Homogeneous Selection and Dispersal Limitation Drive Phyllosphere Fungal Community Assembly in Constructed Wetland Ecosystems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. High-Throughput Sequencing and Bioinformatics Analyses
2.3. Physicochemical Properties Analyses
2.4. Statistical Analyses
3. Results
3.1. Alpha Diversity Index of Phyllosphere Fungi
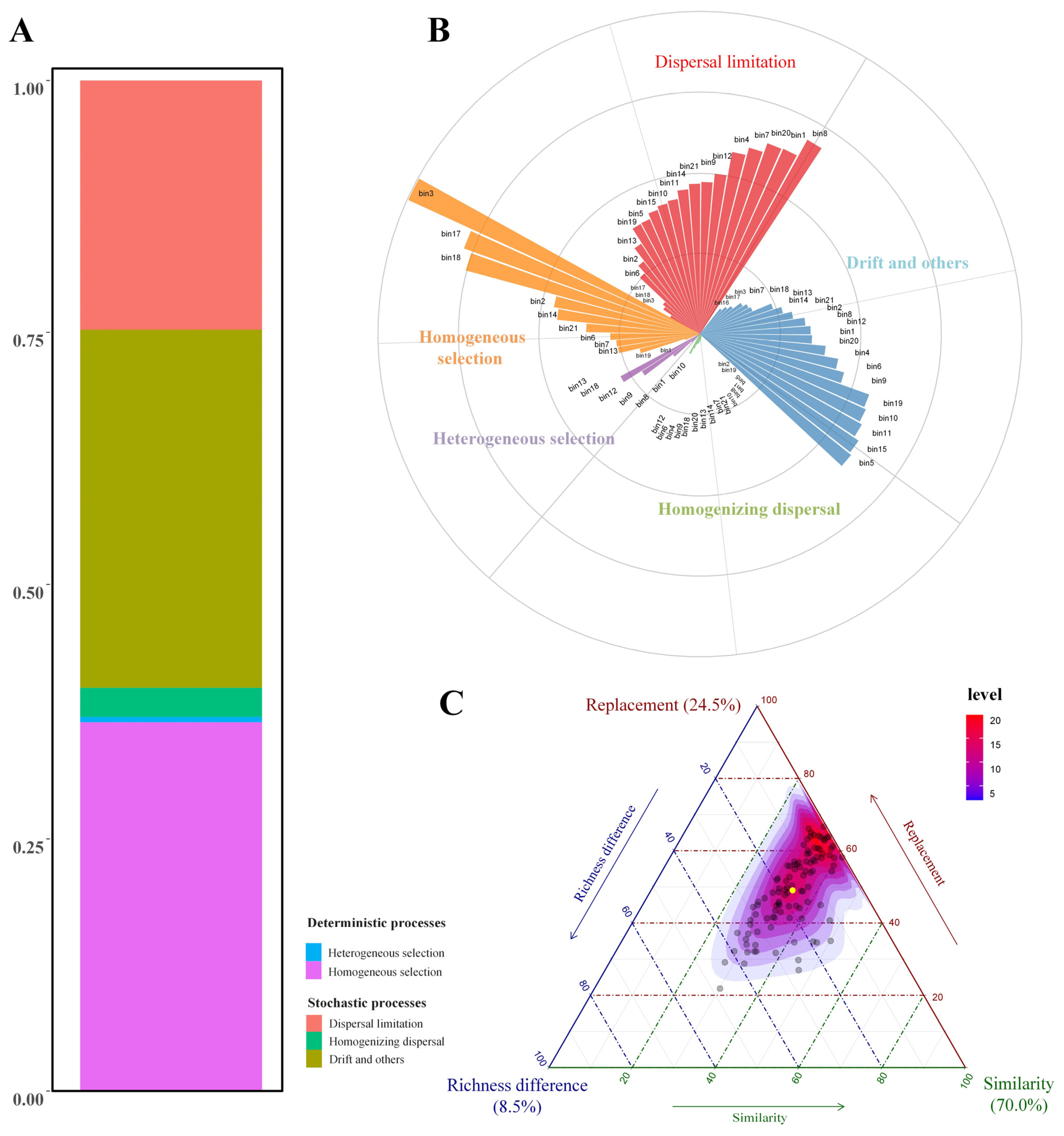
3.2. Fungal Community Assembly Processes
3.3. Phyllosphere Fungi Distribution Patterns
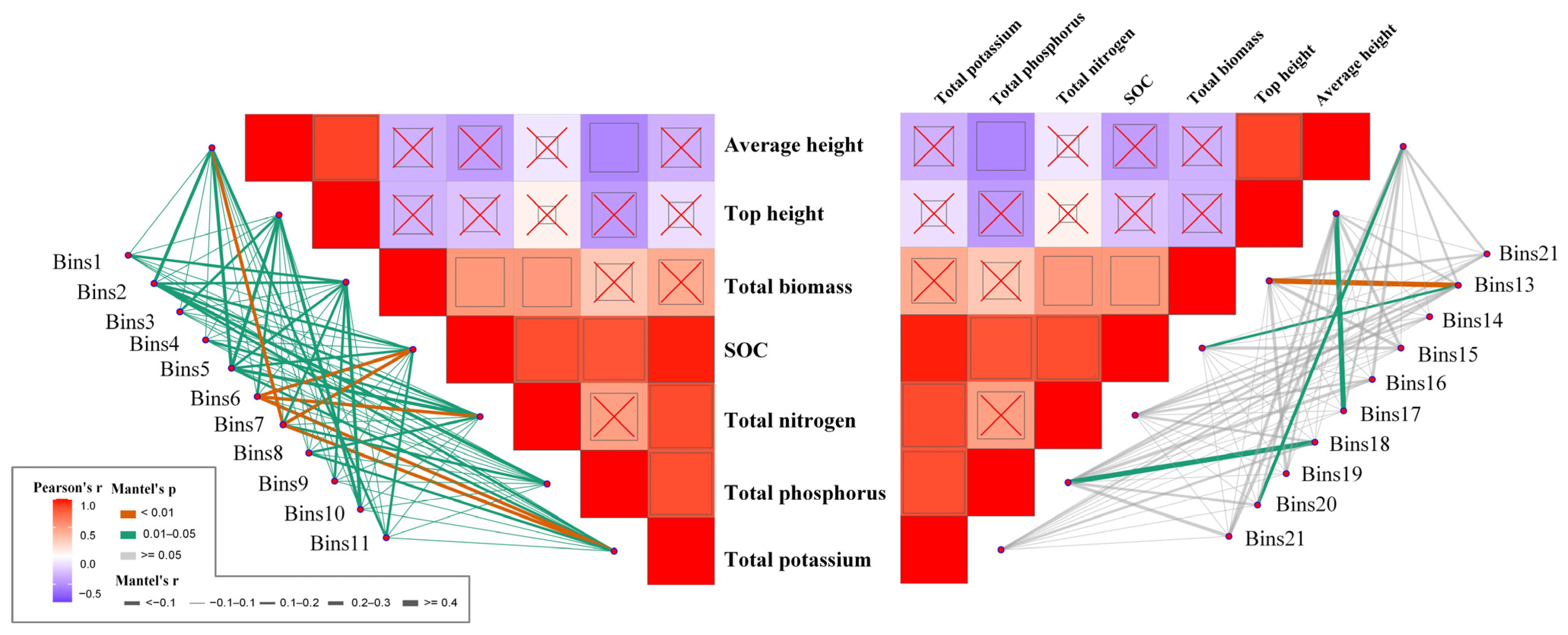
3.4. Network Structure and Topological Roles of Phyllosphere Fungal Communities
4. Discussion
4.1. Plant Type Drives Leaf Surface Fungal Community Diversity and Differentiation
4.2. Multiple Driving Mechanisms of Leaf Surface Fungal Community Assembly
4.3. Ecological Implications of Network Structure and Topological Roles in Phyllosphere Fungal Communities
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Messer, T.L.; Burchell, M.R.; Birgand, F.; Broome, S.W.; Chescheir, G. Nitrate removal potential of restored wetlands loaded with agricultural drainage water: A mesocosm scale experimental approach. Ecol. Eng. 2017, 106, 541–554. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Burchell, M.R.; Krauss, K.W.; Broome, S.W.; Birgand, F. Carbon storage potential in a recently created brackish marsh in eastern North Carolina, USA. Ecol. Eng. 2019, 127, 579. [Google Scholar] [CrossRef]
- He, J.Z.; Zhang, L.M. Advances in ammonia-oxidizing microorganisms and global nitrogen cycle. Acta Ecol. Sin. 2009, 29, 407–413. [Google Scholar]
- Sica, Y.V.; Quintana, R.D.; Radeloff, V.C.; Gavier-Pizarro, G.I. Wetland loss due to land use change in the Lower Paraná River Delta, Argentina. Sci. Total Environ. 2016, 568, 967–978. [Google Scholar]
- Junk, W.J.; An, S.; Finlayson, C.M.; Gopal, B.; Kvt, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current state of knowledge regarding the world’s wetlands and their future under global climate change: A synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar]
- Zhang, T.; Xu, D.; He, F.; Zhang, Y.; Wu, Z. Application of constructed wetland for water pollution control in China during 1990–2010. Ecol. Eng. 2012, 47, 189–197. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, W.; Wang, W.; Yin, W.; Zha, J. A review on China’s constructed wetlands in recent three decades: Application and practice. J. Environ. Sci. 2020, 104, 53–68. [Google Scholar]
- Wang, Q.; Wang, Z.; Wu, L.; Yang, D.; Ding, C.; Wang, M.; Wang, R.; Fu, X.; Wang, Z. Conversion of wetlands to farmland and forests reduces soil microbial functional diversity and carbon use intensity. Appl. Ecol. Environ. Res. 2022, 20, 4553–4564. [Google Scholar] [CrossRef]
- Moreno-Mateos, D.; Power, M.E.; Comín, F.A.; Yockteng, R. Structural and functional loss in restored wetland ecosystems. PLoS Biol. 2012, 10, e1001247. [Google Scholar]
- Zedler, J.B.; Kercher, S. Wetland resources: Status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 2005, 30, 39–74. [Google Scholar] [CrossRef]
- Shi, Q.; Kaur, P.; Gan, J. Harnessing the potential of phytoremediation for mitigating the risk of emerging contaminants. Curr. Opin. Environ. Sci. Health 2023, 32, 100448. [Google Scholar] [CrossRef]
- Fu, J.; Zhao, Y.; Yang, Y.; Yao, Q.; Ji, B.; Chen, S.; Dai, Y.; Tao, R.; Zhang, X. A glance of configuration-operational strategies and intensification of constructed wetland towards land-effective occupation. J. Water Process Eng. 2023, 56, 104473. [Google Scholar] [CrossRef]
- Rani, A.; Chauhan, M.; Sharma, P.K.; Kumari, M.; Mitra, D.; Joshi, S. Microbiological dimensions and functions in constructed wetlands: A review. Microelectron. J. 2024, 7, 100311. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; He, Z.; Wilson, M.J.; Campbell, C.D. Microbial biomass and community structure in a sequence of soils with increasing fertility and changing land use. Microb. Ecol. 2000, 40, 223–237. [Google Scholar] [CrossRef]
- Bardgett, R.D.; van der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Requena, N.; Perez-Solis, E.; Azcón-Aguilar, C.; Jeffries, P.; Barea, J.M. Management of indigenous plant-microbe symbioses aids restoration of desertified ecosystems. Appl. Environ. Microbiol. 2001, 67, 495–498. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Müller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The plant microbiota: Systems-level insights and perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef]
- Schloter, M.; Dilly, O.; Munch, J.C. Indicators for evaluating soil quality. Agric. Ecosyst. Environ. 2003, 98, 255–262. [Google Scholar] [CrossRef]
- Rodriguez, P.A.; Rothballer, M.; Chowdhury, S.P.; Nussbaumer, T.; Falter-Braun, P. Systems biology of plant-microbiome interactions. Mol. Plant 2019, 12, 804–821. [Google Scholar] [CrossRef]
- Adamo, I.; Piuela, Y.; Bonet, J.A.; Castao, C.; Alday, J.G. Sampling forest soils to describe fungal diversity and composition. Which is the optimal sampling size in Mediterranean pure and mixed pine oak forests? Fungal Biol. 2021, 125, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Lu, X.; Zhao, M.; Ding, X.; Lv, H.; Wang, L.; Wu, L. The impact of external plant carbon sources on nitrogen removal and microbial community structure in vertical flow constructed wetlands. Front. Environ. Sci. 2023, 11, 1233422. [Google Scholar] [CrossRef]
- Li, C.; Zhang, D.; Xu, G.; Yan, R.; Huang, Y.; Feng, L.; Yi, J.; Xue, Y.; Liu, H. Effects of alpine grassland degradation on soil microbial communities in Qilian Mountains of China. J. Soil. Sci. Plant Nutr. 2022, 23, 912–923. [Google Scholar] [CrossRef]
- Hartman, W.H.; Richardson, C.J.; Vilgalys, R.; Bruland, G.L. Environmental and anthropogenic controls over bacterial communities in wetland soils. Proc. Natl. Acad. Sci. USA 2008, 105, 17842–17847. [Google Scholar] [CrossRef]
- Peñuelas, J.; Terradas, J. The foliar microbiome. Trends Plant Sci. 2014, 19, 278–280. [Google Scholar] [CrossRef]
- Agler, M.T.; Ruhe, J.; Kroll, S.; Morhenn, C.; Kim, S.T.; Weigel, D.; Kemen, E.M. Microbial hub taxa link host and abiotic factors to plant microbiome variation. PLoS Biol. 2016, 14, e1002352. [Google Scholar] [CrossRef]
- Sapkota, R.; Knorr, K.; Jørgensen, L.N.; O’Hanlon, K.A.; Nicolaisen, M. Host genotype is an important determinant of the cereal phyllosphere mycobiome. New Phytol. 2015, 207, 1134–1144. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, Y.; Lin, X.; Zhang, H.; Zeng, J.; Hou, J.; Yang, Y.; Yao, T.; Knight, R.; Chu, H. Geographic distance and pH drive bacterial distribution in alkaline lake sediments across Tibetan Plateau. Environ. Microbiol. 2012, 14, 2457–2466. [Google Scholar] [CrossRef]
- Ansola, G.; Arroyo, P.; Sáenz de Miera, L.E. Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Sci. Total Environ. 2014, 473, 63–71. [Google Scholar] [CrossRef]
- Zhou, J.; Ning, D. Stochastic community assembly: Does it matter in microbial ecology? Microbiol. Mol. Biol. Rev. 2017, 81, e00002-17. [Google Scholar] [CrossRef]
- Dini-Andreote, F.; Stegen, J.C.; van Elsas, J.D.; Salles, J.F. Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proc. Natl. Acad. Sci. USA 2015, 112, 1326–1332. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis; Part 2; Soil Science Society of America: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Determination of total nitrogen in plant material. Agron. J. 1973, 65, 109–112. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Knudsen, D.; Peterson, G.A.; Pratt, P.F. Lithium, sodium, and potassium. In Methods of Soil Analysis; Part 2; Soil Science Society of America: Madison, WI, USA, 1982; pp. 225–246. [Google Scholar]
- Walkley, A.J.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil. Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Legendre, P. Interpreting the replacement and richness difference components of beta diversity. Glob. Ecol. Biogeogr. 2014, 23, 1324–1334. [Google Scholar] [CrossRef]
- Steven, W.K. Picante: Integrating Phylogenies and Ecology. 2020. Available online: https://CRAN.R-project.org/package=picante (accessed on 10 June 2020).
- Oksanen, J.F.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Wagner, H. Package ‘vegan’. Community Ecol. Package 2019, 2, 1–295. [Google Scholar]
- Paradis, E.; Schliep, K. ape 5.0: An environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35, 526–528. [Google Scholar] [CrossRef]
- Hamilton, N.E.; Ferry, M. ggtern: Ternary Diagrams Using ggplot2. J. Stat. Softw. 2018, 87, 1–17. [Google Scholar] [CrossRef]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Guimerà, R.; Nunes Amaral, L.A. Functional cartography of complex metabolic networks. Nature 2005, 433, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Antonov, M.; Csárdi, G.; Horvát, S.; Müller, K.; Nepusz, T.; Noom, D.; Salmon, M.; Traag, V.; Welles, B.F.; Zanini, F. igraph enables fast and robust network analysis across programming languages. arXiv 2023, arXiv:2311.10260. [Google Scholar] [CrossRef]
- Guo, C.; Yang, A.; Zhang, W. Host Identity Determines the Bacterial and Fungal Community and Network Structures in the Phyllosphere of Plant Species in a Temperate Steppe. Phytobiomes J. 2024, 8, 143–154. [Google Scholar] [CrossRef]
- Yurkov, A.M. Yeasts of the soil—Obscure but precious. Yeast 2018, 35, 369–378. [Google Scholar]
- Lindow, S.E.; Brandl, M.T. Microbiology of the phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef]
- Bell, T. Experimental tests of the bacterial distance-decay relationship. ISME J. 2010, 4, 1357–1365. [Google Scholar] [CrossRef]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef]
- Morris, C.E.; Sands, D.C.; Vanneste, J.L.; Montarry, J.; Oakley, B.; Guilbaud, C. Inferring the evolutionary history of the plant pathogen Pseudomonas syringae from its biogeography in headwaters of rivers in North America, Europe, and New Zealand. mBio 2010, 1, e00107-10-10. [Google Scholar] [CrossRef]
- Hoffmann, A.; Posirca, A.R.; Lewin, S.; Verch, G.; Büttner, C.; Müller, M.E.H. Environmental filtering drives fungal phyllosphere community in regional agricultural landscapes. Plants 2023, 12, 507. [Google Scholar] [CrossRef]
- Chen, D.; Yang, J.; Wang, S.; Lan, S.; Wang, Y.; Liu, Z.J.; Qian, X. Comparative analysis of community composition and network structure between phyllosphere endophytic and epiphytic fungal communities of Mussaenda pubescens. Microbiol. Spectr. 2025, 13, e0101924. [Google Scholar] [CrossRef]
- Newman, M.E.J. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef]
- Olesen, J.M.; Bascompte, J.; Dupont, Y.L.; Jordano, P. The modularity of pollination networks. Proc. Natl. Acad. Sci. USA 2007, 104, 19891–19896. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Röttjers, L.; Faust, K. From hairballs to hypotheses—Biological insights from microbial networks. FEMS Microbiol. Rev. 2018, 42, 761–780. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.W.G.; Weingarten, E.A.; Jackson, C.R. The role of the phyllosphere microbiome in plant health and function. Annu. Plant Rev. 2018, 1, 1–24. [Google Scholar]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular ecological network analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Jousset, A.; Bienhold, C.; Chatzinotas, A.; Gallien, L.; Gobet, A.; Kurm, V.; Küsel, K.; Rillig, M.C.; Rivett, D.W.; Salles, J.F.; et al. Where less may be more: How the rare biosphere pulls ecosystems strings. ISME J. 2017, 11, 853–862. [Google Scholar] [CrossRef]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Tu, Q.; Zhi, X. Functional molecular ecological networks. mBio 2010, 1, e00169-10. [Google Scholar] [CrossRef]
- Shade, A.; Peter, H.; Allison, S.D.; Baho, D.L.; Berga, M.; Bürgmann, H.; Huber, D.H.; Langenheder, S.; Lennon, J.T.; Martiny, J.B.H. Fundamentals of microbial community resistance and resilience. Front. Microbiol. 2012, 3, 417. [Google Scholar] [CrossRef]
- Fukami, T. Historical contingency in community assembly: Integrating niches, species pools, and priority effects. Annu. Rev. Ecol. Evol. Syst. 2015, 46, 1–23. [Google Scholar] [CrossRef]
- Allison, S.D.; Martiny, J.B.H. Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. 2008, 105, 11512–11519. [Google Scholar] [CrossRef] [PubMed]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; van der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 4841. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- Feng, K.; Zhang, Z.; Cai, W.; Liu, W.; Xu, M.; Yin, H.; Wang, A.; He, Z.; Deng, Y. Biodiversity and species competition regulate the resilience of microbial biofilm community. Mol. Ecol. 2017, 26, 6170–6182. [Google Scholar] [CrossRef]
- Vymazal, J.; Kröpfelová, L. Multistage hybrid constructed wetland for enhanced removal of nitrogen. Ecol. Eng. 2015, 84, 202–208. [Google Scholar] [CrossRef]
- Zhang, D.Q.; Jinadasa, K.B.; Gersberg, R.M.; Liu, Y.; Ng, W.J.; Tan, S.K. Application of constructed wetlands for wastewater treatment in developing countries—A review of recent developments (2000–2013). J. Environ. Manag. 2014, 141, 116–131. [Google Scholar] [CrossRef]
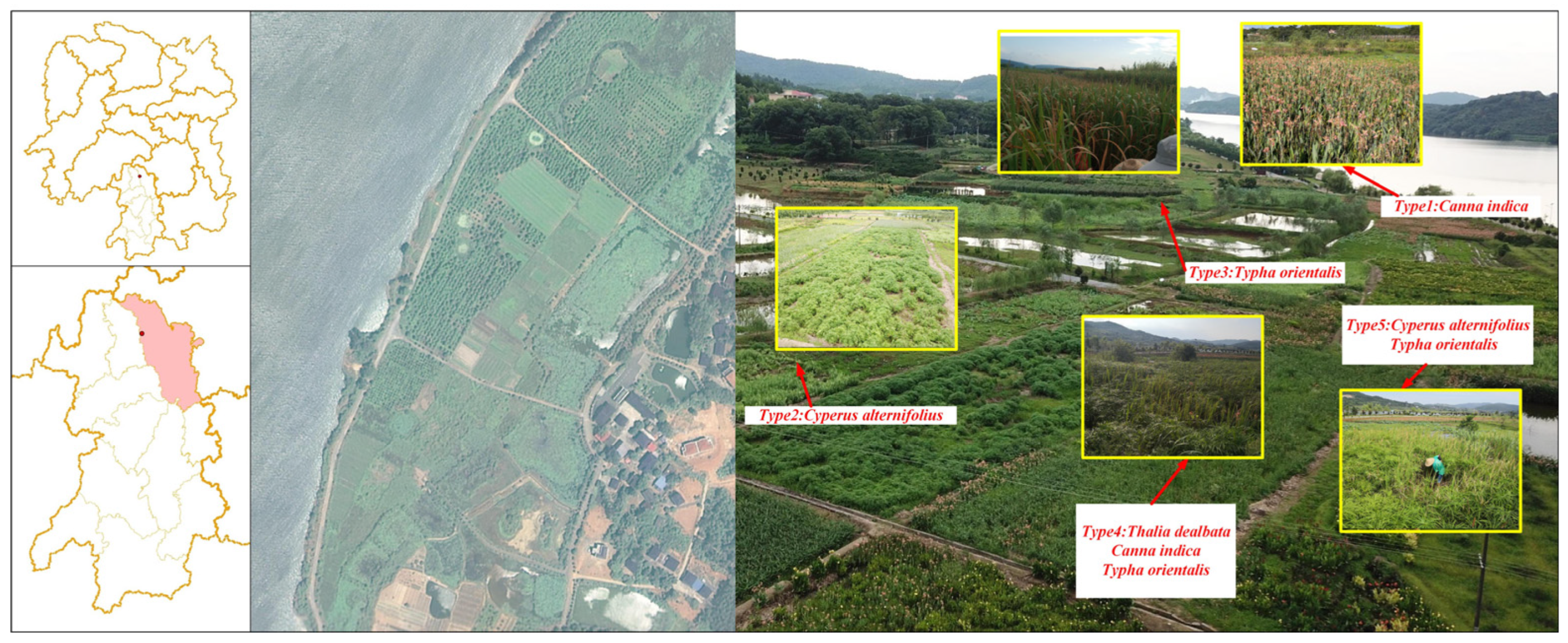


| Type ID | Plant Composition | Cover Contribution (%) |
|---|---|---|
| 1 | Canna indica L. | 90 |
| 2 | Cyperus alternifolius L. | 95 |
| 3 | Typha orientalis Presl | 95 |
| 4 | Thalia dealbata Fraser | 30 |
| Canna indica | 30 | |
| Typha orientalis | 30 | |
| 5 | Cyperus alternifolius | 50 |
| Typha orientalis | 40 |
| Type ID | Chao1 | Good’s Coverage | Observed Species | PD Whole Tree | Shannon | Simpson | Relative Abundance |
|---|---|---|---|---|---|---|---|
| 1 | 802.03 ± 140.01 | 0.99 ± 0.00 | 518.97 ± 107.98 | 119.06 ± 18.42 | 3.96 ± 0.58 | 0.80 ± 0.14 | 0.06 ± 0.09 |
| 2 | 919.40 ± 23.33 | 0.99 ± 0.00 | 635.67 ± 29.11 | 155.03 ± 22.29 | 5.59 ± 0.38 | 0.94 ± 0.02 | 0.09 ± 0.13 |
| 3 | 975.15 ± 128.98 | 0.99 ± 0.00 | 632.90 ± 94.07 | 139.18 ± 13.48 | 4.17 ± 0.39 | 0.83 ± 0.04 | 0.07 ± 0.11 |
| 4 | 712.06 ± 35.96 | 0.99 ± 0.00 | 439.33 ± 10.44 | 103.75 ± 7.56 | 4.79 ± 0.08 | 0.89 ± 0.01 | 0.08 ± 0.11 |
| 5 | 832.98 ± 154.24 | 0.99 ± 0.00 | 573.00 ± 99.33 | 150.11 ± 10.12 | 5.50 ± 0.08 | 0.94 ± 0.00 | 0.04 ± 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, N.; Tian, Y.; Song, Q.; Niu, Y.; Ma, F. Homogeneous Selection and Dispersal Limitation Drive Phyllosphere Fungal Community Assembly in Constructed Wetland Ecosystems. Biology 2025, 14, 1378. https://doi.org/10.3390/biology14101378
Deng N, Tian Y, Song Q, Niu Y, Ma F. Homogeneous Selection and Dispersal Limitation Drive Phyllosphere Fungal Community Assembly in Constructed Wetland Ecosystems. Biology. 2025; 14(10):1378. https://doi.org/10.3390/biology14101378
Chicago/Turabian StyleDeng, Nan, Yuxin Tian, Qingan Song, Yandong Niu, and Fengfeng Ma. 2025. "Homogeneous Selection and Dispersal Limitation Drive Phyllosphere Fungal Community Assembly in Constructed Wetland Ecosystems" Biology 14, no. 10: 1378. https://doi.org/10.3390/biology14101378
APA StyleDeng, N., Tian, Y., Song, Q., Niu, Y., & Ma, F. (2025). Homogeneous Selection and Dispersal Limitation Drive Phyllosphere Fungal Community Assembly in Constructed Wetland Ecosystems. Biology, 14(10), 1378. https://doi.org/10.3390/biology14101378






