Uncovering the Regulatory Role of Proteins in EBSS-Induced Autophagy Using RNA-Seq Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture
2.3. Starvation Inducement of Autophagy
2.4. RNA-Seq Library Preparation and Sequencing
2.5. Quantification and Analysis of RNA-Seq Data
2.6. Immunoblotting Analysis
2.7. Immunofluorescence Microscopy Procedures
2.8. RNA Interference
2.9. Analysis of Data
3. Results
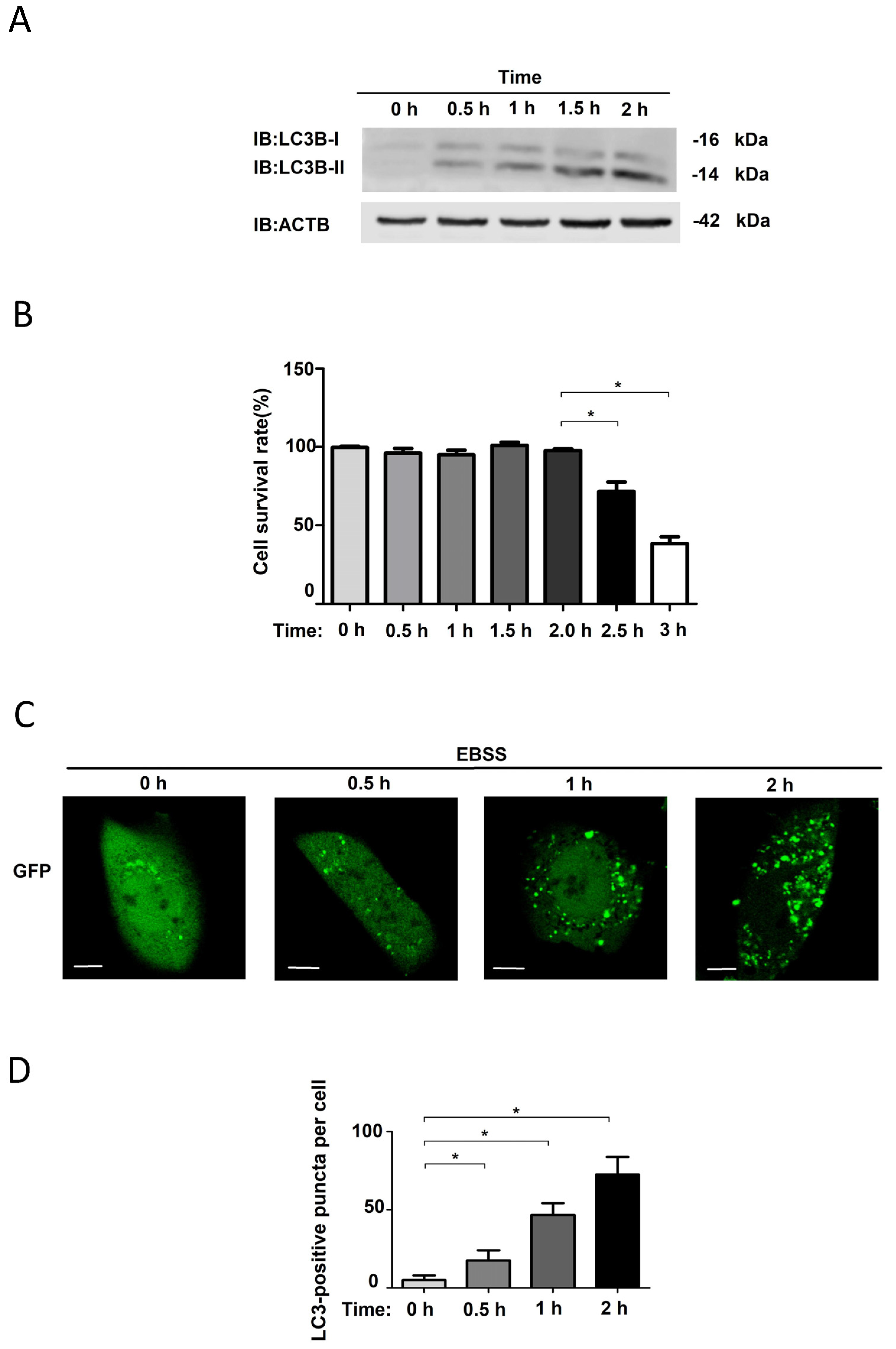
3.1. EBSS-Induced Autophagy in NRK Cells
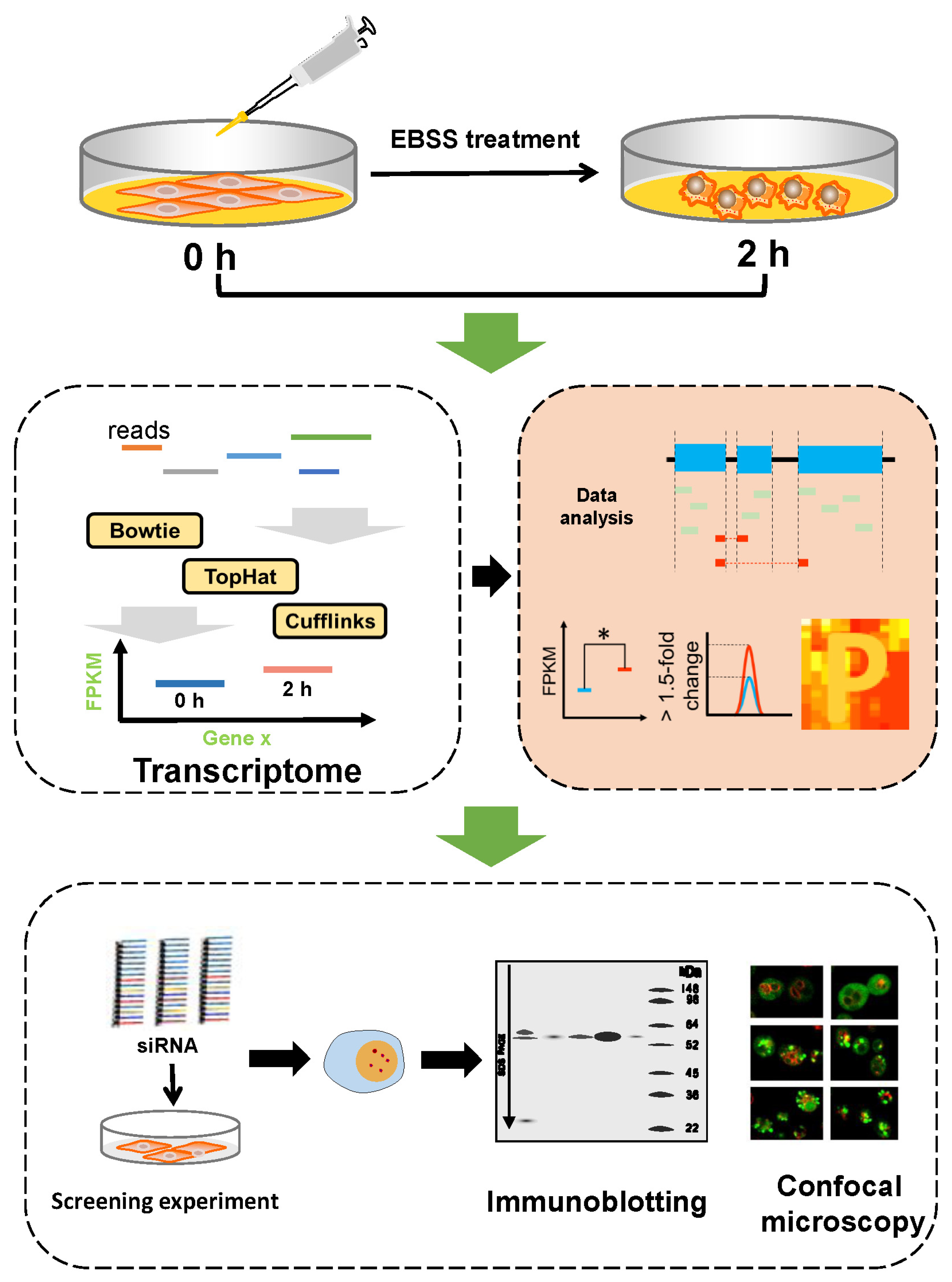
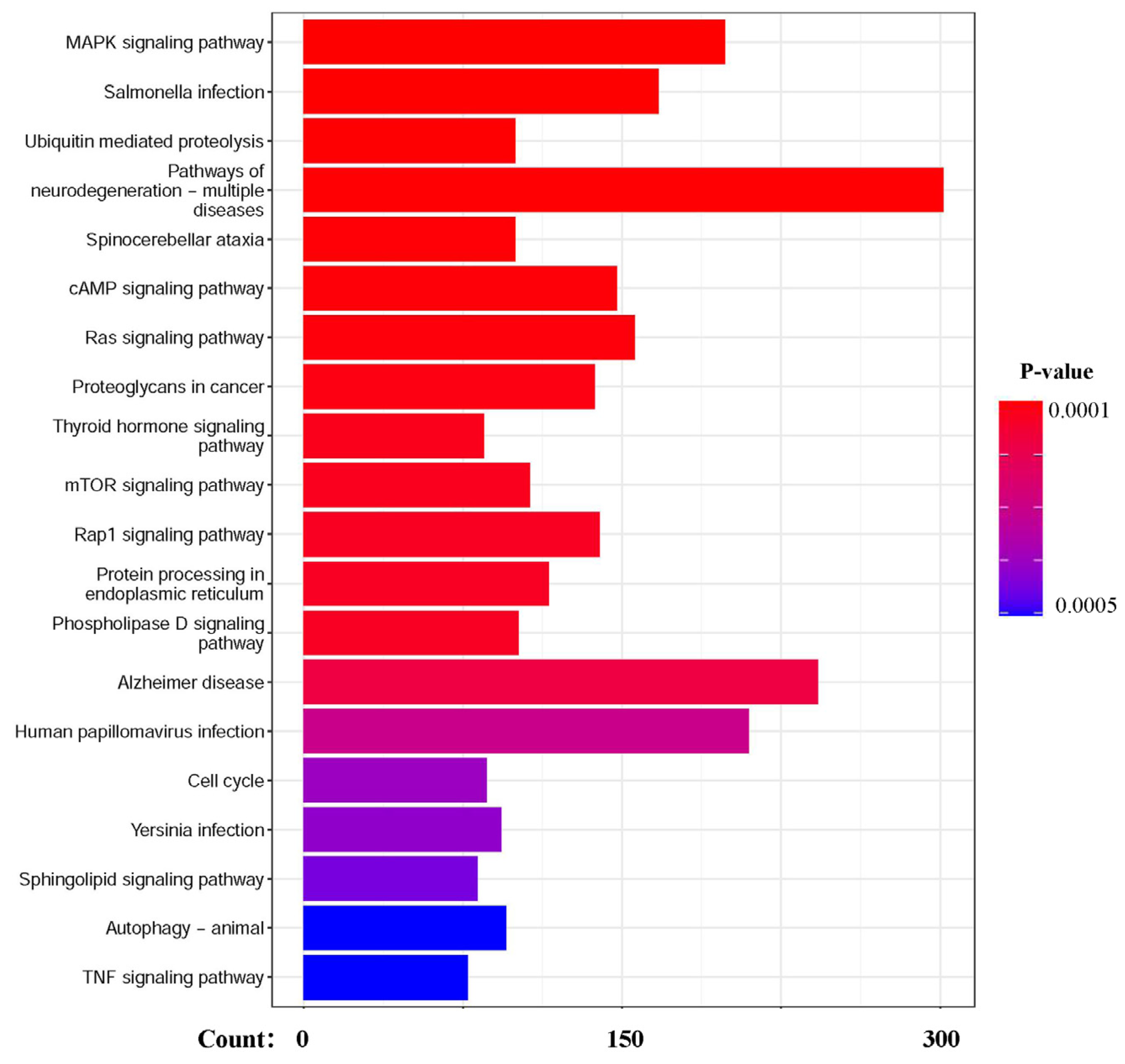
3.2. Quantification and Analysis of RNA-Seq Data for EBSS-Induced Autophagic NRK Cells
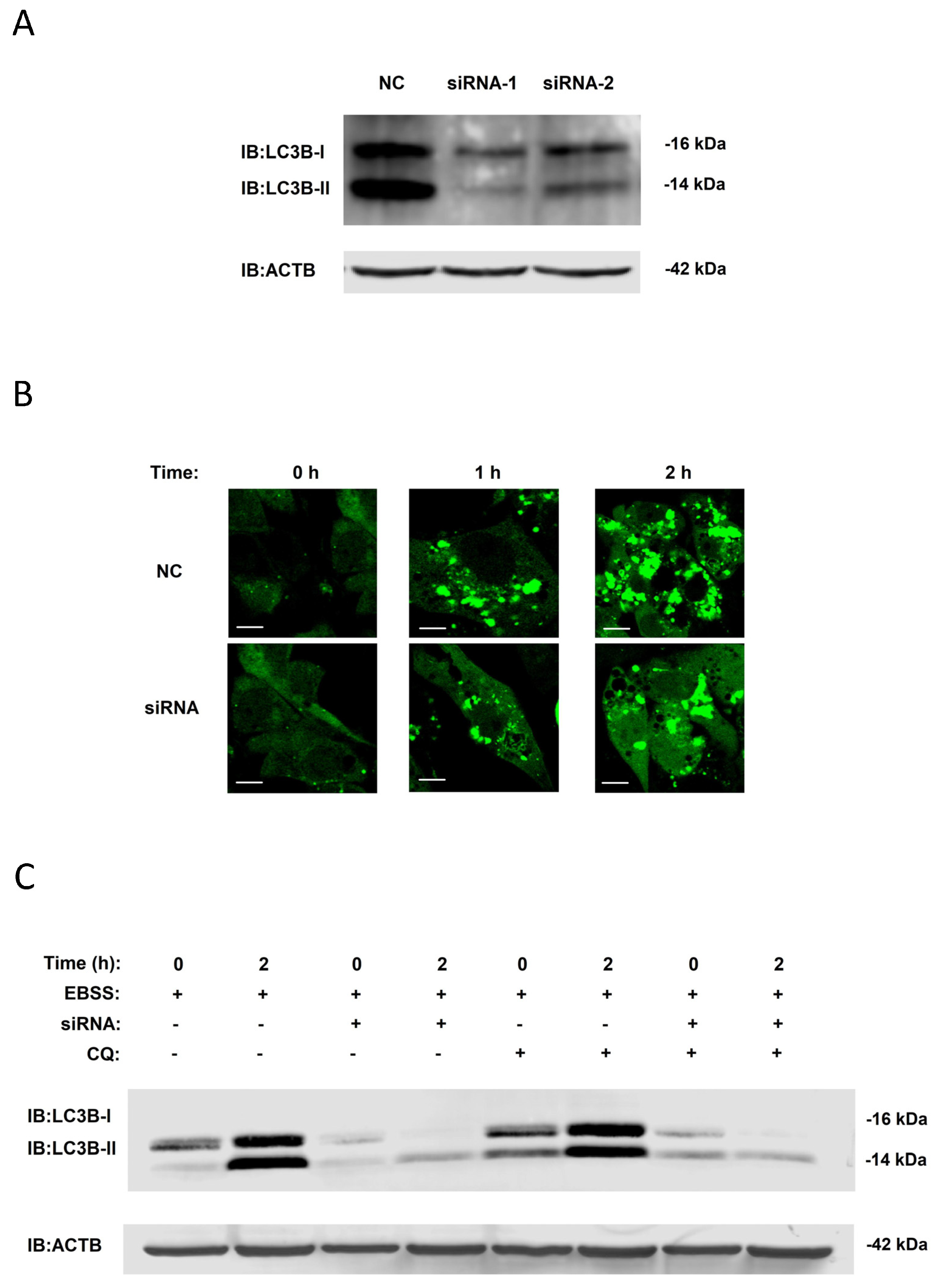
3.3. Validation of Regulatory Function of Candidate Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EBSS | Earle’s balanced salt solution |
| MTOR | mechanistic target of rapamycin kinase |
| NRK | normal rat kidney |
| LC3B | microtubule-associated protein 1 light chain 3 beta |
| ACTB | β-actin |
| FPKM | value of fragments per kilobase of exon model per million mapped fragments |
| GO | gene ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| PPI | protein–protein interaction |
| siRNA | small interfering RNA |
References
- Xie, Y.; Kang, R.; Sun, X.; Zhong, M.; Huang, J.; Klionsky, D.J.; Tang, D. Posttranslational modification of autophagy-related proteins in macroautophagy. Autophagy 2015, 11, 28–45. [Google Scholar] [CrossRef]
- McEwan, D.G.; Dikic, I. The Three Musketeers of Autophagy: Phosphorylation, ubiquitylation and acetylation. Trends Cell Biol. 2011, 21, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Korah, J.; Canaff, L.; Lebrun, J.J. The Retinoblastoma Tumor Suppressor Protein (pRb)/E2 Promoter Binding Factor 1 (E2F1) Pathway as a Novel Mediator of TGFβ-induced Autophagy. J. Biol. Chem. 2016, 291, 2043–2054. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.; Wang, C.; Gong, X.; Zhang, Y.; Deng, W.; Zhou, J.; Huang, D.; Wang, Z.; Zhang, Q.; Guo, A.; et al. An integrative multi-omics approach uncovers the regulatory role of CDK7 and CDK4 in autophagy activation induced by silica nanoparticles. Autophagy 2021, 17, 1426–1447. [Google Scholar] [CrossRef]
- Gao, G.; Chen, W.; Yan, M.; Liu, J.; Luo, H.; Wang, C.; Yang, P. Rapamycin regulates the balance between cardiomyocyte apoptosis and autophagy in chronic heart failure by inhibiting mTOR signaling. Int. J. Mol. Med. 2020, 45, 195–209. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, X.; Zhou, Y.; Wang, H. C2-ceramide induces cell death and protective autophagy in head and neck squamous cell carcinoma cells. Int. J. Mol. Sci. 2014, 15, 3336–3355. [Google Scholar] [CrossRef]
- Jiao, D.; Yang, Z.; Wang, L.; Hu, B.; Wang, J.; Xu, A.; Cheng, W.; Jia, B.; Qing, Y.; Zhao, H.-Y.; et al. Endogenous leptin promotes autophagy in EBSS-induced PFCs. Anim. Cells Syst. 2019, 23, 318–325. [Google Scholar] [CrossRef]
- Mizushima, N.; Kuma, A.; Kobayashi, Y.; Yamamoto, A.; Matsubae, M.; Takao, T.; Natsume, T.; Ohsumi, Y.; Yoshimori, T. Mouse Apg16L, a novel WD-repeat protein, targets to the autophagic isolation membrane with the Apg12-Apg5 conjugate. J. Cell Sci. 2003, 116 Pt 9, 1679–1688. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abeliovich, H.; Agostinis, P.; Agrawal, D.K.; Aliev, G.; Askew, D.S.; Baba, M.; Baehrecke, E.H.; A Bahr, B.; Ballabio, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 2008, 4, 151–175. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef]
- Cai, W.; Xiang, T.; Liu, X.; Fu, C. Multi-omics analysis reveals the role of the autophagy-related gene AGT in chemotherapy resistance in colorectal cancer and the therapeutic potential of its inhibitors. Discov. Oncol. 2024, 15, 674. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhang, Y.; Yuan, L.; Gao, Y.; Tian, X.; Tian, J.; Wan, J.; Li, B.; Wang, X.; Wang, S.; et al. LARS1 lactylation inhibits autophagy by activating mTORC1 to promote podocytes injury in diabetic kidney disease. Cell. Signal. 2025, 134, 111955. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Ruan, C.; Fu, S.; He, C.; Song, J.; Li, H.; Tu, Y.; Tang, D.; Yao, L.; Lin, S.; et al. Atg9-centered multi-omics integration reveals new autophagy regulators in Saccharomyces cerevisiae. Autophagy 2021, 17, 4453–4476. [Google Scholar] [CrossRef]
- Patterson, G.H.; Knobel, S.M.; Sharif, W.D.; Kain, S.R.; Piston, D.W. Use of the green fluorescent protein and its mutants in quantitative fluorescence microscopy. Biophys. J. 1997, 73, 2782–2790. [Google Scholar] [CrossRef]
- Kimura, S.; Noda, T.; Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 2007, 3, 452–460. [Google Scholar] [CrossRef]
- Rekas, A.; Alattia, J.R.; Nagai, T.; Miyawaki, A.; Ikura, M. Crystal structure of venus, a yellow fluorescent protein with improved maturation and reduced environmental sensitivity. J. Biol. Chem. 2002, 277, 50573–50578. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, Q.; Gu, M.; Zhang, K.; Xia, T.; Zhang, S.; Chen, W.; Yin, H.; Yao, H.; Fan, Y.; et al. MIR106A-5p upregulation suppresses autophagy and accelerates malignant phenotype in nasopharyngeal carcinoma. Autophagy 2021, 17, 1667–1683. [Google Scholar] [CrossRef]
- Zhang, Z.; Qian, Q.; Li, M.; Shao, F.; Ding, W.-X.; Lira, V.A.; Chen, S.X.; Sebag, S.C.; Hotamisligil, G.S.; Cao, H.; et al. The unfolded protein response regulates hepatic autophagy by sXBP1-mediated activation of TFEB. Autophagy 2021, 17, 1841–1855. [Google Scholar] [CrossRef]
- Chu, Y.; Kang, Y.; Yan, C.; Yang, C.; Zhang, T.; Huo, H.; Liu, Y. LUBAC and OTULIN regulate autophagy initiation and maturation by mediating the linear ubiquitination and the stabilization of ATG13. Autophagy 2021, 17, 1684–1699. [Google Scholar] [CrossRef]
- Tian, S.; Jin, S.; Wu, Y.; Liu, T.; Luo, M.; Ou, J.; Xie, W.; Cui, J. High-throughput screening of functional deubiquitinating enzymes in autophagy. Autophagy 2021, 17, 1367–1378. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Shu, G.; Liu, G.; Liu, D.; Zhou, J.; Yuan, L.; Zhou, J. Knockdown of p62/sequestosome 1 attenuates autophagy and inhibits colorectal cancer cell growth. Mol. Cell. Biochem. 2014, 385, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cecconi, F.; Choi, A.M.; Chu, C.T.; Codogno, P.; Colombo, M.I.; et al. Molecular definitions of autophagy and related processes. Embo. J. 2017, 36, 1811–1836. [Google Scholar] [CrossRef]
- Dunlop, E.A.; Seifan, S.; Claessens, T.; Behrends, C.; Kamps, M.A.; Rozycka, E.; Kemp, A.J.; Nookala, R.K.; Blenis, J.; Coull, B.J.; et al. FLCN, a novel autophagy component, interacts with GABARAP and is regulated by ULK1 phosphorylation. Autophagy 2014, 10, 1749–1760. [Google Scholar] [CrossRef]
- Tan, S.; Zheng, Z.; Liu, T.; Yao, X.; Yu, M.; Ji, Y. Schisandrin B Induced ROS-Mediated Autophagy and Th1/Th2 Imbalance via Selenoproteins in Hepa1-6 Cells. Front. Immunol. 2022, 13, 857069. [Google Scholar] [CrossRef]
- Jiayong, Z.; Shengchen, W.; Xiaofang, H.; Gang, S.; Shiwen, X. The antagonistic effect of selenium on lead-induced necroptosis via MAPK/NF-κB pathway and HSPs activation in the chicken spleen. Ecotoxicol. Environ. Saf. 2020, 204, 111049. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Chen, B.; Zhao, B.; Gao, X.J. Selenium Deficiency Promotes Oxidative Stress-Induced Mastitis via Activating the NF-κB and MAPK Pathways in Dairy Cow. Biol. Trace Elem. Res. 2022, 200, 2716–2726. [Google Scholar] [CrossRef]
- Chi, Q.; Zhang, Q.; Lu, Y.; Zhang, Y.; Xu, S.; Li, S. Roles of selenoprotein S in reactive oxygen species-dependent neutrophil extracellular trap formation induced by selenium-deficient arteritis. Redox Biol. 2021, 44, 102003. [Google Scholar] [CrossRef]
- Madden, J.A.; Thomas, P.Q.; Keating, A.F. Phosphoramide mustard induces autophagy markers and mTOR inhibition prevents follicle loss due to phosphoramide mustard exposure. Reprod. Toxicol. 2017, 67, 65–78. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, C.; Li, Y.; Wu, R. Uncovering the Regulatory Role of Proteins in EBSS-Induced Autophagy Using RNA-Seq Analysis. Biology 2025, 14, 1373. https://doi.org/10.3390/biology14101373
Ruan C, Li Y, Wu R. Uncovering the Regulatory Role of Proteins in EBSS-Induced Autophagy Using RNA-Seq Analysis. Biology. 2025; 14(10):1373. https://doi.org/10.3390/biology14101373
Chicago/Turabian StyleRuan, Chen, Yuzhu Li, and Ran Wu. 2025. "Uncovering the Regulatory Role of Proteins in EBSS-Induced Autophagy Using RNA-Seq Analysis" Biology 14, no. 10: 1373. https://doi.org/10.3390/biology14101373
APA StyleRuan, C., Li, Y., & Wu, R. (2025). Uncovering the Regulatory Role of Proteins in EBSS-Induced Autophagy Using RNA-Seq Analysis. Biology, 14(10), 1373. https://doi.org/10.3390/biology14101373





