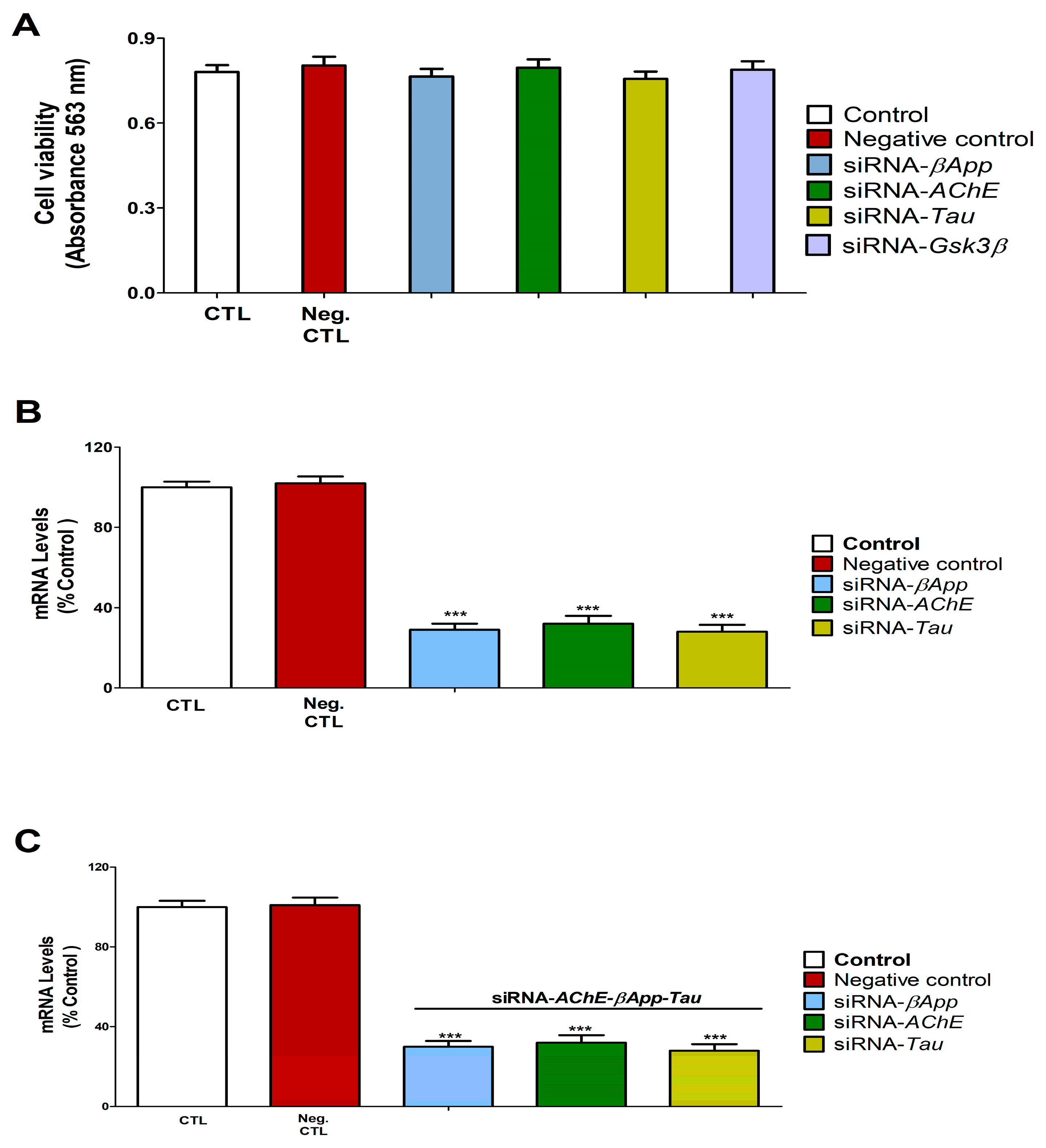
3.7. Gene Knockdown Validation Assessment
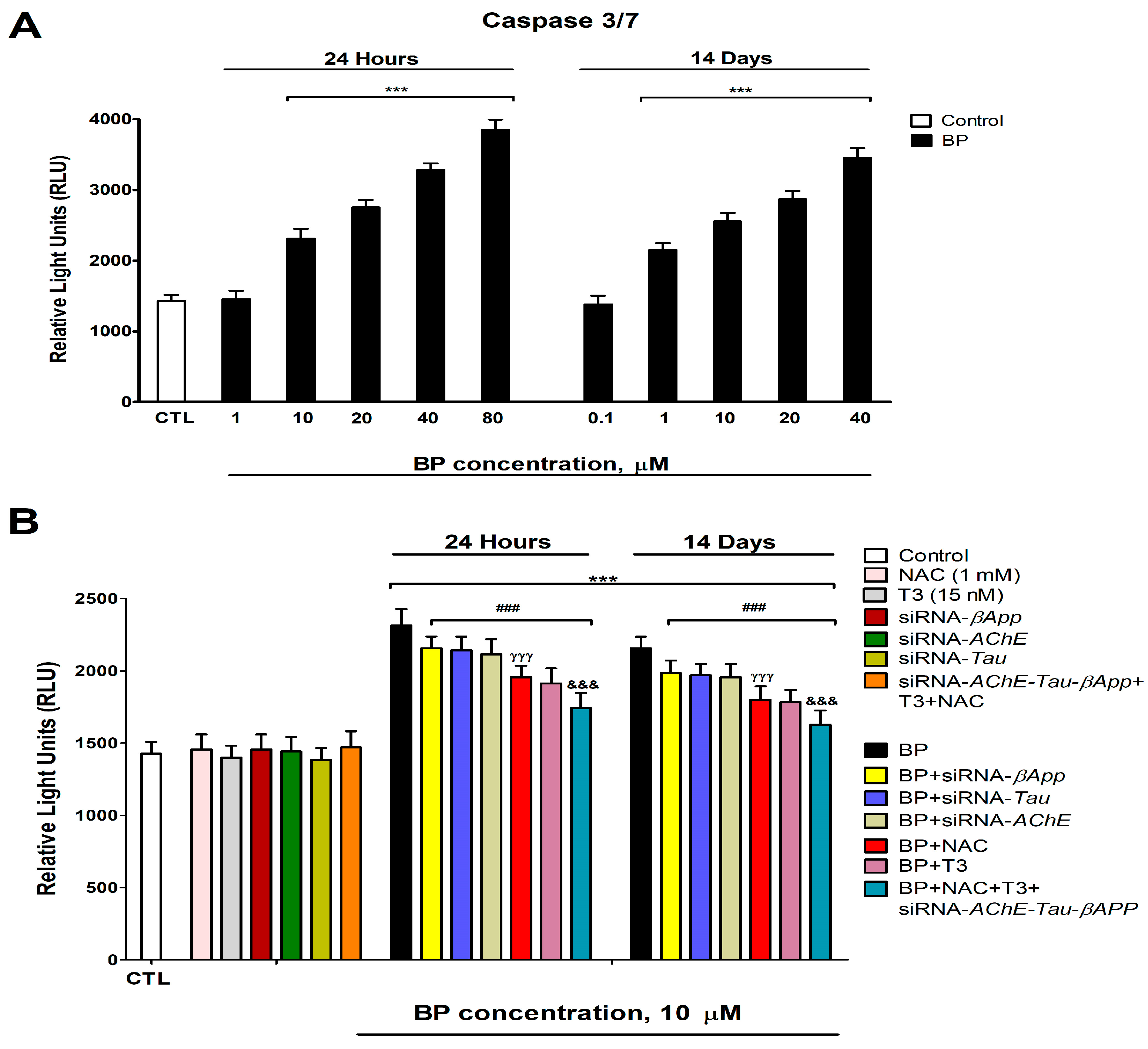
3.8. Cell Viability Assessment and Caspases 3/7 Activation Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| Aβ1-42 | Amyloid-β Peptide 1-42 |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| AChE-R | Acetylcholinesterase “Readthrough” Variant |
| AChE-S | Acetylcholinesterase Synaptic Variant |
| AKT | Protein Kinase B (PKB) |
| ANOVA | Analysis of Variance |
| βApp | β-Amyloid Precursor Protein |
| BDNF | Brain-Derived Neurotrophic Factor |
| BF | Basal Forebrain |
| BFCN | Basal Forebrain Cholinergic Neurons |
| BP | Butylparaben |
| cAMP | Cyclic Adenosine Monophosphate |
| cDNA | Complementary DNA |
| D1 | Type 1 Iodothyronine Deiodinase |
| D3 | Type 3 Iodothyronine Deiodinase |
| DMSO | Dimethyl Sulfoxide |
| DTNB | Dithionitrobenzoic Acid |
| FC | Frontal Cortex |
| FBS | Fetal Bovine Serum |
| GRP78 | Glucose-Regulated Protein 78 |
| GSK3β | Glycogen Synthase Kinase 3 beta |
| HC | Hippocampus |
| HO1 | Heme Oxygenase 1 |
| HRP | Horseradish Peroxidase |
| HSF-1 | Heat Shock Transcription Factor 1 |
| HSP | Heat Shock Protein |
| HSP70 | Heat Shock Protein 70 |
| MDA | Malondialdehyde |
| MED | Minimum Effective Dose |
| MTD | Maximum Tolerated Dose |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| NAC | N-Acetylcysteine |
| NRF2 | Nuclear Factor (erythroid-derived 2)-like 2 |
| OS | Oxidative Stress |
| PBS | Phosphate-Buffered Saline |
| p-GSK3β (Ser9) | Phosphorylated GSK3β (Serine 9) |
| PI3K | Phosphatidylinositol 3-Kinase |
| p-Tau | Phosphorylated-Tau |
| rHSP70 | Recombinant Heat Shock Protein 70 |
| RNAi | RNA Interference |
| ROS | Reactive Oxygen Species |
| siRNA | Small Interfering RNA |
| SOD1 | Superoxide Dismutase 1 |
| T3 | Triiodothyronine |
| Tau | Microtubule-Associated Protein Tau |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
| TRα | Thyroid Hormone Receptor Alpha |
| TRβ | Thyroid Hormone Receptor Beta |
| THs | Thyroid Hormones |
| UPR | Unfolded Protein Response |
References
- Aydemir, D.; Öztaşcı, B.; Barlas, N.; Ulusu, N.N. Effects of butylparaben on antioxidant enzyme activities and histopathological changes in rat tissues. Arh. Hig. Rada Toksikol. 2019, 70, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.; Kim, Y.; Choi, D.; Lee, J.H. Effects of long-term treatment with low concentration butylparaben on prostate organoids. Environ. Pollut. 2025, 366, 125502. [Google Scholar] [CrossRef]
- Kizhedath, A.; Wilkinson, S.; Glassey, J. Assessment of hepatotoxicity and dermal toxicity of butylparaben and methylparaben using HepG2 and HDFn in vitro models. Toxicol. Vitr. 2019, 55, 108–115. [Google Scholar] [CrossRef]
- Zhu, H.; Liao, D.; Mehmood, M.A.; Huang, Y.; Yuan, W.; Zheng, J.; Ma, Y.; Peng, Y.; Tian, G.; Xiao, X.; et al. Systolic heart failure induced by butylparaben in zebrafish is caused through oxidative stress and immunosuppression. Ecotoxicol. Environ. Saf. 2023, 268, 115692. [Google Scholar] [CrossRef]
- Gogoi, P.; Kalita, J.C. Effects of butylparaben exposure on thyroid peroxidase (TPO) and type 1 iodothyronine deiodinase (D1) in female Wistar rats. Toxicology 2020, 443, 152562. [Google Scholar] [CrossRef]
- Singh, M.; Guru, A.; Pachaiappan, R.; Almutairi, B.O.; Arokiyaraj, S.; Gopi, M.; Arockiaraj, J. Impact of butylparaben on beta-cell damage and insulin/PEPCK expression in zebrafish larvae: Protective effects of morin. J. Biochem. Mol. Toxicol. 2024, 38, e23520. [Google Scholar] [CrossRef]
- Huang, L.; Xu, J.; Jia, K.; Wu, Y.; Yuan, W.; Liao, Z.; Cheng, B.; Luo, Q.; Tian, G.; Lu, H. Butylparaben induced zebrafish (Danio rerio) kidney injury by down-regulating the PI3K-AKT pathway. J. Hazard. Mater. 2024, 470, 134129. [Google Scholar] [CrossRef]
- Kim, J.L.; Kim, S.S.; Hwang, K.S.; Park, H.C.; Cho, S.H.; Bae, M.A.; Kim, K.T. Chronic exposure to butylparaben causes photosensitivity disruption and memory impairment in adult zebrafish. Aquat. Toxicol. 2022, 251, 106279. [Google Scholar] [CrossRef]
- Ko, M.Y.; Hyun, S.A.; Jang, S.; Seo, J.W.; Rho, J.; Lee, B.S.; Ka, M. Butylparaben induces the neuronal death through the ER stress-mediated apoptosis of primary cortical neurons. Neurotox. Res. 2022, 40, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Lv, B.R.; Shi, Y.J.; Chen, W.M.; Zhang, J.L. Environmental pollution of paraben needs attention: A study of methylparaben and butylparaben co-exposure trigger neurobehavioral toxicity in zebrafish. Environ. Pollut. 2024, 356, 124370. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hwang, I.; Jeung, E.B. Prenatal and postnatal exposure to butylparaben induces neurodevelopmental disorders in mice offspring. J. Physiol. Pharmacol. 2025, 76, 281–293. [Google Scholar]
- Eickhoff, S.; Franzen, L.; Korda, A.; Rogg, H.; Trulley, V.N.; Borgwardt, S.; Avram, M. The basal forebrain cholinergic nuclei and their relevance to schizophrenia and other psychotic disorders. Front. Psychiatry 2022, 13, 909961. [Google Scholar] [CrossRef] [PubMed]
- Villano, I.; Messina, A.; Valenzano, A.; Moscatelli, F.; Esposito, T.; Monda, V.; Esposito, M.; Precenzano, F.; Carotenuto, M.; Viggiano, A.; et al. Basal forebrain cholinergic system and orexin neurons: Effects on attention. Front. Behav. Neurosci. 2017, 11, 10. [Google Scholar] [CrossRef]
- Grothe, M.J.; Heinsen, H.; Amaro, E.; Grinberg, L.T.; Teipel, S.J. Cognitive correlates of basal forebrain atrophy and associated cortical hypometabolism in mild cognitive impairment. Cereb. Cortex 2016, 26, 2411–2426. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Lite, C.; Guru, A.; Juliet, M.; Arockiaraj, J. Embryonic exposure to butylparaben and propylparaben induced developmental toxicity and triggered anxiety-like neurobehavioral response associated with oxidative stress and apoptosis in the head of zebrafish larvae. Environ. Toxicol. 2022, 37, 1988–2004. [Google Scholar] [CrossRef]
- Huang, Q.; Liao, C.; Ge, F.; Ao, J.; Liu, T. Acetylcholine bidirectionally regulates learning and memory. J. Neurorestoratol. 2022, 10, 100002. [Google Scholar] [CrossRef]
- Chen, Y. Organophosphate-induced brain damage: Mechanisms, neuropsychiatric and neurological consequences, and potential therapeutic strategies. Neurotoxicology 2012, 33, 391–400. [Google Scholar] [CrossRef]
- Moyano, P.; de Frias, M.; Lobo, M.; Anadon, M.J.; Sola, E.; Pelayo, A.; Díaz, M.J.; Frejo, M.T.; Del Pino, J. Cadmium induced ROS alters M1 and M3 receptors, leading to SN56 cholinergic neuronal loss, through AChE variants disruption. Toxicology 2018, 394, 54–62. [Google Scholar] [CrossRef]
- Knorr, D.Y.; Georges, N.S.; Pauls, S.; Heinrich, R. Acetylcholinesterase promotes apoptosis in insect neurons. Apoptosis 2020, 25, 730–746. [Google Scholar] [CrossRef]
- Zhang, X.J.; Yang, L.; Zhao, Q.; Caen, J.P.; He, H.Y.; Jin, Q.H.; Guo, L.H.; Alemany, M.; Zhang, L.Y.; Shi, Y.F. Induction of acetylcholinesterase expression during apoptosis in various cell types. Cell Death Differ. 2002, 9, 790–800. [Google Scholar] [CrossRef]
- Ko, Y.; Kim, E.H.; Kim, D.; Choi, S.; Gil, J.; Park, H.J.; Shin, Y.; Kim, W.; Bae, O.N. Butylparaben promotes phosphatidylserine exposure and procoagulant activity of human red blood cells via increase of intracellular calcium levels. Food Chem. Toxicol. 2023, 181, 114084. [Google Scholar] [CrossRef] [PubMed]
- Khanal, T.; Kim, H.G.; Jin, S.W.; Shim, E.; Han, H.J.; Noh, K.; Park, S.; Lee, D.D.; Kang, W.; Yeo, H.K.; et al. Protective role of metabolism by intestinal microflora in butylparaben-induced toxicity in HepG2 cell cultures. Toxicol. Lett. 2012, 213, 174–183. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Bazer, F.W.; Song, G. Butylparaben promotes apoptosis in human trophoblast cells through increased oxidative stress-induced endoplasmic reticulum stress. Environ. Toxicol. 2018, 33, 436–445. [Google Scholar] [CrossRef]
- Yin, Y.; Xie, Z.; Sun, X.; Wu, X.; Zhang, J.; Shi, H.; Ding, L.; Hong, M. Effect of butylparaben on oxidative stress in the liver of Mauremys sinensis. Toxics 2023, 11, 915. [Google Scholar] [CrossRef]
- Moyano, P.; Sola, E.; Naval, M.V.; Guerra-Menéndez, L.; Fernández, M.C.; Del Pino, J. Neurodegenerative proteinopathies induced by environmental pollutants: Heat shock proteins and proteasome as promising therapeutic tools. Pharmaceutics 2023, 15, 2048. [Google Scholar] [CrossRef]
- Nagar, Y.; Thakur, R.S.; Parveen, T.; Patel, D.K.; Ram, K.R.; Satish, A. Toxicity assessment of parabens in Caenorhabditis elegans. Chemosphere 2020, 246, 125730. [Google Scholar] [CrossRef] [PubMed]
- Akinyemi, A.O.; Simpson, K.E.; Oyelere, S.F.; Nur, M.; Ngule, C.M.; Owoyemi, B.C.D.; Ayarick, V.A.; Oyelami, F.F.; Obaleye, O.; Esoe, D.P.; et al. Unveiling the dark side of glucose-regulated protein 78 (GRP78) in cancers and other human pathology: A systematic review. Mol. Med. 2023, 29, 112. [Google Scholar] [CrossRef] [PubMed]
- Moyano, P.; Flores, A.; San Juan, J.; García, J.; Anadón, M.J.; Plaza, J.C.; Naval, M.V.; Fernández, M.C.; Guerra-Menéndez, L.; Del Pino, J. Imidacloprid unique and repeated treatment produces cholinergic transmission disruption and apoptotic cell death in SN56 cells. Food Chem. Toxicol. 2024, 193, 114988. [Google Scholar] [CrossRef]
- Sharma, V.; Chander Sharma, P.; Reang, J.; Yadav, V.; Kumar Tonk, R.; Majeed, J.; Sharma, K. Impact of GSK-3β and CK-1δ on Wnt signaling pathway in Alzheimer disease: A dual target approach. Bioorg Chem. 2024, 147, 107378. [Google Scholar] [CrossRef]
- Jiang, Q.L.; Li, S.; Zeng, Y.; Zhang, B.T.; Cao, Y.; Li, T.; Jiang, J. High-dose exposure to butylparaben impairs thyroid ultrastructure and function in rats. Sci. Rep. 2024, 14, 4550. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.X.; Wang, C.; Li, X.D.; Guo, W.L.; Liu, G.Y.; Zhang, H.B.; Sun, Y.; Zhu, D.F.; Xu, Q. Activation of cholinergic basal forebrain neurons improved cognitive functions in adult-onset hypothyroid mice. Biomed. Pharmacother. 2022, 153, 113495. [Google Scholar] [CrossRef]
- Sola, E.; Moyano, P.; Flores, A.; García, J.; García, J.M.; Anadon, M.J.; Frejo, M.T.; Pelayo, A.; de la Cabeza Fernandez, M.; Del Pino, J. Cadmium-induced neurotoxic effects on rat basal forebrain cholinergic system through thyroid hormones disruption. Environ. Toxicol. Pharmacol. 2022, 90, 103791. [Google Scholar] [CrossRef]
- Vasilopoulou, C.G.; Constantinou, C.; Giannakopoulou, D.; Giompres, P.; Margarity, M. Effect of adult onset hypothyroidism on behavioral parameters and acetylcholinesterase isoforms activity in specific brain regions of male mice. Physiol. Behav. 2016, 164, 284–291. [Google Scholar] [CrossRef]
- Todorović, J.; Dinčić, M.; Krstić, D.Z.; Čolović, M.B.; Ostojić, J.N.; Kovačević, S.; Lopičić, S.; Spasić, S.; Brkić, P.; Milovanović, A. The simultaneous action of acute paradoxical sleep deprivation and hypothyroidism modulates synaptosomal ATPases and acetylcholinesterase activities in rat brain. Sleep. Med. 2023, 105, 14–20. [Google Scholar] [CrossRef]
- Sola, E.; Moyano, P.; Flores, A.; García, J.M.; García, J.; Anadon, M.J.; Frejo, M.T.; Pelayo, A.; de la Cabeza Fernandez, M.; Del Pino, J. Cadmium-promoted thyroid hormones disruption mediates ROS, inflammation, Aβ and Tau proteins production, gliosis, spongiosis and neurodegeneration in rat basal forebrain. Chem. Biol. Interact. 2023, 375, 110428. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Stohn, J.P. The Type 3 Deiodinase: Epigenetic Control of Brain Thyroid Hormone Action and Neurological Function. Int. J. Mol. Sci. 2018, 19, 1804. [Google Scholar] [CrossRef] [PubMed]
- Salas-Lucia, F.; Bianco, A.C. T3 levels and thyroid hormone signaling. Front. Endocrinol. 2022, 13, 1044691. [Google Scholar] [CrossRef]
- Medici, M.; Visser, T.J.; Peeters, R.P. Genetics of thyroid function. Best. Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 129–142. [Google Scholar] [CrossRef]
- Gogoi, P.; Kumari, N.; Baishya, J. An in-silico molecular docking study on the interaction of different paraben esters (methyl-, ethyl-, propyl- and butylparaben) with human thyroid hormone receptor α1 (THRα1) and β1 (THRβ1). Uttar Pradesh J. Zool. 2022, 43, 603–615. [Google Scholar] [CrossRef]
- Liang, J.; Yang, X.; Liu, Q.S.; Sun, Z.; Ren, Z.; Wang, X.; Zhang, Q.; Ren, X.; Liu, X.; Zhou, Q.; et al. Assessment of thyroid endocrine disruption effects of parabens using in vivo, in vitro, and in silico approaches. Environ. Sci. Technol. 2022, 56, 460–469. [Google Scholar] [CrossRef]
- Hammond, D.N.; Lee, H.J.; Tonsgard, J.H.; Wainer, B.H. Development and characterization of clonal cell lines derived from septal cholinergic neurons. Brain Res. 1990, 512, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Hudgens, E.D.; Ji, L.; Carpenter, C.D.; Petersen, S.L. The gad2 promoter is a transcriptional target of estrogen receptor (ER)alpha and ER beta: A unifying hypothesis to explain diverse effects of estradiol. J. Neurosci. 2009, 29, 8790–8797. [Google Scholar] [CrossRef]
- Bielarczyk, H.; Jankowska, A.; Madziar, B.; Matecki, A.; Michno, A.; Szutowicz, A. Differential toxicity of nitric oxide, aluminum, and amyloid-β-peptide in SN56 cholinergic cells from mouse septum. Neurochem. Int. 2003, 42, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Szutowicz, A.; Bielarczyk, H.; Gul, S.; Ronowska, A.; Pawełczyk, T.; Jankowska-Kulawy, A. Phenotype-dependent susceptibility of cholinergic neuroblastoma cells to neurotoxic inputs. Metab. Brain Dis. 2006, 21, 149–161. [Google Scholar] [CrossRef]
- Bae, J.S.; Lee, J.D.; Song, S.W.; Shin, H.C.; Choi, Y.K.; Shin, C.Y.; Lee, B.M.; Kim, K.B. Thirteen-week subcutaneous repeated dose toxicity study of butylparaben and its toxicokinetics in rats. Arch. Toxicol. 2021, 95, 2037–2050. [Google Scholar] [CrossRef]
- Ramalho, A.; Vale, A.; Carvalho, F.; Fernandes, E.; Freitas, M. Parabens exposure and its impact on diabesity: A review. Toxicology 2025, 515, 154125. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; He, Y.; Zhang, T.; Zhu, H.; Huang, X.; Bai, X.; Zhang, B.; Kannan, K. Profiles of parabens and their metabolites in paired maternal-fetal serum, urine and amniotic fluid and their implications for placental transfer. Ecotoxicol. Environ. Saf. 2020, 191, 110235. [Google Scholar] [CrossRef]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Bishop, A.M.; Needham, L.L. Urinary concentrations of four parabens in the U.S. population: NHANES 2005–2006. Environ. Health Perspect. 2010, 118, 679–685. [Google Scholar] [CrossRef]
- Seidel, F.; Kappenberg, F.; Fayyaz, S.; Scholtz-Illigens, A.; Cherianidou, A.; Derksen, K.; Nell, P.; Marchan, R.; Edlund, K.; Leist, M.; et al. Risk assessment of parabens in a transcriptomics-based in vitro test. Chem. Biol. Interact. 2023, 384, 110699. [Google Scholar] [CrossRef]
- Reale, M.; de Angelis, F.; di Nicola, M.; Capello, E.; di Ioia, M.; Luca, G.; Lugaresi, A.; Tata, A.M. Relation between pro-inflammatory cytokines and acetylcholine levels in relapsing-remitting multiple sclerosis patients. Int. J. Mol. Sci. 2012, 13, 12656–12664. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Härtl, R.; Gleinich, A.; Zimmermann, M. Dramatic increase in readthrough acetylcholinesterase in a cellular model of oxidative stress. J. Neurochem. 2011, 116, 1088–1096. [Google Scholar] [CrossRef]
- Zimmermann, M.; Grosgen, S.; Westwell, M.S.; Greenfield, S.A. Selective enhancement of the activity of C-terminally truncated, but not intact, acetylcholinesterase. J. Neurochem. 2008, 104, 221–232. [Google Scholar] [CrossRef]
- Moyano, P.; García, J.M.; García, J.; Anadon, M.J.; Naval, M.V.; Frejo, M.T.; Sola, E.; Pelayo, A.; Pino, J.D. Manganese increases Aβ and Tau protein levels through proteasome 20S and heat shock proteins 90 and 70 alteration, leading to SN56 cholinergic cell death following single and repeated treatment. Ecotoxicol. Environ. Saf. 2020, 203, 110975. [Google Scholar] [CrossRef] [PubMed]
- Shaltiel, G.; Hanan, M.; Wolf, Y.; Barbash, S.; Kovalev, E.; Shoham, S.; Soreq, H. Hippocampal microRNA-132 mediates stress-inducible cognitive deficits through its acetylcholinesterase target. Brain Struct. Funct. 2013, 218, 59–72. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Halder, N.; Lal, G. Cholinergic System and Its Therapeutic Importance in Inflammation and Autoimmunity. Front. Immunol. 2021, 12, 660342. [Google Scholar] [CrossRef]
- Kundu, S.; Ray, A.K. Thyroid Hormone Homeostasis in Adult Mammalian Brain: A Novel Mechanism for Functional Preservation of Cerebral T3 Content During Initial Peripheral Hypothyroidism. Al Ameen J. Med. Sci. 2010, 3, 5–20. [Google Scholar]
- Du, H.; Cui, L.; Zhao, X.; Yu, Z.; He, T.; Zhang, B.; Fan, X.; Zhao, M.; Zhu, R.; Zhang, Z.; et al. Butylparaben induces glycolipid metabolic disorders in mice via disruption of gut microbiota and FXR signaling. J. Hazard. Mater. 2024, 474, 134821. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M.; Mostafa, H.; Khojah, H.M.J. Insulin resistance in Alzheimer’s disease: The genetics and metabolomics links. Clin. Chim. Acta 2023, 539, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Jamshidnejad-Tosaramandani, T.; Kashanian, S.; Babaei, M.; Al-Sabri, M.H.; Schiöth, H.B. The Potential Effect of Insulin on AChE and Its Interactions with Rivastigmine In Vitro. Pharmaceuticals 2021, 14, 1136. [Google Scholar] [CrossRef]
- Moyano, P.; Flores, A.; García, J.; García, J.M.; Anadon, M.J.; Frejo, M.T.; Sola, E.; Pelayo, A.; Del Pino, J. Bisphenol A single and repeated treatment increases HDAC2, leading to cholinergic neurotransmission dysfunction and SN56 cholinergic apoptotic cell death through AChE variants overexpression and NGF/TrkA/P75(NTR) signaling disruption. Food Chem. Toxicol. 2021, 157, 112614. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Moyano, P.; Sola, E.; García, J.M.; García, J.; Anadon, M.J.; Frejo, M.T.; Naval, M.V.; Fernadez, M.C.; Pino, J.D. Single and repeated bisphenol A treatment induces ROS, Abeta and hyperphosphorylated-tau accumulation, and insulin pathways disruption, through HDAC2 and PTP1B overexpression, leading to SN56 cholinergic apoptotic cell death. Food Chem. Toxicol. 2022, 170, 113500. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Jung, H.Y.; Kwon, H.J.; Kim, J.W.; Nam, S.M.; Chung, J.Y.; Choi, J.H.; Kim, D.W.; Yoon, Y.S.; Hwang, I.K. Effects of Dendropanax morbifera Léveille extract on hypothyroidism-induced oxidative stress in the rat hippocampus. Food Sci. Biotechnol. 2016, 25, 1761–1766. [Google Scholar] [CrossRef]
- Chakrabarti, S.K.; Ghosh, S.; Banerjee, S.; Mukherjee, S.; Chowdhury, S. Oxidative stress in hypothyroid patients and the role of antioxidant supplementation. Indian. J. Endocrinol. Metab. 2016, 20, 674–678. [Google Scholar] [CrossRef]
- Torres-Manzo, A.P.; Franco-Colín, M.; Blas-Valdivia, V.; Pineda-Reynoso, M.; Cano-Europa, E. Hypothyroidism causes endoplasmic reticulum stress in adult rat hippocampus: A mechanism associated with hippocampal damage. Oxid. Med. Cell. Longev. 2018, 20, 2089404. [Google Scholar] [CrossRef]
- Kasai, S.; Kokubu, D.; Mizukami, H.; Itoh, K. Mitochondrial Reactive Oxygen Species, Insulin Resistance, and Nrf2-Mediated Oxidative Stress Response-Toward an Actionable Strategy for Anti-Aging. Biomolecules 2023, 13, 1544. [Google Scholar] [CrossRef]
- Hurrle, S.; Hsu, W.H. The etiology of oxidative stress in insulin resistance. Biomed. J. 2017, 40, 257–262. [Google Scholar] [CrossRef]
- Rojo, A.I.; Sagarra, M.R.; Cuadrado, A. GSK-3beta down-regulates the transcription factor Nrf2 after oxidant damage: Relevance to exposure of neuronal cells to oxidative stress. J. Neurochem. 2008, 105, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, H.; Zheng, P.; Feng, R.; Wang, X.; Huang, F.; Ma, M.; Tian, Y.; Zhang, G. The alleviative effect of thyroid hormone on cold stress-induced apoptosis via HSP70 and mitochondrial apoptosis signal pathway in bovine Sertoli cells. Cryobiology 2022, 105, 63–70. [Google Scholar] [CrossRef]
- Akhigbe, R.; Ajayi, A.F.; Micheal, L.O.; Grace, A.G.; Omole, A.I.; Adelusi, T.I. Dysthyroidism induces hepatorenal injury by modulating HSP70/HSP90 and VEGF signaling in male Wistar rats. Niger. J. Physiol. Sci. 2021, 36, 33–41. [Google Scholar] [PubMed]
- Bozaykut, P.; Ozer, N.K.; Karademir, B. Nrf2 silencing to inhibit proteolytic defense induced by hyperthermia in HT22 cells. Redox Biol. 2016, 8, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Lavu, N.; Richardson, L.; Radnaa, E.; Kechichian, T.; Urrabaz-Garza, R.; Sheller-Miller, S.; Bonney, E.; Menon, R. Oxidative stress-induced downregulation of glycogen synthase kinase 3 beta in fetal membranes promotes cellular senescence. Biol. Reprod. 2019, 101, 1018–1030. [Google Scholar] [CrossRef]
- Berson, A.; Knobloch, M.; Hanan, M.; Diamant, S.; Sharoni, M.; Schuppli, D.; Geyer, B.C.; Ravid, R.; Mor, T.S.; Nitsch, R.M.; et al. Changes in readthrough acetylcholinesterase expression modulate amyloid-beta pathology. Brain 2008, 131, 109–119. [Google Scholar] [CrossRef]
- Bond, C.E.; Patel, P.; Crouch, L.; Tetlow, N.; Day, T.; Abu-Hayyeh, S.; Williamson, C.; Greenfield, S.A. Astroglia up-regulate transcription and secretion of ‘readthrough’ acetylcholinesterase following oxidative stress. Eur. J. Neurosci. 2006, 24, 381–386. [Google Scholar] [CrossRef]
- Abascal, M.L.; Sanjuan, J.; Moyano, P.; Sola, E.; Flores, A.; Garcia, J.M.; Garcia, J.; Frejo, M.T.; Del Pino, J. Insulin Signaling Disruption and INF-gamma Upregulation Induce Abeta(1-42) and Hyperphosphorylated-Tau Proteins Synthesis and Cell Death after Paraquat Treatment of Primary Hippocampal Cells signaling disruption and inf-gamma upregulation induce Abeta(1-42) and hyperphosphorylated-tau proteins synthesis and cell death after paraquat treatment of primary hippocampal cells. Chem. Res. Toxicol. 2022, 35, 2214–2218. [Google Scholar]
- Gorenberg, E.L.; Chandra, S.S. The role of co-chaperones in synaptic proteostasis and neurodegenerative disease. Front. Neurosci. 2017, 11, 248. [Google Scholar] [CrossRef]
- Bahn, G.; Park, J.S.; Yun, U.J.; Lee, Y.J.; Choi, Y.; Park, J.S.; Baek, S.H.; Choi, B.Y.; Cho, Y.S.; Kim, H.K.; et al. NRF2/ARE pathway negatively regulates BACE1 expression and ameliorates cognitive deficits in mouse Alzheimer’s models. Proc. Natl. Acad. Sci. USA 2019, 116, 12516–12523. [Google Scholar] [CrossRef]
- Nwadiugwu, M.; Onwuekwe, I.; Ezeanolue, E.; Deng, H. Beyond amyloid: A machine learning-driven approach reveals properties of potent GSK-3β inhibitors targeting neurofibrillary tangles. Int. J. Mol. Sci. 2024, 25, 2646. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Ohsako, S.; Kanai, Y.; Kurohmaru, M. Single administration of butylparaben induces spermatogenic cell apoptosis in prepubertal rats. Acta Histochem. 2014, 116, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Cantero, J.L.; Atienza, M.; Lage, C.; Zaborszky, L.; Vilaplana, E.; Lopez-Garcia, S.; Pozueta, A.; Rodriguez-Rodriguez, E.; Blesa, R.; Alcolea, D.; et al. Atrophy of basal forebrain initiates with tau pathology in individuals at risk for Alzheimer’s disease. Cereb. Cortex 2020, 30, 2083–2098. [Google Scholar] [CrossRef] [PubMed]
- Kwakowsky, A.; Potapov, K.; Kim, S.; Peppercorn, K.; Tate, W.P.; Ábrahám, I.M. Treatment of beta amyloid 1-42 (Aβ(1-42))-induced basal forebrain cholinergic damage by a non-classical estrogen signaling activator in vivo. Sci. Rep. 2016, 6, 21101. [Google Scholar] [CrossRef] [PubMed]
- Latina, V.; Caioli, S.; Zona, C.; Ciotti, M.T.; Borreca, A.; Calissano, P.; Amadoro, G. NGF-Dependent Changes in Ubiquitin Homeostasis Trigger Early Cholinergic Degeneration in Cellular and Animal AD-Model. Front. Cell. Neurosci. 2018, 12, 487. [Google Scholar]
- Willis, C.L.; Ray, D.E.; Marshall, H.; Elliot, G.; Evans, J.G.; Kind, C.N. Basal forebrain cholinergic lesions reduce heat shock protein 72 response but not pathology induced by the NMDA antagonist MK-801 in the rat cingulate cortex. Neurosci. Lett. 2006, 407, 112–117. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Li, W.; Zhang, H. Activation of Wnt/β-catenin signalling via GSK3 inhibitors direct differentiation of human adipose stem cells into functional hepatocytes. Sci. Rep. 2017, 7, 40716. [Google Scholar] [CrossRef]
- Moyano, P.; Flores, A.; Fernández, M.C.; García, J.; Sanjuan, J.; Plaza, J.C.; Del Pino, J. Increased levels of phosphorylated-P38α induce WNT/β-Catenin and NGF/P75NTR/TrkA pathways disruption and SN56 cell death following single and repeated chlorpyrifos treatment. Foods 2024, 13, 2427. [Google Scholar] [CrossRef]
- Shekari, A.; Fahnestock, M. Retrograde axonal transport of BDNF and proNGF diminishes with age in basal forebrain cholinergic neurons. Neurobiol. Aging. 2019, 84, 131–140. [Google Scholar]



| Abbreviation | Gene | Forward (F) and Reverse (R) Primers |
|---|---|---|
| AChE-S | Acetylcholinesterase | F-ctgaacctgaagcccttagag R-ccgcctcgtccagagtat |
| AChE-R | Acetylcholinesterase | F-gagcagggaatgcacaag R-ggggaggtaaagaagagag |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyano, P.; Flores, A.; Sanjuan, J.; Plaza, J.C.; Guerra-Menéndez, L.; Abascal, L.; Mateo, O.; del Pino, J. Cholinergic Transmission Dysregulation and Neurodegeneration Induced by Thyroid Signaling Disruption Following Butylparaben Single and Repeated Treatment. Biology 2025, 14, 1380. https://doi.org/10.3390/biology14101380
Moyano P, Flores A, Sanjuan J, Plaza JC, Guerra-Menéndez L, Abascal L, Mateo O, del Pino J. Cholinergic Transmission Dysregulation and Neurodegeneration Induced by Thyroid Signaling Disruption Following Butylparaben Single and Repeated Treatment. Biology. 2025; 14(10):1380. https://doi.org/10.3390/biology14101380
Chicago/Turabian StyleMoyano, Paula, Andrea Flores, Javier Sanjuan, Jose Carlos Plaza, Lucía Guerra-Menéndez, Luisa Abascal, Olga Mateo, and Javier del Pino. 2025. "Cholinergic Transmission Dysregulation and Neurodegeneration Induced by Thyroid Signaling Disruption Following Butylparaben Single and Repeated Treatment" Biology 14, no. 10: 1380. https://doi.org/10.3390/biology14101380
APA StyleMoyano, P., Flores, A., Sanjuan, J., Plaza, J. C., Guerra-Menéndez, L., Abascal, L., Mateo, O., & del Pino, J. (2025). Cholinergic Transmission Dysregulation and Neurodegeneration Induced by Thyroid Signaling Disruption Following Butylparaben Single and Repeated Treatment. Biology, 14(10), 1380. https://doi.org/10.3390/biology14101380






