Deciphering the Fate of Burned Trees After a Forest Fire: A Systematic Review Focused on Conifers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- Population: every worldwide conifer forest, covered by prescribed fire or wildfire in natural or semi-natural areas during the last twenty-five years (from 31 December 1999 to 31 January 2025;
- Intervention: every plant’s physiological proxies used to identify any fire-related stress;
- Control: tree visual assessment related to tree vitality;
- Outcome: any possible observed physiological outcome.
3. Results
3.1. Bibliographic Overview
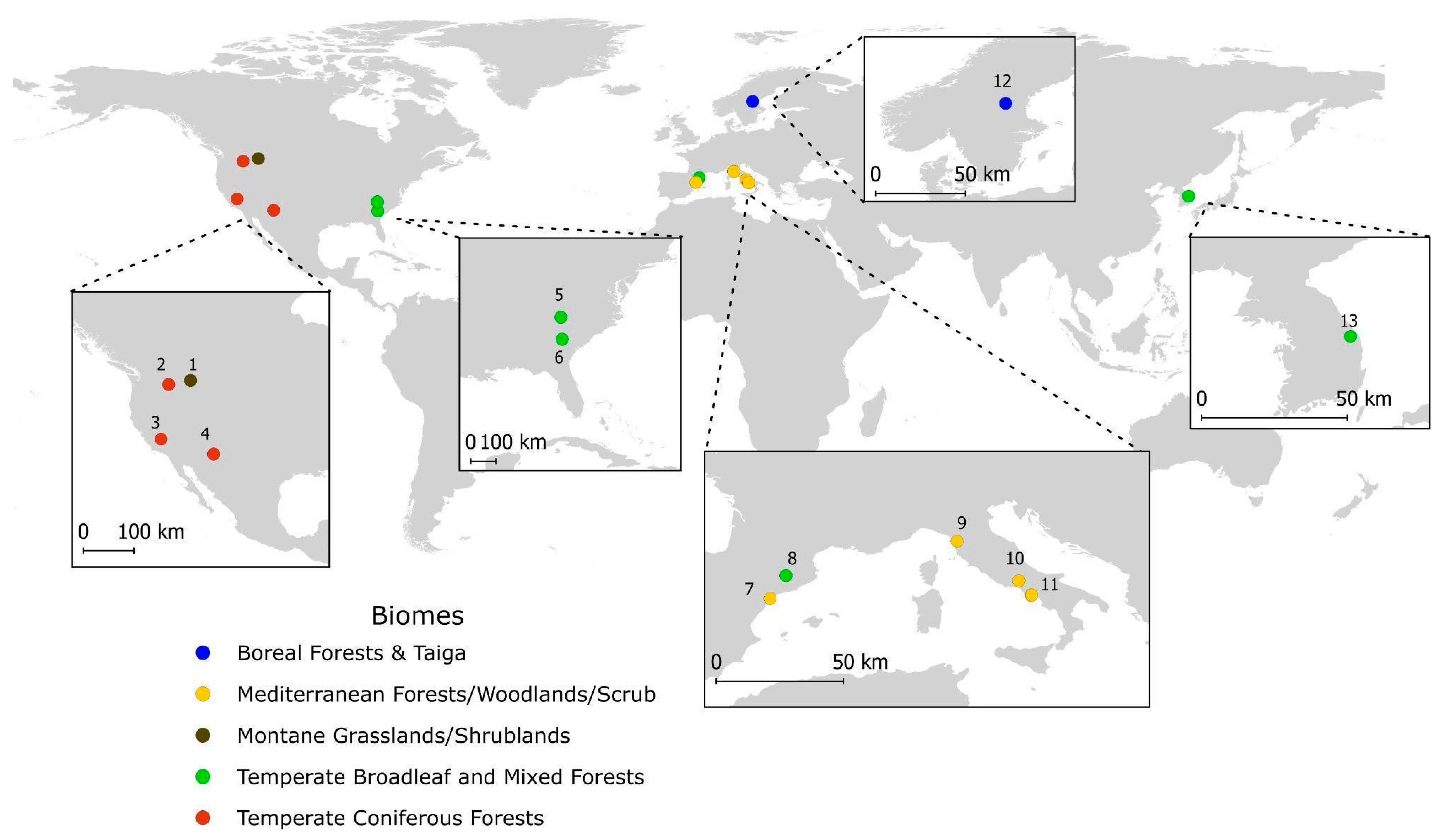
3.2. Mapping of Study Sites
4. Discussion
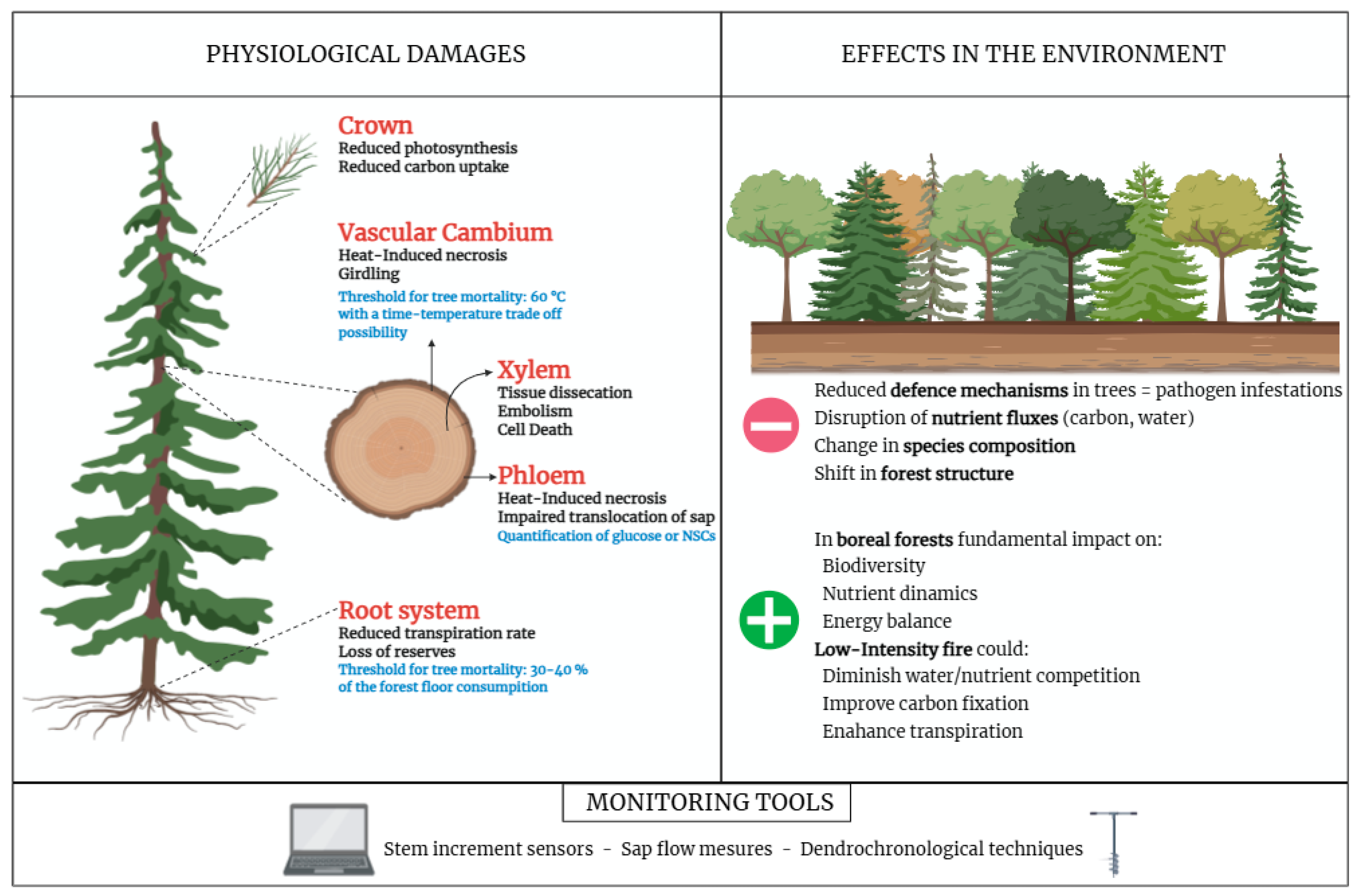
4.1. Effects of Fire on Tree Vitality and Functioning
4.2. Fire Effects in the Environment
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Detailed Location of Study Areas in 14 Papers Considered: Per Each Continent, the Number of References Is Reported
| Europe: Italy, Spain, Sweden |
| North America: USA |
| Asia: South Korea |
- Lombardona mountain, Vicopisano (PI), Tuscany
- Monte Cairo massif, Lazio region, Central Italy
- Vesuvius National Park, southern Italy
- Tirone Alto Vesuvio Nature Reserve, within Vesuvius National Park
- El Perelló locality, southern Catalonia
- Pyrenees, Miravé and Lloreda, northeastern Iberian Peninsula
- Municipality of Ljusdal, central Sweden
- Blue Ridge ecosystem, Southern Appalachians (Georgia, North Carolina, South Carolina, Tennessee)
- Fort Gordon, near Augusta, Georgia
- Wallow Fire footprint, Apache-Sitgreaves National Forests, near Greer, Alpine, and Nutrioso, east-central Arizona
- Marble Fork drainage, Sequoia National Park, California
- Northern Blue Mountains, northeastern Oregon
- Western Montana, Blackfoot-Clearwater Wildlife Management Area
- Mountains around Samcheok, eastern Korea
Appendix A.2. Biome Type [29] 16 Papers Considered: Per Each Biome Type the Number of References Is Reported
| Biome Type | N° | Location |
| Mediterranean Forests/Woodlands/Shrub | 5 | Lombardona mountain, Vicopisano (PI), Tuscany; Monte Cairo massif, Lazio region, Central Italy; Vesuvius National Park, southern Italy; Tirone Alto Vesuvio Nature Reserve, within Vesuvius National Park; El Perelló locality, southern Catalonia |
| Temperate Broadleaf and Mixed Forests | 4 | Pyrenees, Miravé and Lloreda, northeastern Iberian Peninsula; Blue Ridge ecosystem, Southern Appalachians (Georgia, North Carolina, South Carolina, Tennessee); Fort Gordon, near Augusta, Georgia; Mountains around Samcheok, eastern Koreae |
| Temperate Coniferous Forests | 3 | Wallow Fire footprint, Apache-Sitgreaves National Forests, near Greer, Alpine, and Nutrioso, east-central Arizona; Marble Fork drainage, Sequoia National Park, California; Northern Blue Mountains, northeastern Oregon |
| Boreal Forests and Taiga | 1 | Municipality of Ljusdal, central Sweden |
| Mountain Grasslands and Shrublands | 1 | Western Montana, Blackfoot-Clearwater Wildlife Management Area |
Appendix A.3. Köppen–Geiger Climate Classification [46] 16 Papers Considered: Per Each Continent the Number of References Is Reported
| Zone | N° | Location |
| Mediterranean (Csa) | 6 | Lombardona mountain, Vicopisano (PI), Tuscany; Monte Cairo massif, Lazio region, Central Italy; Vesuvius National Park, southern Italy; Tirone Alto Vesuvio Nature Reserve, within Vesuvius National Park; El Perelló locality, southern Catalonia; Marble Fork drainage, Sequoia National Park, California |
| Oceanic/Humid Temperate (Cfb) | 2 | Pyrenees, Miravé and Lloreda, northeastern Iberian Peninsula; Blue Ridge ecosystem, Southern Appalachians (Georgia, North Carolina, South Carolina, Tennessee) |
| Humid Subtropical (Cfa) | 2 | Fort Gordon, near Augusta, Georgia; Mountains around Samcheok, eastern Koreae |
| Cold Semi-Arid (BSk) | 2 | Wallow Fire footprint, Apache-Sitgreaves National Forests, near Greer, Alpine, and Nutrioso, east-central Arizona; Northern Blue Mountains, northeastern Oregon |
| Cold Continental (Dfb, Dwa) | 1 | Western Montana, Blackfoot-Clearwater Wildlife Management Area |
| Subartic/Boreal (Dfc) | 1 | Municipality of Ljusdal, central Sweden |
Appendix A.4. Conifer Species Studied in 16 Papers Considered
| Genus | N° | Species |
| Pinus | 13 | Pinus densiflora Pinus halepensis Pinus lambertiana Pinus nigra Pinus nigra salzmannii Pinus palustris Pinus pinaster Pinus pinea Pinus ponderosa Pinus strobiformis Pinus sylvestris Pinus taeda Pinus virginiana |
| Abies | 1 | Abies concolor |
| Pseudotsuga | 1 | Pseudotsuga menziesii |
| Picea | 1 | Picea engelmannii |
References
- Gajendiran, K.; Kandasamy, S.; Narayanan, M. Influences of Wildfire on the Forest Ecosystem and Climate Change: A Comprehensive Study. Environ. Res. 2024, 240, 117537. [Google Scholar] [CrossRef]
- Harvey, B.J.; Andrus, R.A.; Anderson, S.C. Incorporating Biophysical Gradients and Uncertainty into Burn Severity Maps in a Temperate Fire-prone Forested Region. Ecosphere 2019, 10, e02600. [Google Scholar] [CrossRef]
- Maringer, J.; Hacket-Pain, A.; Ascoli, D.; Garbarino, M.; Conedera, M. A New Approach for Modeling Delayed Fire-induced Tree Mortality. Ecosphere 2021, 12, e03458. [Google Scholar] [CrossRef]
- Nolan, R.H.; Collins, L.; Leigh, A.; Ooi, M.K.J.; Curran, T.J.; Fairman, T.A.; Resco De Dios, V.; Bradstock, R. Limits to Post-fire Vegetation Recovery under Climate Change. Plant Cell Environ. 2021, 44, 3471–3489. [Google Scholar] [CrossRef]
- Hood, S.M.; Varner, J.M.; Van Mantgem, P.; Cansler, C.A. Fire and Tree Death: Understanding and Improving Modeling of Fire-Induced Tree Mortality. Environ. Res. Lett. 2018, 13, 113004. [Google Scholar] [CrossRef]
- Samborska, V.; Ritchie, H. Wildfires. 2024. Available online: https://ourworldindata.org/wildfires (accessed on 5 October 2025).
- Ritchie, H. Deforestation and Forest Loss. 2021. Available online: https://ourworldindata.org/deforestation (accessed on 5 October 2025).
- Trouvé, R.; Oborne, L.; Baker, P.J. The Effect of Species, Size, and Fire Intensity on Tree Mortality within a Catastrophic Bushfire Complex. Ecol. Appl. 2021, 31, e02383. [Google Scholar] [CrossRef]
- Sparks, A.M.; Blanco, A.S.; Wilson, D.R.; Schwilk, D.W.; Johnson, D.M.; Adams, H.D.; Bowman, D.M.J.S.; Hardman, D.D.; Smith, A.M.S. Fire Intensity Impacts on Physiological Performance and Mortality in Pinus Monticola and Pseudotsuga Menziesii Saplings: A Dose–Response Analysis. Tree Physiol. 2023, 43, 1365–1382. [Google Scholar] [CrossRef]
- Bennett, L.T.; Bruce, M.J.; Machunter, J.; Kohout, M.; Krishnaraj, S.J.; Aponte, C. Assessing Fire Impacts on the Carbon Stability of Fire-tolerant Forests. Ecol. Appl. 2017, 27, 2497–2513. [Google Scholar] [CrossRef]
- Sparks, A.M.; Kolden, C.A.; Smith, A.M.S.; Boschetti, L.; Johnson, D.M.; Cochrane, M.A. Fire Intensity Impacts on Post-Fire Temperate Coniferous Forest Net Primary Productivity. Biogeosciences 2018, 15, 1173–1183. [Google Scholar] [CrossRef]
- Hüttnerová, T.; Paczkowski, S.; Neubert, T.; Jirošová, A.; Surový, P. Comparison of Individual Sensors in the Electronic Nose for Stress Detection in Forest Stands. Sensors 2023, 23, 2001. [Google Scholar] [CrossRef]
- Reilly, M.J.; Zuspan, A.; Yang, Z. Characterizing Post-Fire Delayed Tree Mortality with Remote Sensing: Sizing up the Elephant in the Room. Fire Ecol. 2023, 19, 64. [Google Scholar] [CrossRef]
- Frassinelli, N.; Cocozza, C.; Marchi, E.; Foderi, C.; Touloupakis, E.; Neri, F.; Traversi, M.L.; Giovannelli, A. Detecting Glucose in the Phloem to Quickly Define Latent Post-Fire Mortality in Pinus Trees in Northern Italy. Fire 2024, 7, 315. [Google Scholar] [CrossRef]
- Leverkus, A.B.; Buma, B.; Wagenbrenner, J.; Burton, P.J.; Lingua, E.; Marzano, R.; Thorn, S. Tamm review: Does salvage logging mitigate subsequent forest disturbances? For. Ecol. Manag. 2021, 481, 118721. [Google Scholar] [CrossRef]
- Niccoli, F.; Esposito, A.; Altieri, S.; Battipaglia, G. Fire Severity Influences Ecophysiological Responses of Pinus Pinaster Ait. Front. Plant Sci. 2019, 10, 539. [Google Scholar] [CrossRef]
- Hood, S.; Lutes, D. Predicting Post-Fire Tree Mortality for 12 Western US Conifers Using the First Order Fire Effects Model (FOFEM). Fire Ecol. 2017, 13, 66–84. [Google Scholar] [CrossRef]
- Bär, A.; Nardini, A.; Mayr, S. Post-fire Effects in Xylem Hydraulics of Picea Abies, Pinus Sylvestris and Fagus Sylvatica. New Phytol. 2018, 217, 1484–1493. [Google Scholar] [CrossRef]
- Partelli-Feltrin, R.; Smith, A.M.S.; Adams, H.D.; Thompson, R.A.; Kolden, C.A.; Yedinak, K.M.; Johnson, D.M. Death from Hunger or Thirst? Phloem Death, Rather than Xylem Hydraulic Failure, as a Driver of Fire-induced Conifer Mortality. New Phytol. 2023, 237, 1154–1163. [Google Scholar] [CrossRef]
- Thompson, M.T.C.; Koyama, A.; Kavanagh, K.L. Wildfire Effects on Physiological Properties in Conifers of Central Idaho Forests, USA. Trees 2017, 31, 545–555. [Google Scholar] [CrossRef]
- Khanmohammadi, S.; Arashpour, M.; Bazli, M.; Farzanehfar, P. Data-Driven PM2.5 Exposure Prediction in Wildfire-Prone Regions and Respiratory Disease Mortality Risk Assessment. Fire 2024, 7, 277. [Google Scholar] [CrossRef]
- Van Mantgem, P.J.; Caprio, A.C.; Stephenson, N.L.; Das, A.J. Does Prescribed Fire Promote Resistance to Drought in Low Elevation Forests of the Sierra Nevada, California, USA? Fire Ecol. 2016, 12, 13–25. [Google Scholar] [CrossRef]
- Bottero, A.; D’Amato, A.W.; Palik, B.J.; Kern, C.C.; Bradford, J.B.; Scherer, S.S. Influence of Repeated Prescribed Fire on Tree Growth and Mortality in Pinus Resinosa Forests, Northern Minnesota. For. Sci. 2017, 63, 94–100. [Google Scholar] [CrossRef]
- Abella, S.R.; Sprow, L.A.; Schetter, T.A. Delayed Tree Mortality After Prescribed Fires in Mixed Oak Forests in Northwestern Ohio. For. Sci. 2021, 67, 412–418. [Google Scholar] [CrossRef]
- Pontes-Lopes, A.; Silva, C.V.J.; Barlow, J.; Rincón, L.M.; Campanharo, W.A.; Nunes, C.A.; De Almeida, C.T.; Silva Júnior, C.H.L.; Cassol, H.L.G.; Dalagnol, R.; et al. Drought-Driven Wildfire Impacts on Structure and Dynamics in a Wet Central Amazonian Forest. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210094. [Google Scholar] [CrossRef]
- Robbins, Z.J.; Loudermilk, E.L.; Reilly, M.J.; O’Brien, J.J.; Jones, K.; Gerstle, C.T.; Scheller, R.M. Delayed Fire Mortality Has Long-term Ecological Effects across the Southern Appalachian Landscape. Ecosphere 2022, 13, e4153. [Google Scholar] [CrossRef]
- Ministero dell’Università e della Ricerca. Developing Innovative Methods to Assess Tree Vitality After a Wildfire Through Analyses of Cambium Sugars Metabolism (DIVAS Project, Prot. P2022Z5742). In PRIN 2022—Progetti di Ricerca di Rilevante Interesse Nazionale (PNRR); Ministero dell’Università e della Ricerca: Rome, Italy, 2022. [Google Scholar]
- Higgins, J.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, UK: The Cochrane Collaboration; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’Amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on Earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Beck, H.E.; McVicar, N.T.R.; Vergopolan, A.; Berg, N.J.; Lutsko, A.; Dufour, Z.; Zeng, X.; Jiang, A.I.J.M. van Dijk, D.G. Miralles. High-resolution (1 km) Köppen-Geiger maps for 1901–2099 based on constrained CMIP6 projections. Sci. Data 2023, 10, 724. [Google Scholar] [CrossRef]
- Whittaker, R.H. Communities and Ecosystems, 2nd ed.; Macmillan Publishing Co. Collier Macmillan Publishers: New York, NY, USA, 1975. [Google Scholar]
- Dukat, P.; Kelly, J.; Doerr, S.H.; Edvardsson, J.; Hölttä, T.S.; Lehner, I.; Lindroth, A.; Santín, C.; Kljun, N. Boreal Forest Tree Growth and Sap Flow after a Low-Severity Wildfire. Agric. For. Meteorol. 2024, 347, 109899. [Google Scholar] [CrossRef]
- Guo, D.; Mitchell, R.J.; Withington, J.M.; Fan, P.; Hendricks, J.J. Endogenous and Exogenous Controls of Root Life Span, Mortality and Nitrogen Flux in a Longleaf Pine Forest: Root Branch Order Predominates. J. Ecol. 2008, 96, 737–745. [Google Scholar] [CrossRef]
- Reed, C.C.; Hood, S.M. Nonstructural Carbohydrates Explain Post-Fire Tree Mortality and Recovery Patterns. Tree Physiol. 2024, 44, tpad155. [Google Scholar] [CrossRef]
- Niccoli, F.; Pacheco-Solana, A.; Delzon, S.; Kabala, J.P.; Asgharinia, S.; Castaldi, S.; Valentini, R.; Battipaglia, G. Effects of Wildfire on Growth, Transpiration and Hydraulic Properties of Pinus Pinaster Aiton Forest. Dendrochronologia 2023, 79, 126086. [Google Scholar] [CrossRef]
- Michaletz, S.T.; Johnson, E.A. How Forest Fires Kill Trees: A Review of the Fundamental Biophysical Processes. Scand. J. For. Res. 2007, 22, 500–515. [Google Scholar] [CrossRef]
- Valor, T.; González-Olabarria, J.R.; Piqué, M.; Casals, P. The Effects of Burning Season and Severity on the Mortality over Time of Pinus Nigra Spp. Salzmannii (Dunal) Franco and P. Sylvestris L. For. Ecol. Manag. 2017, 406, 172–183. [Google Scholar] [CrossRef]
- Niccoli, F.; Altieri, S.; Kabala, J.P.; Battipaglia, G. Fire Affects Tree Growth, Water Use Efficiency and Carbon Sequestration Ecosystem Service of Pinus Nigra Arnold: A Combined Satellite and Ground-Based Study in Central Italy. Forests 2023, 14, 2023. [Google Scholar] [CrossRef]
- Roccaforte, J.P.; Sánchez Meador, A.; Waltz, A.E.M.; Gaylord, M.L.; Stoddard, M.T.; Huffman, D.W. Delayed Tree Mortality, Bark Beetle Activity, and Regeneration Dynamics Five Years Following the Wallow Fire, Arizona, USA: Assessing Trajectories towards Resiliency. For. Ecol. Manag. 2018, 428, 20–26. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, S.; Kim, J.; Kang, W.; Park, K.-H.; Kim, C.-B.; Girona, M.M. Predicting Post-Fire Tree Mortality in a Temperate Pine Forest, Korea. Sustainability 2021, 13, 569. [Google Scholar] [CrossRef]
- Nesmith, J.C.B.; Das, A.J.; O’Hara, K.L.; Van Mantgem, P.J. The Influence of Prefire Tree Growth and Crown Condition on Postfire Mortality of Sugar Pine Following Prescribed Fire in Sequoia National Park. Can. J. For. Res. 2015, 45, 910–919. [Google Scholar] [CrossRef]
- Valor, T.; Casals, P.; Altieri, S.; González-Olabarria, J.R.; Piqué, M.; Battipaglia, G. Disentangling the Effects of Crown Scorch and Competition Release on the Physiological and Growth Response of Pinus Halepensis Mill. Using δ13C and δ18O Isotopes. For. Ecol. Manag. 2018, 424, 276–287. [Google Scholar] [CrossRef]
- O’Brien, J.J.; Kevin Hiers, J.; Mitchell, R.J.; Varner, J.M.; Mordecai, K. Acute Physiological Stress and Mortality Following Fire in a Long-Unburned Longleaf Pine Ecosystem. Fire Ecol. 2010, 6, 1–12. [Google Scholar] [CrossRef]
- Filip, G.M.; Schmitt, C.L.; Scott, D.W.; Fitzgerald, S.A. Understanding and Defining Mortality in Western Conifer Forests. West. J. Appl. For. 2007, 22, 105–115. [Google Scholar] [CrossRef]
- Youngblood, A.; Grace, J.B.; McIver, J.D. Delayed Conifer Mortality after Fuel Reduction Treatments: Interactive Effects of Fuel, Fire Intensity, and Bark Beetles. Ecol. Appl. 2009, 19, 321–337. [Google Scholar] [CrossRef]
- Beck, H.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Lutsko, N.J.; Dufour, A.; Zeng, Z.; Jiang, X.; van Dijk, A.I.J.M.; Miralles, D.G. High-resolution (1 km) Köppen-Geiger maps for 1901–2099 based on constrained CMIP6 projections. Sci. Data 2023, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizzarri, A.; Paladini, M.; Frassinelli, N.; Marchi, E.; Zampieri, R.M.; Giovannelli, A.; Cocozza, C. Deciphering the Fate of Burned Trees After a Forest Fire: A Systematic Review Focused on Conifers. Biology 2025, 14, 1372. https://doi.org/10.3390/biology14101372
Bizzarri A, Paladini M, Frassinelli N, Marchi E, Zampieri RM, Giovannelli A, Cocozza C. Deciphering the Fate of Burned Trees After a Forest Fire: A Systematic Review Focused on Conifers. Biology. 2025; 14(10):1372. https://doi.org/10.3390/biology14101372
Chicago/Turabian StyleBizzarri, Alessandro, Margherita Paladini, Niccolò Frassinelli, Enrico Marchi, Raffaella Margherita Zampieri, Alessio Giovannelli, and Claudia Cocozza. 2025. "Deciphering the Fate of Burned Trees After a Forest Fire: A Systematic Review Focused on Conifers" Biology 14, no. 10: 1372. https://doi.org/10.3390/biology14101372
APA StyleBizzarri, A., Paladini, M., Frassinelli, N., Marchi, E., Zampieri, R. M., Giovannelli, A., & Cocozza, C. (2025). Deciphering the Fate of Burned Trees After a Forest Fire: A Systematic Review Focused on Conifers. Biology, 14(10), 1372. https://doi.org/10.3390/biology14101372










