Integration of Light and Circadian Signaling in Plant Gene Regulatory Networks: Implications for Photomorphogenesis and Stress Adaptation
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Importance of Light and Circadian Rhythms in Plants
1.2. Evolutionary Context
1.3. Scope and Objectives
2. Molecular Components of Light and Circadian Systems
2.1. Photoreceptors and Light Signaling
2.2. Core Circadian Clock Machinery
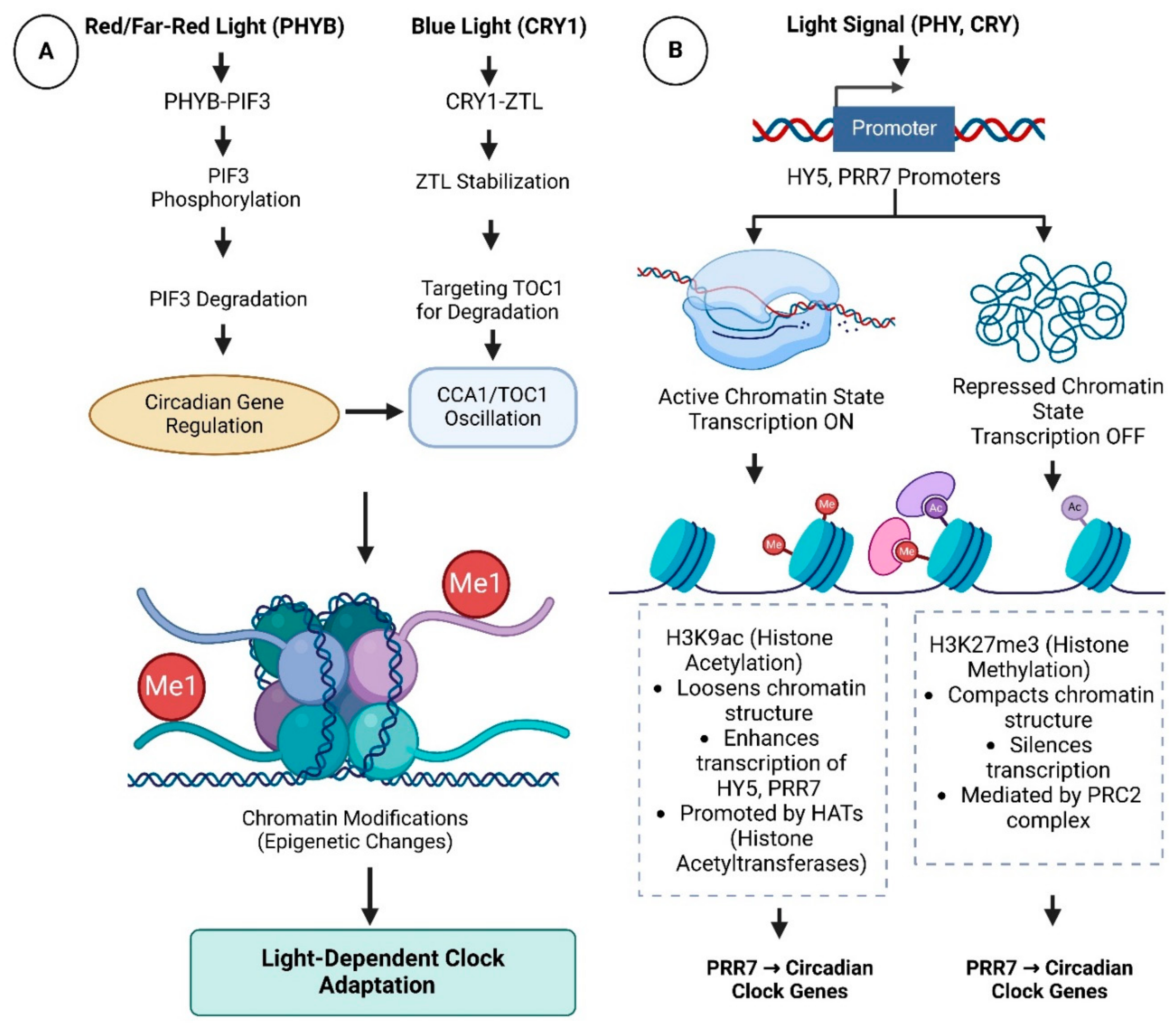
3. Mechanisms of Light–Clock Integration
3.1. Light Inputs to the Circadian Clock
3.2. Clock Regulation of Light Signaling
3.3. Shared Transcriptional Hubs
4. Functional Outcomes of Light–Clock Crosstalk
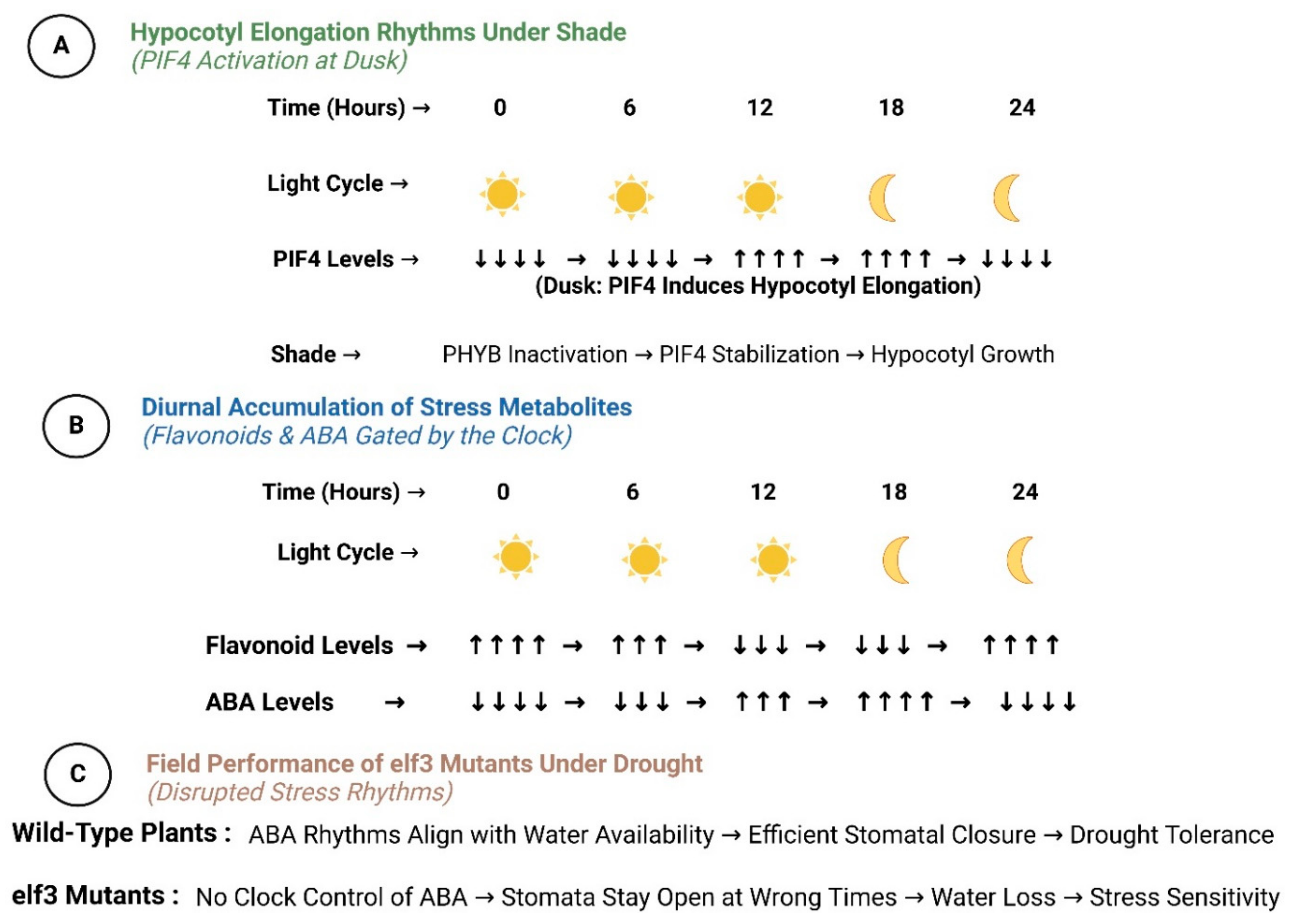
4.1. Photomorphogenesis and Shade Avoidance
4.2. Light–Circadian Networks with Environmental Stress
4.3. Photoperiodic Flowering
4.4. Abiotic Stress Resilience
4.5. Metabolic Regulation
4.6. Controlled Environment and Photoperiodic Stress
5. Emerging Technologies and Approaches
5.1. Systems Biology Tools
5.2. CRISPR-Based Functional Studies
5.3. Deciphering Spatiotemporal Complexity Through Advanced Technologies
6. Climate Change and Agricultural Implications
6.1. Impact of Changing Light and Temperature Regimes
6.2. Breeding Climate-Resilient Crops
| Crop | Target Gene | Engineering Approach | Outcome | References |
|---|---|---|---|---|
| Rice | Ghd7 | CRISPR editing | Photoperiod insensitivity, delayed flowering | [131] |
| Wheat | ELF3 | Allele mining | Improved heat tolerance and crop | [132] |
| Tomato | HY5 | CRISPR knockout | Enhanced fruit yield under low light | [133] |
| Barley | Ppd-H1 | Marker-assisted selection | Early flowering, adaptation to long days | [134] |
| Maize | ZmCCT | CRISPR editing | Delayed flowering, improved biomass | [135] |
| Soybean | E1 | RNA interference (RNAi) | Extended flowering period, higher yield | [136] |
| Potato | StSP6A | CRISPR editing | Tuber formation under long-day conditions | [137] |
| Arabidopsis | TOC1 | CRISPR editing | Altered circadian rhythms for stress adaptation | [42] |
| Sorghum | SbPRR37 | CRISPR editing | Delayed flowering, improved drought tolerance | [138] |
| Sugar beet | BvBTC1 | CRISPR editing | Bolting resistance, improved root yield | [139] |
| Grapevine | VvCO | Overexpression | Improved berry size and ripening timing | [140] |
| Strawberry | FaTFL1 | RNAi | Continuous flowering, higher fruit yield | [141] |
6.3. Policy and Technological Integration
7. Challenges and Future Directions
7.1. Unresolved Questions in Light–Clock Crosstalk
7.2. Interdisciplinary Synergies for Sustainable Agriculture
7.3. Integrating Multi-Omics with Computational Modeling
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Creux, N.; Harmer, S. Circadian rhythms in plants. Cold Spring Harb. Perspect. Biol. 2019, 11, a034611. [Google Scholar] [CrossRef] [PubMed]
- McClung, C.R. Circadian clock components offer targets for crop domestication and improvement. Genes 2021, 12, 374. [Google Scholar] [CrossRef] [PubMed]
- Legris, M.; Nieto, C.; Sellaro, R.; Prat, S.; Casal, J.J. Perception and signalling of light and temperature cues in plants. Plant J. 2017, 90, 683–697. [Google Scholar] [CrossRef]
- Razzaq, K.; Du, J. Phytohormonal regulation of plant development in response to fluctuating light conditions. J. Plant Growth Regul. 2025, 44, 1903–1936. [Google Scholar] [CrossRef]
- Wu, W.; Wu, H.; Liang, R.; Huang, S.; Meng, L.; Zhang, M.; Xie, F.; Zhu, H. Light regulates the synthesis and accumulation of plant secondary metabolites. Front. Plant Sci. 2025, 16, 1644472. [Google Scholar] [CrossRef]
- Leong, R.; He, X.; Beijen, B.S.; Sakai, T.; Goncalves, J.; Ding, P. Unlocking gene regulatory networks for crop resilience and sustainable agriculture. Nat. Biotechnol. 2025, 43, 1254–1265. [Google Scholar] [CrossRef]
- Guo, Y.-W.; Liu, Y.; Huang, P.-C.; Rong, M.; Wei, W.; Xu, Y.-H.; Wei, J.-H. Adaptive Changes and Genetic Mechanisms in Organisms Under Controlled Conditions: A Review. Int. J. Mol. Sci. 2025, 26, 2130. [Google Scholar] [CrossRef]
- Yadav, A.; Singh, D.; Lingwan, M.; Yadukrishnan, P.; Masakapalli, S.K.; Datta, S. Light signaling and UV-B-mediated plant growth regulation. J. Integ. Plant Biol. 2020, 62, 1270–1292. [Google Scholar] [CrossRef]
- Wang, P.; Abid, M.A.; Qanmber, G.; Askari, M.; Zhou, L.; Song, Y.; Liang, C.; Meng, Z.; Malik, W.; Wei, Y.J.E.; et al. Photomorphogenesis in plants: The central role of phytochrome interacting factors (PIFs). Environ. Exp. Bot. 2022, 194, 104704. [Google Scholar] [CrossRef]
- Fraser, O.J. Crosstalk Between the Circadian Clock and the Immune System in Arabidopsis. Ph.D. Thesis, The University of Edinburgh, Edinburgh, UK, 2025. [Google Scholar]
- Amaral, J.; Lobo, A.K.; Carmo-Silva, E. Regulation of Rubisco activity in crops. New Phytol. 2024, 241, 35–51. [Google Scholar] [CrossRef]
- Sarfraz, Z.; Zarlashat, Y.; Ambreen, A.; Mujahid, M.; Iqbal, M.S.; Fatima, S.A.; Iqbal, M.S.; Iqbal, R.; Fiaz, S. Plant Biochemistry in the Era of Omics: Integrated Omics Approaches to Unravel the Genetic Basis of Plant Stress Tolerance. Plant Breed. 2025, 0, 1–23. [Google Scholar] [CrossRef]
- Oakenfull, R.J.; Davis, S.J. Shining a light on the Arabidopsis circadian clock. Plant Cell Environ. 2017, 40, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Y.; Zhang, H.; Xu, J.; Gao, X.; Zhang, T.; Liu, X.; Guo, L.; Zhao, D. Environmental F actors coordinate circadian clock function and rhythm to regulate plant development. Plant Signal. Behav. 2023, 18, 2231202. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yuan, L.; Yang, X.; Zhang, X.; Wang, L.; Xie, Q. Circadian clock in plants: Linking timing to fitness. J. Integr. Plant Biol. 2022, 64, 792–811. [Google Scholar] [CrossRef] [PubMed]
- Pashkovskiy, P.; Khalilova, L.; Vereshchagin, M.; Voronkov, A.; Ivanova, T.; Kosobryukhov, A.A.; Allakhverdiev, S.I.; Kreslavski, V.D.; Kuznetsov, V.V. Impact of varying light spectral compositions on photosynthesis, morphology, chloroplast ultrastructure, and expression of light-responsive genes in Marchantia polymorpha. Plant Physiol. Biochem. 2023, 203, 108044. [Google Scholar] [CrossRef]
- Clayton, W.A.; Albert, N.W.; Thrimawithana, A.H.; McGhie, T.K.; Deroles, S.C.; Schwinn, K.E.; Warren, B.A.; McLachlan, A.R.; Bowman, J.L.; Jordan, B.R. UVR8-mediated induction of flavonoid biosynthesis for UVB tolerance is conserved between the liverwort Marchantia polymorpha and flowering plants. Plant J. 2018, 96, 503–517. [Google Scholar] [CrossRef]
- Patnaik, A.; Alavilli, H.; Rath, J.; Panigrahi, K.C.; Panigrahy, M. Variations in circadian clock organization & function: A journey from ancient to recent. Planta 2022, 256, 91. [Google Scholar] [CrossRef]
- Lee, S.-J.; Kang, K.; Lim, J.-H.; Paek, N.-C. Natural alleles of CIRCADIAN CLOCK ASSOCIATED1 contribute to rice cultivation by fine-tuning flowering time. Plant Physiol. 2022, 190, 640–656. [Google Scholar] [CrossRef]
- Fei, J.; Jiang, Q.; Guo, M.; Lu, J.; Wang, P.; Liu, S.; Qu, J.; Ma, Y.; Guan, S. Fine mapping and functional research of key genes for photoperiod sensitivity in maize. Front. Plant Sci. 2022, 13, 890780. [Google Scholar] [CrossRef]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef]
- Michael, T.P. Core circadian clock and light signaling genes brought into genetic linkage across the green lineage. Plant Physiol. 2022, 190, 1037–1056. [Google Scholar] [CrossRef]
- Sanchez, S.E.; Rugnone, M.L.; Kay, S.A. Light perception: A matter of time. Mol. Plant 2020, 13, 363–385. [Google Scholar] [CrossRef]
- Laosuntisuk, K.; Elorriaga, E.; Doherty, C.J. The game of timing: Circadian rhythms intersect with changing environments. Annu. Rev. Plant Biol. 2023, 74, 511–538. [Google Scholar] [CrossRef] [PubMed]
- Loyola, R.; Herrera, D.; Mas, A.; Wong, D.C.J.; Höll, J.; Cavallini, E.; Amato, A.; Azuma, A.; Ziegler, T.; Aquea, F. The photomorphogenic factors UV-B RECEPTOR 1, ELONGATED HYPOCOTYL 5, and HY5 HOMOLOGUE are part of the UV-B signalling pathway in grapevine and mediate flavonol accumulation in response to the environment. J. Exp. Bot. 2016, 67, 5429–5445. [Google Scholar] [CrossRef] [PubMed]
- Gaillochet, C.; Develtere, W.; Jacobs, T.B. CRISPR screens in plants: Approaches, guidelines, and future prospects. Plant Cell 2021, 33, 794–813. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Ranieri, A.; Castagna, A. Anything new under the sun? An update on modulation of bioactive compounds by different wavelengths in agricultural plants. Plants 2021, 10, 1485. [Google Scholar] [CrossRef]
- Foster, R. Fundamentals of circadian entrainment by light. Light. Res. Technol. 2021, 53, 377–393. [Google Scholar] [CrossRef]
- Wang, J.; Wen, W.; Hanif, M.; Xia, X.; Wang, H.; Liu, S.; Liu, J.; Yang, L.; Cao, S.; He, Z. TaELF3-1DL, a homolog of ELF3, is associated with heading date in bread wheat. Mol. Breed. 2016, 36, 161. [Google Scholar] [CrossRef]
- Wong, K.Y.; Fernandez, F.-X. Circadian responses to light-flash exposure: Conceptualization and new data guiding future directions. Front. Neurol. 2021, 12, 627550. [Google Scholar] [CrossRef]
- Petersen, J.; Rredhi, A.; Szyttenholm, J.; Mittag, M. Evolution of circadian clocks along the green lineage. Plant Physiol. 2022, 190, 924–937. [Google Scholar] [CrossRef]
- Kharshiing, E.V.; Mawphlang, O.I.L.; Lama, V.; Bhattacharjee, R.; Sahoo, L. Manipulation of light environment for optimising photoreceptor activity towards enhancing plant traits of agronomic and horticultural importance in crops. J. Hortic. Sci. Biotechnol. 2022, 97, 535–551. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Liu, Y.; Fan, S.; Ma, Q. Progress of research on the regulatory pathway of the plant shade-avoidance syndrome. Front. Plant Sci. 2020, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Huq, E.; Lin, C.; Quail, P.H. Light signaling in plants—A selective history. Plant Physiol. 2024, 195, 213–231. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; He, L.; Huang, Y.; Zhou, Y. Optophysiology: Illuminating cell physiology with optogenetics. Physiol. Rev. 2022, 102, 1263–1325. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Samtani, H.; Laxmi, A. Molecular dialogue between light and temperature signalling in plants: From perception to thermotolerance. J. Exp. Bot. 2024, 76, 677–694. [Google Scholar] [CrossRef]
- Legris, M.; Boccaccini, A. Stem phototropism toward blue and ultraviolet light. Physiol. Plant. 2020, 169, 357–368. [Google Scholar] [CrossRef]
- Laad, P.; Patel, P.; Guruprasad, K. UVR8 Signaling, Mechanism, and Integration with Other Pathways. In UV-B Radiation and Crop Growth; Springer: Berlin/Heidelberg, Germany, 2023; pp. 193–221. [Google Scholar]
- Mulekar, J.J.; Huq, E. Expanding roles of protein kinase CK2 in regulating plant growth and development. J. Exp. Bot. 2014, 65, 2883–2893. [Google Scholar] [CrossRef]
- Zhou, H.; Zhu, W.; Wang, X.; Bian, Y.; Jiang, Y.; Li, J.; Wang, L.; Yin, P.; Deng, X.W.; Xu, D. A missense mutation in WRKY32 converts its function from a positive regulator to a repressor of photomorphogenesis. New Phytol. 2022, 235, 111–125. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, T.; Hiltbrunner, A. Phytochrome higher order mutants reveal a complex set of light responses in the moss Physcomitrium patens. New Phytol. 2023, 239, 1035–1050. [Google Scholar] [CrossRef]
- Dwivedi, S.L.; Quiroz, L.F.; Spillane, C.; Wu, R.; Mattoo, A.K.; Ortiz, R. Unlocking allelic variation in circadian clock genes to develop environmentally robust and productive crops. Planta 2024, 259, 72. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.; Mishra, S.; Khurana, P.; Khurana, J.P. CRY2 gene of rice (Oryza sativa subsp. indica) encodes a blue light sensory receptor involved in regulating flowering, plant height and partial photomorphogenesis in dark. Plant Cell Rep. 2023, 42, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Portolés, S.; Ren, Y.; Sun, G.; Wang, X.-F.; Zhang, H.; Guo, S. The key clock component ZEITLUPE (ZTL) negatively regulates ABA signaling by degradation of CHLH in Arabidopsis. Front. Plant Sci. 2022, 13, 995907. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Irfan, M.; Kumar, A.; Kumar, P.; Datta, A. Recent insights into plant circadian clock response against abiotic stress. J. Plant Growth Regul. 2022, 41, 3530–3543. [Google Scholar] [CrossRef]
- Laosuntisuk, K.; Desai, J.S.; Doherty, C.J. An Arabidopsis Cell Culture With Weak Circadian Rhythms Under Constant Light Compared With Constant Dark Can Be Rescued by ELF3. Plant Direct 2024, 8, e70028. [Google Scholar] [CrossRef]
- Xu, Y.; Zhu, Z. PIF4 and PIF4-interacting proteins: At the nexus of plant light, temperature and hormone signal integrations. Int. J. Mol. Sci. 2021, 22, 10304. [Google Scholar] [CrossRef]
- Sharma, A.; Samtani, H.; Sahu, K.; Sharma, A.K.; Khurana, J.P.; Khurana, P. Functions of Phytochrome-Interacting Factors (PIFs) in the regulation of plant growth and development: A comprehensive review. Int. J. Biol. Macromol. 2023, 244, 125234. [Google Scholar] [CrossRef]
- Sorkin, M.L.; Nusinow, D.A. Time will tell: Intercellular communication in the plant clock. Trends Plant Sci. 2021, 26, 706–719. [Google Scholar] [CrossRef]
- de Assis, L.V.M.; Oster, H. The circadian clock and metabolic homeostasis: Entangled networks. Cel. Mol. Life Sci. 2021, 78, 4563–4587. [Google Scholar] [CrossRef]
- Niwa, Y.; Ito, S.; Nakamichi, N.; Mizoguchi, T.; Niinuma, K.; Yamashino, T.; Mizuno, T. Genetic linkages of the circadian clock-associated genes, TOC1, CCA1 and LHY, in the photoperiodic control of flowering time in Arabidopsis thaliana. Plant Cell Physiol. 2007, 48, 925–937. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, J.; Lin, C.; Zhang, J.; Yan, H.; Guan, Z.; Zhang, C. Single-cell transcriptomics applied in plants. Cells 2024, 13, 1561. [Google Scholar] [CrossRef]
- Gray, J.A.; Shalit-Kaneh, A.; Chu, D.N.; Hsu, P.Y.; Harmer, S.L. The REVEILLE clock genes inhibit growth of juvenile and adult plants by control of cell size. Plant Physiol. 2017, 173, 2308–2322. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yuan, L.; Xie, Q. The circadian clock ticks in plant stress responses. Stress Biol. 2022, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.; Fasano, C.; Perrella, G.; Facella, P. Cryptochromes and the circadian clock: The story of a very complex relationship in a spinning world. Genes 2021, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Wang, Y.; Yu, Y.; He, Y.; Wang, L. Coordinative regulation of plants growth and development by light and circadian clock. Abiotech 2021, 2, 176–189. [Google Scholar] [CrossRef]
- Gil Rodríguez, S. Functional Characterization of the Connection Between the Circadian Clock and the DNA Damage and Repair Response in Arabidopsis Thaliana. Ph.D. Thesis, Universitat Autònoma de Barcelona, Bellaterra, Spain, 2019. [Google Scholar]
- Tolsma, J.S.; Torres, J.J.; Richards, J.T.; Perera, I.Y.; Doherty, C.J. Evaluating the effects of the circadian clock and time of day on plant gravitropic responses. In Plant Gravitropism: Methods and Protocols; Springer: New York, NY, USA, 2022; pp. 301–319. [Google Scholar]
- Kim, H.; Kim, J.; Choi, G. Epidermal phyB requires RRC1 to promote light responses by activating the circadian rhythm. New Phytol. 2023, 238, 705–723. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H. Revisiting the role and mechanism of ELF3 in circadian clock modulation. Gene 2024, 913, 148378. [Google Scholar] [CrossRef]
- Nieto, C.; Catalán, P.; Luengo, L.M.; Legris, M.; López-Salmerón, V.; Davière, J.M.; Casal, J.J.; Ares, S.; Prat, S. COP1 dynamics integrate conflicting seasonal light and thermal cues in the control of Arabidopsis elongation. Sci. Adv. 2022, 8, eabp8412. [Google Scholar] [CrossRef]
- Ramkisoensing, A.; Meijer, J.H. Synchronization of biological clock neurons by light and peripheral feedback systems promotes circadian rhythms and health. Front. Neurol. 2015, 6, 128. [Google Scholar] [CrossRef]
- Facella, P.; Lopez, L.; Carbone, F.; Galbraith, D.W.; Giuliano, G.; Perrotta, G. Diurnal and circadian rhythms in the tomato transcriptome and their modulation by cryptochrome photoreceptors. PLoS ONE 2008, 3, e2798. [Google Scholar] [CrossRef]
- Nozue, K.; Harmer, S.L.; Maloof, J.N. Genomic analysis of circadian clock-, light-, and growth-correlated genes reveals PHYTOCHROME-INTERACTING FACTOR5 as a modulator of auxin signaling in Arabidopsis. Plant Physiol. 2011, 156, 357–372. [Google Scholar] [CrossRef]
- Haydon, M.J.; Li, X.; Ting, M.K. Temporal control of plant-environment interactions by the circadian clock. Annu. Plant Rev. 2019, 2, 1–32. [Google Scholar]
- Jing, Y.; Lin, R. Transcriptional regulatory network of the light signaling pathways. New Phytol. 2020, 227, 683–697. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Araki, T.; Nagatani, A. Tissue-specific regulation of flowering by photoreceptors. Cell. Mol. Life Sci. 2016, 73, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.Y.; Harmer, S.L. Wheels within wheels: The plant circadian system. Trends Plant Sci. 2014, 19, 240–249. [Google Scholar] [CrossRef]
- Nagel, D.H.; Kay, S.A. Complexity in the wiring and regulation of plant circadian networks. Curr. Biol. 2012, 22, R648–R657. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dwivedi, S.; Bhagavatula, L.; Datta, S. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep. 2023, 42, 829–841. [Google Scholar] [CrossRef]
- Singh, R.K.; Bhalerao, R.P.; Eriksson, M.E. Growing in time: Exploring the molecular mechanisms of tree growth. Tree Physiol. 2021, 41, 657–678. [Google Scholar] [CrossRef]
- Grundy, J.; Stoker, C.; Carré, I.A. Circadian regulation of abiotic stress tolerance in plants. Front. Plant Sci. 2015, 6, 648. [Google Scholar] [CrossRef]
- Liu, Y.; Jafari, F.; Wang, H. Integration of light and hormone signaling pathways in the regulation of plant shade avoidance syndrome. Abiotech 2021, 2, 131–145. [Google Scholar] [CrossRef]
- Bao, Y.; Liu, X.; Feng, C.-H.; Niu, M.-X.; Liu, C.; Wang, H.-L.; Yin, W.; Xia, X. Light and light signals regulate growth and development in woody plants. Forests 2024, 15, 523. [Google Scholar] [CrossRef]
- Roeder, A.H.; Shi, Y.; Yang, S.; Abbas, M.; Sasidharan, R.; Yanovsky, M.J.; Casal, J.J.; Ruffel, S.; Von Wirén, N.; Assmann, S.M. Translational insights into abiotic interactions: From Arabidopsis to crop plants. Plant Cell 2025, 37, koaf140. [Google Scholar] [CrossRef]
- Junior, C.A.S.; D’Amico-Damião, V.; Carvalho, R.F. Phytochrome type B family: The abiotic stress responses signaller in plants. Ann. Appl. Biol. 2021, 178, 135–148. [Google Scholar] [CrossRef]
- Liang, T.; Yu, S.; Pan, Y.; Wang, J.; Kay, S.A. The interplay between the circadian clock and abiotic stress responses mediated by ABF3 and CCA1/LHY. Proc. Natl. Acad. Sci. USA 2024, 121, e2316825121. [Google Scholar] [PubMed]
- Yang, T.; He, Y.; Niu, S.; Yan, S.; Zhang, Y. Identification and characterization of the CONSTANS (CO)/CONSTANS-like (COL) genes related to photoperiodic signaling and flowering in tomato. Plant Sci. 2020, 301, 110653. [Google Scholar] [CrossRef] [PubMed]
- Martignago, D.; Siemiatkowska, B.; Lombardi, A.; Conti, L. Abscisic acid and flowering regulation: Many targets, different places. Int. J. Mol. Sci. 2020, 21, 9700. [Google Scholar] [CrossRef]
- Liu, J.; Yi, Q.; Dong, G.; Chen, Y.; Guo, L.; Gao, Z.; Zhu, L.; Ren, D.; Zhang, Q.; Li, Q.; et al. Improving Rice Quality by Regulating the Heading Dates of Rice Varieties without Yield Penalties. Plants 2024, 13, 2221. [Google Scholar] [CrossRef]
- Cao, S.; Luo, X.; Xu, D.; Tian, X.; Song, J.; Xia, X.; Chu, C.; He, Z. Genetic architecture underlying light and temperature mediated flowering in Arabidopsis, rice, and temperate cereals. New Phytol. 2021, 230, 1731–1745. [Google Scholar] [CrossRef]
- Capovilla, G.; Schmid, M.; Posé, D. Control of flowering by ambient temperature. J. Exp. Bot. 2015, 66, 59–69. [Google Scholar] [CrossRef]
- Yari Kamrani, Y.; Shomali, A.; Aliniaeifard, S.; Lastochkina, O.; Moosavi-Nezhad, M.; Hajinajaf, N.; Talar, U. Regulatory role of circadian clocks on ABA production and signaling, stomatal responses, and water-use efficiency under water-deficit conditions. Cells 2022, 11, 1154. [Google Scholar] [CrossRef]
- Cha, J.-Y.; Kim, J.; Jeong, S.Y.; Shin, G.-I.; Ji, M.G.; Hwang, J.-W.; Khaleda, L.; Liao, X.; Ahn, G.; Park, H.-J. The Na+/H+ antiporter SALT OVERLY SENSITIVE 1 regulates salt compensation of circadian rhythms by stabilizing GIGANTEA in Arabidopsis. Proc. Nat. Acad. Sci. 2022, 119, e2207275119. [Google Scholar] [CrossRef]
- Nitschke, S.; Cortleven, A.; Iven, T.; Feussner, I.; Havaux, M.; Riefler, M.; Schmülling, T. Circadian stress regimes affect the circadian clock and cause jasmonic acid-dependent cell death in cytokinin-deficient Arabidopsis plants. Plant Cell 2016, 28, 1616–1639. [Google Scholar] [CrossRef]
- Roeber, V.M.; Schmülling, T.; Cortleven, A. The photoperiod: Handling and causing stress in plants. Front. Plant Sci. 2022, 12, 781988. [Google Scholar] [CrossRef]
- Smith, A.M.; Zeeman, S.C. Starch: A flexible, adaptable carbon store coupled to plant growth. Annu. Rev. Plant Biol. 2020, 71, 217–245. [Google Scholar] [CrossRef]
- Hou, X.; Alagoz, Y.; Welsch, R.; Mortimer, M.D.; Pogson, B.J.; Cazzonelli, C. Reducing PHYTOENE SYNTHASE activity fine-tunes the abundance of a cis-carotene-derived signal that regulates the PIF3/HY5 module and plastid biogenesis. J. Exp. Bot. 2024, 75, 1187–1204. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.B.; Bianchetti, R.E.; Alves, F.R.R.; Purgatto, E.; Peres, L.E.P.; Rossi, M.; Freschi, L. Light, ethylene and auxin signaling interaction regulates carotenoid biosynthesis during tomato fruit ripening. Front. Plant Sci. 2018, 9, 1370. [Google Scholar] [CrossRef] [PubMed]
- Divekar, P.A.; Narayana, S.; Divekar, B.A.; Kumar, R.; Gadratagi, B.G.; Ray, A.; Singh, A.K.; Rani, V.; Singh, V.; Singh, A.K. Plant secondary metabolites as defense tools against herbivores for sustainable crop protection. Int. J. Mol. Sci. 2022, 23, 2690. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.N.; Vetoshkina, D.V.; Marenkova, T.V.; Borisova-Mubarakshina, M.M. Antioxidants of non-enzymatic nature: Their function in higher plant cells and the ways of boosting their biosynthesis. Antioxidants 2023, 12, 2014. [Google Scholar] [CrossRef]
- Abuelsoud, W.; Cortleven, A.; Schmülling, T. Photoperiod stress induces an oxidative burst-like response and is associated with increased apoplastic peroxidase and decreased catalase activities. J. Plant Physiol. 2020, 253, 153252. [Google Scholar] [CrossRef]
- Cortleven, A.; Roeber, V.M.; Frank, M.; Bertels, J.; Lortzing, V.; Beemster, G.T.; Schmülling, T. Photoperiod stress in Arabidopsis thaliana induces a transcriptional response resembling that of pathogen infection. Front. Plant Sci. 2022, 13, 838284. [Google Scholar] [CrossRef]
- Warner, R.; Wu, B.-S.; MacPherson, S.; Lefsrud, M. How the distribution of photon delivery impacts crops in indoor plant environments: A review. Sustainability 2023, 15, 4645. [Google Scholar] [CrossRef]
- Liu, X.; Sun, Q.; Wang, Z.; He, J.; Liu, X.; Xu, Y.; Li, Q. Innovative Application Strategies of Light-Emitting Diodes in Protected Horticulture. Agriculture 2025, 15, 1630. [Google Scholar] [CrossRef]
- Badia-i-Mompel, P.; Wessels, L.; Müller-Dott, S.; Trimbour, R.; Ramirez Flores, R.O.; Argelaguet, R.; Saez-Rodriguez, J. Gene regulatory network inference in the era of single-cell multi-omics. Nat. Rev. Genet. 2023, 24, 739–754. [Google Scholar] [CrossRef]
- Andres, J.; Blomeier, T.; Zurbriggen, M.D. Synthetic switches and regulatory circuits in plants. Plant Physiol. 2019, 179, 862–884. [Google Scholar] [CrossRef] [PubMed]
- Gulyás, Z.; Székely, A.; Kulman, K.; Kocsy, G. Light-dependent regulatory interactions between the redox system and miRNAs and their biochemical and physiological effects in plants. Int. J. Mol. Sci. 2023, 24, 8323. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Narsai, R.; He, C.; Wang, Y.; Berkowitz, O.; Whelan, J.; Liew, L.C. Coordinated regulation of the mitochondrial retrograde response by circadian clock regulators and ANAC017. Plant Commun. 2023, 4, 100501. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Lan, Y.; Gong, F.; Li, C.; Xia, F.; Li, Y.; Fang, C. Comparative physiology and transcriptome response patterns in cold-tolerant and cold-sensitive varieties of Solanum melongena. BMC Plant Biol. 2024, 24, 256. [Google Scholar] [CrossRef]
- Wang, K.; Abid, M.A.; Rasheed, A.; Crossa, J.; Hearne, S.; Li, H. DNNGP, a deep neural network-based method for genomic prediction using multi-omics data in plants. Mol. Plant 2023, 16, 279–293. [Google Scholar] [CrossRef]
- Zhu, T.; Li, T.; Lü, P.; Li, C. Single-cell omics in plant biology: Mechanistic insights and applications for crop improvement. Adv. Biotechnol. 2025, 3, 20. [Google Scholar] [CrossRef]
- Ko, D.K.; Rohozinski, D.; Song, Q.; Taylor, S.H.; Juenger, T.E.; Harmon, F.G.; Chen, Z.J. Temporal shift of circadian-mediated gene expression and carbon fixation contributes to biomass heterosis in maize hybrids. PLoS Genet. 2016, 12, e1006197. [Google Scholar] [CrossRef]
- Coll, N.S.; Moreno-Risueno, M.; Strader, L.C.; Goodnight, A.V.; Sozzani, R. Advancing our understanding of root development: Technologies and insights from diverse studies. Plant Physiol. 2025, 197, kiae605. [Google Scholar] [CrossRef]
- Sessa, G.; Carabelli, M.; Possenti, M.; Morelli, G.; Ruberti, I. Multiple pathways in the control of the shade avoidance response. Plants 2018, 7, 102. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Jha, U.C.; Siddique, K.H.; Prasad, P.V.; Kumar, S.; Nayyar, H. Genomics for physiological traits in lentil under stressed environments. In The Lentil Genome; Elsevier: Amsterdam, The Netherlands, 2024; pp. 267–306. [Google Scholar]
- Silva, T.N.; Vermerris, W. High-biomass sorghums as a feedstock for renewable fuels and chemicals. In Sorghum in the 21st Century: Food–Fodder–Feed–Fuel for a Rapidly Changing World; Springer: Singapore, 2021; pp. 723–754. [Google Scholar]
- Thapliyal, G.; Bhandari, M.S.; Vemanna, R.S.; Pandey, S.; Meena, R.K.; Barthwal, S. Engineering traits through CRISPR/cas genome editing in woody species to improve forest diversity and yield. Crit. Rev. Biotechnol. 2023, 43, 884–903. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Pandey, D.M.; Dwivedi, A. A review on modeling approaches for the transcriptional regulatory network intricacies of circadian clock genes in plants. Planta 2025, 262, 17. [Google Scholar] [CrossRef] [PubMed]
- Torii, K.; Inoue, K.; Bekki, K.; Haraguchi, K.; Kubo, M.; Kondo, Y.; Suzuki, T.; Kubota, A.; Uemoto, K.; Shimizu, H. A guiding role of the Arabidopsis circadian clock in cell differentiation revealed by time-series single-cell RNA sequencing. Cell Rep. 2022, 40, 111059. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Hameed, A.; Rehman, A.; Sarfraz, S.; Rajput, N.A.; Atiq, M. CRISPR System Discovery, History, and Future Perspective. In OMICs-Based Techniques for Global Food Security; Wiley: Hoboken, NJ, USA, 2024; pp. 159–170. [Google Scholar]
- Chen, F.; Li, B.; Li, G.; Charron, J.-B.; Dai, M.; Shi, X.; Deng, X.W. Arabidopsis phytochrome A directly targets numerous promoters for individualized modulation of genes in a wide range of pathways. Plant Cell 2014, 26, 1949–1966. [Google Scholar] [CrossRef]
- Alharbi, S.; Al-Dakhil, M.; Alotaibi, F.S. Precise Genome Editing of Plants Through Base and Prime Editor. In OMICs-Based Techniques for Global Food Security; Wiley: Hoboken, NJ, USA, 2024; pp. 269–286. [Google Scholar]
- Buch, K.; Kaushik, A.; Mishra, U.; Beese, S.; Samanta, S.; Singh, R. Unravelling the complexity of plant breeding through modern genetic techniques and tools: A review. Int. J. Plant Soil Sci. 2023, 35, 97–105. [Google Scholar] [CrossRef]
- Baranov, D.; Timerbaev, V. Recent advances in studying the regulation of fruit ripening in tomato using genetic engineering approaches. Int. J. Mol. Sci. 2024, 25, 760. [Google Scholar] [CrossRef]
- Yetgin, A. Revolutionizing multi-omics analysis with artificial intelligence and data processing. Quant. Biol. 2025, 13, e70002. [Google Scholar] [CrossRef]
- Naik, Y.D.; Bahuguna, R.N.; Garcia-Caparros, P.; Zwart, R.S.; Reddy, M.S.; Mir, R.R.; Jha, U.C.; Fakrudin, B.; Pandey, M.K.; Challabathula, D. Exploring the multifaceted dynamics of flowering time regulation in field crops: Insight and intervention approaches. Plant Genome 2025, 18, e70017. [Google Scholar] [CrossRef]
- Sedivy, E. Molecular Characterization of Signaling Mechanisms in Flowering Transition in Arabidopsis and Identification of Novel Flowering QTL in Soybean; University of Illinois at Urbana-Champaign: Champaign, IL, USA, 2013. [Google Scholar]
- de Los Reyes, P.; Serrano-Bueno, G.; Romero-Campero, F.J.; Gao, H.; Romero, J.M.; Valverde, F. CONSTANS alters the circadian clock in Arabidopsis thaliana. Mol. Plant 2024, 17, 1204–1220. [Google Scholar] [CrossRef]
- Osnato, M.; Cota, I.; Nebhnani, P.; Cereijo, U.; Pelaz, S. Photoperiod control of plant growth: Flowering time genes beyond flowering. Front. Plant Sci. 2022, 12, 805635. [Google Scholar] [CrossRef]
- Tripathi, A.; Tripathi, D.K.; Chauhan, D.; Kumar, N.; Singh, G. Paradigms of climate change impacts on some major food sources of the world: A review on current knowledge and future prospects. Agric. Ecosyst. Environ. 2016, 216, 356–373. [Google Scholar] [CrossRef]
- Schulte-Römer, N.; Meier, J.; Söding, M.; Dannemann, E. The LED paradox: How light pollution challenges experts to reconsider sustainable lighting. Sustainability 2019, 11, 6160. [Google Scholar] [CrossRef]
- Singh, V.P.; Singh, S.; Prasad, S.M.; Parihar, P. UV-B Radiation: From Environmental Stressor to Regulator of Plant Growth; Wiley: Hoboken, NJ, USA, 2017. [Google Scholar]
- Susila, H.; Nasim, Z.; Ahn, J.H. Ambient temperature-responsive mechanisms coordinate regulation of flowering time. Int. J. Mol. Sci. 2018, 19, 3196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.; Jiang, Y.; Zhang, H.; Wang, S.; Wang, F.; Zhu, Y. Genetic control and high temperature effects on starch biosynthesis and grain quality in rice. Front. Plant Sci. 2021, 12, 757997. [Google Scholar] [CrossRef]
- Jiang, C.; Kan, J.; Gao, G.; Dockter, C.; Li, C.; Wu, W.; Yang, P.; Stein, N. Barley2035: A decadal vision for barley research and breeding. Mol. Plant 2025, 18, 195–218. [Google Scholar] [CrossRef]
- Maple, R.; Zhu, P.; Hepworth, J.; Wang, J.-W.; Dean, C. Flowering time: From physiology, through genetics to mechanism. Plant Physiol. 2024, 195, 190–212. [Google Scholar] [CrossRef]
- Kishchenko, O.; Zhou, Y.; Jatayev, S.; Shavrukov, Y.; Borisjuk, N. Gene editing applications to modulate crop flowering time and seed dormancy. Abiotech 2020, 1, 233–245. [Google Scholar] [CrossRef]
- Shoaib, N.; Pan, K.; Mughal, N.; Raza, A.; Liu, L.; Zhang, J.; Wu, X.; Sun, X.; Zhang, L.; Pan, Z. Potential of UV-B radiation in drought stress resilience: A multidimensional approach to plant adaptation and future implications. Plant Cell Environ. 2024, 47, 387–407. [Google Scholar] [CrossRef]
- Ebrahimi Naghani, S.; Šmeringai, J.; Pleskačová, B.; Dobisová, T.; Panzarová, K.; Pernisová, M.; Robert, H.S. Integrative phenotyping analyses reveal the relevance of the phyB-PIF4 pathway in Arabidopsis thaliana reproductive organs at high ambient temperature. BMC Plant Biol. 2024, 24, 721. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, M.; Xu, Z.; Xu, Q. Assessment of the effect of ten heading time genes on reproductive transition and yield components in rice using a CRISPR/Cas9 system. Theor. Appl. Genet. 2019, 132, 1887–1896. [Google Scholar] [CrossRef]
- Wittern, L.; Steed, G.; Taylor, L.J.; Ramirez, D.C.; Pingarron-Cardenas, G.; Gardner, K.; Greenland, A.; Hannah, M.A.; Webb, A.A. Wheat EARLY FLOWERING 3 affects heading date without disrupting circadian oscillations. Plant Physiol. 2023, 191, 1383–1403. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Li, J.; Dong, H.; Hu, Z.; Xia, X.; Yu, J.; Zhou, Y. Manipulating the Light Systemic Signal HY5 Greatly Improve Fruit Quality in Tomato. Adv. Sci. 2025, 12, e2500110. [Google Scholar] [CrossRef]
- Rossi, N.; Powell, W.; Mackay, I.J.; Hickey, L.; Maurer, A.; Pillen, K.; Halliday, K.; Sharma, R. Investigating the genetic control of plant development in spring barley under speed breeding conditions. The. Appl. Genet. 2024, 137, 115. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhao, X.; Guo, S.; Dong, S.; Wen, Y.; Han, Z.; Jin, W.; Chen, Y. ZmCCA1a on chromosome 10 of maize delays flowering of Arabidopsis thaliana. Front. Plant Sci. 2020, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Zhai, H.; Wu, H.; Xu, K.; Watanabe, S.; Harada, K. The synchronized efforts to decipher the molecular basis for soybean maturity loci E1, E2, and E3 that regulate flowering and maturity. Front. Plant Sci. 2021, 12, 632754. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Arif, Y.; Siddiqui, H.; Upadhyaya, C.P.; Pichtel, J.; Hayat, S. Critical factors responsible for potato tuberization. Bot. Rev. 2023, 89, 421–437. [Google Scholar] [CrossRef]
- Liu, F.; Wodajo, B.; Xie, P. Decoding the genetic blueprint: Regulation of key agricultural traits in sorghum. Adv. Biotechnol. 2024, 2, 31. [Google Scholar] [CrossRef]
- De Lucchi, C.; Biancardi, E.; Skaracis, G.; De Biaggi, M.; Pavli, O.; Ravi, S.; Chiodi, C.; Broccanello, C.; Stevanato, P. Sugar beet (Beta vulgaris Ssp. Vulgaris L.) improvement with next-generation breeding technology. In Advances in Plant Breeding Strategies: Vegetable Crops: Volume 8: Bulbs, Roots Tubers; Springer: Cham, Switzerland, 2021; pp. 305–343. [Google Scholar]
- Fechter, I.; Hausmann, L.; Zyprian, E.; Daum, M.; Holtgräwe, D.; Weisshaar, B.; Töpfer, R. QTL analysis of flowering time and ripening traits suggests an impact of a genomic region on linkage group 1 in Vitis. Theor. Appl. Genet. 2014, 127, 1857–1872. [Google Scholar] [CrossRef]
- Darby, E.; Islam, T. Environmental and molecular regulation of flowering in cultivated strawberry (Fragaria x ananassa). Hortic. Res. 2025, 12, uhae309. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, A.; Patel, R.; Kumar, V. Genetically modified crop regulations: Scope and opportunity using the CRISPR-Cas9 genome editing approach. Mol. Biol. Rep. 2021, 48, 4851–4863. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.S.; Riaz, S.; Helou, M.A.; Khan, F.S.; Abid, A.; Alvi, A. Internet of things in greenhouse agriculture: A survey on enabling technologies, applications, and protocols. IEEE Access 2022, 10, 53374–53397. [Google Scholar] [CrossRef]
- Slovak, R.; Ogura, T.; Satbhai, S.B.; Ristova, D.; Busch, W. Genetic control of root growth: From genes to networks. Ann. Bot. 2016, 117, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Waheed, S.; Zeng, L. The critical role of miRNAs in regulation of flowering time and flower development. Genes 2020, 11, 319. [Google Scholar] [CrossRef]
- Xia, S.; Chen, J.; Arsala, D.; Emerson, J.; Long, M. Functional innovation through new genes as a general evolutionary process. Nat. Genet. 2025, 57, 295–309. [Google Scholar] [CrossRef]
- Cornelio, C.; Dash, S.; Austel, V.; Josephson, T.; Goncalves, J.; Clarkson, K.; Megiddo, N.; Khadir, B.E.; Horesh, L. AI Descartes: Combining data and theory for derivable scientific discovery. arXiv 2021, arXiv:2109.01634. [Google Scholar] [CrossRef]
- Nohales, M.A.; Liu, W.; Duffy, T.; Nozue, K.; Sawa, M.; Pruneda-Paz, J.L.; Maloof, J.N.; Jacobsen, S.E.; Kay, S.A. Multi-level modulation of light signaling by GIGANTEA regulates both the output and pace of the circadian clock. Dev. Cell 2019, 49, 840–851.e848. [Google Scholar] [CrossRef]
- Saddem-Yagoubi, R.; Naud, O.; Godary-Dejean, K.; Crestani, D. Model-checking precision agriculture logistics: The case of the differential harvest. Discret. Event Dyn. Syst. 2020, 30, 579–604. [Google Scholar] [CrossRef]
- Patel, V.R.; Eckel-Mahan, K.; Sassone-Corsi, P.; Baldi, P. CircadiOmics: Integrating circadian genomics, transcriptomics, proteomics and metabolomics. Nat. Methods 2012, 9, 772–773. [Google Scholar] [CrossRef]
- Chavhan, R.L.; Hinge, V.R.; Wankhade, D.J.; Deshmukh, A.S.; Mahajan, N.; Kadam, U.S. Bioinformatics for molecular breeding and enhanced crop performance: Applications and perspectives. In Bioinformatics for Plant Research and Crop Breeding; Wiley: Hoboken, NJ, USA, 2024; pp. 21–74. [Google Scholar]
- Favero, D.S. Mechanisms regulating PIF transcription factor activity at the protein level. Physiol. Plant. 2020, 169, 325–335. [Google Scholar] [CrossRef]
- Matus, J.T. Transcriptomic and metabolomic networks in the grape berry illustrate that it takes more than flavonoids to fight against ultraviolet radiation. Front. Plant Sci. 2016, 7, 1337. [Google Scholar] [CrossRef]
- Kapazoglou, A.; Tani, E.; Papasotiropoulos, V.; Letsiou, S.; Gerakari, M.; Abraham, E.; Bebeli, P.J. Molecular Responses to Abiotic Stress in Key Woody Perennial Fruit Crops: Genetic, Epigenetic and Microbiota Insights for Crop Resilience and Sustainability in Times of Climate Change. Preprints 2025, 2025020876. [Google Scholar]
- Serrano-Ron, L.; Cabrera, J.; Perez-Garcia, P.; Moreno-Risueno, M.A. Unraveling root development through single-cell omics and reconstruction of gene regulatory networks. Front. Plant Sci. 2021, 12, 661361. [Google Scholar] [CrossRef]


| Component | Function | Mutant Phenotype | Evolutionary Conservation | Ref. |
|---|---|---|---|---|
| PHYB | Red light sensing | Elongated hypocotyl | PHY homologs in moss | [41] |
| CCA1 | Morning oscillator | Arrhythmic growth | CCA1 homologs in rice | [42] |
| HY5 | Light-responsive transcription factor | Reduced photomorphogenesis | HY5 homologs in algae and higher plants | [25] |
| LHY | Morning oscillator | Arrhythmic growth | LHY homologs in angiosperms | [42] |
| TOC1 | Evening oscillator | Altered circadian rhythms | TOC1 homologs in Arabidopsis and rice | [2] |
| CRY1 | Blue light sensing | Hypersensitive to blue light | CRY homologs in algae and higher plants | [43] |
| CRY2 | Blue light sensing | Delayed flowering | CRY homologs in algae and higher plants | [43] |
| ZTL | Circadian clock regulator | Altered circadian period | ZTL homologs in angiosperms | [44] |
| PRR7 | Circadian clock regulator | Altered circadian rhythms | PRR7 homologs in Arabidopsis and rice | [45] |
| PRR9 | Circadian clock regulator | Altered circadian rhythms | PRR9 homologs in Arabidopsis and rice | [45] |
| ELF3 | Light input to clock | Early flowering, arrhythmic growth | ELF3 homologs in monocots and dicots | [46] |
| ELF4 | Light input to clock | Altered circadian rhythms | ELF4 homologs in monocots and dicots | [31] |
| PIF4 | Light signaling and growth | Reduced hypocotyl elongation | PIF4 homologs in angiosperms | [47] |
| PIF5 | Light signaling and growth | Reduced hypocotyl elongation | PIF5 homologs in angiosperms | [48] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mujahid, M.; Ambreen, A.; Zarlashat, Y.; Sarfraz, Z.; Iqbal, M.S.; Waheed, A.; Iqbal, M.S. Integration of Light and Circadian Signaling in Plant Gene Regulatory Networks: Implications for Photomorphogenesis and Stress Adaptation. Biology 2025, 14, 1375. https://doi.org/10.3390/biology14101375
Mujahid M, Ambreen A, Zarlashat Y, Sarfraz Z, Iqbal MS, Waheed A, Iqbal MS. Integration of Light and Circadian Signaling in Plant Gene Regulatory Networks: Implications for Photomorphogenesis and Stress Adaptation. Biology. 2025; 14(10):1375. https://doi.org/10.3390/biology14101375
Chicago/Turabian StyleMujahid, Muhammad, Alia Ambreen, Yusra Zarlashat, Zareen Sarfraz, Muhammad Sajid Iqbal, Abdul Waheed, and Muhammad Shahid Iqbal. 2025. "Integration of Light and Circadian Signaling in Plant Gene Regulatory Networks: Implications for Photomorphogenesis and Stress Adaptation" Biology 14, no. 10: 1375. https://doi.org/10.3390/biology14101375
APA StyleMujahid, M., Ambreen, A., Zarlashat, Y., Sarfraz, Z., Iqbal, M. S., Waheed, A., & Iqbal, M. S. (2025). Integration of Light and Circadian Signaling in Plant Gene Regulatory Networks: Implications for Photomorphogenesis and Stress Adaptation. Biology, 14(10), 1375. https://doi.org/10.3390/biology14101375






