eDNA- and eRNA-Based Detection of 2-Methylisoborneol-Producing Cyanobacteria and Intracellular Synthesis Dynamics in Freshwater Ecosystem
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
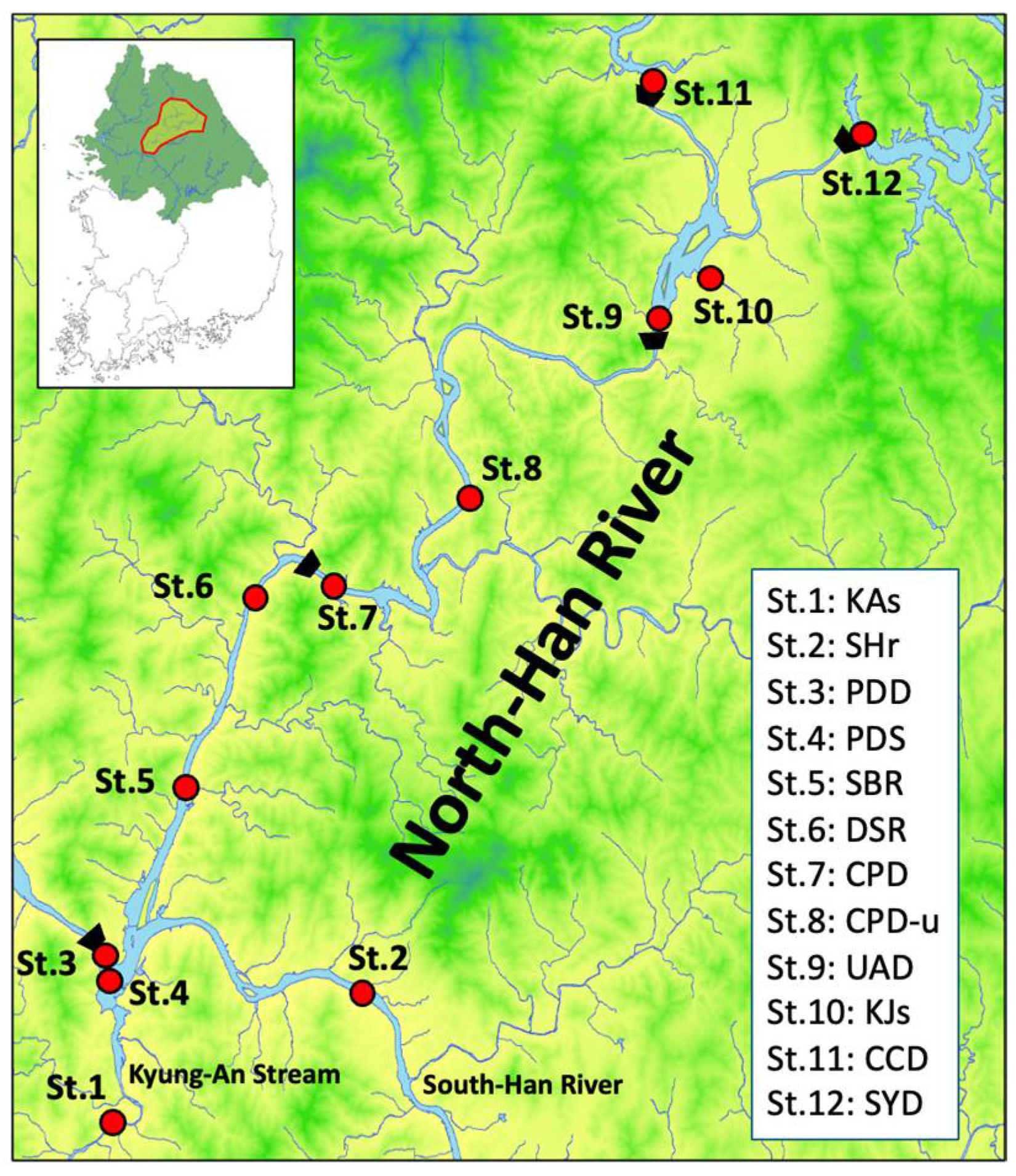
2.1. Study Sites and Sample Collection
2.2. eDNA and eRNA Extraction
2.3. Quantitative Analysis of eDNA and eRNA
2.4. Cyanobacterial Cell Density and 2-MIB Analysis
2.5. Statistical Analysis
3. Results
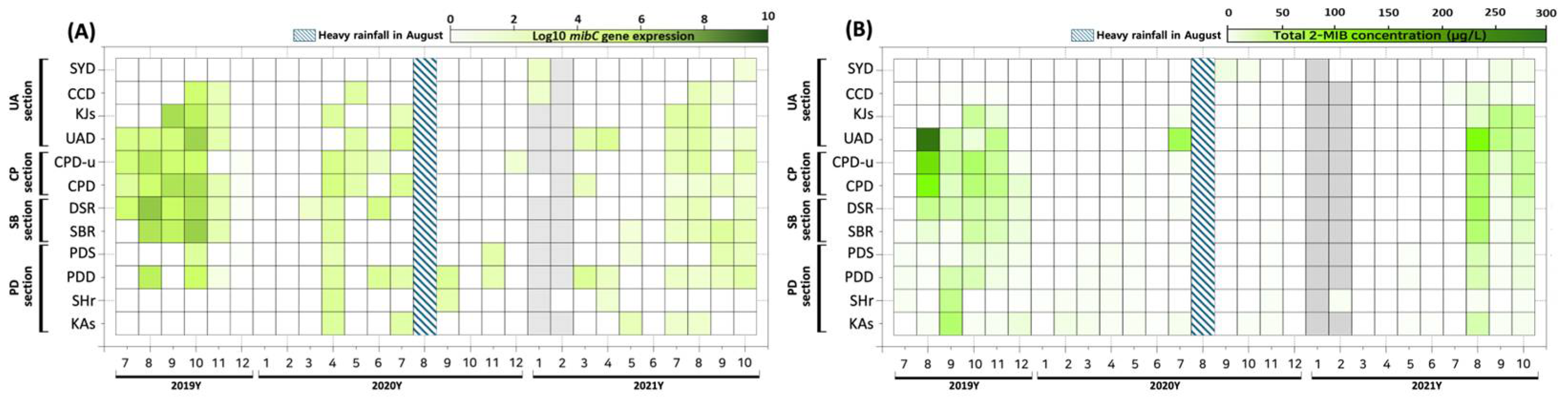
3.1. Spatiotemporal Variation in mibC Gene DNA Copy Numbers
3.2. Spatiotemporal Variation in mibC Gene Expression (RNA)
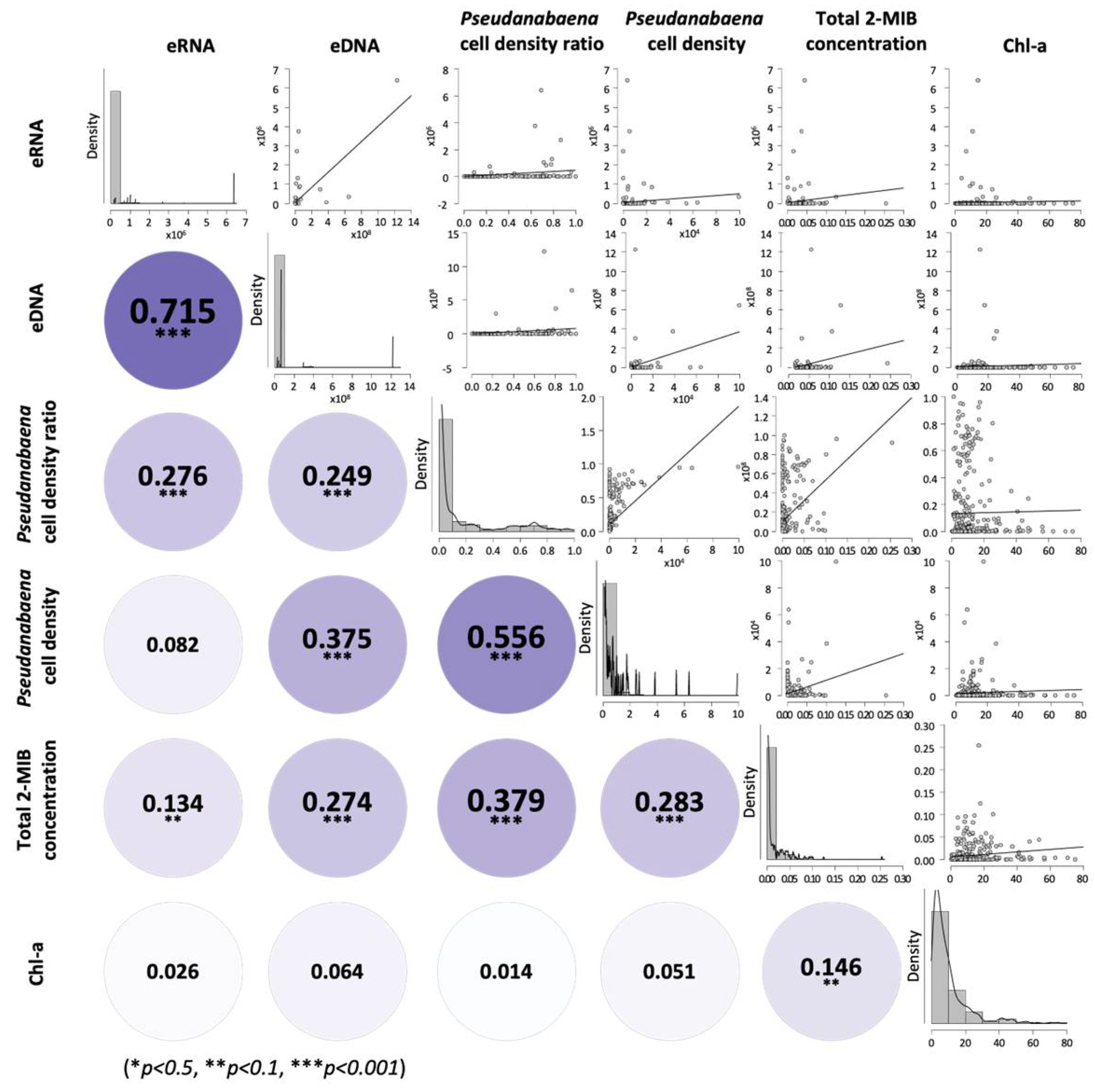
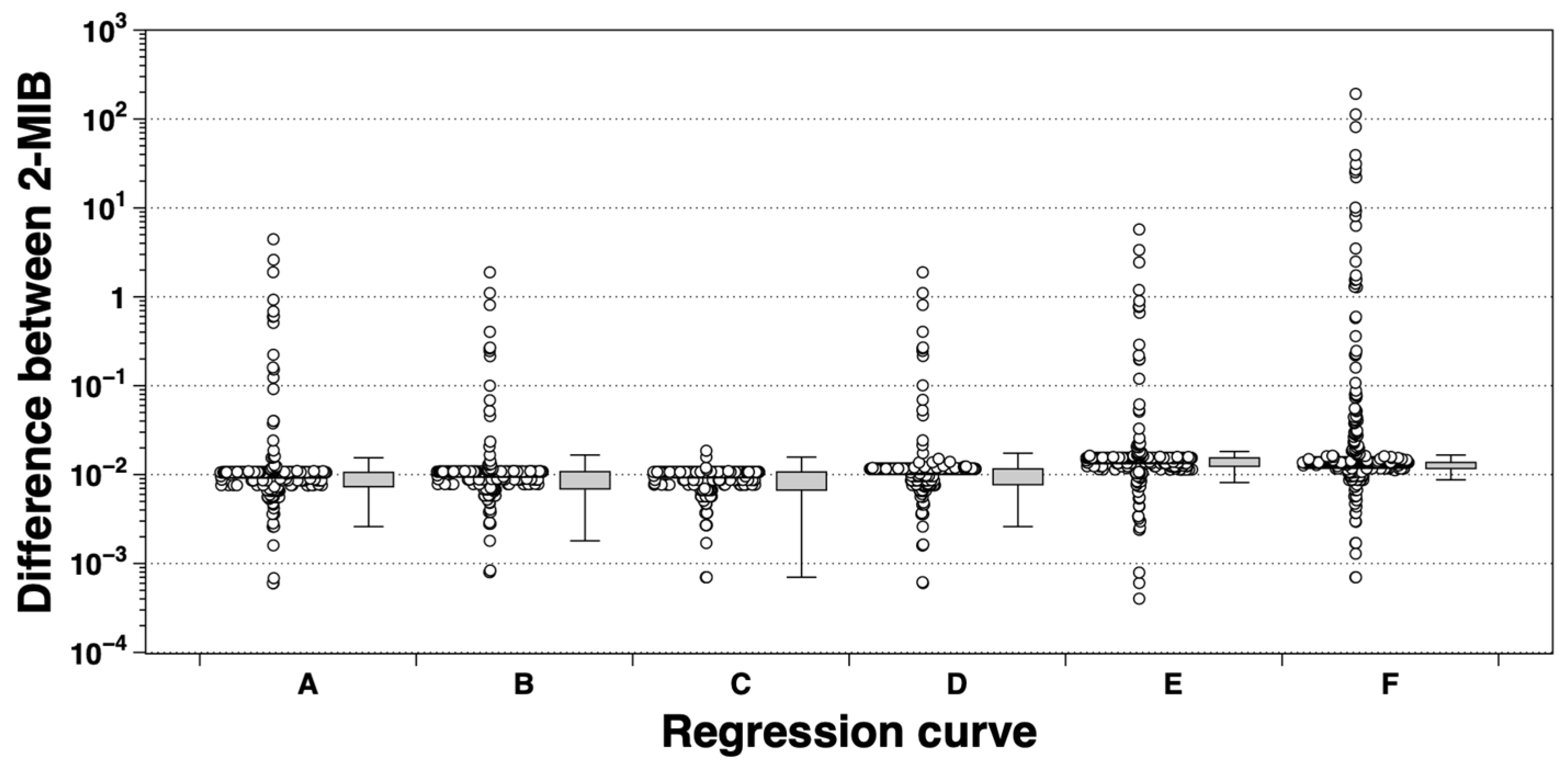
3.3. Relationship Between eRNA Expression and 2-MIB Concentration
4. Discussion
4.1. Cyanobacterial Abundance and 2-MIB Occurrence
4.2. Predictive Value of mibC Expression
4.3. Environmental and Methodological Influences
4.4. Limitations and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Grimm, C.C.; Zimba, P.V. Applications of an Instrumental Method for the Analysis of Off-Flavors in Fresh Water Aquaculture; ACS Publications: Washington, DC, USA, 2003; Volume 848, pp. 209–222. [Google Scholar] [CrossRef]
- Watson, S.B.; Brownlee, B.; Satchwill, T.; Hargesheimer, E.E. Quantitative analysis of trace levels of geosmin and MIB in source and drinking water using headspace SPME. Water Res. 2000, 34, 2818–2828. [Google Scholar] [CrossRef]
- Watson, S.B.; Brownlee, B.; Satchwill, T.; McCauley, E. The use of solid phase microextraction (SPME) to monitor for major organoleptic compounds produced by chrysophytes in surface waters. Water Sci. Technol. 1999, 40, 251–256. [Google Scholar] [CrossRef]
- Watson, S.B.; Ridal, J.; Boyer, G.L. Taste and odour and cyanobacterial toxins: Impairment, prediction, and management in the Great Lakes. Can. J. Fish. Aquat. Sci. 2008, 65, 1779–1796. [Google Scholar] [CrossRef]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.-H.; Kwon, E.E. The role of algae and cyanobacteria in the production and release of odorants in water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Xu, X.; Shi, C.; Liu, X.; Wang, G. Response of taste and odor compounds to elevated cyanobacteria biomass and temperature. Bull. Environ. Contam. Toxicol. 2018, 101, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, C.; Yoon, Y.; Hwang, S.-J. Harmful cyanobacterial material production in the North-Han River (South Korea): Genetic potential and temperature-dependent properties. Int. J. Environ. Res. Public Health 2018, 15, 444. [Google Scholar] [CrossRef]
- Zhang, R.; Qi, F.; Liu, C.; Zhang, Y.; Wang, Y.; Song, Z.; Kumirska, J.; Sun, D. Cyanobacteria-derived taste and odor characteristics in various lakes in China: Songhua Lake, Chaohu Lake and Taihu Lake. Ecotoxicol. Environ. Saf. 2019, 181, 499–507. [Google Scholar] [CrossRef]
- Vezie, C.; Brient, L.; Sivonen, K.; Bertru, G.; Lefeuvre, J.-C.; Salkinoja-Salonen, M. Variation of microcystin content of cyanobacterial blooms and isolated strains in Lake Grand-Lieu (France). Microb. Ecol. 1998, 35, 126–135. [Google Scholar] [CrossRef]
- Vaitomaa, J.; Rantala, A.; Halinen, K.; Rouhiainen, L.; Tallberg, P.; Mokelke, L.; Sivonen, K. Quantitative real-time PCR for determination of microcystin synthetase E copy numbers for Microcystis and Anabaena in lakes. Appl. Environ. Microbiol. 2003, 69, 7289–7297. [Google Scholar] [CrossRef]
- Moustafa, A.; Loram, J.E.; Hackett, J.D.; Anderson, D.M.; Plumley, F.G.; Bhattacharya, D. Origin of saxitoxin biosynthetic genes in cyanobacteria. PLoS ONE 2009, 4, e5758. [Google Scholar] [CrossRef]
- Boopathi, T.; Wang, H.; Lee, M.-D.; Ki, J.-S. Seasonal changes in cyanobacterial diversity of a temperate freshwater Paldang Reservoir (Korea) explored by using pyrosequencing. Korean J. Environ. Biol. 2018, 36, 424–437. [Google Scholar] [CrossRef]
- Jüttner, F.; Watson, S.B. Biochemical and ecological control of geosmin and 2-methylisoborneol in source waters. Appl. Environ. Microbiol. 2007, 73, 4395–4406. [Google Scholar] [CrossRef]
- Boopathi, T.; Ki, J.-S. Impact of environmental factors on the regulation of cyanotoxin production. Toxins 2014, 6, 1951–1978. [Google Scholar] [CrossRef]
- Saker, M.L.; Vale, M.; Kramer, D.; Vasconcelos, V.M. Molecular techniques for the early warning of toxic cyanobacteria blooms in freshwater lakes and rivers. Appl. Microbiol. Biotech. 2007, 75, 441–449. [Google Scholar] [CrossRef]
- Al-Tebrineh, J.; Mihali, T.K.; Pomati, F.; Neilan, B.A. Detection of saxitoxin-producing cyanobacteria and Anabaena circinalis in environmental water blooms by quantitative PCR. Appl. Environ. Microbiol. 2010, 76, 7836–7842. [Google Scholar] [CrossRef]
- Baker, L.; Sendall, B.C.; Gasser, R.B.; Menjivar, T.; Neilan, B.A.; Jex, A.R. Rapid, multiplex-tandem PCR assay for automated detection and differentiation of toxigenic cyanobacterial blooms. Mol. Cell. Probes 2013, 27, 208–214. [Google Scholar] [CrossRef]
- Oikawa, T.; Tsunoda, T.; Nakahigashi, H.; Shimoriku, M.; Kanami, T.; Kimura, S. Musty odor producing benthic cyanobacteria in the Tama River (Japan) and identification of species by genetic analysis. J. Water Supply Res. Technol.—Aqua. 2015, 64, 839–846. [Google Scholar] [CrossRef]
- Shizuka, K.; Ikenaga, M.; Murase, J.; Nakayama, N.; Matsuya, N.; Kakino, W.; Taruya, H.; Maie, N. Diversity of 2-MIB-producing cyanobacteria in Lake Ogawara: Microscopic and molecular ecological approaches. Aquacult. Sci. 2020, 68, 9–23. [Google Scholar]
- Wu, D.; Chen, M.; Shen, A.; Shi, Y. Spatiotemporal dynamics of 2-methylisoborneol produced by filamentous cyanobacteria and associated driving factors in Lake Taihu, China. Harmful Algae 2024, 138, 102703. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Park, R.; Yu, M.; Byeon, M.; Kang, T. qPCR-based monitoring of 2-methylisoborneol/geosmin-producing cyanobacteria in drinking water reservoirs in South Korea. Microorganisms 2023, 11, 2332. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Song, G.; Li, Y.; Yu, G.; Hou, X.; Gan, Z.; Li, R. The diversity, origin, and evolutionary analysis of geosmin synthase gene in cyanobacteria. Sci. Total Environ. 2019, 689, 789–796. [Google Scholar] [CrossRef]
- Ludwig, F.; Medger, A.; Börnick, H.; Opitz, M.; Lang, K.; Göttfert, M.; Röske, I. Identification and expression analyses of putative sesquiterpene synthase genes in Phormidium sp. and prevalence of geoA-like genes in a drinking water reservoir. Appl. Environ. Microbiol. 2007, 73, 6988–6993. [Google Scholar] [CrossRef]
- Martins, A.; Vasconcelos, V. Use of qPCR for the study of hepatotoxic cyanobacteria population dynamics. Arch. Microbiol. 2011, 193, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lim, J.; Lee, C. Quantitative real-time PCR approaches for microbial community studies in wastewater treatment systems: Applications and considerations. Biotechnol. Adv. 2013, 31, 1358–1373. [Google Scholar] [CrossRef]
- Wu, D.; Duirk, S.E. Quantitative analysis of earthy and musty odors in drinking water sources impacted by wastewater and algal derived contaminants. Chemosphere 2013, 91, 1495–1501. [Google Scholar] [CrossRef]
- Tsao, H.-W.; Michinaka, A.; Yen, H.-K.; Giglio, S.; Hobson, P.; Monis, P.; Lin, T.-F. Monitoring of geosmin producing Anabaena circinalis using quantitative PCR. Water Res. 2014, 49, 416–425. [Google Scholar] [CrossRef]
- Chiu, Y.-T.; Yen, H.-K.; Lin, T.-F. An alternative method to quantify 2-MIB-producing cyanobacteria in drinking water reservoirs: Method development and field applications. Environ. Res. 2016, 151, 618–627. [Google Scholar] [CrossRef]
- Yoccoz, N.G. The future of environmental DNA in ecology. Mol. Ecol. 2012, 21, 2031–2038. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.F.; Willerslev, E. Environmental DNA—An emerging tool in conservation for monitoring past and present biodiversity. Biol. Conserv. 2015, 183, 4–18. [Google Scholar] [CrossRef]
- Taberlet, P.; Bonin, A.; Zinger, L.; Coissac, E. Environmental DNA: For Biodiversity Research and Monitoring; Oxford University Press: Oxford, UK, 2018. [Google Scholar] [CrossRef]
- Barnes, M.A.; Turner, C.R. The ecology of environmental DNA and implications for conservation genetics. Conserv. Genet. 2016, 17, 1–17. [Google Scholar] [CrossRef]
- Wang, Z.; Song, G.; Shao, J.; Tan, W.; Li, Y.; Li, R. Establishment and field applications of real-time PCR methods for the quantification of potential MIB-producing cyanobacteria in aquatic systems. J. Appl. Phycol. 2016, 28, 325–333. [Google Scholar] [CrossRef]
- Kim, K.; Yoon, Y.; Cho, H.; Hwang, S.-J. Molecular probes to evaluate the synthesis and production potential of an odorous compound (2-methylisoborneol) in cyanobacteria. Int. J. Environ. Res. Public Health 2020, 17, 1933. [Google Scholar] [CrossRef] [PubMed]
- Yates, M.C.; Derry, A.M.; Cristescu, M.E. Environmental RNA: A revolution in ecological resolution? Trends Ecol. Evol. 2021, 36, 601–609. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, Y.; Liu, K.; Zhong, W.; Yang, J.; Altermatt, F.; Zhang, X. Evaluating eDNA and eRNA metabarcoding for aquatic biodiversity assessment: From bacteria to vertebrates. Environ. Sci. Ecotechnol. 2024, 21, 100441. [Google Scholar] [CrossRef]
- Laroche, O.; Wood, S.A.; Tremblay, L.A.; Lear, G.; Ellis, J.I.; Pochon, X. Metabarcoding monitoring analysis: The pros and cons of using co-extracted environmental DNA and RNA data to assess offshore oil production impacts on benthic communities. PeerJ 2017, 5, e3347. [Google Scholar] [CrossRef]
- Cristescu, M.E.; Hebert, P.D. Uses and misuses of environmental DNA in biodiversity science and conservation. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 209–230. [Google Scholar] [CrossRef]
- Ahi, E.P.; Schenekar, T. The promise of environmental RNA research beyond mRNA. Mol. Ecol. 2025, 34, e17787. [Google Scholar] [CrossRef]
- Veilleux, H.D.; Misutka, M.D.; Glover, C.N. Environmental DNA and environmental RNA: Current and prospective applications for biological monitoring. Sci. Total Environ. 2021, 782, 146891. [Google Scholar] [CrossRef]
- Hechler, R.M. Environmental Transcriptomics for the Biological Monitoring of Aquatic Biodiversity. Master’s Thesis, McGill University, Montreal, QC, Canada, 2022. [Google Scholar]
- Minamoto, T.; Miya, M.; Sado, T.; Seino, S.; Doi, H.; Kondoh, M.; Nakamura, K.; Takahara, T.; Yamamoto, S.; Yamanaka, H. An illustrated manual for environmental DNA research: Water sampling guidelines and experimental protocols. Environ. DNA 2021, 3, 8–13. [Google Scholar] [CrossRef]
- Oh, K.H. Screening of Conserved DNA Sequences in Microcystin-Producing Cyanobacteria and Application of the Sequences to Monitor Microcystins in Water Reservoirs. Master’s Thesis, Graduate School of Seoul National University, Seoul National University, Seoul, Republic of Korea, 2009. [Google Scholar]
- Tajima, N.; Kanesaki, Y.; Sato, S.; Yoshikawa, H.; Maruyama, F.; Kurokawa, K.; Ohta, H.; Nishizawa, T.; Asayama, M.; Sato, N. Complete genome sequence of the nonheterocystous cyanobacterium Pseudanabaena sp. ABRG5-3. Genome Announc. 2018, 6, e01617. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.-H.; Jeong, D.-H.; Cho, Y.-C. Quantification of toxigenic Microcystis spp. in freshwaters by quantitative real-time PCR based on the microcystin synthetase A gene. J. Microbiol. 2013, 51, 18–24. [Google Scholar] [CrossRef]
- Jung, J. Illustration of Korea Freshwater Phytoplankton; Academy Publishing: Seoul, Republic of Korea, 1993. [Google Scholar]
- Park, J.-G. Algal Flora of Korea—Freshwater Cyanobacteria (II); Ministry of Environment, National Institute of Biological Resources: Incheon, Republic of Korea, 2012; p. 126. [Google Scholar]
- Park, J.-G. Algal Flora of Korea—Freshwater Cyanobacteria (I); Ministry of Environment, National Institute of Biological Resources: Incheon, Republic of Korea, 2012; p. 88. [Google Scholar]
- Ding, Z.; Peng, S.; Xia, W.; Zheng, H.; Chen, X.; Yin, L. Analysis of five earthy–musty odorants in environmental water by HS-SPME/GC–MS. Int. J. Analyti. Chem. 2014, 2014, 697260. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Trapp, S.C.; Croteau, R.B. Genomic organization of plant terpene synthases and molecular evolutionary implications. Genetics 2001, 158, 811–832. [Google Scholar] [CrossRef]
- Smith, J.L.; Boyer, G.L.; Zimba, P.V. A review of cyanobacterial odorous and bioactive metabolites: Impacts and management alternatives in aquaculture. Aquaculture 2008, 280, 5–20. [Google Scholar] [CrossRef]
- Srinivasan, R.; Sorial, G.A. Treatment of taste and odor causing compounds 2-methylisoborneol and geosmin in drinking water: A critical review. J. Environ. Sci. 2011, 23, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, S.; Seo, K.; Hwang, S.-J. Molecular identification of Pseudanabaena strains and analysis of 2-MIB production potential in the North-Han River system. Ecol. Environ. 2020, 53, 344–354. [Google Scholar] [CrossRef]
- Komatsu, M.; Tsuda, M.; Ōmura, S.; Oikawa, H.; Ikeda, H. Identification and functional analysis of genes controlling biosynthesis of 2-methylisoborneol. Proc. Natl. Acad. Sci. USA 2008, 105, 7422–7427. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-M.; Cane, D.E. Biochemistry and molecular genetics of the biosynthesis of the earthy odorant methylisoborneol in Streptomyces coelicolor. J. Am. Chem. Soc. 2008, 130, 8908–8909. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Shao, J.; Wang, J.; Li, R. Genes associated with 2-methylisoborneol biosynthesis in cyanobacteria: Isolation, characterization, and expression in response to light. PLoS ONE 2011, 6, e18665. [Google Scholar] [CrossRef]
- Park, R.; Yu, M.-N.; Park, J.-H.; Kang, T.; Lee, J.-E. Effect of culture temperature on 2-methylisoborneol production and gene expression in two strains of Pseudanabaena sp. Cells 2024, 13, 1386. [Google Scholar] [CrossRef]
- Manganelli, M.; Testai, E.; Tazart, Z.; Scardala, S.; Codd, G.A. Co-occurrence of taste and odor compounds and cyanotoxins in cyanobacterial blooms: Emerging risks to human health? Microorganisms 2023, 11, 872. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Zhang, Y.; Mi, W.; Song, G.; Bi, Y. Producers and drivers of odor compounds in a large drinking-water source. Front. Ecol. Evol. 2023, 11, 1216567. [Google Scholar] [CrossRef]
- Mohd Zebaral Hoque, J.; Ab Aziz, N.A.; Alelyani, S.; Mohana, M.; Hosain, M. Improving water quality index prediction using regression learning models. Int. J. Environ. Res. Public Health 2022, 19, 13702. [Google Scholar] [CrossRef]
- Nouraki, A.; Alavi, M.; Golabi, M.; Albaji, M. Prediction of water quality parameters using machine learning models: A case study of the Karun River, Iran. Environ. Sci. Pollut. Res. 2021, 28, 57060–57072. [Google Scholar] [CrossRef]
- Pacheco, A.B.F.; Guedes, I.A.; Azevedo, S.M. Is qPCR a reliable indicator of cyanotoxin risk in freshwater? Toxins 2016, 8, 172. [Google Scholar] [CrossRef]
- Devi, A.; Chiu, Y.-T.; Hsueh, H.-T.; Lin, T.-F. Quantitative PCR based detection system for cyanobacterial geosmin/2-methylisoborneol (2-MIB) events in drinking water sources: Current status and challenges. Water Res. 2021, 188, 116478. [Google Scholar] [CrossRef]
- Sanders, R.; Mason, D.J.; Foy, C.A.; Huggett, J.F. Considerations for accurate gene expression measurement by reverse transcription quantitative PCR when analysing clinical samples. Anal. Bioanal. Chem. 2014, 406, 6471–6483. [Google Scholar] [CrossRef]
- Verwilt, J.; Mestdagh, P.; Vandesompele, J. Artifacts and biases of the reverse transcription reaction in RNA sequencing. RNA 2023, 29, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Hooper, A.; Kille, P.; Watson, S.; Christofides, S.; Perkins, R. The importance of nutrient ratios in determining elevations in geosmin synthase (geoA) and 2-MIB cyclase (MIC) resulting in taste and odour events. Water Res. 2023, 232, 119693. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, K.; Park, C.; Kim, H.; Hwang, S.-J. Temperature effect on the growth and odorous material (2-MIB) production of Pseudanabaena redekei. Korean J. Ecol. Environ. 2023, 56, 151–160. [Google Scholar] [CrossRef]
- Kim, J. Effect of Environmental Variables on the Growth and Off-Flavor Material (2-MIB) Production of Pseudanabaena. Master’s Thesis, Graduate School of Konkuk University, Konkuk University, Seoul, Republic of Korea, 2023. [Google Scholar]
- Jeong, J.-Y.; Lee, S.-H.; Yun, M.-R.; Oh, S.-E.; Lee, K.-H.; Park, H.-D. 2-Methylisoborneol (2-MIB) excretion by Pseudanabaena yagii under low temperature. Microorganisms 2021, 9, 2486. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.G.; Jeong, H.I.; Moon, J.E.; Kim, C.S.; Park, S.Y.; Bae, G.M.; Jeong, S.A.; Lee, H.S.; Lee, G.R.; et al. Study on the Identification of Causes and Management Strategies for Odor and Taste Compound Occurrence in the Bukhan River Basin; K-Water, Han River Basin Management Committee: Daejeon, Republic of Korea, 2022; p. 1430. [Google Scholar]
- Zimba, P.V.; Dionigi, C.P.; Millie, D.F. Evaluating the relationship between photopigment synthesis and 2-methylisoborneol accumulation in cyanobacteria. J. Phycol. 1999, 35, 1422–1429. [Google Scholar] [CrossRef]
- Grant, L.; Botelho, D.; Rehman, A. Early detection methods for toxic cyanobacteria blooms. Pathogens 2024, 13, 1047. [Google Scholar] [CrossRef] [PubMed]
- Pindilli, E.J.; Loftin, K. What’s it worth? Estimating the potential value of early warnings of cyanobacterial harmful algal blooms for managing freshwater reservoirs in Kansas, United States. Front. Environ. Sci. 2022, 10, 805165. [Google Scholar] [CrossRef]
- Watson, S.B. Aquatic taste and odor: A primary signal of drinking-water integrity. J. Toxicol. Environ. Health A 2004, 67, 1779–1795. [Google Scholar] [CrossRef]
- Wang, F.; Li, X.; Liu, T.; Li, X.; Cui, Y.; Xu, L.; Huo, S.; Zou, B.; Qian, J.; Ma, A. Removal of taste and odor compounds from water: Methods, mechanism and prospects. Catalysts 2023, 13, 1356. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Park, C.; Kim, N.-Y.; Hwnag, S.-J. eDNA- and eRNA-Based Detection of 2-Methylisoborneol-Producing Cyanobacteria and Intracellular Synthesis Dynamics in Freshwater Ecosystem. Biology 2025, 14, 1377. https://doi.org/10.3390/biology14101377
Kim K, Park C, Kim N-Y, Hwnag S-J. eDNA- and eRNA-Based Detection of 2-Methylisoborneol-Producing Cyanobacteria and Intracellular Synthesis Dynamics in Freshwater Ecosystem. Biology. 2025; 14(10):1377. https://doi.org/10.3390/biology14101377
Chicago/Turabian StyleKim, Keonhee, Chaehong Park, Nan-Young Kim, and Soon-Jin Hwnag. 2025. "eDNA- and eRNA-Based Detection of 2-Methylisoborneol-Producing Cyanobacteria and Intracellular Synthesis Dynamics in Freshwater Ecosystem" Biology 14, no. 10: 1377. https://doi.org/10.3390/biology14101377
APA StyleKim, K., Park, C., Kim, N.-Y., & Hwnag, S.-J. (2025). eDNA- and eRNA-Based Detection of 2-Methylisoborneol-Producing Cyanobacteria and Intracellular Synthesis Dynamics in Freshwater Ecosystem. Biology, 14(10), 1377. https://doi.org/10.3390/biology14101377







